Abstract
Background
The aging rate in Taiwan is the second highest in the world. As the population ages quickly, the prevalence of dementia increases rapidly. There are some studies that have explored the association between air pollution and cognitive decline, but the association between air pollution and dementia has not been directly evaluated.
Methods
This was a case-control study comprising 249 Alzheimer's disease (AD) patients, 125 vascular dementia (VaD) patients, and 497 controls from three teaching hospitals in northern Taiwan from 2007 to 2010. Data of particulate matter <10 μm in diameter (PM10) and ozone were obtained from the Taiwan Environmental Protection Administration for 12 and 14 years, respectively. Blood samples were collected to determine the apolipoprotein E (APOE) ɛ4 haplotype. Bayesian maximum entropy was used to estimate the individual exposure level of air pollutants, which was then tertiled for analysis. Conditional logistic regression models were used to estimate adjusted odds ratios (AORs) and 95% confidence intervals between the association of PM10 and ozone exposure with AD and VaD risk.
Results
The highest tertile of PM10 (≥49.23 μg/m3) or ozone (≥21.56 ppb) exposure was associated with increased AD risk (highest vs. lowest tertile of PM10: AOR = 4.17; highest vs. lowest tertile of ozone: AOR = 2.00). Similar finding was observed for VaD. The association with AD and VaD risk remained for the highest tertile PM10 exposure after stratification by APOE ɛ4 status and gender.
Conclusions
Long-term exposure to the highest tertile of PM10 or ozone was significantly associated with an increased risk of AD and VaD.
Keywords: Air pollutant, Particulate matter, Ozone, Alzheimer's disease, Vascular dementia, The elderly
1. Introduction
Every 4 seconds a new dementia case occurs around the world, and the number of dementia cases doubles every 20 years [1]. In the United States, Alzheimer's disease (AD) is the leading type of dementia, and it was ranked the fifth leading cause of death in the elderly in 2010 [2]. From 1999 to 2004, the mortality rate of AD increased by 31% [3], which might also be a result of improved reporting. In Taiwan, the prevalence of dementia was 8.04% in the elderly (age ≥65 years) based on a recent National Survey in 2011 to 2012 [4]. As the aging rate increases rapidly worldwide, dementia has become an important health issue in the elderly.
Several factors have been related to dementia risk, for example, age, sex, education, apolipoprotein E (APOE) ɛ4 status, lifestyle, and environment factors [5]. Environmental factors may play an important role in dementia; however, studies are sparse because of its wide spectrum and difficulty in objectively assessing the cumulative exposure of environmental exposure. The exhaust from motor vehicles is the major source of air pollution in Taiwan [6] and has been associated with respiratory and cardiovascular diseases [7]. Particulate matter <10 μm in diameter (PM10) and ozone are especially important as they are the major pollutants for estimating the index of polluted alert region, that is, Pollutants Standard Index, in Taiwan [8], [9]. PM10 refers to solid and liquid particles composed by mixed compound of chemicals and suspends in the air [10]. Animal studies indicated that PM can be transferred from the upper respiratory tract to the brain, leading to brain inflammation—an important pathological evidence of dementia [11], [12]. Ozone is a strong oxidizing agent formed in the troposphere from a series of complex reactions via sunlight on nitrogen dioxide from the exhaust. In rat's hippocampus, exposure to ozone causes oxidative stress and the subsequent progressive neurodegeneration [13]; this seems analogous to that observed in AD patients.
Some studies have explored the relationship between the long-term exposure to traffic-related air pollutants and impaired cognitive function in the elderly [14], [15], [16], [17], [18]. These studies found that PM or black carbon (BC) was related to cognitive impairment/decline (PM10: [15], PM2.5-10: [17], PM2.5: [17], [18], BC: [16]). Similarly, ozone exposure was also related to lower cognitive function [14], [18]. However, without considering the effects of air pollutants, only 1.6% to 6.8% people in the community and 1.9% to 9.6% people in the clinic with cognitive impairment progress to dementia annually [19]. Therefore, it is important to clarify the role of air pollutants on dementia occurrence, and studies evaluating this association are lacking.
Long-term exposure to PM10 or to ozone on dementia risk remains unclear. Therefore, this study aimed to explore this association over an average duration of 13 years. Because APOE ɛ4 status and gender are important confounding factors for dementia risk, this study further evaluated how they modified this association. A powerful and new statistical approach, Bayesian maximum entropy (BME), which simultaneously considers spatial and temporal estimation with soft data, was used to estimate the long-term exposure to air pollutants.
2. Method
2.1. Study population
This case-control study recruited 483 dementia cases from the neurology clinics of three teaching hospitals in northern Taiwan between 2007 and 2010. Healthy controls (n = 565) were recruited from the elderly health check-up program and volunteers of the hospital during the same time period. All participants were aged ≥60 years. Participants with any of the following conditions were excluded: depression, Parkinson's disease, hemorrhagic stroke, cerebral infarction, brain tumor, or dementia subtypes other than AD or vascular dementia (VaD). Participants without blood sample and those who resided outside the Taipei-Keelung metropolitan area were also excluded. After exclusion, 249 AD patients, 125 VaD patients, and 497 controls were included for data analysis. This study was approved by the Institutional Review Boards of National Taiwan University Hospital, En Chu Kong Hospital, and Cardinal Tien's Hospital. Written informed consent was obtained from all participants. For patients with serious cognitive impairment, their consent was obtained from the legal guardian/next of kin/caregiver, who also helped with the verification of information collected from the questionnaire.
A self-reported questionnaire was administered to collect information on demography, vascular risk factors (hypertension, type 2 diabetes mellitus [DM], and hyperlipidemia, and body mass index [BMI] at age 40s), lifestyle, and family history of diseases. A blood sample was collected in tubes containing sodium ethylenediamine tetraacetic acid from each participant. After centrifugation, genomic DNA was extracted from buffy coat by using QuickGene-Mini80 system (Fujifilm, Tokyo, Japan) and stored at −80°C.
2.2. Evaluation of AD and VaD
At each hospital, one neurologist performed the clinical examination to screen potential dementia cases. Mini-Mental State Examination was used to assess their cognitive function. The diagnosis of dementia was evaluated by Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria [20]. Head magnetic resonance imaging (about 90% of dementia cases) and computed tomography (about 10% of dementia cases) were performed to exclude participants with organic lesions. Diagnosis of probable (typical AD presentation) AD was based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association Alzheimer's Criteria [21]. Diagnosis of VaD was made according to the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria [22]. Because of different etiology between large and small vessel dementia, only VaD patients with small vessel-related stroke (e.g., lacunar infarction and leukoaraiosis) were recruited. Controls with a completely independent activity of daily living and instrumental activity of daily living were assessed by Short Portable Mental Status Questionnaire [23] to exclude participants with possible cognitive impairment and other mental disorders.
2.3. Exposure assessment
Ambient monitoring data of PM10 and ozone were obtained from Taipei–Keelung metropolitan area including 24 monitoring stations from the Department of Taiwan Air Quality Monitoring Network, Environmental Protection Administration (EPA) [8] between 1993 and 2006. BME method was used to estimate the spatiotemporal distribution of PM10 and ozone concentration [24]. Because the elderly tend to live at the same place after they retired (the average retirement age was 54.8 in 2005), we assume that the residential place corresponded to the estimated spatiotemporal distribution for each individual. Exposures of 12-year PM10 and 14-year ozone were estimated because these were the longest exposure data of these two pollutants that we can retrieve from Taiwan EPA. The duration of each pollutant is restricted to the data available. The annual average exposures of PM10 and ozone were all below the ambient air quality standard in Taiwan (Supplementary Material). For analysis using multiple regression, the estimated mean annual exposures to PM10 and ozone levels were tertiled into the lowest tertile (T1: PM10: <44.95 μg/m3; ozone: <20.20 [parts per billion, ppb]), the medium tertile (T2: PM10: 44.95–49.23 μg/m3; ozone: 20.20–21.56 ppb), and the highest tertile (T3: PM10: >49.23 μg/m3; ozone: >21.56 ppb) groups based on exposure data from the controls.
2.4. Spatial estimation
BME is an epistemic framework, which distinguishes the general and specific knowledge of the spatiotemporal processes and generates the more informative spatiotemporal maps for the variables of interest compared with land-use regression model [25]. The details of BME method and its applications can be referred to previous publications [26], [27], [28].
The process of spatiotemporal air pollutants can be characterized by spatiotemporal trend and covariance. Nested spatiotemporal covariance models were used to characterize the spatiotemporal dependence of the air pollutants to reveal the spatiotemporal processes at different space-time scales [27], [29]. To account for the impact of air pollutants to AD occurrence, BME method generated the annual cumulative level of PM10 and ozone at the corresponding residential place for each participant between 1993 and 2006 for further analysis. SEKS-GUI v.1.0.0 package was used for BME analysis [30], [31].
2.5. Statistical analysis
After stepwise model selection (Slentry = 0.15, Slstay = 0.15), selected variables and those with biological importance were adjusted in the models as potential confounders. For AD, the following variables were adjusted in the model: age, gender, APOE ɛ4 status, PM10 level, ozone level, education years, and BMI. For VaD, the following variables were adjusted: age, gender, APOE ɛ4 status, PM10 level, ozone level, education years, and alcohol consumption. To control for the confounding effect of age, study participants were stratified by 5-year age interval and compared cases and controls within each stratum in the multivariable analysis. Age was adjusted in all multivariable regression models to control for residual confounding due to age variation within each age stratum. Conditional logistic regression models were used to estimate adjusted odds ratios (AORs) and 95% confidence intervals (CIs) between the association of PM10 and ozone exposure with AD and VaD risk.
Because APOE ɛ4 and female gender are important risk factors for late-onset AD, we also performed a stratification analysis to evaluate how they modified the association between air pollutants and the risk of AD or VaD, respectively. The likelihood ratio test was used to assess the interactions by comparing a model with terms for main effects and interaction terms to the model with terms for main effects only. Cochran-Armitage trend test was performed to assess if there is a dose-response relationship between air pollutants (PM10 and ozone) and dementia risk. SAS version 9.3 (SAS Institute, Cary, NC) was used for statistical analyses. All statistical tests were two sided.
3. Results
3.1. Characteristics of the study population
This study included 249 AD cases, 125 VaD cases, and 497 controls. Compared with controls, AD cases were older (79.1 vs. 72.9 years), had a higher BMI at age 40s (24.0 vs. 22.8 kg/m2), included more women (66% vs. 52%), had a lower education level (≤6 years: 48% vs. 12%), more had type 2 DM (18% vs. 13%), fewer had the history of hypertension (40% and 54%) and hyperlipidemia (21% vs. 30%), and more were APOE ɛ4 carriers (41% vs. 15%, Table 1). The smoking status, alcohol consumption, and the history of cardiovascular disease were similar between the AD cases and controls.
Table 1.
Characteristics of the study population
| Variables | AD, N = 249 | VaD, N = 125 | Control, N = 497 |
|---|---|---|---|
| Mean ± SD | |||
| Age (yrs old) | 79.1 ± 6.9* | 79.9 ± 7.0* | 72.9 ± 6.1 |
| BMI at age 40 (kg/m2) | 24.0 ± 3.0* | 23.6 ± 3.4 | 22.8 ± 3.5 |
| MMSE | 18.0 ± 6.1 | 14.9 ± 6.4 | NA |
| N (%) | |||
| Female | 164 (66)* | 70 (56) | 256 (52) |
| Education (yrs) | |||
| ≦6 | 119 (48)* | 70 (56)* | 59 (12) |
| 6–12 | 92 (37)* | 42 (34)* | 200 (40) |
| >12 | 38 (15)* | 13 (10)* | 238 (48) |
| Ever smoker | 54 (22) | 40 (32)* | 83 (17) |
| Alcohol consumption | 27(11) | 23 (18)* | 52 (10) |
| Type 2 DM | 46 (18)* | 38 (30)* | 63 (13) |
| Hypertension | 99 (40)* | 83 (66)* | 268 (54) |
| Hyperlipidemia | 52 (21)* | 29 (23) | 148 (30) |
| Cardiovascular disease | 60 (24) | 40 (32) | 150 (30) |
| APOE ɛ4 carriers | 96 (41)* | 23 (22) | 67 (15) |
Abbreviations: AD, Alzheimer's disease; VaD, vascular dementia; SD, standard deviation; BMI, body mass index; MMSE, Mini-Mental State Examination; NA, not applicable; DM, diabetes mellitus; APOE ɛ4, apolipoprotein E ɛ4.
NOTE. Chi-square tests (for categorical variables), Mann-Whitney U-tests, and t tests (for nonnormally and normally distributed continuous variables) to compare the distribution between cases (AD or VaD) and controls.
P < .05 indicating statistical significance (and in bold).
As compared with controls, VaD cases were older (79.9 vs. 72.9 years), had a lower education level (≤6 years: 56% vs. 12%), more were ever smokers (32% vs. 17%), more reported alcohol consumption (18% vs. 10%), and more had a history of type 2 diabetes mellitus (DM) (30% vs. 13%) and hypertension (66% vs. 54%, Table 1). There were no significant differences in BMI, gender, the history of hyperlipidemia and cardiovascular disease, and APOE ɛ4 carriers between the VaD cases and controls.
3.2. Spatial and temporal distribution of PM10 and ozone
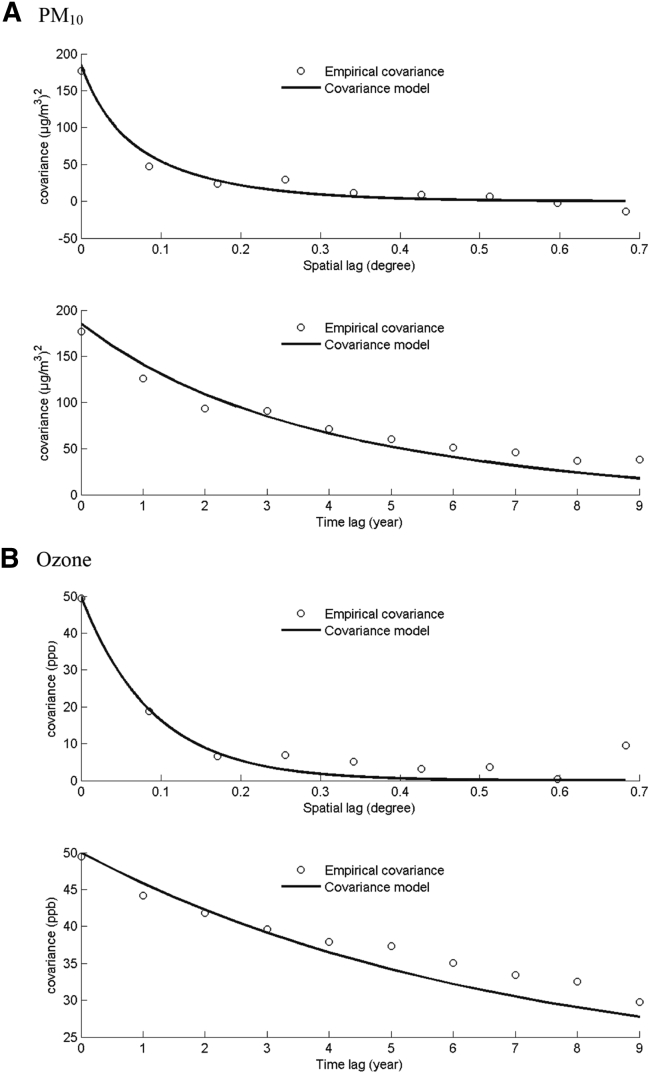
The spatial and temporal covariance models (Fig. 1) were used in BME framework, respectively. As the spatial and time lag increased, the spatial and temporal covariance both became smaller. Sills of both spatial and temporal covariance models were larger for ozone than those for PM10.
Fig. 1.
The spatial (upper)/temporal (lower) covariance model fitting used for Bayesian maximum entropy (BME) estimation: (A) particulate matter <10 μm in diameter (PM10); (B) ozone. X-axis indicates the spatial/time lag (degree/years); Y-axis indicates the covariance of each air pollutant. Circles are estimated empirical covariance. Curved lines are fitted covariance models, which characterize the spatiotemporal dependence for the annual PM10 and ozone exposure.
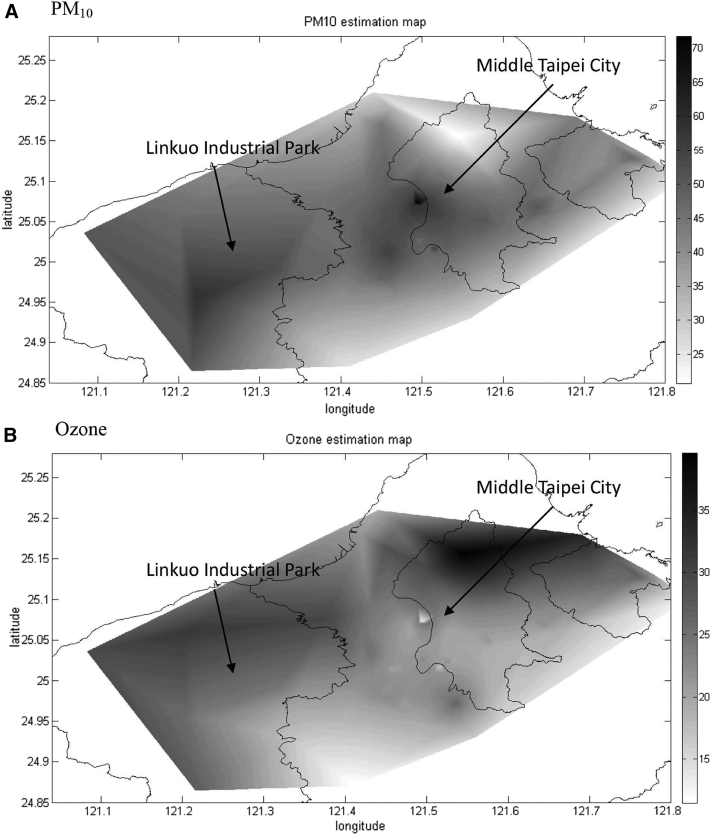
The averaged spatial distribution was different between PM10 and ozone, as shown in Fig. 2. Elevated PM10 was located in the middle Taipei city and Linkou Industrial Park as a result of traffic and industrial emission (Fig. 2A). In contrast, average ozone concentration was elevated in areas near Yangmingshan National Park and Linkou Thermal Power Plant (Fig. 2B).
Fig. 2.
Fitted covariance model used for Bayesian maximum entropy (BME) estimation in northern Taiwan: (A) the average annual particulate matter <10 μm in diameter (PM10) exposure over 12 years, (B) the average annual ozone exposure over 14 years. X-axis indicates the longitude of the study area; Y-axis indicates the latitude of the study area.
3.3. Association of PM10 and ozone with dementia risk
The highest tertile of PM10 exposure was significantly associated with increased AD risk (highest vs. lowest tertile: AOR = 4.17, 95% CI = 2.31–7.54, Table 2). Increased risk was also observed for those with ozone exposure (highest vs. lowest tertile: AOR = 2.00, 95% CI = 1.14–3.50). Similar results were observed for VaD (highest vs. lowest tertile of PM10: AOR = 3.61, 95% CI = 1.67–7.81; highest vs. lowest tertile of ozone: AOR = 2.09, 95% CI = 1.01–4.33). Linear trend (Ptrend < .05) was found in both PM10 and ozone for AD and VaD. Medium-level PM10 or ozone exposure was not associated with AD or VaD risk.
Table 2.
The association between air pollutants (PM10 or ozone) and the risk of dementia (AD or VaD)
| Level of air pollutants |
Ptrend | ||||||
|---|---|---|---|---|---|---|---|
| T1∗ |
T2∗ |
T3∗ |
|||||
| N (case/control) | AOR (95% CI) | N (case/control) | AOR (95% CI) | N (case/control) | AOR (95% CI) | ||
| AD† | |||||||
| PM10‡ | 82/199 | 1.00 | 68/145 | 1.68 (0.94–3.00) | 99/153 | 4.17 (2.31–7.54) | <.0001 |
| Ozone§ | 92/202 | 1.00 | 51/125 | 0.60 (0.33–1.09) | 106/170 | 2.00 (1.14–3.50) | .03 |
| VaD‖ | |||||||
| PM10‡ | 41/199 | 1.00 | 35/145 | 1.86 (0.89–3.90) | 49/153 | 3.61 (1.67–7.81) | .004 |
| Ozone § | 36/202 | 1.00 | 32/125 | 0.62 (0.28–1.38) | 57/170 | 2.09 (1.01–4.33) | .05 |
Numbers in bold indicated significant findings, that is, AOR not including 1 or P < 0.05.
Abbreviations: PM10, particulate matter <10 μm in diameter; AD, Alzheimer's disease; VaD, vascular dementia; AOR, adjusted odds ratio; CI, confidence interval; ppb, parts per billion.
The level of each air pollutant was tertiled (T1, T2, and T3).
Models of AD were adjusted for age, gender, apolipoprotein E (APOE) ɛ4 status, PM10 level, ozone level, education years, and body mass index (kg/m2).
Groups for PM10 exposure, lowest tertile (T1: <44.95 μg/m3), medium tertile (T2: 44.95–49.23 μg/m3), highest tertile (T3: >49.23 μg/m3).
Groups for ozone exposure, lowest tertile (T1: <20.20 ppb), medium tertile (T2: 20.20–21.56 ppb), highest tertile (T3: >21.56 ppb).
Models of VaD were adjusted for age, gender, APOE ɛ4 status, PM10 level, ozone level, education years, and alcohol consumption.
3.4. Effect modification by APOE ɛ4 status
No significant interaction was found between APOE ɛ4 status and PM10 exposure for AD risk (Pinteraction > .05, Table 3). Significant association was found in some subgroups after stratification by APOE ɛ4 status. The highest tertile of PM10 exposure was significantly associated with AD risk in APOE ɛ4 noncarriers (highest vs. lowest tertile: AOR = 4.24, 95% CI = 2.11–8.54, Table 3) and APOE ɛ4 carriers (highest vs. lowest tertile: AOR = 3.50, 95% CI = 1.08–11.29). No significant associations were observed in other subgroups.
Table 3.
The association between air pollutants (PM10 or ozone) and the risk of dementia (AD or VaD) by APOE ɛ4 status
| Variables |
APOE ɛ4 noncarriers |
APOE ɛ4 carriers |
Pinteraction | ||
|---|---|---|---|---|---|
| Case/control | AOR (95% CI) | Case/control | AOR (95% CI) | ||
| AD∗ | |||||
| Overall | 140/392 | 1.00 | 96/66 | 4.95 (2.99–8.18) | |
| PM10† | .17 | ||||
| T1 | 45/159 | 1.00 | 33/33 | 1.00 | |
| T2 | 33/114 | 1.19 (0.57–2.48) | 31/19 | 2.67 (0.92–7.71) | |
| T3 | 62/119 | 4.24 (2.11–8.54) | 32/14 | 3.50 (1.08–11.29) | |
| Ozone‡ | .65 | ||||
| T1 | 55/159 | 1.00 | 44/29 | 1.00 | |
| T2 | 28/101 | 0.48 (0.23–1.00) | 20/14 | 0.85 (0.28–2.52) | |
| T3 | 57/132 | 1.80 (0.92–3.55) | 32/23 | 2.55 (0.88–7.35) | |
| VaD§ | |||||
| Overall | 81/392 | 1.00 | 23/66 | 2.07 (1.01–4.24) | |
| PM10† | .32 | ||||
| T1 | 27/159 | 1.00 | 7/33 | 1.00 | |
| T2 | 24/114 | 1.45 (0.65–3.24) | 6/19 | NA | |
| T3 | 30/119 | 2.60 (1.12–6.02) | 10/14 | NA | |
| Ozone‡ | .56 | ||||
| T1 | 26/159 | 1.00 | 5/23 | 1.00 | |
| T2 | 19/101 | 0.50 (0.21–1.21) | 3/14 | 0.82 (0.03–21.68) | |
| T3 | 36/132 | 1.47 (0.67–3.22) | 15/29 | NA | |
Numbers in bold indicated significant findings, that is, AOR not including 1 or P < 0.05.
Abbreviations: PM10, particulate matter <10 μm in diameter; AD, Alzheimer's disease; VaD, vascular dementia; APOE, apolipoprotein E; AOR, adjusted odds ratio; CI, confidence interval; NA, not applicable.
Models of AD were adjusted for age, gender, APOE ɛ4 status, PM10 level, ozone level, education years, and body mass index (kg/m2).
Groups for PM10 exposure, lowest tertile (T1: <44.95 μg/m3), medium tertile (T2: 44.95–49.23 μg/m3), and highest tertile (T3: >49.23 μg/m3).
Groups for ozone exposure, lowest tertile (T1: <20.20 ppb), medium tertile (T2: 20.20–21.56 ppb), and highest tertile (T3: >21.56 ppb).
Models of VaD were adjusted for age, gender, APOE ɛ4 status, PM10 level, ozone level, education years, and alcohol consumption.
APOE ɛ4 status did not significantly modify the association between PM10 or ozone exposure and VaD risk (Pinteraction > .05, Table 3). The highest tertile of PM10 exposure was associated with VaD risk in APOE ɛ4 noncarriers (highest vs. lowest tertile: AOR = 2.60, 95% CI = 1.12–6.02). No significant association was observed in other subgroups.
3.5. Effect modification by gender
Interaction between gender and PM10 exposure did not reach statistical significance for AD or VaD risk (Ptrend > .05, Table 4). After stratification by gender, significant association was found in some subgroups. The highest tertile of PM10 exposure was significantly associated with increased AD risk in men (highest vs. lowest tertile: AOR = 4.50, 95% CI = 1.85–10.95) and in women (highest vs. lowest tertile: AOR = 3.75 95% CI = 1.60–8.81, Table 4). Results were not significant for ozone exposure and AD risk.
Table 4.
The association between air pollutants (PM10 or ozone) and the risk of dementia (AD or VaD) by gender
| Variables | Men |
Women |
Pinteraction | ||
|---|---|---|---|---|---|
| Case/control | AOR (95% CI) | Case/control | AOR (95% CI) | ||
| AD∗ | |||||
| Overall | 85/241 | 0.95 (0.60–1.51) | 164/256 | 1.00 | |
| PM10† | .95 | ||||
| T1 | 28/99 | 1.00 | 54/100 | 1.00 | |
| T2 | 25/85 | 1.93 (0.81–4.58) | 46/70 | 1.48 (0.64–3.38) | |
| T3 | 32/57 | 4.50 (1.85–10.95) | 64/86 | 3.75 (1.60–8.81) | |
| Ozone‡ | .48 | ||||
| T1 | 29/79 | 1.00 | 61/82 | 1.00 | |
| T2 | 24/87 | 0.50 (0.20–1.27) | 51/88 | 0.70 (0.30–1.64) | |
| T3 | 32/75 | 2.24 (0.97–5.18) | 52/86 | 1.93 (0.86–4.34) | |
| VaD§ | |||||
| Overall | 55/241 | 1.39 (0.76–2.56) | 70/256 | 1.00 | |
| PM10† | .78 | ||||
| T1 | 16/99 | 1.00 | 25/100 | 1.00 | |
| T2 | 16/85 | 1.62 (0.54–4.84) | 20/70 | 2.11 (0.71–6.32) | |
| T3 | 23/57 | 3.46 (1.11–10.78) | 25/86 | 2.90 (0.94–8.98) | |
| Ozone‡ | .70 | ||||
| T1 | 16/79 | 1.00 | 18/82 | 1.00 | |
| T2 | 24/87 | 0.54 (0.17–1.68) | 28/88 | 0.85 (0.27–2.70) | |
| T3 | 15/75 | 1.58 (0.57–4.37) | 24/86 | 2.28 (0.75–6.94) | |
Numbers in bold indicated significant findings, that is, AOR not including 1 or P < 0.05.
Abbreviations: PM10, particulate matter <10 μm in diameter; AD, Alzheimer's disease; VaD, vascular dementia; AOR, adjusted odds ratio; CI, confidence interval.
Models of AD were adjusted for age, gender, APOE ɛ4 status, PM10 level, ozone level, education years, and body mass index (kg/m2).
Groups for PM10 exposure, lowest tertile (T1: <44.95 μg/m3), medium tertile (T2: 44.95–49.23 μg/m3), and highest tertile (T3: >49.23 μg/m3).
Groups for ozone exposure, lowest tertile (T1: <20.20 ppb), medium tertile (T2: 20.20–21.56 ppb), and highest tertile (T3: >21.56 ppb).
Models of VaD were adjusted for age, gender, APOE ɛ4 status, PM10 level, ozone level, education years, and alcohol consumption.
No significant interaction was found between gender and PM10 or ozone exposure for VaD risk (Ptrend > .05, Table 4). Significant increased VaD risk was observed only in men exposed to the highest tertile of PM10 (highest vs. lowest tertile: AOR = 3.46, 95% CI = 1.11–10.78). No significant association was found between ozone exposure and VaD risk after stratified by gender.
4. Discussion
Previous reports have linked exposure to traffic-related PM [14], [15], [17], [18] and BC [16] to cognitive decline in the elderly. However, only a small portion of cognitively impaired elderly progress to dementia [19]; therefore, studies on cognitive impairment have been unable to fully explain the association between exposure to air pollutants and dementia risk. To the best of our knowledge, this is the first case-control study which assessed the association between longitudinal air pollution (PM10 and ozone) exposure with clinically diagnosed dementia (AD and VaD). We found that the elevated long-term PM10 level was significantly associated with an increased risk of AD and VaD in the elderly. Animal studies have shown that air pollutants may go to olfactory bulb and trigger inflammation response in the brain [28], [32] as a result of the accumulation of amyloid-β (Aβ) 42. Aβ 42, an important early biomarker of AD, has been related to inflammation response in the brain [33] and the subsequent dysfunction of blood-brain barrier, neural degeneration, cerebrovascular pathologic signs, and apoptosis in glial cells [12]. Therefore, long-term PM10 exposure increased AD risk may be explained by the inflammation response. Similarly, the highest tertile of ozone exposure was related to increased AD and VaD risk. Some animal studies found that oxidative stress caused by ozone can induce the loss of brain repair in the hippocampus, which then affects memory and leads to AD occurrence [34], [35].
APOE gene regulates cholesterol/lipid metabolism and the ɛ4 haplotype is a well-known risk factor of AD. This study found that both APOE ɛ4 carriers and noncarriers with the highest tertile of PM10 exposure have increased AD risk (AOR = 3.50 and 4.24, respectively).
This study has some limitations. First, this study explored only two air pollutants: ozone and PM10. This was done because there is more complete long-term data and they have been linked to different health outcomes in humans, for example, respiratory and cardiovascular disease and memory change in rats [35]. For example, PM2.5 data from Taiwan EPA is available from 2005, which may be too short a period to explain its effects on dementia risk as the pathological evidence of AD tends to start a decade before the diagnosis. Because PM10 and PM2.5 have a high correlation (0.81) in our study, PM10 may serve as a surrogate of PM2.5. In addition, it is not easy to keep track of the address of each participant for a long time. Therefore, it was assumed that these participants have lived in the same place for 12 to 14 years for estimating their exposure to air pollutants. Our study participants are quite old (average age is 79 years). Because the average retirement age was around 55 years in 2005, most of the participants should have retired even though the exposure was estimated from 12 to 14 years ago. Therefore, we assumed that these participants tended to live in the same places after retirement compared with young people. Last, people who did not survive for 12 to 14 years did not have an opportunity to be counted, so our results have some survival bias.
This study has several strengths. First, different spatiotemporal mapping techniques, for example, inverse distance weighted, kriging, spline, and BME, have been used for predicting or estimating the exposure to air pollutants (PM10 and PM2.5) [24], [29], [36]. Also, this study used BME approach [24], which estimated the distribution of the air pollutants via simultaneous consideration of the spatial and temporal variations compared with other approaches such as land-use regression [16]. In addition, previous studies assessed the association between air pollutants and cognitive impairment of which only a small portion of old people with cognitive impairment progress to AD [18]. Therefore, this study provides new findings on air pollutants and the risk of AD.
In summary, we observed a dose-response relationship between PM10 and the risk of AD and small-vessel VaD, which has not been reported before. Future studies are warranted to explore the role of other air pollutants in the etiology of AD and VaD.
Research in context.
-
1.
Systematic review: Some studies have explored the relationship between long-term exposure to traffic-related air pollutants and cognitive impairment in the elderly. These studies found that particulate matter (PM) or black carbon (BC) was related to cognitive decline in either gender. However, without considering the effects of air pollutants, only 1.6% to 6.8% people in the community and 1.9% to 9.6% people in the clinic with cognitive impairment progress to dementia annually. Therefore, it is important to clarify the role of air pollutants on dementia occurrence, and studies evaluating this association are lacking.
-
2.
Interpretation: In this study, we found that long-term exposure to PM10 or ozone was associated with an increased risk of Alzheimer's disease (AD) and vascular dementia (VaD).
-
3.
Future directions: Future studies are warranted to explore the role of other air pollutants in the etiology of AD and VaD.
Footnotes
Conflicts of interest and source of funding: There is no conflict interest. Funding for the study was provided by National Science Council grants (96-2314-B-002-197) and (97-2314-B-002-168-MY3) and Department of Health (100-TD-PH-14).
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2014.11.015.
Supplementary data
References
- 1.World Health Organization. Dementia: a public health priority, 2012. Available at: http://whqlibdoc.who.int/publications/2012/9789241564458_eng.pdf. Accessed March 13, 2014.
- 2.Murphy S.L., Xu J., Kochanek K.D. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61:1–117. [PubMed] [Google Scholar]
- 3.Steenland K., MacNail J., Vega I., Levey A. Recent trends in Alzheimer's disease mortality in the United States, 1999–2004. Alzheimer Dis Assoc Disord. 2009;23:165. doi: 10.1097/wad.0b013e3181902c3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taiwan Alzheimer's Disease Association. 2013. Available at: http://www.alz.co.uk/sites/default/files/conf2013/oc002.pdf. Accessed March 13, 2014.
- 5.Chen J.H., Lin K.P., Chen Y.C. 2009. Risk factors for dementia. J Formos Med Assoc. 2009;108:754–764. doi: 10.1016/S0929-6646(09)60402-2. [DOI] [PubMed] [Google Scholar]
- 6.Taiwan Ministry of Transportation and Communication. 2011. Available at: http://www.motc.gov.tw. Accessed March 13, 2014.
- 7.Brunekreef B., Holgate S.T. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 8.Taiwan Environmental Protection Administration Executive Yuan. 2000. Available at: http://www.epa.gov.tw/. Accessed March 13, 2014.
- 9.Khanna N. Measuring environmental quality: an index of pollution. Ecol Econ. 2000;35:191–202. [Google Scholar]
- 10.Davidson C.I., Phalen R.F., Solomon P.A. Airborne particulate matter and human health: a review. Aerosol Sci Technol. 2005;39:737–749. [Google Scholar]
- 11.Block M.L., Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderon-Garciduenas L., Reed W., Maronpot R.R., Henriquez-Roldán C., Delgado-Chavez R., Calderón-Garcidueñas A. Brain inflammation and Alzheimer's-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–658. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- 13.Moulton P.V., Yang W. Air pollution, oxidative stress, and Alzheimer's disease. J Environ Public Health. 2012;2012:472751. doi: 10.1155/2012/472751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J.C., Schwartz J. Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology. 2009;30:231–239. doi: 10.1016/j.neuro.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Ranft U., Schikowski T., Sugiri D., Krutmann J., Krämer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109:1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Power M.C., Weisskopf M.G., Alexeeff S.E., Coull B.A., Spiro A., 3rd, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119:682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weuve J., Puett R.C., Schwartz J., Yanosky J.D., Laden F., Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172:219–227. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatto N.M., Henderson V.W., Hodis H.N., St John J.A., Lurmann F., Chen J.C. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology. 2014;40:1–7. doi: 10.1016/j.neuro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell A.J., Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association . 4th ed. American Psychiatric Association; Washington DC: 1994. Diagnostic and statistical manual of mental disorders: DSM-IV. [Google Scholar]
- 21.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu H.-L., Chen J.-C., Christakos G., Jerrett M. BME estimation of residential exposure to ambient PM10 and ozone at multiple time scales. Environ Health Perspect. 2009;117:537–544. doi: 10.1289/ehp.0800089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu H.L., Wang C.H., Liu M.C., Kuo Y.M. Estimation of fine particulate matter in Taipei using landuse regression and Bayesian maximum entropy methods. Int J Environ Res Public Health. 2011;8:2153–2169. doi: 10.3390/ijerph8062153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christakos G., Hristopulos D.T. Kluwer Academic; Boston: 1998. Spatiotemporal environmental health modelling: a tractatus stochasticus. [Google Scholar]
- 27.Christakos G., Serre M.L. BME analysis of spatiotemporal particulate matter distributions in North Carolina. Atmos Environ. 2000;34:3393–3406. [Google Scholar]
- 28.Christakos G., Bogaert P., Serre M.L. Springer; New York: 2002. Temporal GIS: advanced functions for field-based applications. [Google Scholar]
- 29.Yu H.L., Wang C.H. Retrospective prediction of intraurban spatiotemporal distribution of PM2.5 in Taipei. Atmos Environ. 2010;44:3053–3065. [Google Scholar]
- 30.Kolovos A., Yu H.L., Christakos G. Department of Geography, San Diego State University; San Diego, CA: 2006. SEKS-GUI v.0.6. User's manual-06 Ed. [Google Scholar]
- 31.Yu H.L., Kolovos A., Christakos G., Chen J.C., Warmerdam S., Dev B. Interactive spatiotemporal modelling of health systems: the SEKS–GUI framework. Stoch Environ Res Risk Assess. 2007;21:555–572. [Google Scholar]
- 32.Campbell A., Oldham M., Becaria A., Bondy S.C., Meacher D., Sioutas C. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26:133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Naslund J., Haroutunian V., Mohs R., Davis K.L., Davies P., Greengard P. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 34.Dorado-Martinez C., Paredes-Carbajal C., Mascher D., Borgonio-Pérez G., Rivas-Arancibia S. Effects of different ozone doses on memory, motor activity and lipid peroxidation levels, in rats. Int J Neurosci. 2001;108:149–161. doi: 10.3109/00207450108986511. [DOI] [PubMed] [Google Scholar]
- 35.Pope C.A., Burnett R.T., Thurston G.D., Thun M.J., Calle E.E., Krewski D. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 36.Kebaili Bargaoui Z., Chebbi A. Comparison of two kriging interpolation methods applied to spatiotemporal rainfall. J Hydrol. 2009;365:56–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.