Abstract
Background
Amyloid imaging using fluorine 18–labeled tracers florbetapir, florbetaben, and flutemetamol has recently been reported in Alzheimer's disease (AD).
Methods
We systematically searched MEDLINE and EMBASE for relevant studies published from January 1980 to March 2014. Studies comparing imaging findings in AD and normal controls (NCs) were pooled in a meta-analysis, calculating pooled weighted sensitivity, specificity, and diagnostic odds ratio (OR) using the DerSimonian-Laird random-effects model.
Results
Nineteen studies, investigating 682 patients with AD, met inclusion criteria. Meta-analysis demonstrated a sensitivity of 89.6%, a specificity of 87.2%, and an OR of 91.7 for florbetapir in differentiating AD patients from NCs, and a sensitivity of 89.3%, a specificity of 87.6%, and a diagnostic OR of 69.9 for florbetaben. There were insufficient data to complete analyses for flutemetamol.
Conclusions
Results suggest favorable sensitivity and specificity of amyloid imaging with fluorine 18–labeled tracers in AD. Prospective studies are required to determine optimal imaging analysis methods and resolve outstanding clinical uncertainties.
Keywords: Alzheimer's, Dementia, Amyloid, Positron emission tomography, Florbetapir, Florbetaben, Flutemetamol, Sensitivity, Specificity
1. Introduction
Timely diagnosis of dementia obviates prolonged uncertainty, unnecessary investigations, delays in initiation of symptomatic treatments, and recruitment of poorly characterized patients into research trials. Definitive diagnosis may only be achieved by histopathological examination of invasive brain biopsy or postmortem tissue, or molecular genetic testing in a minority with inherited dementia. Clinical diagnostic criteria in recent use for Alzheimer's disease (AD) often fail to robustly differentiate accurately between AD and non-AD pathology with up to 40% of patients diagnosed with non-AD dementias identified as having pathology consistent with AD at postmortem in some series [1]. Clinical diagnosis either provides good diagnostic sensitivity at the expense of specificity or vice versa (pooled averages 81% sensitive and 70% specific) [2].
When considering the pathophysiology of AD, it has been proposed that the presymptomatic phase is characterized by an early rise in amyloid accumulation, followed later by synaptic dysfunction, tau-mediated neuronal injury, reduction in brain volume, and finally emergence of cognitive symptoms, followed by a clinical syndrome of frank dementia [3]. This suggests a sensitive and specific biomarker of brain amyloid deposition, such as amyloid imaging, would be a useful diagnostic tool, perhaps as an adjunct to investigation of cerebrospinal fluid (CSF) biomarkers (including amyloid and tau), cerebral hypometabolism ascertained by 18F-fluorodeoxyglucose positron emission tomography, hippocampal volume, tractography, and clinical cognitive assessments.
The most widely studied radiolabeled amyloid ligand, 11C-labeled Pittsburgh compound B (11C-PiB), demonstrates high affinity and selective amyloid binding. Imaging using 11C-PiB has been reported to successfully differentiate between patients with AD and healthy controls [4], predict the progression of mild cognitive impairment (MCI) to symptomatic AD [5], and correlate with underlying amyloid neuropathology [6]. Disadvantages include a radioactive decay half-life of only 20 minutes and a scanning time of 30 minutes, rendering it limited to use in centers with cyclotrons, which are expensive and impractical, thereby limiting its utility in clinical settings.
The proven efficacy of amyloid imaging and impracticalities of 11C-PiB triggered a search to identify compounds with desirable qualities of high affinity for amyloid, rapid brain uptake and washout from non–amyloid-containing tissues, short imaging time, and long stable pseudoequilibrium allowing flexibility in imaging acquisition time. Amyloid imaging using three novel fluorine 18–labeled (18F) tracers, 18F-florbetapir, 18F-florbetaben, and 18F-flutemetamol, with a longer half-life than 11C-PIB of 110 minutes has recently been investigated in a number of studies, including phase 2 clinical trials. These agents have recently gained US Food and Drug Administration approval for use in clinical practice. The benefits of identifying amyloid in vivo extend to monitoring disease progression, and, with the advent of treatments targeting amyloid deposition, potentially also as a surrogate biomarker of treatment efficacy.
In this systematic review and meta-analysis, we (1) assess the quality of recent studies investigating amyloid imaging using novel fluorine-labeled tracers, (2) investigate their pooled reported sensitivity and specificity for diagnosis of AD, and (3) consider the utility of amyloid imaging using these agents in the differential diagnosis of dementia syndromes.
2. Methods
2.1. Search strategy and study selection
We searched the Medical Literature Analysis and Retrieval System Online (MEDLINE) and the Excerpta Medica Database (EMBASE) via OVID for human studies published from January 1980 to March 2014, inclusive of all languages. The search terms used were (1) “dementia,” (2) “amyloid” or “positron emission tomography,” (3) “florbetapir” or “florbetaben” or “flutemetamol,” and (3) “sensitivity” and “specificity” or “diagnosis.” The inclusion criteria were as follows: (1) original study; (2) study of diagnostic accuracy of florbetapir/florbetaben/flutemetamol amyloid imaging; (3) the study compares clinically or pathologically diagnosed dementia (AD, frontotemporal dementia [FTD], vascular dementia [VD], dementia with Lewy bodies [DLB) with each other or with normal controls; (4) amyloid imaging was not interpreted with other imaging modality; (5) total subjects in the study of at least 10; and (6) sufficient data were reported to enable a 2 × 2 contingency table to be formulated for the calculation of sensitivity and specificity. Two authors applied these inclusion criteria independently in identifying the studies.
2.2. Data extraction
We extracted data on the author's name, year of publication, amount of ligand injected per scan, method of image analysis, criteria used for defining a positive amyloid scan, criteria used for clinical or pathological diagnosis, study population characteristics (mean age, mean Mini-Mental State Examination [MMSE] score), internal and external validity score, and constructed a 2 × 2 contingency table for each study. (1) True positive was defined as patients with AD who had positive amyloid imaging, (2) false negative: patients with AD who had negative amyloid imaging, (3) true negative: diagnostic comparison group patients with negative amyloid imaging, and (4) false positive: diagnostic comparison group patients with positive amyloid imaging. In studies where there were more than one relevant data set, we chose the data set with (1) the most relevant clinical diagnosis (probable and definite AD, as opposed to possible AD), (2) quantitative analysis, rather than visual analysis, (3) age-matched normal controls (NCs) (when there were both young and older normal controls), and (4) data set in which intermediate result was reported.
2.3. Quality assessment
Our study is reported in accordance with the PRISMA guidelines; a completed PRISMA checklist is available as Appendix 1 [7]. Our assessment of the internal and external validity of individual studies is detailed in Appendix 2; assessment was based on the STARD checklist, and in particular items 3 to 5, 7, 11 to 13, 15, 16, 18, 21, 22, and 25 [8].
2.4. Data synthesis and statistical analysis
We calculated the sensitivity and specificity of the relevant diagnostic comparison groups for each included study. We also pooled the studies comparing amyloid imaging in AD patients and NCs for florbetapir and florbetaben in a meta-analysis using Meta-DiSc (Javier Zamora, Boston, MA, USA) software for statistical analysis [9]. For this, we calculated the pooled weighted sensitivity, specificity, positive and negative likelihood ratios (LRs), and diagnostic odds ratio (OR) for AD patients versus NC using DerSimonian-Laird random-effects model. This model recognizes both between- and within-study heterogeneity and considers the different effect sizes of the studies, thus allowing the results to be applied to a wider range of studies [10]. For the log OR of any 2 × 2 table with zero, the software would add 0.5 to each cell. We assessed for between-study heterogeneity using I2 and χ2 test, where an I2 >50% and a P value <.05 indicate a significant amount of heterogeneity unlikely to be explained by random variation alone [11]. We further investigated the heterogeneity using a summary receiver operating characteristic curve to describe a set of operating characteristics across the studies and used Spearman correlation coefficient to look for a threshold effect. A positive Spearman correlation coefficient between logit of sensitivity and logit of 1 − specificity would suggest the possibility of a threshold effect, caused by the different studies using different diagnostic thresholds to determine a positive or negative result. We also used single-factor meta-regression analysis to look for possible sources of heterogeneity using the following variates as predictor variables: (1) mean age; (2) mean MMSE score; (3) male-female distribution; and (4) method of image analysis (visual vs. quantitative). A variate is considered to be explanatory if the regression coefficient was statistically significant (P < .05).
3. Results
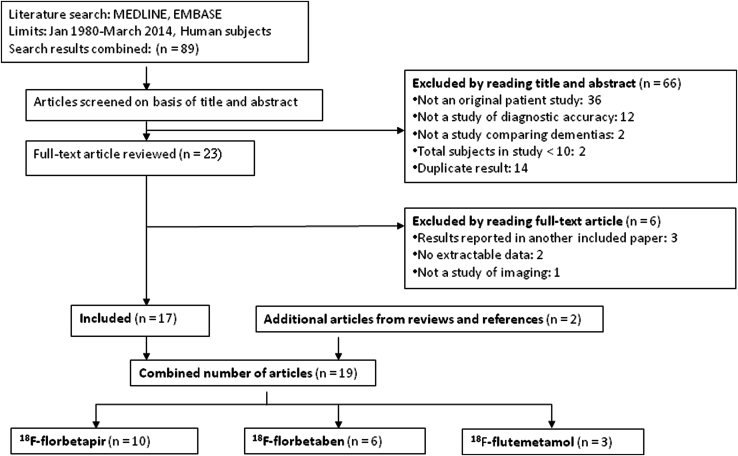
Our literature search produced a total of 86 articles (Fig. 1). We excluded 66 articles after reading the abstracts and reviewed the remaining 23 full-text articles, after which a further six articles were excluded. We identified two additional articles from reviews and references, bringing the review to a total of 19 articles. Table 1 summarizes the number and mean age of subjects in the included studies. Table 2 specifies the data collected from each study. The review revealed that most of the available studies' diagnostic comparison groups were AD patients versus NCs; therefore, we performed a meta-analysis investigating these groups. Relevant data pertaining to other dementias were also included for the consideration of diagnostic utility (Table 2). Table 3 summarizes the pooled weighted sensitivity, specificity, LRs, and diagnostic ORs for AD patients versus NCs for florbetapir and florbetaben. The pooled diagnostic ORs and positive LRs and their confidence intervals (CIs) were all above 1, suggesting that florbetapir and florbetaben amyloid imaging have diagnostic utility in this group.
Fig. 1.
Flow chart of study selection.
Table 1.
Summary of all studies
| Amyloid imaging | Number of studies | Alzheimer's disease |
Non–Alzheimer's disease |
||
|---|---|---|---|---|---|
| Number | Combined mean age, y (95% CI) | Number | Combined mean age, y (95% CI) | ||
| Florbetapir | 10 | 343 | 72.5 (68.2–76.8) | 348 | 68.2 (64.5–71.9) |
| Florbetaben | 6 | 277 | 70.7 (68.2–73.2) | 253 | 68.5 (66.0–71.1) |
| Flutemetamol | 3 | 62 | 69.3 (67.1–71.4) | 38 | 66.6 (62.3–71.0) |
Abbreviation: CI, confidence interval.
Table 2.
Characteristics of individual studies
| Study | Year | Quality∗ |
Imaging method |
Diagnosis |
Study population |
Outcome measure |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Internal | External | Amount MBq | Image analysis | Definition positive amyloid scan SUVR threshold | Diagnostic criteria | Number | Mean age (SD) | Mean MMSE (SD) | Male/female | Sensitivity (%) | Specificity (%) | ||
| Florbetapir | |||||||||||||
| Camus et al.[12] | 2012 | 9 | 4 | 259 ± 57 | Quantitative | >1.122 | NINCDS, DSM-IV | 13 AD | 67.8 (6.5) | 23.0 (3.6) | 4/9 | 92.3 | 90.5 |
| 21 NC | 66.2 (4.3) | 29.0 (1.3) | 9/12 | ||||||||||
| Clark et al.[13], [14] | 2012 | 9 | 3 | 370 | Quantitative | >1.10 | Pathological | 39 AD | All 79.4 (12.6) | NR | NR | 97.4 | 100 |
| 10 NC | |||||||||||||
| Doraiswamy et al. [15] | 2012 | 9 | 4 | 370 | Visual | Cortical uptake | NINCDS, MMSE≤24 | 31 AD | 76.7 | 21.4 | 28/41 | 67.7 | 85.5 |
| 69 NC | 69.8 | 29.6 | 18/13 | ||||||||||
| Fleisher[16] | 2011 | 9 | 4 | 370 | Quantitative | ≥1.17 | NINCDS, M10-24 | 68 AD | 74.6 (9.5) | 19.9 (4.4) | 16/33 | 80.9 | 79.3 |
| 82 NC | 71.8 (10) | 29.6 (0.5) | 10/45 | ||||||||||
| Fleisher et al. [17] | 2013 | 9 | 4 | 370 | Quantitative | >1.083 | NINCDS, M10-24 | 45 AD | 74.7 (9.0) | 20.6 (4.3) | 22/23 | 84 | 75.4 |
| 61 NC | 67.9 (11.3) | 29.6 (0.5) | 27/34 | ||||||||||
| Grundman et al. [18] | 2013 | 9 | 2 | 370 | Visual | NR | Clinical judgment | 86 AD | NR | NR | NR | 61.6 | 57.1 |
| 21 non-AD | |||||||||||||
| Joshi et al.[19] | 2012 | 9 | 4 | 370 | Visual | Cortical uptake | NINCDS, M10-24 | 9 AD | 70.3 | 20.9 | 2/8 | 100 | 100 |
| 11 NC | 44.4 | 29.7 | 6/4 | ||||||||||
| La Joie et al.[20] | 2012 | 9 | 3 | 4 MBq/kg | Quantitative | >1.1 | NINCDS | 22 AD | NR | NR | NR | 90.9 | 91.9 |
| 37 NC | |||||||||||||
| Newberg et al.[21] | 2012 | 9 | 3 | 370 | Visual | Cortical uptake | NINCDS | 19 AD | 73 (9) | 20 (7) | 8/11 | 94.7 | 95.2 |
| 21 NC | 67 (13) | 29 (1) | 13/8 | ||||||||||
| Wong et al.[22] | 2010 | 9 | 2 | 370 | Visual | Cortical uptake | NINCDS, M10-24 | 11 AD | NR | NR | NR | 90.9 | 86.7 |
| 15 NC | |||||||||||||
| Florbetaben | |||||||||||||
| Barthel et al.[23] | 2011 | 9 | 3 | 300 | Visual | Cortical uptake | NINCDS, DSM-IV | 10 AD | 69 (7) | 19 (7) | 8/2 | 90 | 90 |
| 10 NC | 67 (8) | 29 (1) | 8/2 | ||||||||||
| Barthel et al.[24], phase II trial | 2011 | 9 | 4 | 300 | Quantitative | Linear discriminate function analysis of regional SUVRs | NINCDS, DSM-IV | 81 AD | 70.7 (7.8) | 22.6 (2.3) | 47/34 | 85 | 91 |
| 69 NC | 68.2 (6.9) | 29.3 (0.8) | 30/39 | ||||||||||
| Schipke et al. [25] | 2012 | 9 | 3 | NR | NR | NR | NINCDS, DSM-IV | 121 AD, 80 NC | NR | NR | NR | 81.8 | 82.5 |
| Tiepolt et al. [26] | 2012 | 9 | 3 | 300 ± 60 | Visual | Cortical uptake | NINCDS, DSM-IV | 25 AD | 70.9 (8.0) | 22.5 (2.2) | 14/11 | 80 | 96 |
| 25 NC | 67.1 (7.7) | 29.2 (0.8) | 14/11 | ||||||||||
| Villemagne et al.[27], [28] | 2011 | 9 | 4 | 300 | Visual | Cortical uptake | NINCDS, Consensus criteria FTD | 30 AD | 72.0 (9.2) | 22.8 (3.7) | 14/16 | ||
| 32 NC | 70.7 (6.3) | 29.6 (0.7) | 19/13 | 96.7 | 84.4 | ||||||||
| 11 FTD | 63.5 (7.0) | 24.5 (2.9) | 7/4 | 96.7 | 90.9 | ||||||||
| 7 DLB | 71.7 (5.7) | 24.0 (6.6) | 7/0 | 96.7 | 71.4 | ||||||||
| 4 VD | 73.0 (11.0) | 27.8 (2.1) | 0/4 | 96.7 | 75.0 | ||||||||
| 5 PD | 72.6 (6.5) | 27.4 (2.7) | 5/0 | 96.7 | 100 | ||||||||
| Villemagne et al.[29] | 2012 | 9 | 4 | 300 | Visual | Cortical uptake | NINCDS | 10 AD | 70.8 (9.6) | 22.7 (3.9) | 8/2 | 100 | 70 |
| 10 NC | 70.4 (7.2) | 29.6 (0.7) | 6/4 | ||||||||||
| Flutemetamol | |||||||||||||
| Duara et al. [30] | 2013 | 9 | 4 | 185 | Quantitative | >1.56 | NINCDS, DSM-IV | 27 AD | 69.6 (7.0) | 23.3 (2.2) | 12/15 | 92.6 | 93.3 |
| >1.40 | 15 NC | 68.7 (7.6) | 28.8 (1.0) | 9/6 | 92.6 | 80 | |||||||
| Vandenberghe et al.[31], phase II | 2010 | 9 | 3 | 185 | Visual | Cortical uptake | NINCDS, DSM-IV | 27 AD | 69.6 (7.0) | 23.3 (2.2) | 12/15 | 92.6 | 96 |
| 15 NC | 68.7 (7.6) | 29.4 (1.0) | 9/6 | ||||||||||
| Nelissen et al.[32], phase I | 2009 | 9 | 2 | 180 | Visual, Quantitative | Cortical uptake | NINCDS, DSM-IV | 8 AD | 68.6 (6.9) | 23.1 (1.9) | 6/2 | 75.0 | 87.5 |
| 8 NC | 62.5 (7.1) | 28.9 (1.0) | 5/3 | ||||||||||
Abbreviations: SUVR, standard uptake value ratio; SD, standard deviation; MMSE, Mini-Mental State Examination; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders; AD, Alzheimer's disease; NC, normal control; NR, not reported; FTD, frontotemporal dementia; DLB, dementia with Lewy bodies; VD, vascular dementia; PD, Parkinson's disease.
NOTE. Studies in bold were included in the meta-analysis.
Please see Appendix 2.
Table 3.
Summary of pooled weighted sensitivity, specificity, positive and negative likelihood ratios, and diagnostic odds ratio for Alzheimer's disease versus normal controls
| Sensitivity (%) | Specificity (%) | LR+ | LR− | Diagnostic OR | |
|---|---|---|---|---|---|
| Florbetapir (n = 7) | |||||
| Pooled estimates | 89.6 | 87.2 | 7.90 | 0.108 | 91.7 |
| 95% CI | 84.2–93.6 | 81.7–91.6 | 4.23–14.8 | 0.055–0.213 | 26.7–315 |
| I2 value, % | 46.2 | 50.3 | 38.9 | 35.7 | 53.5 |
| Chi-squared (P value) | 11.2 (P = .084) | 12.1 (P = .060) | 9.82 (P = .132) | 9.34 (P = .155) | 12.9 (P = .045) |
| Florbetaben | |||||
| Pooled estimates | 89.3 | 87.6 | 6.06 | 0.141 | 69.9 |
| 95% CI | 82.7–94.0 | 80.4–92.9 | 3.36–10.9 | 0.087–0.228 | 29.6–165 |
| I2 value, % | 48.6 | 13.4 | 34.8 | 0.0 | 0.0 |
| Chi-squared (P value) | 5.83 (P = .120) | 3.46 (P = .326) | 4.60 (P = .203) | 2.61 (P = .456) | 0.68 (P = .879) |
Abbreviations: LR+, positive likelihood ratio, LR−, negative likelihood ratio; OR, odds ratio; CI, confidence interval.
3.1. Florbetapir
There were 10 studies identified in this group, with nine studies comparing the diagnostic utility of AD versus NC and one study comparing AD versus non-AD (which included FTD, VD, alcohol-related dementia, corticobasal degeneration, depression, structural causes, and dementia of uncertain etiology). Two studies, Clark et al. [13] and Doraiswamy et al. [15], were prospective cohort studies, whereas the rest were case-control studies. The study by Clark et al. was the only one with a neuropathological diagnosis, demonstrating a very favorable sensitivity of 97.5% and specificity of 100% in differentiating AD patients from NCs. Fleisher et al. [16], Fleisher et al. [17], Doraiswamy et al. [15], and Grundman et al. [18] recruited patients from the same multicenter nonrandomized phase I and II trials of florbetapir PET imaging; therefore, only the study by Fleisher et al. [16] with the most complete number of patients was included in the meta-analysis for AD patients versus NCs.
For the meta-analysis, seven studies were included, with a total number of 181 patients with AD (combined mean age, 71.8 years [95% CI: 67.4–76.2], combined mean MMSE score, 21.0 [95% CI: 18.7–23.1]) and 197 NCs (combined mean age, 68.3 years [95% CI: 64.5–72.2], combined mean MMSE score, 29.2 [95% CI: 28.8–29.6]). The meta-analysis revealed a pooled weighted sensitivity of 89.6%, a pooled weighted specificity of 87.2%, and a pooled diagnostic OR of 91.7 for differentiating AD patients from NCs. For the pooled diagnostic OR, heterogeneity was present between the studies, and although the CI did not contain the value of 1, it was wide, suggesting that more studies with larger sample sizes are required for a more definite conclusion on the strength of this diagnostic utility. Spearman correlation coefficient was −0.927 (P = .003) suggesting the absence of a threshold effect. Single-factor meta-regression analysis for other possible sources of heterogeneity did not show any significant association between the variates described previously and log OR. This heterogeneity may contribute to an underestimation of the discriminatory ability of florbetapir.
3.2. Florbetaben
Six case-control studies were identified in this group. Barthel et al. [24] reported on the use of florbetaben PET imaging in a multicenter phase II trial in patients with AD and NCs, finding a sensitivity of 85% and a specificity of 91%. It found that regional standard uptake value ratios (SUVRs) were significantly higher in the frontal, temporal, parietal, occipital, and anterior and posterior cingulate cortices in patients with AD compared with NCs. An ongoing phase III trial on florbetaben PET imaging for detecting in vivo β-amyloid using histopathology as the gold standard is estimated to be completed later this year [33]. Schipke et al. [25] and Tiepolt et al. [26] recruited patients from the phase II trial and were therefore excluded from the meta-analysis.
For the meta-analysis, four studies were included, with a total number of 131 patients with AD (combined mean age, 70.6 years [95% CI: 67.9–73.3], combined mean MMSE score, 21.8 [95% CI: 19.2–24.3]) and 121 NCs (combined mean age, 69.1 years [95% CI: 66.3–71.9], combined mean MMSE score, 29.4 [95% CI: 29.0–29.8]). The meta-analysis resulted in a pooled weighted sensitivity of 89.3%, a pooled weighted specificity of 87.6%, and a pooled diagnostic OR of 69.9, with no significant heterogeneity (all I2 <50% and P values < .05). However, the wide CI for the diagnostic OR again suggests the need for further and larger studies to be conducted.
3.3. Flutemetamol
There were three studies in this group, with the study by Nelissen et al. [32] being a phase I trial and the study by Vandenberghe et al. [31] being a phase II trial for flutemetamol PET imaging in AD. Results were encouraging, with the phase II trial demonstrating a sensitivity of 92.6% and specificity of 96.0% in differentiating AD patients from NCs. The study by Duara et al. [30] was an extension of the phase II trial, which demonstrated the additive value of structural magnetic resonance imaging to the diagnostic classification of prodromal and probable AD. Flutemetamol is structurally identical to 11C-PiB except for the presence of 3′ 18F-fluorine, making it unique compared to the other fluorine tracers. A trial is ongoing on the distribution of 11C-PiB and flutemetamol in regions of cerebral amyloid deposition in AD, with the hypothesis that there will be no significant difference between the two [34].
4. Discussion
Clinical diagnostic criteria in recent use for AD (including iterations of the Diagnostic and Statistical Manual of Mental Disorders and International Classification of Diseases) have variable specificity and sensitivity with pooled averages of 70% and 81%, respectively, when compared with postmortem data [2]. The National Institute on Aging and the Alzheimer's Association work groups have recently revised the NINCDS-ADRDA criteria for AD to incorporate biomarkers in an attempt to improve diagnostic accuracy [35]. A dynamic change in biomarker profiles during the evolution of AD progression has been postulated with early deposition of β-amyloid followed by neuronal degeneration [3]. Cerebral PET amyloid imaging uses tracers that bind to fibrillar β-amyloid plaques and is able to estimate neuritic amyloid plaque burden [4]. This is the first systematic review and meta-analysis to investigate the diagnostic utility of florbetapir, florbetaben, and flutemetamol as biomarkers of amyloid deposition in AD.
Our results demonstrate a pooled weighted sensitivity of 89.6%, a pooled weighted specificity of 87.2%, and a pooled diagnostic OR of 91.7 for florbetapir in differentiating AD patients from NCs. Investigation of florbetaben demonstrated similar results with a pooled weighted sensitivity of 89.3%, a specificity of 87.6%, and a pooled diagnostic OR of 69.9. There were insufficient data to provide pooled statistical analysis of the diagnostic utility of flutemetamol. These results therefore suggest favorable sensitivity and specificity of fluorine-based tracers when compared to clinical diagnosis and other biomarkers commonly used in practice [21] and are comparable to 11C-PiB imaging, with, in addition, good patient tolerability demonstrated [23]. When considering the pooled diagnostic OR, heterogeneity was present between studies, with wide CIs suggesting the need for more and better powered studies. All the studies assessed had good internal validity, reflecting that a causal relationship has been satisfactorily demonstrated and that confounding factors were controlled for as far as possible.
There are, however, a number of important limitations in studies evaluated in this systematic review which must be considered before it can be concluded that amyloid imaging with fluorine-based tracers should be introduced as a routine in the investigation of suspected AD. Although all studies scored highly on measures of internal validity, 10 studies did not score maximum points on external validity; most of these did not report on comorbidities and exclude patients accordingly. The number of subjects included in each study was low, and the number of studies per tracer was limited (Table 2), most notably in the flutemetamol group. Different diagnostic criteria were applied across the studies, adding to interstudy heterogeneity. Pharmaceutical funding of included studies may feasibly have introduced bias.
Characterization of cognitive impairment was variable and somewhat limited in most studies, with MMSE reported in the majority. MMSE is notably easy to administer with high test-retest reliability but lacks diagnostic specificity in AD [36], as demonstrated by the overlap of MMSE scores between those diagnosed with MCI and healthy controls [30] and those with AD and MCI [27]. Some of the studies did use more detailed cognitive batteries, but details of results are not reported in sufficient detail to allow evaluation in a meta-analysis [19], [23], [26], [29].
Only one study correlated imaging findings with histopathological confirmation of AD at postmortem [13], demonstrating a high sensitivity and specificity of 92% and 100%, respectively, for florbetapir. Although these results are encouraging, study inclusion criteria required a predicted life expectancy of less than 6 months, limiting generalizability of these results.
APOE ε4 status is a known risk factor for cerebral amyloid deposition and for the development of AD. APOE ε4 status was demonstrated to significantly influence amyloid imaging results [12], [17], [24] (with Camus et al. [12] demonstrating this as the only significant variable). Reporting of APOE ε4 status was incomplete across studies, and no statistical adjustment was made for results. Furthermore, the significance of amyloid deposition in asymptomatic APOE ε4–positive individuals remains extremely uncertain because of a lack of longitudinal data on natural history in such cases.
A fundamental limitation of amyloid imaging is the lack of specificity of amyloid burden in AD. Amyloid deposition has been implicated in a range of neurodegenerative disorders, including common differential diagnoses for AD, such as DLB and some pathological subtypes of primary progressive aphasia. Villemagne et al. [27] investigated the potential of amyloid imaging in differentiating between patients with FTDs, VD, DLB, Parkinson's disease, MCI, and healthy controls. Differences were only demonstrated between AD and healthy controls (and MCI and healthy controls). Because of small numbers in the subgroup analyses, robust conclusions cannot be drawn about the role of these tracers in the differential diagnosis of dementia without further clarification from larger and better powered studies.
Methods for scan interpretation differed between studies evaluated in this meta-analysis and included visual and quantitative analysis (Table 2). Although quantitative image analysis should feasibly provide a more objective outcome measure, SUVR cutoff varied, with only one study using a SUVR cutoff calculated from patients with postmortem-proven AD and healthy APOE ε4 negative as controls (Fleisher et al. [16]). Visual interpretation varied between studies, with Camus et al. [12] reporting 31% specificity for AD; however, Clark et al. [13] demonstrated a strong correlation between visual review of images and histopathological diagnosis.
Results from our meta-analysis do not enable recommendations to be made regarding transformation of MCI to AD in clinical practice. Doraiswamy et al. [15] provide limited longitudinal data to address this question with a suggestion that positive amyloid imaging in patients with MCI confers a greater risk of progression in MMSE scores. Follow-up, however, was limited to a period of 18 months, and therefore, further prospective longitudinal studies are required to clarify this observation. It is also unknown whether the sensitivity of amyloid imaging varies according to disease stage. Duara et al. [30] found a lower rate of positive amyloid scans among patients with MCI compared with those with AD (50% vs. 93%). Without standardized SUVR reference ranges or further studies assessing predementia phases of AD, it is currently not possible to conclude whether disease stage–specific SUVR thresholds are required to improve the detection of early-phase AD.
The mean age of subjects with AD in the florbetapir, florbetaben, and flutemetamol groups was 72.5, 70.7, and 69.3 years, respectively. Results from this systematic review and meta-analysis cannot, therefore, be easily extrapolated for use of amyloid imaging in early-onset AD in whom symptoms often present atypically including with aphasia, apraxia, agnosia, and visual disturbance, rather than typical amnestic syndromes. Predominant neocortical pathology, sparing the hippocampi, may manifest with a different pattern of biomarkers to disease in older age. The diagnostic utility of amyloid imaging in early-onset disease, therefore, requires separate investigation. Other areas requiring further investigation are the effectiveness, including cost-effectiveness, of amyloid imaging as compared to other currently available diagnostic tools (including CSF biomarkers, 18F-fluorodeoxyglucose positron emission tomography, and single photon emission computed tomography imaging), which are currently lacking.
5. Conclusion
This systematic review and meta-analysis has demonstrated favorable sensitivity and specificity of amyloid imaging with novel fluorine tracers in diagnosis of AD, supporting their use as an adjunct in clinical practice. It has, however, also highlighted a number of areas of uncertainty suggesting the need for further well-powered longitudinal studies including correctly phenotyped patients, correlating with structural imaging and CSF biomarkers, neuropsychology assessment, APOE ε4 status, and ultimately, histopathological disease confirmation, before they can be recommended for routine and widespread use.
Research in context.
-
1.
Systematic review: We searched the Medical Literature Analysis and Retrieval System Online (MEDLINE) and the Excerpta Medica Database (EMBASE) for human studies published from January 1980 to March 2014, inclusive of all languages. Search terms were “dementia”; “amyloid” or “positron emission tomography”; “florbetapir” or “florbetaben” or “flutemetamol”; “sensitivity” and “specificity” or “diagnosis.” Articles fulfilling study inclusion criteria were systematically evaluated using the STARD checklist.
-
2.
Interpretation: To our knowledge, this is the first systematic review and meta-analysis investigating the diagnostic utility of 18F-labeled tracers in Alzheimer's disease (AD); our study demonstrated favorable sensitivity and specificity, supporting their use in clinical practice.
-
3.
Future directions: Our study has highlighted areas of uncertainty suggesting the need for well-powered prospective studies with postmortem histopathology as a diagnostic gold standard. Further investigation is required to evaluate uncertainties including, whether APOE ε4 status influences imaging results and if 18F imaging can successfully discriminate between different dementias.
Acknowledgments
S.P. is funded by a fellowship from NHS Research Scotland.
Footnotes
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.dadm.2014.11.004.
Supplementary data
References
- 1.Beach T.G., Monsell S.E., Philips L.E., Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centres 2005–2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knopman D.S., DeKosky S.T., Cummings J.L., Chui H., Corey-Bloom J., Relkin N. Practice parameter: Diagnosis of dementia (an evidence-based review), report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 3.Jack C.R., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe C.C., Ng S., Ackermann U., Gong S.J., Pike K., Savage G. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 5.Villemagne V.L., Pike K.E., Chetelat G., Ellis K.A., Mulligan R.S., Bourgeat P. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikonomovic M.D., Klunk W.E., Abrahamson E.E., Mathis C.A., Price J.C., Tsopelas N.D. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131(Pt 6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bossuyt P.M., Reitsma J.B., Bruns D.E., Gatsonis C.A., Glasziou P.P., Irwig L.M. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamora J., Abraira V., Muriel A., Khan K.S., Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwig L., Macaskill P., Glasziou P., Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol. 1995;48:119–130. doi: 10.1016/0895-4356(94)00099-c. discussion 131–2. [DOI] [PubMed] [Google Scholar]
- 11.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Camus V., Payoux P., Barré L., Desgranges B., Voisin T., Tauber C. Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. Eur J Nucl Med Mol Imaging. 2012;39:621–631. doi: 10.1007/s00259-011-2021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark C.M., Pontecorvo M.J., Beach T.G., Bedell B.J., Cloeman R.E., Doraiswamy P.M. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 14.Clark C.M., Schneider J.A., Bedell B.J., Beach T.G., Bilker W.B., Mintun M.A. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doraiswamy P.M., Sperling R.A., Coleman R.E., Johnson K.A., Reiman E.M., Davis M.D. Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study. Neurology. 2012;79:1636–1644. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleisher A.S., Chen K., Liu X., Roontiva A., Thiyyagura P., Ayutyanont N. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–1411. doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 17.Fleisher A.S., Chen K., Liu X., Ayutyanont N., Roontiva A., Thiyyagura P. Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging. 2013;34:1–12. doi: 10.1016/j.neurobiolaging.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Grundman M., Pontecorvo M.J., Salloway S.P., Doraiswamy P.M., Fleisher A.S., Sadowsky C.H. Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord. 2013;27:4–15. doi: 10.1097/WAD.0b013e318279d02a. [DOI] [PubMed] [Google Scholar]
- 19.Joshi A.D., Pontecorvo M.J., Clark C.M., Carpenter A.P., Jennings D.L., Sadowsky C.H., Florbetapir F 18 Study Investigators Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer's disease and cognitively normal subjects. J Nucl Med. 2012;53:378–384. doi: 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- 20.La Joie R., Perrotin A., Barré L., Hommet C., Mézenge F., Ibazizene M. Region-specific hierarchy between atrophy, hypometabolism, and β-amyloid (Aβ) load in Alzheimer's disease dementia. J Neurosci. 2012;32:16265–16273. doi: 10.1523/JNEUROSCI.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newberg A.B., Arnold S.E., Wintering N., Rovner B.W., Alavi A. Initial clinical comparison of 18F-florbetapir and 18F-FDG PET in patients with Alzheimer disease and controls. J Nucl Med. 2012;53:902–907. doi: 10.2967/jnumed.111.099606. [DOI] [PubMed] [Google Scholar]
- 22.Wong D.F., Rosenberg P.B., Zhou Y., Kumar A., Raymont V., Ravert H.T. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18) J Nucl Med. 2010;51:913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barthel H., Luthardt J., Becker G., Patt M., Hammerstein E., Hartwig K. Individualized quantification of brain β-amyloid burden: results of a proof of mechanism phase 0 florbetaben PET trial in patients with Alzheimer's disease and healthy controls. Eur J Nucl Med Mol Imaging. 2011;38:1702–1714. doi: 10.1007/s00259-011-1821-1. [DOI] [PubMed] [Google Scholar]
- 24.Barthel H., Gertz H.J., Dresel S., Peters O., Bartenstein P., Buerger K. Cerebral amyloid-β PET with florbetaben (18F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011;10:424–435. doi: 10.1016/S1474-4422(11)70077-1. [DOI] [PubMed] [Google Scholar]
- 25.Schipke C.G., Peters O., Heuser I., Grimmer T., Sabbagh M.N., Sabri O. Impact of beta-amyloid-specific florbetaben PET imaging on confidence in early diagnosis of Alzheimer's disease. Dement Geriatr Cogn Disord. 2012;33:416–422. doi: 10.1159/000339367. [DOI] [PubMed] [Google Scholar]
- 26.Tiepolt S., Barthel H., Butzke D., Hesse S., Patt M., Gerz H.J. Influence of scan duration on the accuracy of β-amyloid PET with florbetaben in patients with Alzheimer's disease and healthy volunteers. Eur J Nucl Med Mol Imaging. 2013;40:238–244. doi: 10.1007/s00259-012-2268-8. [DOI] [PubMed] [Google Scholar]
- 27.Villemagne V.L., Ong K., Mulligan R.S., Holl G., Pejoska S., Jones G. Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J Nucl Med. 2011;52:1210–1217. doi: 10.2967/jnumed.111.089730. [DOI] [PubMed] [Google Scholar]
- 28.Rowe C.C., Ackerman U., Browne W., Mulligan R., Pike K.L., O'Keefe G. Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 29.Villemagne V.L., Mulligan R.S., Pejoska S., Ong K., Jone G., O'Keefe G. Comparison of 11C-PiB and 18F-florbetaben for Aβ imaging in ageing and Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2012;39:983–989. doi: 10.1007/s00259-012-2088-x. [DOI] [PubMed] [Google Scholar]
- 30.Duara R., Loewenstein D.A., Shen Q., Barker W., Potter E., Varon D. Amyloid positron emission tomography with (18)F-flutemetamol and structural magnetic resonance imaging in the classification of mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2013;9:295–301. doi: 10.1016/j.jalz.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenberghe R., Van Laere K., Ivanoiu A., Salmon E., Bastin C., Triau E. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 32.Nelissen N., Van Laere K., Thurfjell L., Owenius R., Vandenbulcke M., Koole M. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med. 2009;50:1251–1259. doi: 10.2967/jnumed.109.063305. [DOI] [PubMed] [Google Scholar]
- 33.ClinicalTrials.gov. Phase III study of florbetaben (BAY94–9172) PET imaging for detection/exclusion of cerebral β-amyloid compared to histopathology. NCT01020838.
- 34.ClinicalTrials.gov. Bridging study of C11 PiB and F18 flutemetamol brain PET. NCT01607476.
- 35.Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark C., Sheppard L., Fillenbaum G.G., Galasko D., Morris J.C., Koss E. Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease—a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol. 1999;56:857–862. doi: 10.1001/archneur.56.7.857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.