Abstract
Background
Screening could improve recognition of dementia in primary care. We sought to determine the effect of screening for dementia in primary care practices on the formal diagnosis rate; the distribution of differential diagnoses; and the factors associated with receiving a formal diagnosis.
Methods
The “Dementia: life- and person-centered help in Mecklenburg-Western Pomerania” is an ongoing general practitioner (GP)-based, randomized, controlled intervention trial. A total of 4064 community dwelling patients (aged ≥70 years) were screened for dementia in 108 GP practices. Of these patients, 692 (17%) had positive screening results (DemTect score <9). Of these 692 patients, 406 (59%) provided informed consent. The analyses included the data from 243 patients with a complete baseline assessment (preliminary data; January 2014).
Results
Of 146 patients without a formal diagnosis of dementia, 72 (49%) received a formal diagnosis after a positive screening outcome (69% with “unspecified dementia”). Female sex was significantly associated with receiving a formal diagnosis (multivariate analyses).
Conclusion
Screening improved the identification of dementia considerably. Because of the risk of receiving a false-positive diagnosis, additional diagnostic assessment should be mandatory.
Keywords: Dementia, Screening, Recognition, Early diagnosis, Differential diagnosis, Primary care, Diagnosis rate, DelpHi trial
1. Introduction
A timely diagnosis of dementia is considered of major importance to ensure adequate access to information, evidence-based treatment, care, and support for people with dementia [1]. However, even in high-income countries with advanced medical care systems, 50% to 80% of all prevalent dementia cases are not formally diagnosed [1], [2], [3]. The screening of older patients for cognitive impairment could be a method to improve the recognition of dementia. Previous studies have shown that screening for dementia in general practitioners' (GPs) practices did increase the diagnosis rates [4], [5]. Nevertheless, routine screening for dementia has been controversial and is not presently recommended in the dementia guidelines owing to the lack of evidence that patients benefit from it [6], [7], [8]. In addition, patients with positive screening results often undergo no additional diagnostic assessment and do not receive specific treatment [4], [5]. For the benefit of patients, a positive screening outcome should initiate an adequate diagnostic assessment and, depending on the results, state of the art treatment and care. Furthermore, screening could result in potential harms [9], [10], [11], [12]. Older patients might avoid visiting their GP because they fear to be diagnosed with dementia. Also, false-positive screening results increase the probability for misdiagnosis, could result in unnecessary examinations and treatments, and might cause anxiety and depression in the affected subjects. Boustani et al [13] showed that the probability of false-positive screening results is relatively high: about 20% of patients who screened positive for dementia did not have dementia at all and another 30% had mild cognitive impairment. Sandholzer et al [5] examined the acceptance of screening among German primary care patients and found that 89% of the screened patients considered the screening to be useful. Only 1.5% of the patients reported that they worried more about their health after the screening. Other surveys revealed that a clear majority of participants would prefer to be diagnosed early and expected to be properly informed about their diagnosis [14], [15].
Initiatives have been implemented to improve the recognition of dementia, such as the “Annual Wellness Visit” for Medicare enrollees in the United States that includes the detection of any cognitive impairment [16]. Also, the proactive “dementia case finding scheme” was initiated by the government of the United Kingdom, aiming to increase the diagnosis rate of dementia and to improve dementia treatment and care on the population level [17]. No recent data are available about the effect that screening for dementia would have on the diagnostic process in German primary care practice. Thus, the present research report aims to contribute empirical findings to (1) the effect of a screening test for dementia in German primary care practices on the rate of a formal diagnosis of dementia; (2) the distribution of etiologies of newly assigned diagnoses after a screening for dementia; and (3) the factors associated with receiving a formal diagnosis of dementia after a positive dementia screening test.
2. Methods
2.1. Study design
The present cross-sectional analyses were performed on data derived from the ongoing GP-based, randomized, controlled intervention trial, Dementia: life- and person-centered help in Mecklenburg-Western Pomerania (DelpHi-MV). The details of the study have been previously reported [18]. The eligible patients (aged >70 years and living at home) were screened for dementia at participating GP practices using DemTect [19]. This personal interview-based instrument includes five tasks (recall of a word list, number transcoding task, word fluency task, digit span reverse, delayed recall of word list) and is widely used for dementia screening in general practices in Germany [20]. The patients who meet the inclusion criteria for the DelpHi trial (DemTect score <9) were informed by their GPs about the study, invited to participate, and asked to provide written informed consent. If the patient named a caregiver, the caregiver was asked to participate in the study. When patients were unable to give written informed consent, their legal representative was asked to sign the consent form on their behalf (as approved by the Ethical Committee of the Chamber of Physicians of Mecklenburg-Western Pomerania, registry number BB 20/11). The study physicians received allowances for performing the screening test (10€ per patient) and study enrollment (100€ per patient).
Study enrollment into the main study began January 1, 2012. The participants and their caregivers were assigned to an intervention or a control group, depending on whether the treating GP practice had been randomized to the control or intervention group. In both groups, identical, standardized, computer-assisted baseline assessments were conducted as face-to-face interviews at the participants' homes. After the baseline assessment, the intervention group received the “DelpHi-Intervention” (for a detailed description of the DelpHi-Intervention, see Eichler et al [21]). In contrast, the control group received “care as usual.” The ClinicalTrials.gov identifier is NCT01401582.
2.2. Study population
A total of 4064 patients were screened for dementia in 108 participating GP practices. Of these, 692 patients (17%) were eligible for the DelpHi trial, and 406 patients (59%) agreed to participate in the study. However, 90 participants either did not start the baseline assessment or had not finished all parts of the assessment at the time of the analyses.
Fifty-eight patients were withdrawn from the analyses owing to withdrawal of informed consent (n = 31), death (n = 20), relocation (n = 3) or other reasons (n = 4). No statistically significant differences were found between the patients included in the analyses and those who withdrew for DemTect score, age, and sex (see Supplemental Table 1, available online).
Fifteen patients were excluded because the psychometric instruments could not be used (the patients were unable to answer the questions because of the severity of dementia or the patients had refused to answer for other reasons). The patients included in the analyses had significantly higher DemTect scores than those patients excluded because of missing data (mean 5.84 ± 2.02 versus 3.73 ± 2.34; Welch's t test, t(15.50) = 3.41; P < .01). Regarding age, sex, and a formal diagnosis of dementia, we found no significant differences (see Supplemental Table 2, available online).
The complete baseline assessment regarding relevant variables was available for 243 patients (January 1, 2014, preliminary data). Of the 243 patients, 97 (40%) had been formally diagnosed with dementia by their GP before the screening (53% unspecified dementia; 24% vascular dementia; 19% Alzheimer's disease). Patients with a formal diagnosis had a significantly lower score on the DemTect (mean 5.43 ± 2.09 versus 6.11 ± 1.94; Welch's t test, t(196.42) = 2.57, P = .01) and the Mini Mental State Examination (MMSE) [22] than patients not previously diagnosed with dementia (mean 20.34 ± 5.88 versus 23.12 ± 4.96; Welch's t test, t(181.49) = 3.84, P < .001). A detailed comparison of the patients with and without a formal diagnosis of dementia before screening can be found in the study by Eichler et al [3]. The presented analyses were based on data from 146 patients without a formal diagnosis of dementia before screening.
2.3. Procedures and instruments
For the sample description, patient age, sex, living situation (alone versus not alone), cognitive status, depression, and functional status were analyzed. Cognitive status was assessed using the German version of the MMSE [22]. In the analyses, we used the total MMSE score and a categorization indicating “no cognitive impairment” (score 27–30), “mild” (score 20–26), “moderate” (score 10–19), and “severe cognitive impairment” (score 0–9). Depression was operationalized using the score of the Geriatric Depression Scale [23], [24] as a dichotomized variable (“no depression,” score 0–5; “depression,” score 6–15). The functional status was assessed using the Bayer Activities of Daily Living Scale [25], [26], which yields a mean score of 1 to 10, where 1 indicates the lowest possible impairment and 10 the highest possible impairment. For all patients who had provided the respective informed consent, the formal medical diagnoses (International Classification of Disease, version 10, codes F00, F01, F02, F03, G30, and G31) were retrieved from the medical records in the GP practice (including the exact date of the initial diagnosis).
2.4. Statistical analysis
We summarized the variables that describe the sample using descriptive statistics. To assess the effect of screening on the formal diagnosis rate, we categorized the patients into two groups: patients diagnosed after screening (all patients who had received a formal diagnosis of dementia between the day of screening and the baseline assessment) and patients not diagnosed after screening (all patients who had not received a formal diagnosis between the day of screening and the baseline assessment). To test for the differences between those groups, we used Welch's t test (robust to unequal variances) for continuous and Fisher's exact test for categorized variables. To identify the factors associated with the assignment of a diagnosis of dementia after screening, we fitted a logistic regression model, including age, sex, living situation, cognitive impairment, depression, and functional status. Because DelpHi-MV is a cluster-randomized trial, the dependency of data from participants who belonged to the same cluster (i.e., the same treating GP) had to be considered. Therefore, we applied a conditional (fixed effect) logistic regression model, which offers consistent estimates in cases of clustered data [27], [28]. Before running the final regression model, we checked for nonlinear relations using the multivariate fractional polynomial procedure [29]. We found no indication for nonlinear relations; thus, these predictors were included in linear terms. The chosen estimation procedure excluded 60 observations because of the invariance in the outcome variable in certain clusters (i.e., all or none of the patients treated by the same GP were formally diagnosed with dementia). We found no significant differences between the included and these excluded cases regarding the analyzed variables (see Supplemental Table 3, available online). The final regression analysis was performed on the remaining 86 patients assigned to 15 clusters (the clusters were unbalanced). Standard errors of the regression coefficients were estimated using the jackknife method, which allows one to estimate standard errors in complex samples [30]. Statistical analyses were performed using Stata/IC [31].
3. Results
3.1. Sociodemographic and clinical characteristics
The sociodemographic and clinical characteristic of the study sample are listed in Table 1.
Table 1.
Sociodemographic and clinical characteristics of the study sample
| Variable | Total sample (n = 146) | Formally diagnosed after screening |
t | df | P value | |
|---|---|---|---|---|---|---|
| No (n = 74) | Yes (n = 72) | |||||
| Female sex | 91 (62) | 38 (51) | 53 (74) | — | — | <.01∗ |
| Age (y) | 79.58 ± 5.32 | 79.30 ± 4.98 | 79.88 ±5.66 | 0.65 | 142.56 | .51† |
| Living alone | 66 (45) | 31 (42) | 35 (49) | — | — | .51∗ |
| Cognitive impairment (MMSE) | 23.14 ± 4.96 | 24.26 ± 4.83 | 22.00 ± 4.87 | 2.81 | 145.81 | <.01† |
| None (score 27–30) | 52 (37) | 35 (47) | 17 (24) | <.02∗ | ||
| Mild (score 20–26) | 56 (38) | 24 (32) | 32 (44) | |||
| Moderate (score 10–19) | 32 (22) | 13 (18) | 19 (26) | |||
| Severe (score 0–9) | 6 (4) | 2 (3) | 4 (6) | |||
| Depression (GDS ≥6) | 36 (25) | 18 (24) | 18 (25) | — | — | 1.00∗ |
| Functional status (B-ADL) | 3.26 ± 2.23 | 2.73 ± 2.01 | 3.80 ± 2.33 | 2.97 | 141.54 | <.01† |
Abbreviations: MMSE, Mini Mental State Examination (range 0–30; higher score indicates better cognitive functioning); B-ADL, Bayer Activities of Daily Living Scale (range 0–10; lower score indicates better performance); GDS, Geriatric Depression Scale (sum score 0–15; score ≥6 indicates depression).
NOTE. Data presented as n (%) or mean ± standard deviation.
Fisher's exact test.
Welch's t test.
3.2. Effect of screening on receiving a formal diagnosis
Of 146 patients who had not been formally diagnosed before screening, 72 (49%) received at least one formal diagnosis of dementia after a positive DemTect screening. Of these, 38 (53%) received the formal diagnosis on the day of the screening.
3.3. Distribution of differential diagnoses
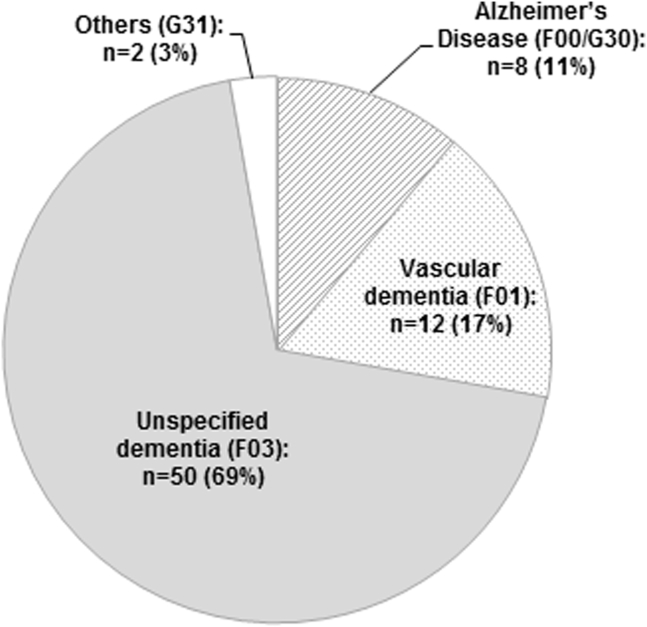
The distribution of newly assigned differential diagnoses after screening is shown in Figure 1. “Unspecified dementia” was by far the most often assigned diagnosis; 69% of the patients received this diagnosis. This proportion was still higher in the patients who received their diagnosis at the day of screening (74%).
Fig. 1.
Differential diagnoses of dementia in primary care patients formally diagnosed after they have screened positive for dementia (n = 72).
3.4. Factors associated with receiving a formal diagnosis of dementia after positive DemTect screening
Univariate statistical analyses revealed that patients who had received a formal diagnosis of dementia after screening were significantly more often women (74% versus 51%) and were significantly more often severely impaired regarding their cognitive (MMSE) and functional status (Bayer Activities of Daily Living Scale; Table 1).
In the multivariate conditional logistic regression analyses, the only factor significantly associated with receiving a formal diagnosis of dementia after screening was female sex [F(6,14) = 3.68, P < .05]. The chance of receiving a formal diagnosis after a positive DemTect screening was more than five times greater for women than for men (odds ratio 5.42; P < .01; 95% confidence interval 1.93–15.22). The results are listed in Table 2.
Table 2.
Factors associated with receiving a formal diagnosis of dementia after screening
| Variable | Formally diagnosed after screening |
OR | t | P value | 95% CI | |
|---|---|---|---|---|---|---|
| No (n = 38) | Yes (n = 48) | |||||
| Age (y) | 80.08 ± 5.35 | 80.13 ± 5.15 | 0.92 | 0.85 | .41 | 0.74–1.14 |
| Female sex | 17 (45) | 37 (77) | 5.42 | 3.51 | <.01 | 1.93–15.22 |
| Living alone | 11 (29) | 25 (52) | 1.02 | 0.03 | .98 | 0.19–5.53 |
| Cognitive impairment (MMSE) | 24.00 ± 5.28 | 21.87 ± 4.63 | 0.93 | 1.23 | .24 | 0.82–1.05 |
| Functional status (B-ADL) | 2.97 ± 2.17 | 3.68 ± 2.22 | 1.35 | 1.54 | .15 | 0.89–2.04 |
| Depression (GDS) | 10 (26) | 11 (23) | 0.65 | 0.40 | .69 | 0.07–6.42 |
Abbreviations: OR, odds ratio; CI, confidence interval; MMSE, Mini Mental State Examination (range 0–30; higher score indicates better cognitive functioning); B-ADL, Bayer Activities of Daily Living Scale (range 0–10; lower score indicates better performance); GDS, Geriatric Depression Scale (sum score 0–15; score ≥6 indicates depression).
NOTE. Data presented as mean ± standard deviation or n (%). Conditional fixed effect logistic regression analysis (n = 86 patients assigned to n = 15 clusters): F(6,14) = 3.68, P < .05.
4. Discussion
The present analysis revealed a strong effect of screening for dementia among German primary care patients on the probability of receiving a formal diagnosis of dementia. Almost 50% of previously undiagnosed patients received a formal diagnosis of dementia after they had screened positive at their GP practice. The overall diagnosis rate in primary care patients who had screened positive for dementia increased from 40% before screening [3] to 70% after screening. Thus, our findings indicate that a considerable number of patients with cognitive impairment might not be recognized by their GP without a cognitive test. The results of the multivariate analysis showed that the chance of receiving a formal diagnosis after a positive DemTect screening was more than five times higher in woman than in men; thus, women, in particular, might benefit from screening.
Previous research has demonstrated that physicians often do not recognize cognitive impairment, even in those with severe dementia [1], [13], [32], [33]. For German primary care patients, Sandholzer et al [5] showed that the prevalence of cognitive impairment in patients aged 70 years or older was 4.6% according to the GP diagnosis but 21% according to the MMSE score.
Our results have provided evidence that routine screening can improve the identification of dementia considerably. To the best of our knowledge, this study is the first to demonstrate such a profound effect of screening, although previous studies reported substantially smaller effects. Sandholzer et al [5] found that only 6% of the German primary care patients who had screened positive for dementia had received any diagnostic assessment or treatment. Borson et al [4] showed that relevant physician behavior (i.e., a formal diagnosis of dementia, specialist referral, or cognitive enhancing medication) occurred for only 17% of the positively screened patients. A reason for the considerably high effect of screening in the present study could have been the active involvement of the participating GPs in a dementia intervention trial that aimed to optimize treatment and care for people with dementia. The GPs did not only receive information about the result of the cognitive test. Furthermore, they informed all positively screened patients about the DelpHi trial and had obtained their informed consent for study participation. This might have increased the perceived importance of a positive screening outcome, which could have led to an increased probability that the respective patients would receive a formal diagnosis of dementia. This might have been a sort of clinical “Hawthorne effect” such that the GPs might have changed their behavior because they were participating in an intervention study [34], [35].
According to the medical files of the participating GP practices, more than 50% of the newly diagnosed patients received their formal diagnosis on the day of the screening. In addition, the proportion of the diagnosis of “unspecified dementia” as the only diagnosis was considerably high (69% of all patients formally diagnosed after screening and 74% of patients formally diagnosed at the day of screening). This could imply that some GPs might assign a formal diagnosis to patients because of a positive screening result. However, our data did not provide any insight into the process of assigning a formal diagnosis. A desired consequence would be that a positive screening result would initiate additional differential diagnostic assessments of the patients. Without an adequate diagnostic assessment, the risk of assigning a false-positive diagnosis of dementia is high [13]. Reversible causes of cognitive impairment, such as depression, vitamin B12 deficiency, or hypothyroidism might remain unrecognized and therefore untreated.
In the present sample, 24% of the patients who screened positive for dementia on the DemTect (score <9) received a formal diagnosis of dementia but were not cognitively impaired according to their MMSE result (score 27–30; assessed at baseline). Some of these patients might have been misdiagnosed. Because previous studies showed that the MMSE is less sensitive for detecting milder forms of cognitive impairment (43%–43%) [36] than the DemTect (80%–100%) [19], [37], the rate of false-positive diagnoses in our study might have been lower than 24%. However, we were unable to validate the GP diagnoses with our data. Nevertheless, analyzing our follow-up data will allow more detailed information about which formal diagnosis will be confirmed or changed in the course of the disease and what processes of additional differential diagnostic and treatment will be followed after the initial diagnosis.
The potential adverse effects of a false-positive diagnosis must be weighed against the harm of overlooking dementia. Recognizing and diagnosing dementia is an inevitable prerequisite for providing adequate information, treatment, and care. Thus, although screening for dementia in elderly patients has not yet been adopted in the dementia guidelines [6], [7], [8], it could be a possibility to reduce the high rates of unrecognized dementia. The requirements for an adequate procedure to detect cognitive impairment in routine care are currently being discussed. The Alzheimer's Association has proposed an algorithm to detect cognitive impairment during the Medicare “Annual Wellness Visit” in a primary care setting in the United States [16] that could also be a possible strategy for primary care settings in other countries. Because screening entails the risk that patients will receive a false-positive diagnosis of dementia after they have screened positive, additional diagnostic assessments should be mandatory. Also, the proactive provision of adequate treatment and care in the case of a confirmed diagnosis should be fostered.
Currently, little evidence is available that shows improved health outcomes for patients who have been formally diagnosed with dementia [9], [38]. Regarding the potential adverse effects of screening, no studies have yet directly addressed the adverse psychological effects of screening or the adverse effects of false-positive test results [38]. Thus, the potential positive and negative consequences of screening for dementia require systematic and comprehensive evaluation. Analyzing our follow-up data will enable us to provide additional evidence. In the DelpHi trial [18], [21], the patient outcomes of the control group receiving “care as usual” will be compared with the outcomes of the intervention group receiving a collaborative dementia care management after screening positive for dementia. Thus, we will be able to determine the effect of receiving a formal diagnosis of dementia after a positive screening result on various outcomes (e.g., treatment and care, quality of life, psychological well being, and health outcomes) in both groups.
Research in context.
-
1.
Systematic review: We searched PubMed for articles with the following search terms: dementia and screening; recognition; early or timely diagnosis; differential diagnosis; primary care; or diagnosis rate. In addition, we reviewed the reference lists of articles identified.
-
2.
Interpretation: We provided evidence that the screening of elderly patients for dementia in primary care practices can improve recognition of dementia considerably. However, there is a risk that patients receive a formal diagnosis of dementia based on a positive screening result (without further differential diagnostic assessment).
-
3.
Future directions: To reduce the risk of false-positive diagnoses, future studies should implement screening procedures that include further differential diagnostic. Future research needs to provide evidence that screening for dementia improves health outcomes for patients who are formally diagnosed with dementia after they have been screened positive for dementia.
Acknowledgments
Author contributions: T.E. drafted the manuscript. J.R.T., the study coordinator, contributed substantially to the overall design and implementation of the DelpHi trial. J.H. (statistical analyses), B.M. (health economy), D.W. (pharmacy), A.D. (nursing science), I.K. (neurology), and S.T. (psychiatry) contributed to the DelpHi trial and the report in accordance with their areas of expertise. W.H., the principal investigator of the study, contributed substantially to the concept of the DelpHi trial and the final version of the report. All authors read and approved the final report.
Additional contributions: The DelpHi trial was developed and established as a result of input from the following experts in their respective fields: Aniela Angelow, Grit Aßmann, Georgia Böwing, Adina Dreier, Thomas Fiß, Daniel Fredrich, Leonore Köhler, and Steffen Richter. An experienced field study team provided support with data collection and data management: Ines Abraham, Kerstin Albuerne, Vaska Böhmann, Kathleen Dittmer, Sarah Gardzella, Jana Hubert, Ulrike Kempe, Viktoriya Kim, Katja Lübke, Andrea Pooch, Saskia Moll, Sabine Schmidt, Christine Winckler, and Paula Winter. We also thank all participating patients and their general practitioners for their most valued collaboration.
The study was funded by the German Center for Neurodegenerative Diseases (DZNE) and the University Medicine Greifswald.
Conflict of interest disclosure: The authors declare no conflicts of interest.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2014.11.007.
Supplementary data
References
- 1.Prince M., Bryce R., Ferri C. Alzheimer's Disease International (ADI); London: 2011. World Alzheimer Report 2011—The Benefits of Early Diagnosis and Intervention. [Google Scholar]
- 2.Relkin N. Screening and early diagnosis of dementia. Am J Manag Care. 2000;6(22 Suppl):S1111–S1118. [PubMed] [Google Scholar]
- 3.Eichler T., Thyrian J., Hertel J., Köhler L., Wucherer D., Dreier A. Rates of formal diagnosis in people screened positive for dementia in primary care: Results of the DelpHi-trial. J Alzheimers Dis. 2014;42:451–458. doi: 10.3233/JAD-140354. [DOI] [PubMed] [Google Scholar]
- 4.Borson S., Scanlan J., Hummel J., Gibbs K., Lessig M., Zuhr E. Implementing routine cognitive screening of older adults in primary care: Process and impact on physician behavior. J Gen Intern Med. 2007;22:811–817. doi: 10.1007/s11606-007-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandholzer H., Breull A., Fischer G.C. Early diagnosis and early treatment of cognitive disorders: A study of geriatric screening of an unselected patient population in general practice. Z Gerontol Geriatr. 1999;32:172–178. doi: 10.1007/s003910050102. [DOI] [PubMed] [Google Scholar]
- 6.UK National Screening Committee (UK NSC). The UK NSC policy on Alzheimer's Disease screening in adults. Available at: http://www.screening.nhs.uk/dementia (published 2010). Accessed August 12, 2014.
- 7.U.S. Preventive Services Task Force. Screening for cognitive impairment in older adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdeme.htm (published March 2014). Accessed August 12, 2014.
- 8.Royal Australian College of General Practitioners . 8 ed. Royal Australian College of General Practitioners; East Melbourne, Australia: 2012. Guidelines for Preventive Activities in General Practice. [Google Scholar]
- 9.Borson S., Frank L., Bayley P.J., Boustani M., Dean M., Lin P.J. Improving dementia care: The role of screening and detection of cognitive impairment. Alzheimers Dement. 2013;9:151–159. doi: 10.1016/j.jalz.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Couteur D.G., Doust J., Creasey H., Brayne C. Political drive to screen for pre-dementia: Not evidence based and ignores the harms of diagnosis. BMJ. 2013;347:f5125. doi: 10.1136/bmj.f5125. [DOI] [PubMed] [Google Scholar]
- 11.Boustani M.A., Justiss M.D., Frame A., Austrom M.G., Perkins A.J., Cai X. Caregiver and noncaregiver attitudes toward dementia screening. J Am Geriatr Soc. 2011;59:681–686. doi: 10.1111/j.1532-5415.2011.03327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunet M.D., McCartney M., Heath I., Tomlinson J., Gordon P., Cosgrove J. There is no evidence base for proposed dementia screening. BMJ. 2012;345:e8588. doi: 10.1136/bmj.e8588. [DOI] [PubMed] [Google Scholar]
- 13.Boustani M., Callahan C.M., Unverzagt F.W., Austrom M.G., Perkins A.J., Fultz B.A. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20:572–577. doi: 10.1111/j.1525-1497.2005.0126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luck T., Luppa M., Sieber J., Schomerus G., Werner P., Konig H.H. Attitudes of the German general population toward early diagnosis of dementia—Results of a representative telephone survey. PLoS One. 2012;7:e50792. doi: 10.1371/journal.pone.0050792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinner G., Bouman W.P. Attitudes of patients with mild dementia and their carers towards disclosure of the diagnosis. Int Psychogeriatr. 2003;15:279–288. doi: 10.1017/s1041610203009530. [DOI] [PubMed] [Google Scholar]
- 16.Cordell C.B., Borson S., Boustani M., Chodosh J., Reuben D., Verghese J. Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9:141–150. doi: 10.1016/j.jalz.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Prime Minister’s challenge on dementia. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215101/dh_133176.pdf (published March 2012). Accessed August 12, 2014.
- 18.Thyrian J.R., Fiss T., Dreier A., Bowing G., Angelow A., Lueke S. Life- and person-centred help in Mecklenburg-Western Pomerania, Germany (DelpHi): Study protocol for a randomised controlled trial. Trials. 2012;13:56. doi: 10.1186/1745-6215-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalbe E., Kessler J., Calabrese P., Smith R., Passmore A.P., Brand M. DemTect: A new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry. 2004;19:136–143. doi: 10.1002/gps.1042. [DOI] [PubMed] [Google Scholar]
- 20.Thyrian J.R., Hoffmann W. Dementia care and general physicians—A survey on prevalence, means, attitudes and recommendations. Cent Eur J Public Health. 2012;20:270–275. doi: 10.21101/cejph.a3751. [DOI] [PubMed] [Google Scholar]
- 21.Eichler T., Thyrian J.R., Dreier A., Wucherer D., Kohler L., Fiss T. Dementia care management: Going new ways in ambulant dementia care within a GP-based randomized controlled intervention trial. Int Psychogeriatr. 2014;26:247–256. doi: 10.1017/S1041610213001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessler J., Markowitsch H.J., Denzler P. Beltz Test GmbH; Göttingen: 1990. Mini-Mental-Status-Test (MMST) [German Version] [Google Scholar]
- 23.Gauggel S., Birkner B. Validity and reliability of a German version of the Geriatric Depression Scale (GDS) Zeitschrift Klin Psychologie. 1999;28:18–27. [Google Scholar]
- 24.Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 25.Erzigkeit H., Lehfeld H., Pena-Casanova J., Bieber F., Yekrangi-Hartmann C., Rupp M. The Bayer-Activities of Daily Living Scale (B-ADL): Results from a validation study in three European countries. Dement Geriatr Cogn Disord. 2001;12:348–358. doi: 10.1159/000051280. [DOI] [PubMed] [Google Scholar]
- 26.Hindmarch I., Lehfeld H., de J.P., Erzigkeit H. The Bayer Activities of Daily Living Scale (B-ADL) Dement Geriatr Cogn Disord. 1998;9(Suppl 2):20–26. doi: 10.1159/000051195. [DOI] [PubMed] [Google Scholar]
- 27.Breslow N., Day N. International Agency for Research on Cancer; Lyon: 1980. Statistical Methods in Cancer Research. Vol. 1. The Analysis of Case-Control Studies. (IARC Scientific Publication no. 32) [PubMed] [Google Scholar]
- 28.Hosmer D., Lemeshow S., Sturdivant R. 3rd ed. John Wiley & Sons; New Jersey: 2013. Applied Logistic Regression. [Google Scholar]
- 29.Royston P., Sauerbrei W. 1st ed. John Wiley & Sons; New York: 2008. Multivariable Model-Building—A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Continuous Variables. [Google Scholar]
- 30.Efron B., Tibshirani R. Bootstrap measures for standard errors, confidence intervals and other measures of statistical accuracy. Statist Sci. 1986;1:54–75. [Google Scholar]
- 31.StataCorp . StataCorp LP; College Station, TX: 2009. Stata Statistical Software: Release 11. [Google Scholar]
- 32.Connolly A., Gaehl E., Martin H., Morris J., Purandare N. Underdiagnosis of dementia in primary care: Variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health. 2011;15:978–984. doi: 10.1080/13607863.2011.596805. [DOI] [PubMed] [Google Scholar]
- 33.Boustani M., Peterson B., Hanson L., Harris R., Lohr K.N. Screening for dementia in primary care: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:927–937. doi: 10.7326/0003-4819-138-11-200306030-00015. [DOI] [PubMed] [Google Scholar]
- 34.Roethlisberger F.J., Dickson W.J. Harvard University Press; Cambridge: 1939. Management and the Worker. [Google Scholar]
- 35.McCarney R., Warner J., Iliffe S., van Haselen R., Griffin M., Fisher P. The Hawthorne effect: A randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackin R.S., Ayalon L., Feliciano L., Arean P.A. The sensitivity and specificity of cognitive screening instruments to detect cognitive impairment in older adults with severe psychiatric illness. J Geriatr Psychiatry Neurol. 2010;23:94–99. doi: 10.1177/0891988709358589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohn N., Kalbe E., Georg H., Kessler J. Vergleich MMST und DemTect: Spezifität und Sensitivität bei primär kognitiven Störungen. Akt Neurol. 2007;34:P672. [Google Scholar]
- 38.Lin J.S., O'Connor E., Rossom R.C., Perdue L.A., Eckstrom E. Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:601–612. doi: 10.7326/0003-4819-159-9-201311050-00730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.