Abstract
Introduction
There are no blood-based biomarkers for cognitive decline in aging, or mild cognitive impairment (MCI) and Alzheimer's disease (AD). Cumulative evidence suggests that apolipoproteins, complement system, and transthyretin are involved in AD pathogenesis by sequestration of amyloid β. However, there is no clinical study to assess the utility of “sequester proteins” in risk assessment and/or diagnosis of MCI and AD.
Methods
Serum levels of sequester proteins and their clinical potential in cognitive decline assessment were analyzed by longitudinal and cross-sectional studies using independent cohorts and were confirmed by a prospective study.
Results
A combination of apolipoprotein A1, complement C3, and transthyretin achieved an area under the curve of 0.89 (sensitivity 91% and specificity 80%) in MCI versus healthy controls and also discriminated individuals with mild cognitive decline from healthy controls.
Discussion
A set of sequester proteins could be blood-based biomarkers for assessment of early stages of cognitive decline.
Keywords: Alzheimer's disease, Apolipoprotein, Biomarker, Complement system, Diagnostics, Mild cognitive impairment, Transthyretin
1. Introduction
Biomarker discovery for neurologic disorders is challenging but rewarding because it facilitates early diagnosis, monitors disease progression, and assesses response to medical treatment. Currently, the number of people who have been diagnosed with dementia worldwide is 35.6 million, and it is predicted that this rate will increase to >100 million people in 2050 [1]. In fact, 15% of individuals (4.86 million) aged ≥65 years have been affected in Japan [2]. Diagnosis and intervention at early stages of dementia may greatly reduce the number of individuals suffering from this debilitating disease.
Alzheimer's disease (AD) is the most common form of dementia with >24 million people suffering from this neurodegenerative disorder worldwide [3]. In the United States, 5.2 million cases of AD were reported in 2014, and this number is predicted to double by 2050 [4]. Mild cognitive impairment (MCI) is a syndrome defined as cognitive decline that is greater than expected for an individual's age and is regarded as a risk group for AD [5]. MCI is considered as a prestage of dementia, and without clinical intervention, 40% of patients with MCI may convert to AD within 4 years of diagnosis [5]. To reduce the number of people with dementia, the development of biomarkers for cognitive decline and intervention, which may prevent the progression of MCI to dementia, is urgently needed.
Histopathologically, AD is distinguished from other neurologic diseases, including other types of dementia and normal aging, by abundant deposits of fibrillar amyloid β (Αβ). Aβ-derived diffusible ligands (ADDLs) cause abnormal spine morphology and a significant decrease in spine density [6]. ADDLs trigger synaptic dysfunction, which may lead to memory loss in AD [7].
In healthy individuals, Aβ may be removed by clearance mechanisms such as phagocytosis by microglia. Complement proteins sequestrate Aβ via an innate immune response in the brain [8]. Aβ has been shown to act as a proinflammatory agent causing the activation of the complement system. C3, C4, and complement factor H were seen to be present in Aβ plaques in AD [9]. Overexpression of chemokines, such as macrophage inflammatory protein 4 (MIP-4) [10], in microglia and astrocytes has suggested the involvement of inflammation in the brain. The presence of α2-macroglobulin is also a genetic risk factor for AD, which may be involved in Aβ clearance [11].
Another group of sequester proteins, such as apolipoproteins (apolipoprotein E [apoE], apolipoprotein A1 [apoA1], and apolipoprotein J [apoJ]), bind to Aβ to prevent its aggregation and reduce its toxicity and are involved in Aβ clearance [12], [13], [14]. Transthyretin (TTR) or pre-albumin has been found in cerebrospinal fluid (CSF) as an Aβ-binding protein and suppresses the toxicity of oligomers [15], [16].
Thus, these sequester proteins have neuroprotective functions and may help delay the pathologic progression of AD via Aβ clearance. Considering the crucial role of these proteins in disease pathogenesis, we hypothesized that sequester proteins have a clinical potential for risk assessment of cognitive impairment and for early diagnosis of MCI and AD.
In this study, we analyzed peripheral biomarkers to identify cognitive decline by focusing on proteins involved in Aβ sequestration and validated the clinical utility of these proteins as biomarkers in identifying MCI and AD by both longitudinal and cross-sectional cohort studies.
2. Methods
2.1. Participants and cohort size
In a longitudinal study, participants were recruited from the “Tone Project” that was performed in Ibaraki, Japan [17]. In the Tone cohort, 1270 available participants who were ≥65 years and cognitively normal in 2001 were set as the baseline. Among them, 1024 participants were followed up in 2005, and 584 participants were followed up in 2008. Serum samples were collected in 2001, 2005, and 2008. Paired blood samples from the same participant in 2005 and 2008 were used in this study. Based on the diagnosis of participants followed up in 2012, we selected participants with MCI due to AD from 2005 to 2008 and excluded participants who progressed to other types of dementia. After the selection of MCI/AD, we randomly selected age-matched nondemented disease control (NDC) subjects and adjusted its number with that of MCI. We carefully chose samples in the NDC group in 2005 and 2008 from individuals who had also been diagnosed as NDC in 2001 and 2005, respectively. The serum samples from participants were grouped as follows: group 1, NDC-NDC (n = 20), comprised individuals who had remained cognitively normal during the follow-up; group 2, NDC-MCI (n = 9), comprised participants who had progressed from normal to MCI; group 3, MCI-stable MCI (sMCI)/AD (n = 6), comprised participants who were diagnosed with MCI in 2005 and MCI or AD in 2008. See Supplementary Methods for details of the Tone cohort.
In a cross-sectional study, patients were from the Tsukuba University Hospital (Tsukuba, Japan) and were set as the Tsukuba cohort. The sample size of the Tsukuba cohort was 441 and included AD, MCI, other types of dementia, and other psychiatric diseases; in particular, we selected from participants with AD, amnestic MCI (aMCI), and NDC. All cases that had the mini-mental state examination (MMSE) score data were included.
In a prospective study, a total of 258 participants from Uji City, Kyoto, Japan, were enrolled to evaluate biomarker suitability for the detection of cognitive decline. APOE genotyping and MMSE were also performed. Of the 258 participants, 64 were recruited for further clinical diagnosis; among these, 23 had MCI. To evaluate sequester proteins as biomarkers for early stage cognitive impairment, we designated as pre-MCI those participants with MMSE scores of 27–29. In addition, regardless of the MMSE score, the participants with decreased cerebral blood flow in the parietal lobe, posterior cingulate gyrus, and precuneus on single-photon emission computed tomography [18] and/or those with atrophy of the hippocampus on magnetic resonance imaging [19] were categorized as pre-MCI.
In all cohort studies, participants with any psychiatric illness according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria were excluded. Written informed consent was obtained from all eligible participants. All protocols in the present work were approved by the ethics committee of each institute.
2.2. Procedures for diagnosis
The team reviewed the functional, medical, neurologic, psychiatric, and neuropsychological data to reach a diagnostic consensus on dementia according to the DSM-IV criteria.
Criteria for MCI were retrospectively applied among the nondemented individuals after the conference. Consistent with the standard criteria, for all subtypes of MCI described in the following, the participants considered to have MCI were required to have (1) objective impairment in one cognitive domain based on the average of scores on neuropsychological measures within that domain, and 1 standard deviation (SD) and 1.5 SD cutoffs derived from normative corrections for age, years of education, and sex; (2) essentially preserved activities of daily living; (3) presence of memory complaints; and (4) no diagnosis of dementia by group consensus.
First, for our subtype of aMCI single, memory impairment was defined as a score <1 or 1.5 SD below the demographically corrected mean on the category-cued recall test; performance on scores from all other cognitive domains was required to fall within normal limits (>1 or 1.5 SD below the demographically corrected mean). Second, aMCI multiple was diagnosed in the presence of memory impairment in one or more cognitive domains. Third, a diagnosis of nonamnestic MCI (naMCI) single required cognitive impairment in a single non-memory domain and normal performance scores in all other cognitive domains. Finally, naMCI multiple was diagnosed if impairment was seen in two of the four non-memory domains and when the memory domain score was within normal limits. Individuals without dementia and MCI were defined as NDC. Diagnostic criteria described previously were applied to all three cohorts.
2.3. Serum sampling
Serum sample stored at −80°C was transferred to the laboratory for analysis, thawed on ice, and 40 μL of aliquots were generated. These aliquots were stored at −80°C for immunoassay. Almost all the serum samples were frozen and thawed two times, and some were frozen and thawed three times. There were no significant changes in protein levels for freeze-thaw cycles less than five (data not shown).
2.4. Immunoassay
Serum levels of sequester proteins from all participants were analyzed by a multi-immunoassay. Protein concentrations were determined using HNDG1-36K (complement C3 [C3], TTR, apoE, apoA1) and HNDG2-36K kits (complement C4 [C4], MIP-4; EMD Millipore, Billerica, MA, USA), following the manufacturers' instructions. The inactive form of C3 was proteolytically cleaved during activation, and the multiplex immunoassay specifically detected an inactive form of C3 but not the activated fragment.
2.5. Statistical analysis
A P value of ≤.05 was considered significant. The area under the curve (AUC) of the receiver operating characteristic curve (ROC) was quantified by C-statistics. The closest point to the upper left corner of the ROC curve gave the optimum sensitivity and specificity values. The least absolute shrinkage and selection operator (LASSO) modeling using glmnet package (version 1.9-5) for R (version 3.1.0) was used to evaluate the combination of multiple biomarkers. The softwares Origin (version 7.5) (OriginLab Corp, Northampton, MA, USA) and MedCalc (version 14.8.1) (MedCalc Software, Mariakerke, Belgium) were used to perform statistical analyses. See Supplementary Methods for details of statistical analysis.
3. Results
3.1. Longitudinal analysis of serum apoA1, apoE, C3, C4, TTR, and MIP-4 levels in the Tone cohort
In 2005, 211 of 1024 participants who were followed up were diagnosed with MCI. In 2008, among 584 participants who were followed up, 92 additional participants were diagnosed with MCI. There were no significant distributional differences for age, education, body mass index, and geriatric depression scale [20] among the groups (Supplementary Table 1). Both aMCI and naMCI were included.
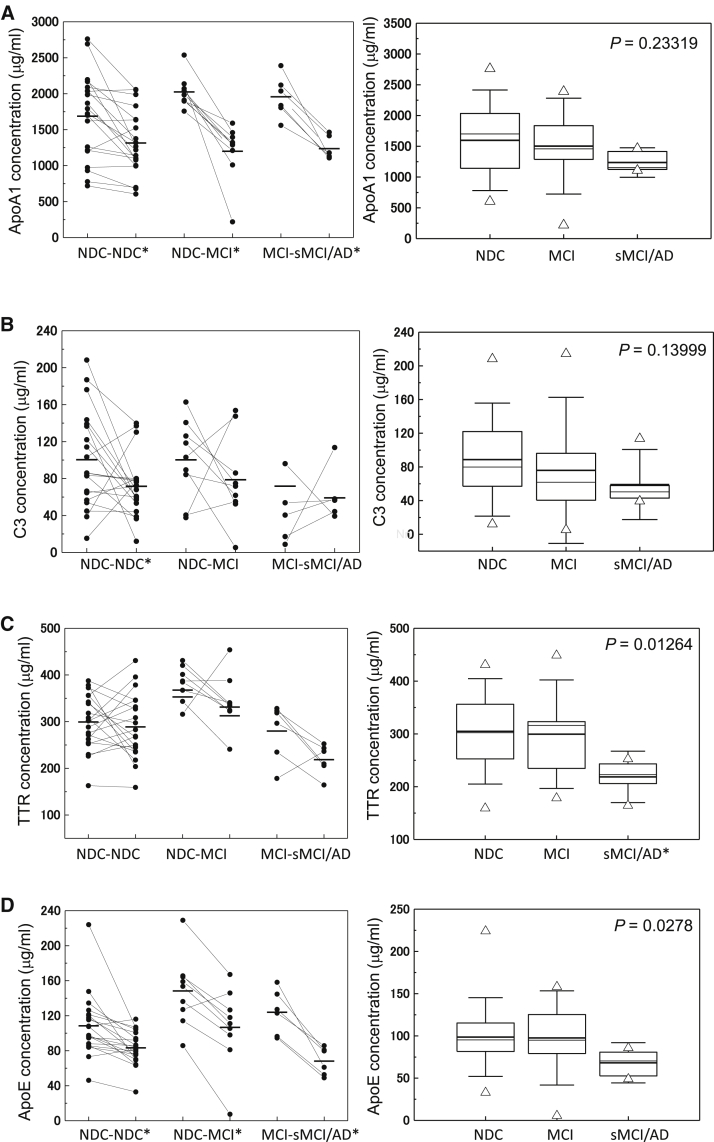
As shown in the left panels of Fig. 1, longitudinal comparison of individuals in group 2 (NDC-MCI) who were NDC in 2005 and had MCI in 2008 showed substantial decrease in serum apoA1 (P = 8.14E-04) and apoE (P = .00102), although normal aging also showed decreasing trends. Moreover, in group 3 (MCI-sMCI/AD), serum levels of apoA1 (P = .00179) and apoE (P = .00161) showed significant decrease during progression of the disease. TTR concentrations showed substantial decrease in the NDC-MCI and MCI-sMCI/AD groups but were not associated with normal aging (Supplementary Table 2). Serum C4 levels increased in individuals with normal aging and those with progression of the disease; MIP-4 levels did not significantly change (Supplementary Fig. 1, left panel).
Fig. 1.
Serum levels of sequester proteins apoA1 (A), C3 (B), TTR (C), and apoE (D) during progression of cognitive impairment from 2005 to 2008 in the longitudinal (left panel) and cross-sectional (right panel) studies from the Tone cohort. In the left panel, asterisks indicate significant changes in protein levels between 2005 and 2008 samples. In the right panel, the bold solid bars within the boxplot represent the median abundance, and the solid bars represent mean abundance for the given group. Open triangles are the highest and lowest values in each group. Error bars represent ±1.5 SD. Significant differences among the three groups are indicated (Kruskal-Wallis test). In the longitudinal analysis, significant changes in protein levels between paired samples (Wilcoxon signed-rank test) are indicated by (*). ApoA1: NDC-NDC (P = .00109), NDC-MCI (P = .00915), MCI-sMCI/AD (P = .03603), apoE: NDC-NDC (P = 4.19E-04), NDC-MCI (P = .01285), and MCI-sMCI/AD (P = .03603). In the cross-sectional analysis, significant changes in protein levels between two groups (Bonferroni test) are likewise indicated (*). TTR: NDC versus sMCI/AD (P = .02025). Abbreviations: TTR, transthyretin; SD, standard deviation; NDC, nondemented disease control; MCI, mild cognitive impairment; sMCI, stable mild cognitive impairment; AD, Alzheimer's disease.
All data of the Tone cohort were also analyzed cross-sectionally (Fig. 1 and Supplementary Fig. 1: right panels and Supplementary Table 3). There was a significant decrease of TTR (P = .01264) and apoE (P = .0278) among the NDC, MCI, and sMCI/AD groups, and TTR levels showed significant differences between NDC and sMCI/AD (P = .02025; Fig. 1C and D). We also found that apoA1 and C3 levels showed a decreasing trend in MCI and sMCI/AD.
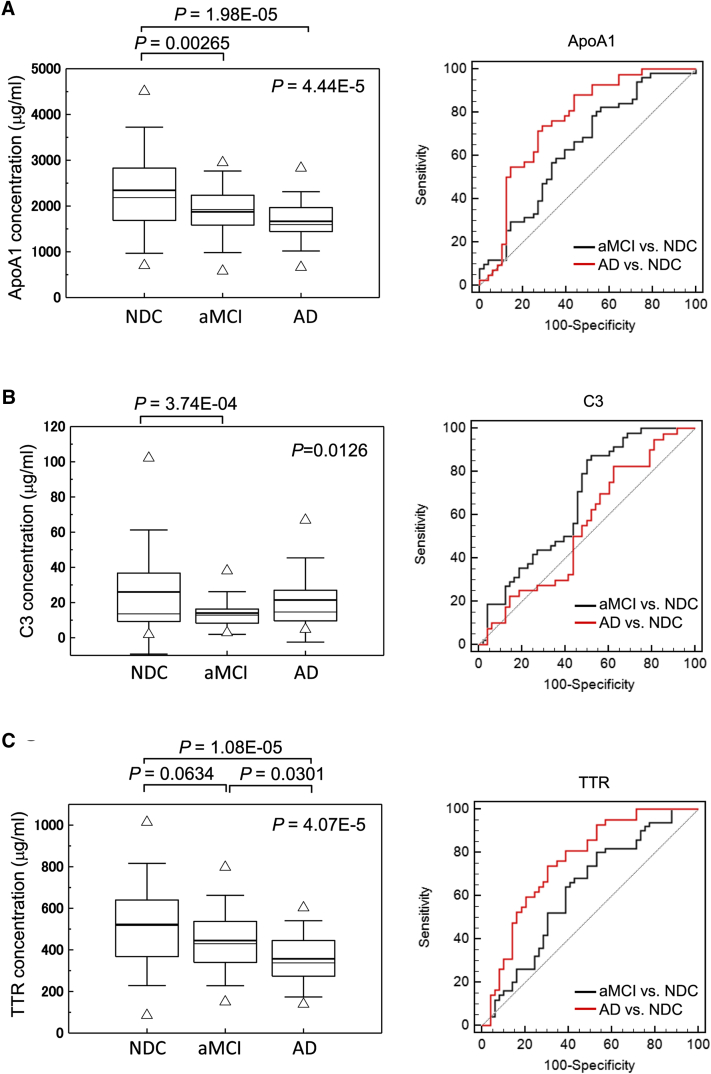
3.2. Cross-sectional analysis of serum apoA1, C3, and TTR levels in the Tsukuba cohort
As shown in Table 1 and Fig. 2A–C(left panel; boxplots), low levels of serum apoA1 (P = 4.44E-05), C3 (P = .0126), and TTR (P = 4.07E-05) were found with progression of cognitive impairment. ApoA1 and TTR seemed to gradually decrease in individuals with aMCI and AD. On the other hand, serum C3 was decreased in the aMCI group and was the same or increased in the AD group. ApoE, C4, and MIP-4 did not show statistically significant differences among the groups (Supplementary Fig. 2).
Table 1.
Characteristics of participants and serum levels of sequester proteins in MCI and AD in the cross-sectional study of the Tsukuba cohort
| Characteristics | NDC (n = 49) | aMCI (n = 51) | AD (n = 42) | P value∗ |
|---|---|---|---|---|
| Age | 69.8 ± 12.4† | 71.2 ± 7.9 | 73.9 ± 7.4 | .27876 |
| Male/female | 33/16 | 21/30 | 10/32 | |
| MMSE score | 28.8 ± 1.6 | 26.7 ± 2.1 | 18.3 ± 5.8 | 2.02E-18 |
| ApoA1‡ | 2345.3 ± 918.0 | 1873.6 ± 594.4§ | 1665.3 ± 431.8|| | 4.44E-05 |
| C3‡ | 28.1 ± 24.1 | 14.0 ± 8.0§ | 21.5 ± 15.9 | .01264 |
| TTR‡ | 520.5 ± 144.8 | 445.0 ± 144.8 | 357.0 ± 122.1|| | 4.07E-05 |
Abbreviations: MCI, mild cognitive impairment; AD, Alzheimer's disease; NDC, nondemented disease control; aMCI, amnestic mild cognitive impairment; MMSE, mini-mental state examination; TTR, transthyretin; SD, standard deviation.
Kruskal-Wallis test. Significant differences among the three groups are indicated.
Mean ± SD.
mg/mL.
Bonferroni test. Significant differences in NDC versus aMCI were observed in apoA1 (P = .00265) and C3 (P = 3.74E-04).
Bonferroni test. Significant differences in NDC versus AD were observed in apoA1 (P = 1.98E-05) and TTR (P = 1.08E-02).
Fig. 2.
Cross-sectional analysis of serum apoA1 (A), C3 (B), and TTR (C) levels and their corresponding levels according to the MMSE scores (D) in the Tsukuba cohort. C-statistics analysis of the sequester proteins in MCI and AD is shown in the right panel. In boxplot, the bold solid bars within the boxplot represent the median abundance, and the solid bars represent mean abundance for the given group. Open triangles are the highest and lowest values in each group. Error bars represent ±1.5 SD. Significant differences among three groups are indicated (Kruskal-Wallis test). Significant difference in protein levels between two groups are also indicated (Bonferroni test). Abbreviations: TTR, transthyretin; MMSE, mini-mental state examination; MCI, mild cognitive impairment; SD, standard deviation; aMCI, amnestic mild cognitive impairment; NDC, nondemented disease control; AD, Alzheimer's disease.
As shown in Fig. 2A–C (right panel; ROC curve), three sequester proteins distinguished MCI from NDC: apoA1 (AUC = 0.64, sensitivity 78%, specificity 48%, P = .0124); C3 (AUC = 0.67, sensitivity 88%, specificity 48%, P = .0033); and TTR (AUC = 0.62, sensitivity 80%, specificity 47%, P = .0298). The AD versus NDC comparison revealed higher sensitivity and specificity in apoA1 (AUC = 0.76, sensitivity 74%, specificity 71%, P < .0001) and TTR (AUC = 0.76, sensitivity 74%, specificity 69%, P < .0001).
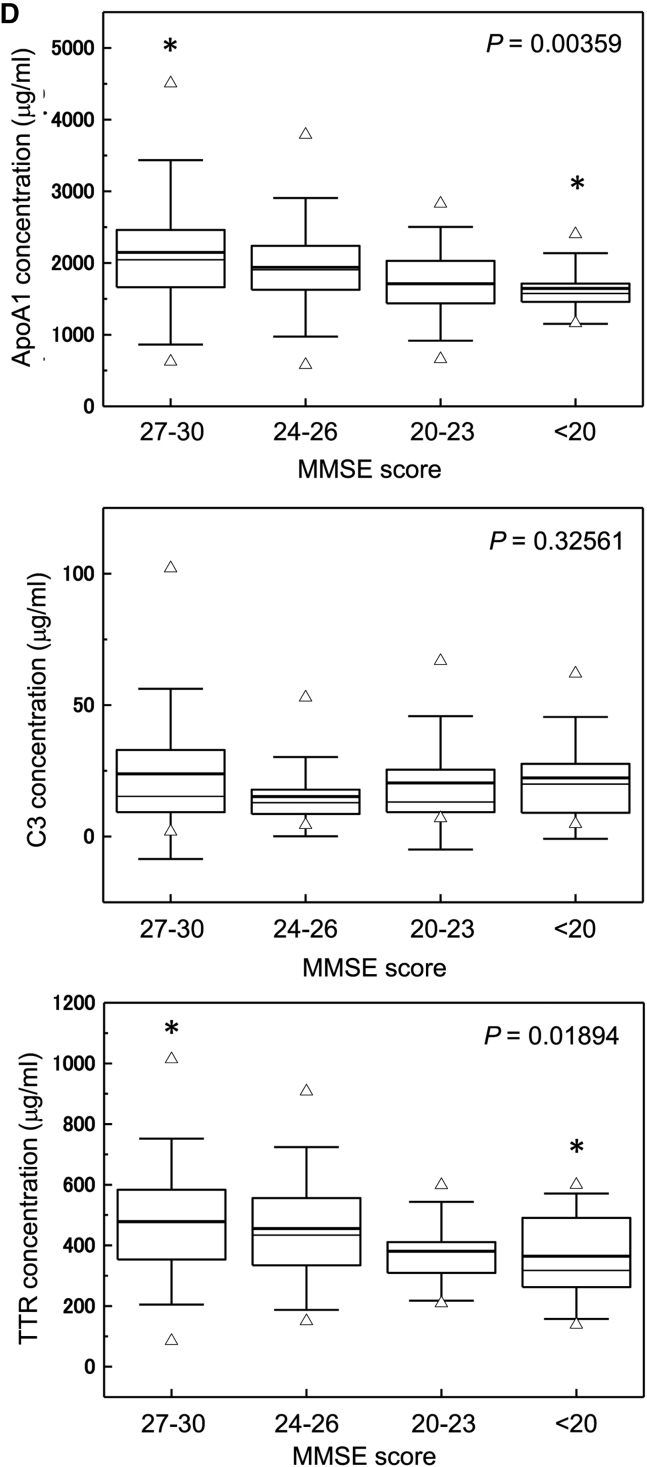
We categorized individuals according to MMSE score into four groups (score 27–30 [n = 71], 24–27 [n = 34], 20–23 [n = 17], and <20 [n = 20]) that were age-matched and compared levels of these three sequester proteins (Fig. 2D, Supplementary Table 4). Individuals with lower MMSE scores had significantly decreased apoA1 (P = .00359) and TTR (P = .01894). Individuals with MMSE scores <20 showed significantly low levels of apoA1 (P = .0396) and TTR (P = .05074) compared with individuals with MMSE scores of 27–30.
C4, apoE, and MIP-4 did not show clinical potential to distinguish aMCI or AD from NDC. C4 and apoE were not significantly different among the groups categorized by the MMSE score (Supplementary Figs. 2 and 3).
3.3. Multivariable statistical analysis of biomarker proteins
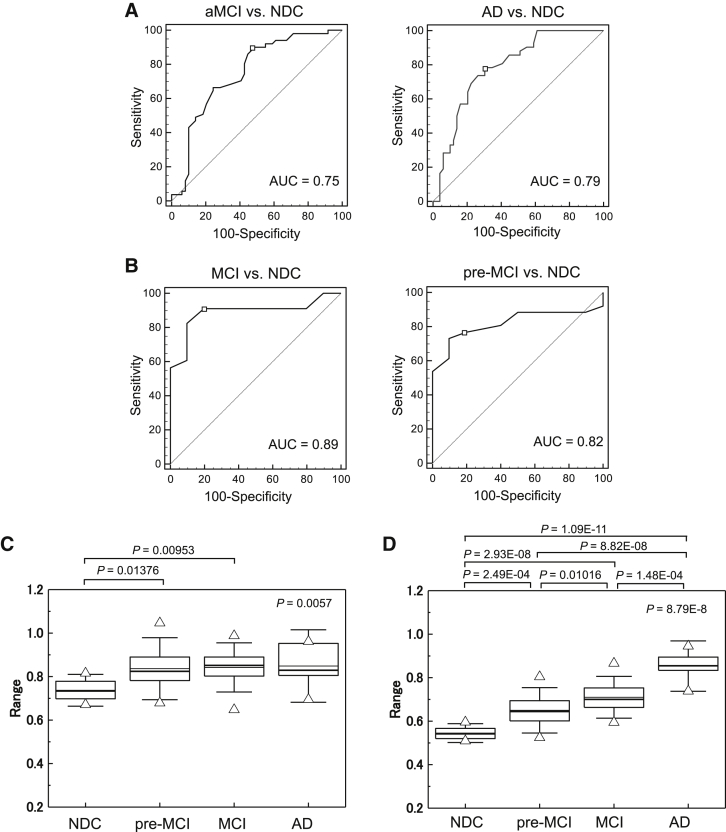
All cross-sectional data from the Tsukuba cohort were used for multivariable statistical analysis (Table 2A and Fig. 3A). In the LASSO regression, the optimal value of parameter λ was obtained by a combination of apoA1, C3, and TTR. In the NDC versus aMCI comparison, AUC was 0.75 (P < .0001) with 90% sensitivity and 53% specificity. In NDC versus AD, AUC was 0.79 (P < .0001) with 79% sensitivity and 69% specificity. Addition of MMSE score improved AUC values up to 0.83 and 1.00 in the MCI versus NDC and AD versus NDC comparisons, respectively. These results indicate that combination of C3, apoA1, and TTR were clinically useful in the assessment of early stages of cognitive impairment.
Table 2.
Clinical potential of a set of three sequester proteins as biomarker for pre-MCI, MCI, and AD analyzed by LASSO regression
| Disease | Biomarker | Sensitivity (%) | Specificity (%) | AUC | 95% CI | P value | Criterion |
|---|---|---|---|---|---|---|---|
| (A) Discrimination of MCI or AD from NDC in the cross-sectional study of the Tsukuba cohort | |||||||
| aMCI versus NDC | ApoA1 | 90 | 53 | 0.75 | 0.654–0.832 | <.0001 | 0.43 |
| C3 | |||||||
| TTR | |||||||
| AD versus NDC | ApoA1 | 79 | 69 | 0.79 | 0.687–0.865 | <.0001 | 0.46 |
| C3 | |||||||
| TTR | |||||||
| aMCI versus NDC | ApoA1 | 82 | 73 | 0.83 | 0.745–0.900 | <.0001 | 0.54 |
| C3 | |||||||
| TTR | |||||||
| MMSE score | |||||||
| AD versus NDC | ApoA1 | 100 | 94 | 1.00 | 0.953–1.000 | <.0001 | 0.26 |
| C3 | |||||||
| TTR | |||||||
| MMSE score | |||||||
| (B) Discrimination of pre-MCI or MCI from NDC in the prospective study of the Uji cohort | |||||||
| pre-MCI versus NDC | ApoA1 | 77 | 80 | 0.82 | 0.655–0.927 | <.0001 | 0.65 |
| C3 | |||||||
| TTR | |||||||
| MCI versus NDC | ApoA1 | 91 | 80 | 0.89 | 0.731–0.971 | <.0001 | 0.65 |
| C3 | |||||||
| TTR | |||||||
| pre-MCI versus NDC | ApoA1 | 88 | 90 | 0.92 | 0.784–0.985 | <.0001 | 0.42 |
| C3 | |||||||
| TTR | |||||||
| MMSE score | |||||||
| MCI versus NDC | ApoA1 | 100 | 100 | 1.00 | 0.745–1.000 | <.0001 | 0.44 |
| C3 | |||||||
| TTR | |||||||
| MMSE score | |||||||
Abbreviations: MCI, mild cognitive impairment; AD, Alzheimer's disease; LASSO, least absolute shrinkage and selection operator; AUC, area under the curve; CI, confidence interval; NDC, nondemented disease control; TTR, transthyretin; aMCI, amnestic mild cognitive impairment.
Fig. 3.
Evaluation of clinical potential of a set of serum apoA1, C3, and TTR in the cross-sectional (A and B; C-statistics) and prospective studies using LASSO regression model (C and D; boxplots). Significant differences were observed in NDC versus pre-MCI and NDC versus MCI (C). Addition of the MMSE score to these protein levels in LASSO analysis increased clinical potential (D; NDC versus pre-MCI [P = 2.49E-04], NDC versus MCI [P = 2.93E-08], NDC versus AD [P = 1.09E-11], pre-MCI versus MCI [P = .01016], MCI versus AD [P = 1.48E-04], and pre-MCI versus AD [P = 8.82E-08]). The bold solid bars within the boxplot (C, D) represent the median abundance, and the solid bars represent mean abundance for the given group. Open triangles are the highest and lowest values in each group. Error bars represent ±1.5 SD. Kruskal-Wallis test was used to determine statistical significance among the groups, and Bonferroni test was used in the two-group comparison. Schematic model of changes of serum sequester protein levels with progression of cognitive impairment. (E). Serum levels of C3 and apoA1 show decreased trends in early stages in cognitive impairment and apoA1 continues to decrease in progression to AD. Serum TTR levels was not changed in cognitive decline at early stage but decreased in MCI and AD. Abbreviations: TTR, transthyretin; LASSO, least absolute shrinkage and selection operator; NDC, nondemented disease control; MCI, mild cognitive impairment; AD, Alzheimer's disease; aMCI, amnestic mild cognitive impairment; AUC, area under the curve.
3.4. Clinical performance of a set of sequester proteins as biomarkers for cognitive decline in a prospective study
In the present study, we hypothesized that a combination of biomarkers, specifically apoA1, C3, and TTR, would lead to improved discrimination between cognitively normal individuals and those with MCI and AD. To confirm the clinical potential of this multivariable statistical test, we examined whether a combination of serum apoA1, C3, and TTR could discriminate cognitive impairment at early stages from NDC in a prospective study. We enrolled 258 participants and performed immunoassay of apoA1, C3 and TTR, and MMSE (Supplementary Table 5).
We next calculated the probability of MCI in the 64 participants who had clinical diagnosis using equations derived from the LASSO regression of the Tsukuba cohorts. Characteristics of participants with clinical diagnosis and serum apoA1, C3, and TTR levels are summarized in Table 3. Surprisingly, a combination of C3, apoA1, and TTR was sufficient in discriminating not only MCI but also pre-MCI from NDC with a high ROC value (AUC = 0.89, sensitivity 91% and specificity 80% in MCI versus NDC; AUC = 0.82, sensitivity 77% and specificity 80% in pre-MCI versus NDC; Table 2B, Fig. 3B–D). Significant differences were observed in NDC versus MCI (P = .00953) and NDC versus pre-MCI (P = .01376). Addition of the MMSE score in the equation resulted in more obvious discriminations.
Table 3.
Characteristics of participants with clinical diagnosis and serum apoA1, C3, and TTR levels in the prospective study for MCI and AD risk analysis
| Characteristics | NDC (n = 10) | Pre-MCI (n = 26) | MCI (n = 23) | AD (n = 5) | P value∗ |
|---|---|---|---|---|---|
| Age | 62.6 ± 8.3† | 65.5 ± 10.5 | 69.9 ± 9.6 | 77.0 ± 3.7 | .11499 |
| Male/female | 2/8 | 7/19 | 14/9 | 1/4 | |
| APOE ε4 carrier, % | 10.0 | 19.2 | 43.5 | 20.0 | |
| MMSE score | 30.0 ± 0.0 | 28.9 ± 1.0 | 27.3 ± 1.6 | 23.4 ± 0.5 | 8.39E-08 |
| ApoA1‡ | 2044.0 ± 235.9 | 1583.6 ± 470.4§ | 1605.1 ± 381.4|| | 1412.1.4 ± 520.4 | .00411 |
| C3‡ | 7.5 ± 2.3 | 5.7 ± 2.2 | 5.0 ± 2.2|| | 6.2 ± 3.2 | .01699 |
| TTR‡ | 425.2 ± 104.2 | 339.8 ± 144.9 | 320.8 ± 126.0 | 373.2 ± 149.9 | .03065 |
Abbreviations: TTR, transthyretin; MCI, mild cognitive impairment; AD, Alzheimer's disease; NDC, nondemented disease control; MMSE, mini-mental state examination; SD, standard deviation.
Kruskal-Wallis test. Significant differences among the three groups (NDC, pre-MCI, and MCI) are indicated.
Mean ± SD.
mg/mL.
Bonferroni test. Significant differences in NDC versus pre-MCI were observed in apoA1 (P = .02298).
Bonferroni test. Significant differences in NDC versus MCI were observed in apoA1 (P = .03907) and C3 (P = .03305).
4. Discussion
The diagnosis of MCI and AD in the early stages is still a challenge for general physicians at clinics and hospitals because of the difficulty in determining the onset of nascent and measurable cognitive impairment and also because of the absence of reliable biomarkers. To our knowledge, this is the first study to show the clinical utility of the Aβ sequester protein as a biomarker in screening individuals with cognitive decline, MCI, and AD.
In the cross-sectional and longitudinal cohort studies, we found a set of sequester proteins, apoA1, C3, and TTR, that were useful for the assessment of cognitive decline (Supplementary Table 6). Multivariable statistical analysis revealed that this set of serum proteins could discriminate not only MCI and AD but also the early stages of cognitive decline from NDC. Serum apoA1 and TTR showed decreased levels in individuals with lower MMSE scores. Because the MMSE score is included in the criteria for the diagnosis of AD, AUC in AD versus NDC was expected to improve. MCI and NDC are in the same range of the MMSE score (24–30) by diagnostic criteria. Interestingly, the MMSE score also improved AUC in NDC versus MCI.
Serum levels of TTR and apoA1 were found to gradually decrease during the progression of cognitive decline in individuals with sMCI and AD. On the other hand, C3 levels were observed to decrease during the early stages of cognitive decline. ApoA1 and TTR showed similar decreasing trends during the progression of cognitive decline. A hypothetical model of dynamic AD biomarkers related to the pathologic cascade was proposed by Jack et al. [21]. A series of pathologic events leading to cognitive impairment occur before atrophy of certain brain areas can be detected by magnetic resonance imaging. Notably, these events occur more than 10–20 years before the onset of AD. The term “preclinical stage” is now generally accepted as the initial process of AD pathogenesis. The serum levels of apoA1, C3, and TTR may decrease during progression from a cognitively normal state to preclinical AD and MCI. Differences in protein levels between NDC and MCI/AD in the Tone cohort seemed to be obscured compared with those in the Tsukuba and Uji cohorts. This may be explained by the fact that sequester proteins tend to decrease with normal aging; thus, the higher average age (by 6–9 years) of the participants in the Tone cohort, compared with those of other two cohorts, may have affected the results.
Diabetes mellitus has been reported to increase the risk for dementia including AD [22], [23]. However, in this study, we did not have sufficient data on fasting serum samples. Further studies would be needed to analyze whether environmental and biological factors affect the levels of these sequester proteins.
ApoA1 is the main protein constituent of high-density lipoproteins and is well-known for its important role in reverse cholesterol transport. Aβ binds to cholesterols, such as apoA1 and apoE, at the blood-brain barrier [24]. Moreover, there are decreased levels of apoA1 in CSF of patients with AD [25], [26]. There was a significant association between the APOE ε4 allele and AD [27]. In this study, there was no significant change in serum levels of apoE during progression of the disease. It should be noted that literature varies on apoE protein levels in CSF and blood of patients with AD [28], [29]. ApoJ was not tested in this study because reproducible results were not obtained in a pilot experiment. ApoJ may cooperatively regulate Aβ clearance and aggregation with apoE [30]; however, levels of these peripheral proteins in AD vary in literature [31], [32].
The complement system consists of a series of proteins that are important co-factors in chronic neurodegenerative disorders such as AD [33]. Innate immunity is recognized to play a major role in an individual's susceptibility to neurodegenerative disorders. Both classic and alternative pathways are associated with Aβ pathogenesis [34]. Decreased levels of inactivated form of C3 were observed in MCI in our cross-sectional studies. Individuals with lower levels of inactive C3 may have susceptibility to cognitive decline because of low Aβ sequestration function, although low C3 levels were also observed in normal aging.
TTR is a 55 kDa homotetrameric protein mainly produced in the liver and choroid plexus of the brain, and it is secreted in the periphery and CSF [35]. Most previous reports have presented results of decreased CSF levels of TTR in AD [36], [37]. Plasma TTR levels are lower in participants with cognitive decline and AD compared with those in cognitive normal controls [38], [39].
Thus, it is possible that decreasing peripheral availability of C3, apoA1, and TTR results in the failure of these proteins to protect against disease progression via Aβ sequestration. Monitoring of the progression of neuropathologic changes in asymptomatic stages of AD by biomarkers is important for early intervention, which may help prevent AD progression or may result to more effective treatment. The proteins analyzed in this study to be involved in the sequestration of Aβ are not directly related to neuropathologic changes of the synapse; rather, they are involved in protection of the synapse against Aβ toxicity during progression of cognitive impairment. Thus, lower levels of sequester proteins may lead to synaptic damage in the preclinical stages of AD pathogenesis.
Sequester proteins other than apoA1, C3, and TTR did not show clinical potential for the assessment of cognitive decline. Serum protein levels of some sequester proteins may vary by circumstances or genetic factors and did not show significant changes. In several studies, serum albumin was shown to bind to Aβ oligomers [40], [41]. We measured the serum albumin concentration among disease groups in the Tone cohort; however, there was no significant difference in the albumin levels, both among the disease (P = .38579) and MMSE score groups (P = .93611; data not shown).
Larger scale population studies are still required to show the clinical utility of these sequester proteins for risk assessment of the progression of cognitive decline at the preclinical stage. We confirmed these results using a conventional in vitro diagnostics assay that is available in most clinical chemistry laboratories (unpublished data). This and other similar studies are crucial for the development of blood-based screening methods to detect not only early MCI but also preclinical AD. Early detection of cognitive impairment may ultimately reduce the incidence of dementia.
Research in context.
-
1.
Systematic review: The authors reviewed literature on PubMed sources and Web sites that described blood-based biomarkers for cognitive decline. Numerous reports described cerebral fluid biomarkers for Alzheimer's disease (AD). Although amyloid β in the blood has been expected to be an AD biomarker, it is not clinically useful in terms of reproducibility.
-
2.
Interpretation: We showed the usefulness of sequester proteins involved in amyloid β clearance to evaluate cognitive decline and progression of cognitive impairment in three cohort studies. The current results are consistent with the hypothesis that individuals with decreased levels or impaired function of the sequester proteins are susceptible to cognitive impairment.
-
3.
Future directions: The article proposes a simple blood test for risk assessment of cognitive decline. Further investigation is required on (1) the clinical usefulness of a set of biomarkers by larger scale population study and (2) the relationship between low levels of sequester proteins and synapse damage during progression of cognitive impairment.
Acknowledgments
The authors thank Tatsumi Korenaga for immunoassay assistance and Tomomi Ogawa and Yoko Okabe for their help in clinical sample collection. They thank all the participants in this study for their commitment and help in advancing research. This study was supported in part by the Grants-in-Aid from the Ministry of Health, Labor, and Welfare of Japan (Research on Dementia; grant no. H23-dementia-003).
Footnotes
With regard to potential conflicts of interest, K.U. serves as a board member of MCBI, Inc.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2015.04.003.
Supplementary data
References
- 1.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Asada T. 2013. Report of Health and Labor Sciences Research Grants (201218011A) Tokyo. [Google Scholar]
- 3.Leow A.D., Yanovsky I., Parikshak N., Hua X., Lee S., Toga A.W. Alzheimer's disease neuroimaging initiative: a one-year follow up study using tensor-based morphometry correlating degenerative rates, biomarkers and cognition. Neuroimage. 2009;45:645–655. doi: 10.1016/j.neuroimage.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer's Association Alzheimer's Association Report: 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10:e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 6.Lacor P.N., Buniel M.C., Furlow P.W., Clemente A.S., Velasco P.T., Wood M. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lesne S., Koh M.T., Kotilinek L., Kayed R., Glabe C.G., Yang A. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 8.Aiyaz M., Lupton M.K., Proitsi P., Powell J.F., Lovestone S. Complement activation as a biomarker for Alzheimer's disease. Immunobiology. 2012;217:204–215. doi: 10.1016/j.imbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Strohmeyer R., Ramirez M., Cole G.J., Mueller K., Rogers J. Association of factor H of the alternative pathway of complement with agrin and complement receptor 3 in the Alzheimer's disease brain. J Neuroimmunol. 2002;131:135–146. doi: 10.1016/s0165-5728(02)00272-2. [DOI] [PubMed] [Google Scholar]
- 10.Chang C.Y., Lee Y.H., Leu S.J., Wang C.Y., Wei C.P., Hung K.S. CC-chemokine ligand 18/pulmonary activation-regulated chemokine expression in the CNS with special reference to traumatic brain injuries and neoplastic disorders. Neuroscience. 2010;165:1233–1243. doi: 10.1016/j.neuroscience.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 11.Qiu Z., Strickland D.K., Hyman B.T., Rebeck G.W. α2-macroglobulin enhances the clearance of endogenous soluble β-amyloid peptide via low-density lipoprotein receptor-related protein in cortical neurons. J Neurochem. 1999;73:1393–1398. doi: 10.1046/j.1471-4159.1999.0731393.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.Y., Tse W., Smith J.D., Landreth G.E. Apolipoprotein E promotes β-amyloid trafficking and degradation by modulating microglial cholesterol levels. J Biol Chem. 2012;287:2032–2044. doi: 10.1074/jbc.M111.295451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paula-Lima A.C., Tricerri M.A., Brito-Moreira J., Bomfim T.R., Oliveira F.F., Magdesian M.H. Human apolipoprotein A-I binds amyloid-β and prevents Aβ-induced neurotoxicity. Int J Biochem Cell Biol. 2009;41:1361–1370. doi: 10.1016/j.biocel.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Yu J.T., Tan L. The role of clusterin in Alzheimer's disease: Pathways, pathogenesis, and therapy. Mol Neurobiol. 2012;45:314–326. doi: 10.1007/s12035-012-8237-1. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzman A.L., Gregori L., Vitek M.P., Lyubski S., Strittmatter W.J., Enghilde J.J. Transthyretin sequesters amyloid β protein and prevents amyloid formation. Proc Natl Acad Sci U S A. 1994;91:8368–8372. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buxbaum J.N., Ye Z., Reixach N., Friske L., Levy C., Das P. Transthyretin protects Alzheimer's mice from the behavioral and biochemical effects of Aβ toxicity. Proc Natl Acad Sci U S A. 2008;105:2681–2686. doi: 10.1073/pnas.0712197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamoto M., Kodama C., Kinoshita T., Yamashita F., Hidaka S., Mizukami K. Dementia and mild cognitive impairment among non-responders to a community survey. J Clin Neurosci. 2009;16:270–276. doi: 10.1016/j.jocn.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda H., Mizumura S., Nagao T., Ota T., Iizuka T., Nemoto K. An easy Z-score imaging system for discrimination between very early Alzheimer's disease and controls using brain perfusion SPECT in a multicentre study. Nucl Med Commun. 2007;28:199–205. doi: 10.1097/MNM.0b013e328013eb8b. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda H., Mizumura S., Nemoto K., Yamashita F., Imabayashi E., Sato N. Automatic voxel-based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer Disease. AJNR Am J Neuroradiol. 2012;33:1109–1114. doi: 10.3174/ajnr.A2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 21.Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biessels G.J., Staekenborg S., Brunner E., Brayne C., Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 23.Ott A., Stolk R.P., van Harskamp F., Pols H.A., Hofman A., Breteler M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 24.Jaeger S., Pietrzik C.U. Functional role of lipoprotein receptors in Alzheimer's disease. Curr Alzheimer Res. 2008;5:15–25. doi: 10.2174/156720508783884675. [DOI] [PubMed] [Google Scholar]
- 25.Castano E.M., Roher A.E., Esh C.L., Kokjohn T.A., Beach T. Comparative proteomics of cerebrospinal fluid in neuropathologically-confirmed Alzheimer's disease and non-demented elderly subjects. Neurol Res. 2006;28:155–163. doi: 10.1179/016164106X98035. [DOI] [PubMed] [Google Scholar]
- 26.Roher A.E., Maarouf C.L., Sue L.I., Hu Y., Wilson J., Beach T.G. Proteomics-derived cerebrospinal fluid markers of autopsy-confirmed Alzheimer's disease. Biomarkers. 2009;14:493–501. doi: 10.3109/13547500903108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertram L., McQueen M.B., Mullin K., Blacker D., Tanzi R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 28.Scacchi R., Gambina G., Ruggeri M., Martini M.C., Ferrari G., Silvestri M. Plasma levels of apolipoprotein E and genetic markers in elderly patients with Alzheimer's disease. Neurosci Lett. 1999;259:33–36. doi: 10.1016/s0304-3940(98)00889-1. [DOI] [PubMed] [Google Scholar]
- 29.Slooter A.J., de Knijff P., Hofman A., Cruts M., Breteler M.M., Van Broeckhoven C. Serum apolipoprotein E level is not increased in Alzheimer's disease: The Rotterdam study. Neurosci Lett. 1998;248:21–24. doi: 10.1016/s0304-3940(98)00339-5. [DOI] [PubMed] [Google Scholar]
- 30.DeMattos R.B., Cirrito J.R., Parsadanian M., May P.C., O'Dell M.A., Taylor J.W. ApoE and clusterin cooperatively suppress Aβ levels and deposition: Evidence that ApoE regulates extracellular Aβ metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- 31.IJsselstijn L., Dekker L.J., Koudstaal P.J., Hofman A., Sillevis Smitt P.A., Breteler M.M. Serum clusterin levels are not increased in presymptomatic Alzheimer's disease. J Proteome Res. 2011;10:2006–2010. doi: 10.1021/pr101221h. [DOI] [PubMed] [Google Scholar]
- 32.Thambisetty M., Simmons A., Velayudhan L., Hye A., Campbell J., Zhang Y. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010;67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonifati D.M., Kishore U. Role of complement in neurodegeneration and neuroinflammation. Mol Immunol. 2007;44:999–1010. doi: 10.1016/j.molimm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Veerhuis R., Nielsen H.M., Tenner A.J. Complement in the brain. Mol Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sousa J.C., Cardoso I., Marques F., Saraiva M.J., Palha J.A. Transthyretin and Alzheimer's disease: Where in the brain? Neurobiol Aging. 2007;28:713–718. doi: 10.1016/j.neurobiolaging.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Biroccio A., Del Boccio P., Panella M., Bernardini S., Di Ilio C., Gambi D. Differential post-translational modifications of transthyretin in Alzheimer's disease: A study of the cerebral spinal fluid. Proteomics. 2006;6:2305–2313. doi: 10.1002/pmic.200500285. [DOI] [PubMed] [Google Scholar]
- 37.Gloeckner S.F., Meyne F., Wagner F., Heinemann U., Krasnianski A., Meissner B. Quantitative analysis of transthyretin, tau and amyloid-β in patients with dementia. J Alzheimers Dis. 2008;14:17–25. doi: 10.3233/jad-2008-14102. [DOI] [PubMed] [Google Scholar]
- 38.Han S.H., Jung E.S., Sohn J.H., Hong H.J., Hong H.S., Kim J.W. Human serum transthyretin levels correlate inversely with Alzheimer's disease. J Alzheimers Dis. 2011;25:77–84. doi: 10.3233/JAD-2011-102145. [DOI] [PubMed] [Google Scholar]
- 39.Velayudhan L., Killick R., Hye A., Kinsey A., Guntert A., Lynham S. Plasma transthyretin as a candidate marker for Alzheimer's disease. J Alzheimers Dis. 2012;28:369–375. doi: 10.3233/JAD-2011-110611. [DOI] [PubMed] [Google Scholar]
- 40.Milojevic J., Raditsis A., Melacini G. Human serum albumin inhibits Aβ fibrillization through a “monomer-competitor” mechanism. Biophys J. 2009;97:2585–2594. doi: 10.1016/j.bpj.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanyon H.F., Viles J.H. Human serum albumin can regulate amyloid-β peptide fiber growth in the brain interstitium: Implications for Alzheimer disease. J Biol Chem. 2012;287:28163–28168. doi: 10.1074/jbc.C112.360800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.