Abstract
Background
Our objectives were (1) to test the association between the report of subjective cognitive decline (SCD) and prospective objective cognitive performance in high age individuals and (2) to study the course of longitudinal cognitive performance before and after the first report of SCD.
Methods
Cognitively normal elderly participants of the German Study on Ageing, Cognition, and Dementia study (N = 2330) with SCD (subjective decline in memory with and without associated concerns) and without SCD at baseline were assessed over 8 years with regard to immediate and delayed verbal recall, verbal fluency, working memory, and global cognition. Baseline performance and cognitive trajectories were compared between groups. In addition, cognitive trajectories before and after the initial report of SCD (incident SCD) were modelled in those without SCD at baseline.
Results
Baseline performance in the SCD group was lower and declined more steeply in immediate and delayed verbal recall than in the control group (no SCD at baseline). This effect was more pronounced in the SCD group with concerns. Incident SCD was preceded by decline in immediate and delayed memory and word fluency.
Conclusions
SCD predicts future memory decline. Incident SCD is related to previous cognitive decline. The latter finding supports the concept of SCD indicating first subtle decline in cognitive performance that characterizes preclinical Alzheimer's disease.
Keywords: Subjective cognitive decline, Preclinical Alzheimer's disease, Early detection
1. Introduction
Evidence from clinical and epidemiological studies suggests that subjective cognitive decline (SCD) may represent the initial symptomatic manifestation of Alzheimer's disease (AD) before mild cognitive impairment (MCI) [1]. A number of studies found that subjective cognitive decline (SCD) in the cognitively normal elderly is associated with an increased risk of dementia [2], [3], [4] particularly in cases where individuals report concern about memory decline [5]. Hypothetically, SCD may represent the self-experience of subtle cognitive decline, before impairment on cognitive tests occurs [1].
Cross-sectional studies in epidemiological samples, however, often did not find an association between SCD and objective memory performance [6], [7], [8]. In large cohorts, weak associations between SCD and memory performance in unimpaired elderly individuals have been observed [9], [10], [11], [12]. One potential explanation for the small magnitude of the cross-sectional association of SCD with cognitive performance is that SCD relates to individual cognitive trajectories (decline) rather than to cross-sectional abilities.
Only a few studies have addressed the association of SCD with trajectories of cognitive decline. Those with follow-up periods of less than 5 years did not find such associations [13], [14]. However, Jorm et al. [15] assessed 331 elderly nondemented individuals over 70 years of age three times over 7.6 years and showed that memory complaints were associated with past memory performance and future memory decline. In that study, anxiety and depression were the strongest predictors of memory complaints. In a population cohort aged 62 to 85 years, (N = 1168), Dik [16] found that baseline memory complaints were associated with a decline in delayed memory, information processing speed, and overall cognition on the Mini-Mental State Examination (MMSE) over 6 years. Hohman et al. [17] repeatedly assessed 98 cognitively normal subjects (mean age = 75 years) with various cognitive instruments including the Cognitive Failures Questionnaire (CFQ) during an average of 11.5 years. Higher CFQ values, aggregated over several follow-ups, were associated with the speed of decline in immediate and delayed verbal memory. This relationship was not present in figural memory or executive function.
These studies with extended follow-up suggest that in elderly subjects SCD may be associated with accelerated memory decline. It is not known, however, how specific concerns (worries) associated with SCD affect the risk of cognitive decline as opposed to SCD without concerns. Also it is not known to what extent the decline in individual cognitive domains occurs before the first report of SCD.
In this study, we examine the relationship of subjective decline in memory, as one particular type of SCD, in association with and without concerns with future and preceding performance in different cognitive domains. We assessed a large cohort of unimpaired elderly subjects over 8 years and conducted growth curve modeling (GCM) of cognitive performance data.
2. Methods
2.1. Sample
The German Study on Ageing, Cognition, and Dementia (AgeCoDe) in primary care patients is an ongoing multicenter prospective study in elderly individuals with a focus on the identification of risk factors and predictors of cognitive decline and dementia. Details about the sampling method and selection process are described in previous publications [5]. A total of 3327 subjects free of dementia at baseline were recruited from general practitioner (GP) registries and assessed with structured clinical interviews and cognitive tests. Main inclusion criteria were ages greater than 75 years, native German language, absence of severe hearing or vision impairments, and residing at home rather than in an institution. The approval of this study was provided by the local ethics committees of the Universities of Bonn, Hamburg, Düsseldorf, Heidelberg/Mannheim, Leipzig, and Munich. All subjects gave written informed consent before the participation in this study.
2.2. Assessment
Subjects were interviewed in their home environment by trained psychologists or physicians at baseline and at all follow-up examinations, which were 18 months apart.
Subjective memory capacity was assessed with the question: “Do you feel like your memory is becoming worse?” The possible answers were “no,” “yes, but this does not worry me,” or “yes, this worries me.” The same question was asked at each of five follow-up visits, which occurred at 18 months intervals.
A 10-item word list learning task and a semantic verbal fluency task from the Consortium to Establish a Registry of Alzheimer's Disease (CERAD) neuropsychological battery [18], was applied at baseline and at all follow-ups. The CERAD 10-item word list consists of three immediate recall trials. Immediate recall performance equals the sum of recalled words across three presentations of the list. Delayed recall refers to the free recall of the 10-item word list after a delay of approximately 10 minutes filled with other tasks. The CERAD verbal fluency test consists of a 1-minute task for naming animals. The number of correct names given, without duplicates, is used as the score.
In addition, we administered the Structured Interview for the diagnosis of dementia of the Alzheimer type, multi-infarct dementia and dementia of other etiology according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition and International Classification of Diseases, Tenth Revision (ICD-10) (SIDAM) [19]. The cognitive assessment of the SIDAM (SISCO) contains the Mini-Mental State Examination (MMSE) [20] and additional items for the assessment of four different areas of cognition: orientation, memory, intellectual abilities, and higher cognitive functions (subscales verbal working memory, constructional abilities, aphasia, and apraxia). For this study, a verbal working memory composite score was used, which consists of seven items, including subtraction calculations (e.g. 9-3 = ?), backward spelling of a word, and backward digit span. The MMSE served as a measure of global cognition in the present analyses.
Depressive symptoms were assessed with the 15-item version of the Geriatric Depression Scale (GDS) [21]. Level of education was categorized as low, middle, or high using the Comparative Analysis of Social Mobility in Industrial Nations educational classification instrument [22]. apolipoprotein (APOE) ε4 genotyping was performed in all subjects.
At follow-up, dementia was diagnosed according to DSM-IV criteria, applying the diagnostic algorithm of the SIDAM that makes use of the SISCO score as a cognitive measure and impairment in activities of daily living scale (SIDAM ADL scale). The diagnosis of AD-type dementia was made according to the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria. All diagnoses were made in consensus by the interviewer, experienced geriatric psychiatrists, or geriatricians.
For those subjects, who could not be interviewed in person at follow-up the Global Deterioration Scale [23] and the subscales “Changes in Performance of Everyday Activities” and “Changes in Habits” of the Blessed Dementia Scale [24] were completed by the interviewer with an informant (spouse, relative, caregiver) and/or with the GP. Based on this information the diagnosis of dementia was established.
2.3. Group definitions
Only cognitively normal subjects (n = 2330), performing within 1 standard deviation (SD) of the normative SISCO domain scores, derived in an independent study [25], at baseline were included in the present analyses.
Subjects with a negative response to the SCD question (see section 2.2) served as controls (CO, n = 993), and were compared with subjects reporting a memory decline, either without associated concerns (worries) (SCD-C, n = 965), or with associated concerns (SCD+C, n = 372).
2.4. Follow-up assessment rates
Five follow-up waves with 18 months intervals after baseline are the basis for the present analyses. The number of personal interviews was 2049 (87.9%) at follow-up 1, 1825 (78.3%) at follow-up 2, 1505 (64.6%) at follow-up 3, 1259 (54%) at follow-up 4, and 1037 (44.5%) at follow-up 5.
The main reasons for not obtaining a personal interview were (1) refusal of a personal visit because of several reasons, including, but not limited to bad medical conditions (follow-up 1: 58.7%, follow-up 2: 47.4%, follow-up 3: 47.2%, follow-up 4: 24.2%, follow-up 5: 15.4%) and (2) death (follow-up 1: 32%, follow-up 2: 48%, follow-up 3: 41.5%, follow-up 4:36.7%, follow-up 5: 36.5%). Informant-based information on those participants without personal interview was obtained on 273 participants at follow-up 1, on 222 at follow-up 2, on 316 at follow-up 3, on 197 at follow-up 4, and on 180 at follow-up 5. The combined follow-up rates (personal interview, informant-based information only) were 99.7% at follow-up 1, 87.9% at follow-up 2, 78.2% at follow-up 3, 62.5% at follow-up 4, and 52.2% at follow-up 5. Note that individuals were not followed-up anymore in the case of incident dementia or informant-based information only at one follow-up.
2.5. Statistical analyses and modeling
In the first set of analyses, we modeled cross-sectional and longitudinal group differences (according to SCD status at baseline) in each cognitive domain (verbal immediate and delayed recall, verbal fluency, working memory, global cognition) of those subjects with personal interview including all follow-ups. In addition, we conducted analyses with reduced number of follow-ups to investigate the minimal time span after which differential decline became significant.
GCM were estimated with Mplus 7 [26]. A quadratic term was included where it improved the models over fitting only a linear trend [27]. Participant attrition and missing data were addressed with the full information maximum likelihood method [28]. Time was treated with fixed time scores as intervals between measurements were approximately of equal distance. The Akaike Information Criterion, Bayesian information criterion (BIC), sample size adjusted BIC, root mean square error of approximation (RMSEA) [29], chi square fit index, and comparative fit index (CFI) [30] were used as indices of model fit. A CFI value greater than 0.95 and an RMSEA value of 0.04 or less indicate a very good model fit [31], [32]. The maximum likelihood with robust standard errors method was used for model estimation, allowing for robust estimation even if the assumption of normal distribution was challenged. We report maximum likelihood parameter estimates and significance values.
Following the general GCM recommendations of McArdle and Grimm [33], we used an unconditional model to estimate the dependent variables without covariates (Model 1). If the amount of variance unexplained by Model 1 remained significant, age, gender, years of education, GDS score (dichotomized at the conventional cut-off score of 6 suggestive of depression), and APOE ε4 genotype (yes/no) were added as covariates (Model 2). Because a significant amount of residual variance remained after adding the covariates, Model 3, which included the groups (CO, SCD-C, SCD+C) as predictor variables, was generated. To reduce the complexity of this final model, only variables indicative of a significant trend in Model 2 (P < .1) remained in Model 3 [34]. To derive a plot of the three group trajectories, a multigroup analysis with stratification by group was conducted.
The CO group was compared with the SCD+C and SCD-C groups using group contrasts adjusted for significant covariates. Three latent factors—intercept (baseline performance), linear slope (change rate), and quadratic slope (quadratic change rate)—were investigated and tested for significance.
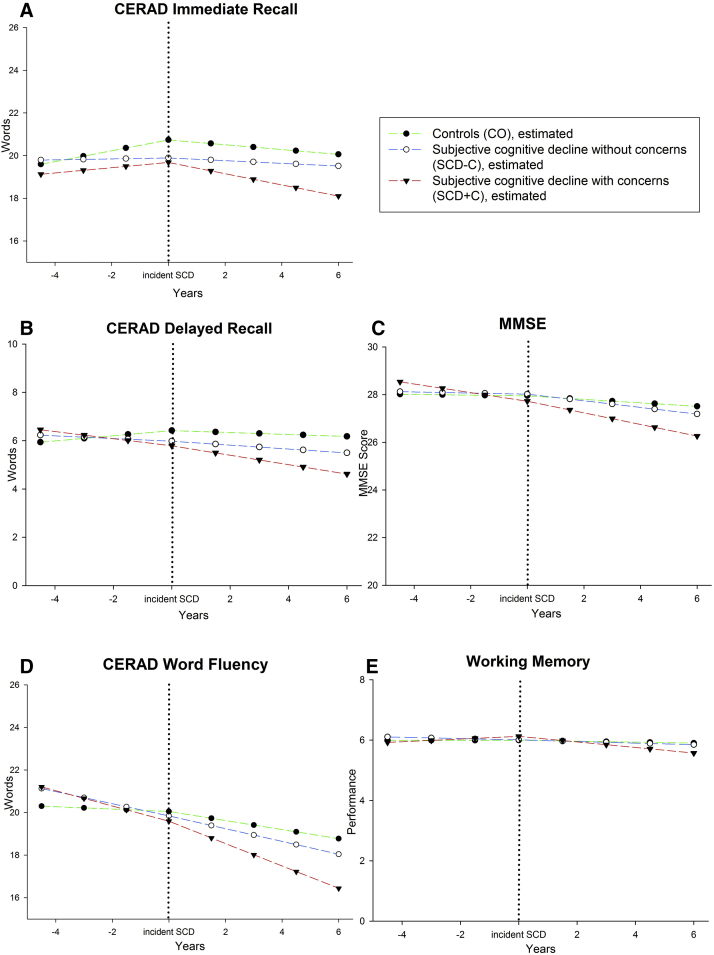
In the second set of analyses, only CO subjects (no SCD at baseline) were included. In those, who reported SCD at some point during follow-up, the initial report of SCD-C or SCD+C was defined as incident SCD. Subjects were classified as incident SCD-C if they did report SCD-C, but did not report SCD+C at any follow-up. They were categorized as SCD+C if they additionally showed a concern regarding memory at any follow-up. Thus, a subject reporting no SCD at baseline, SCD-C at follow-up 1, and SCD+C at follow-up 2 was classified as incident SCD+C, with an onset of SCD at follow-up 1. We modeled the trajectories of each cognitive domain before and after incident SCD. We compare stable CO subjects (who never reported SCD) to subjects with incident SCD-C or SCD+C. The time point of incident SCD at follow-up was recoded as zero to estimate cognitive trajectories. In this analysis, the time point zero is incident SCD. The previous time point was recoded as −1, −2, −3, etc., whereas the time points after the incident SCD were recoded as +1, +2, +3, etc. Each time point represents an interval of 18 months (Fig. 2).
Fig. 2.
Trajectories of estimated means for stable controls (CO), converters into SCD without concerns (SCD-C) and converters into SCD with concerns (SCD+C), controlling for age, gender, education apolipoprotein ε4-genotype and depression (at baseline).
By overlaying the trajectories at time point 0, we obtained group trajectories with up to five time points before and after incident SCD. The stable CO group trajectories during follow-up were randomly assigned to the starting time points by computing a uniformly distributed random integer in the range between one and five, similar to the proportional distribution of incident SCD. Sample sizes at the extremes were not sufficient for statistical modeling (n < 20). Thus, the analysis was restricted to three time points before and four time points after incident SCD. The preprocessed data were fitted with piecewise linear growth models [35]. Mean change over time from time point −3 to incident SCD was represented as the linear slope before SCD. Incident SCD to time point +4 was represented as the slope after SCD. The time point zero (incident SCD) was the common intercept of the two slopes. The stable CO group was compared with the incident SCD+C and incident SCD-C groups using group contrasts adjusted for covariates.
To control for false positive results we considered only results with P < .01 to be significant.
2.6. Predicting incident dementia hazard
In addition to the cognitive trajectories, Cox proportional hazards regression analysis was performed with SPSS 21 (IBM) to model the risk of incident Alzheimer' dementia and of all dementia types as a function of group membership and of the covariates age, gender, education, depression, and APOE ε4 genotype.
3. Results
The three groups differed significantly in terms of gender and education, but not in age, APOE ε4 genotype, and MMSE (Table 1). Follow-up rates did not differ between groups (χ2 (8) = 7.814; P = .452 n.s.).
Table 1.
Sample description for groups with and without subjective cognitive decline at baseline
| Groups |
Total sample | Group differences between the three groups | Significant post-hoc tests | |||
|---|---|---|---|---|---|---|
| CO | SCD-C | SCD+C | ||||
| n | 993 | 965 | 372 | 2330 | ||
| Rate of follow-up in % at follow-up1 | 85.7 | 88.8 | 83.5 | χ2 (8, N = 2330) = 7.814, P = .452, n.s. | ||
| Rate of follow-up in % at follow-up2 | 73.1 | 76.6 | 72.9 | |||
| Rate of follow-up in % at follow-up3 | 58.4 | 62.7 | 58.0 | |||
| Rate of follow-up in % at follow-up4 | 47.6 | 51.3 | 48.5 | |||
| Rate of follow-up in % at follow-up5 | 36.6 | 40.6 | 35.8 | |||
| MMSE: mean (SD) | 27.97 (1.49) | 27.95 (1.51) | 27.88 (1.55) | 27.95 (1.51) | P = .672 | |
| Age in years: mean (SD) | 79.39 (3.40) | 79.73 (3.56) | 79.72 (3.60) | 79.58 (3.50) | P = .065 | |
| Female, n (%) | 661 (66.6) | 566 (58.7) | 263 (70.7) | 1490 (63.9) | χ2 (2, N = 2330) = 22.04, P < .0001 | ∗,‡ |
| Level of education | χ2 (4, N = 2330) = 16.26, P < .003 | ∗,†,‡ | ||||
| Low, n (%) | 675 (68.0) | 618 (64.0) | 262 (70.4) | 1555 (66.7) | ||
| Middle, n (%) | 241 (24.3) | 233 (24.1) | 67 (18.0) | 541 (23.2) | ||
| High, n (%) | 77 (7.8) | 114 (11.8) | 43 (11.6) | 234 (10.0) | ||
| APOE ε4+, n (%) | 195 (19.6) | 201 (20.8) | 78 (21.0) | 0.20 (20.3) | χ2 (2, N = 2330) = 0.54, P = .765 | |
| Incident Alzheimer's dementia within five follow-ups: n | 51 | 88 | 53 | 192 | ||
| Risk, incident Alzheimer's dementia§ hazard ratio, P-value (CI) | 1.0 |
1.64 P = .005 (1.16–2.32) |
2.89 P = .000 (1.96–4.26) |
- | ||
| Incident dementia within five follow-ups: n | 88 | 129 | 87 | 304 | ||
| Risk, incident dementia§ hazard ratio, P-value (CI) | 1.0 |
1.41 P = .014 (1.07–1.85) |
2.63 P < .001 (1.95–3.55) |
- | ||
Abbreviations: CO, controls (without subjective cognitive decline); SCD-C, SCD (subjective cognitive decline) without concerns; SCD+C, SCD with concerns; SD, standard deviation; APOE ε4, apolipoprotein ε4; FU, follow-up interval was 18 months; MMSE, Mini-Mental State Examination; CI, confidence interval; n.s., non-significant.
NOTE. Covariates: age, sex, education (low, medium, high), depressive symptoms (Geriatric Depression Scale scores <6 points or ≥6).
Bolded text indicates significant hazard ratio. Italicized text indicates significant P-values.
CO vs. SCD-C.
CO vs. SCD+C.
SCD-C vs. SCD+C.
CO group = reference group.
In both sets of analyses, the final models of the cognitive trajectories had a good fit throughout (RMSEA < 0.04, CFI > 0.97) and outperformed the unconditional (Model 1) and covariate (Model 2) models. Further details on model fit can be provided by the corresponding author on request.
3.1. Baseline cognitive performance
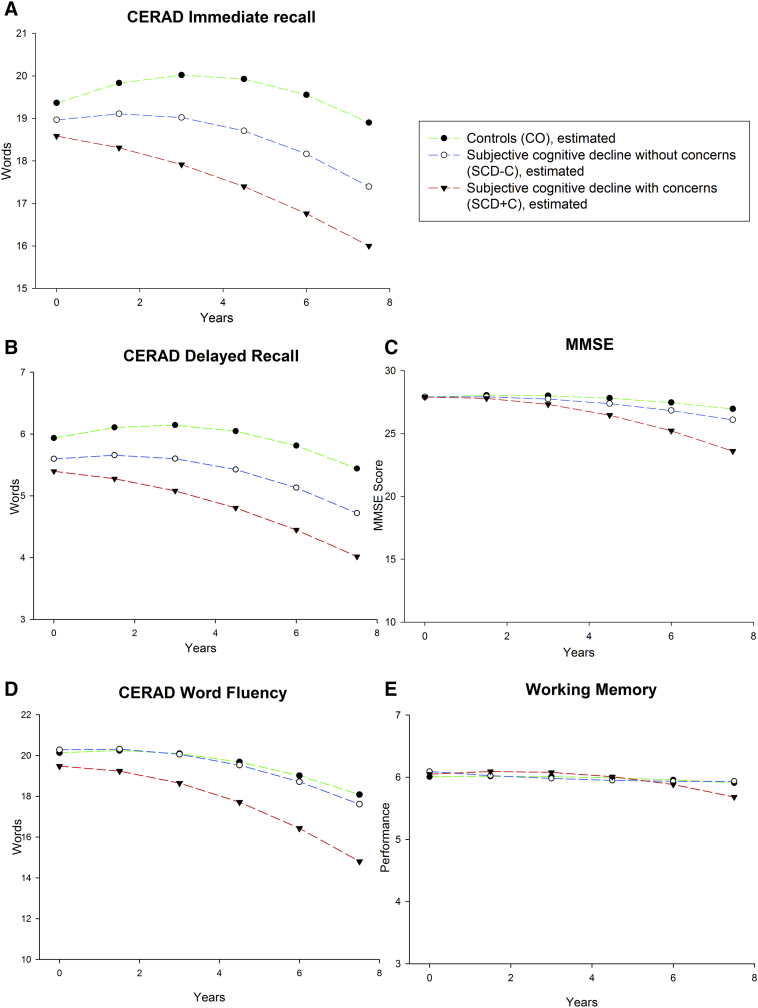
All groups differed significantly from each other in delayed recall performance with the CO group performing the best, followed by the SCD-C and SCD+C groups (see Fig. 1 and Table 2). In addition, the adjusted group contrasts between CO and SCD+C, and SCD-C and SCD+C, were significant for immediate recall and for the word fluency task. There were no significant differences in working memory and global cognition between groups.
Fig. 1.
Trajectories of estimated means for controls (CO), SCD without concerns (SCD-C) and SCD with concerns (SCD+C) controlling for age, gender, education apolipoprotein ε4 -genotype and depression at baseline.
Table 2.
Results of the growth factor estimates with regard to baseline groups
| Immediate recall |
Delayed recall |
Word fluency |
Working memory |
MMSE |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | P-value | Estimate | P-value | Estimate | P-value | Estimate | P-value | Estimate | P-value | |
| Intercept | ||||||||||
| CO vs. SCD-C | −0.352 | .080 | −0.073 | .004 | 0.015 | .947 | 0.040 | .315 | −0.010 | .869 |
| CO vs. SCD+C | −0.389 | .000 | −0.252 | .000 | −0.313 | .034 | 0.026 | .376 | 0.064 | .125 |
| SCD- vs SCD+C | −0.504 | .020 | −0.271 | .029 | −0.713 | .020 | 0.019 | .732 | 0.132 | .118 |
| Slope | ||||||||||
| CO vs. SCD-C | −0.352 | .008 | −0.093 | .060 | 0.018 | .915 | −0.017 | .218 | −0.110 | .005 |
| CO vs. SCD+C | −0.410 | .000 | −0.157 | .000 | −0.130 | .238 | −0.008 | .359 | −0.180 | .000 |
| SCD- vs. SCD+C | 0.412 | .024 | −0.182 | .044 | −0.267 | .233 | −0.007 | .742 | −0.246 | .000 |
| Quadratic slope | ||||||||||
| CO vs. SCD-C | 0.027 | .354 | 0.051 | .358 | −0.029 | .404 | ∗ | ∗ | ||
| CO vs. SCD+C | 0.035 | .079 | 0.014 | .142 | −0.027 | .246 | ||||
| SCD- vs SCD+C | 0.033 | .393 | 0.014 | .451 | −0.024 | .606 | ||||
Abbreviations: CO, controls (without subjective cognitive decline); SCD-C, SCD (subjective cognitive decline) without concerns; SCD+C, SCD with concerns; MMSE, Mini-Mental State Examination.
NOTE. In all cognitive domains, P-values are corrected for age, gender, education, depression, and apolipoprotein ε4 genotype.
Bold text indicates significant group contrasts.
Only linear slope was fitted to the data.
3.2. Cognitive decline trajectories
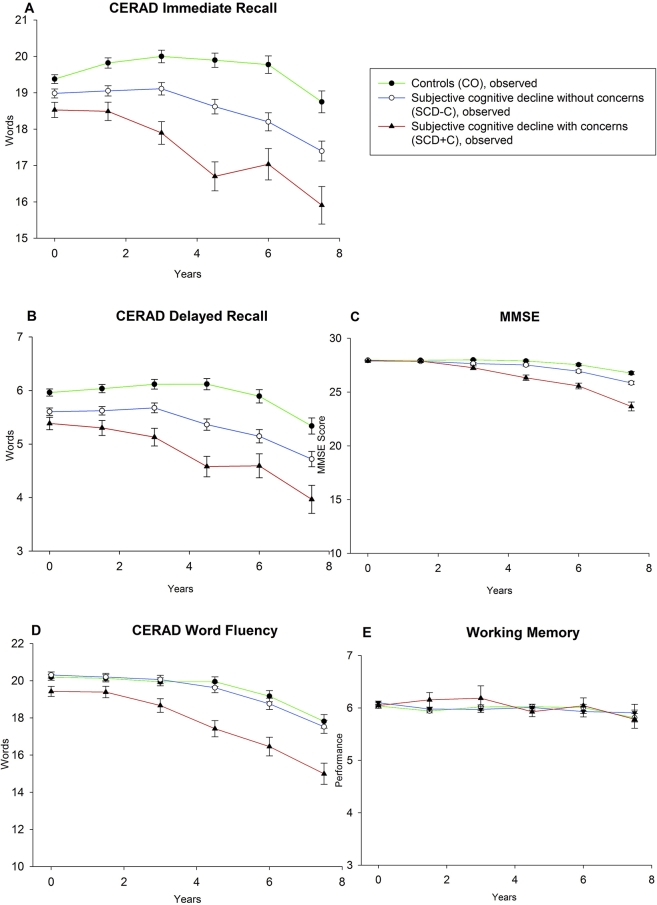
Fig. 1 displays the estimated means of cognitive trajectories (see Supplementary Material for observed means) for the three groups.
In delayed recall, the adjusted group contrasts of the linear slope revealed significant differences in decline over time between CO and SCD+C, at a trend level between CO and SCD-C, and between SCD-C and SCD+C (see Table 2 and Fig. 1B).
The adjusted group contrasts for immediate recall also showed significant differences between the CO and SCD-C, CO and SCD+C groups, and SCD-C and SCD+C groups. In global cognition, there were significant differences between CO and SCD-C groups, CO and SCD+C groups, and SCD-C and SCD+C groups. There were no significant differences of slope in the group contrasts for word fluency and working memory.
Models with reduced follow-up time revealed that the rates of decline in all domains were not significantly different before follow-up 4 (respectively, 6 years). Thus, SCD-associated cognitive decline became apparent only 6 years after baseline.
There was no group difference in the quadratic slope in any of the cognitive domains.
3.3. Covariate effects
Covariate effects for the first set of analyses are presented in the Supplementary Material.
3.4. Cognitive performance and incident SCD
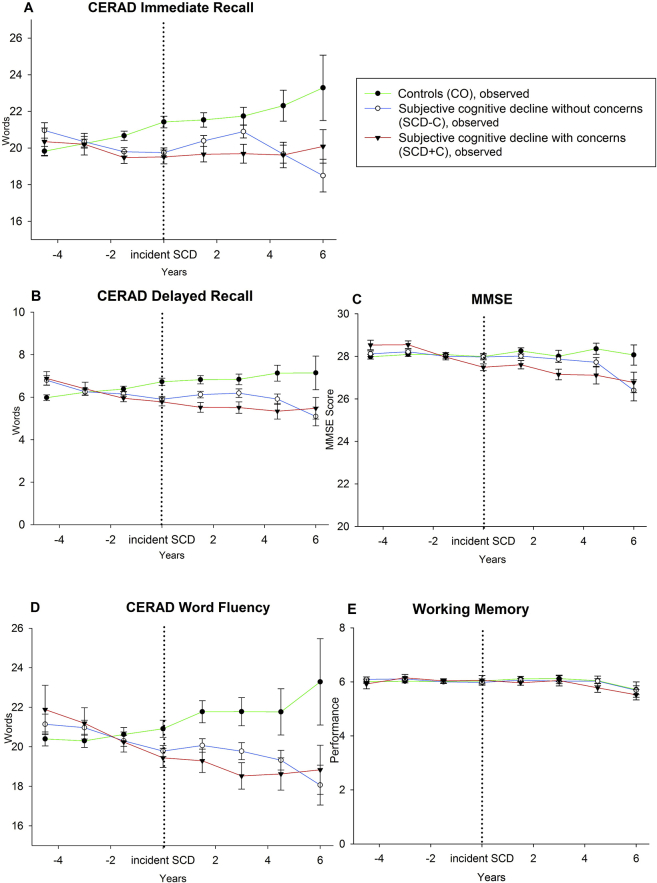
Of the 993 individuals free of SCD at baseline, 361 subjects reported SCD without concerns and 146 reported SCD with concerns some time during the follow-up period. The median distance of first SCD report from baseline was four assessments (6 years). Fig. 2 shows the cognitive trajectories (estimated means, see Supplementary Material for observed means) before and after incident SCD. Table 3 lists the statistical comparisons.
Table 3.
Results of the growth factor estimates with regard to incident SCD
| Immediate recall |
Delayed recall |
Word fluency |
Working memory |
MMSE |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | P-value | Estimate | P-value | Estimate | P-value | Estimate | P-value | Estimate | P-value | |
| Intercept—incident SCD | ||||||||||
| Stable CO vs. converters into SCD-C | −0.724 | .034 | −0.338 | .060 | −0.321 | .440 | −0.005 | .949 | 0.111 | .413 |
| Stable CO vs. converters into SCD+C | −0.615 | .005 | −0.332 | .003 | −0.287 | .308 | 0.027 | .633 | −0.148 | .106 |
| Converters into SCD-C vs. converters into SCD+C | −0.490 | .242 | −0.329 | .139 | −0.268 | .589 | 0.082 | .451 | −0.389 | .009 |
| Slope before SCD | ||||||||||
| Stable CO vs. converters into SCD-C | −0.370 | .005 | −0.234 | .001 | −0.399 | .012 | −0.035 | .378 | 0.006 | .922 |
| Stable CO vs. converters into SCD+C | −0.161 | .105 | −0.174 | .001 | −0.265 | .020 | 0.018 | .587 | −0.137 | .006 |
| Converters into SCD-C vs. converters into SCD+C | 0.038 | .850 | −0.108 | .279 | −0.120 | .612 | 0.070 | .300 | −0.267 | .003 |
| Slope after SCD | ||||||||||
| Stable CO vs. converters into SCD-C | 0.149 | .449 | −0.004 | .970 | −0.137 | .518 | 0.013 | .803 | −0.027 | .796 |
| Stable CO vs. converters into SCD+C | −0.025 | .827 | −0.095 | .101 | −0.252 | .055 | −0.045 | .237 | −0.063 | .384 |
| Converters into SCD-C vs. converters into SCD+C | −0.189 | .342 | −0.184 | .065 | −0.301 | .138 | −0.096 | .115 | −0.118 | .273 |
Abbreviations: CO, controls (without subjective cognitive decline); SCD-C, SCD (subjective cognitive decline) without concerns; SCD+C, SCD with concerns; MMSE, Mini-Mental State Examination.
NOTE. In all cognitive domains, P-Values are corrected for age, gender, education, and apolipoprotein ε4 genotype.
Bold text indicates significant group contrasts.
At the time point of incident SCD, those with incident SCD+C had poorer delayed recall than stable CO. A similar trend was observed for incident SCD-C. There was a significant difference in the slope of delayed recall performance between groups. Although the stable CO increased their performance (possibly due to test repetition effects), the other groups showed a decline in delayed recall preceding their incident SCD (CO vs. SCD-C, CO vs. SCD+C).
A similar pattern was observed for immediate recall: At incident SCD, those with incident SCD+C had poorer immediate recall performance than those with stable CO. The same was observed for incident SCD-C. The immediate recall slopes before incident SCD differed significantly between CO and SCD-C, but not between CO and SCD+C.
For word fluency, there were no significant intercept differences at incident SCD, but before incident SCD there were significant differences in the slopes between the stable CO group and the incident SCD-C and incident SCD+C groups.
There was no significant difference in decline between the groups in working memory either in the intercept at incident SCD or in the slope before incident SCD.
In global cognition, there was a significant difference in performance at incident SCD between SCD-C and SCD+C. Incident SCD+C was preceded by a significant decline in global cognition before incident SCD compared with CO, and also compared with SCD-C.
In none of the studied cognitive domains, there was a group difference in slope after incident SCD.
3.5. Cox regression of SCD as a predictor of incident dementia
In addition to the GCM analyses, we performed a Cox regression analysis to assess the risk of baseline SCD on future dementia of AD-type and of all cause dementia. For AD-type dementia, SCD+C showed a 2.89 times increased risk (P < .001) and SCD-C showed a 1.64 times increased risk (P = .005) for over five follow-ups in comparison with CO. For dementia of all cause, SCD+C showed a 2.63 times increased risk (P < .001), whereas SCD-C showed a 1.4 times increased risk (P = .014).
4. Discussion
Overall, our results show that a report of subjective memory decline, a specific form of SCD, in cognitively normal elderly may predict future objective memory decline and incident dementia. Furthermore, incident subjective memory decline may also reflect past cognitive decline.
As expected, SCD at baseline predicted accelerated decline in episodic memory over 8 years, and the decline was more pronounced in SCD+C. This confirms prior reports [15], [16], [17]. The other cognitive decline measures were largely unrelated to the subjective report on memory, suggesting some degree of specificity regarding the association of subjective and objective decline.
Apart from different cognitive trajectories, we also found baseline differences in episodic memory between groups, particularly in the SCD+C group. As expected, greater age, male gender, lower education, depressive symptoms, and having the APOE ε4 genotype were associated with poorer baseline cognition, but the effects of SCD were independent from these covariates. Known effects on cognitive decline were replicated in our study. In line with Caselli et al. [36], APOE ε4 had an effect on delayed recall, but not on MMSE. Age had a significant effect on the level and shape of the trajectories, in line with Gomeni et al. [37], depression had an effect on baseline, but no effect on verbal memory decline, in line with Royall et al. [38]. Education was associated with better baseline performance in all measures.
A novel finding of this study is that, in subjects free of SCD at baseline, incident SCD was preceded by objective memory decline. For delayed recall and also for verbal fluency, the slope of decline in the 4.5 years before incident SCD was stronger in the SCD-C and SCD+C group. Subtle objective decline therefore precedes, and possibly also gives rise to, the report of SCD, supporting the concept that SCD may indicate a subtle decline in cognitive function [1]. This temporal sequence was evident in group analyses but would probably not be detectable on an individual basis.
It is not possible with the present data to exactly determine the time lag between objective and subjective decline. The answer to this question will, however, also depend on the relative sensitivity of the objective and subjective decline assessments used.
Amieva et al. [39] showed that cognitive complaints, assessed with a questionnaire, increased on an average 7 to 8 years before the diagnosis of Alzheimer's dementia, whereas verbal fluency started to drop 12 years before diagnosis. Amieva et al. did not focus on the SCD individuals themselves but their findings are in line with the sequence proposed by this paper. Stewart et al. [40] found that hippocampal atrophy over 4 years preceded incident SCD and this could also be a cause of the memory decline preceding SCD in the current sample.
We here focused on prevalent SCD and incident SCD. However, like other phenotypical classifications (e.g. MCI), SCD is unlikely to be absolutely stable over time. It will be an interesting issue for further research to study the determinants and consequences of SCD stability over time.
In subjects without SCD at baseline or during follow-up, there is a slight increase in memory test scores over the years, probably due to test repetition effects [41]. However, these test repetition effects appear to be outpaced by memory decline in the SCD groups. Reduced test repetition effects in the CERAD word list learning task have also been described before in patients with mild AD [42].
The strengths of our study include the large sample size, the multicentre design, and long follow-up time, the availability of APOE ε4 genotyping and application of GCM. The limitations of our study include the lack of brain imaging. Our data do not allow us to elucidate the brain changes that underlie incident SCD. However, elderly help-seeking subjects with SCD (but without mild cognitive impairment) show signs of brain atrophy in the entorhinal cortex [43] and hippocampus [44], glucose metabolism changes [45], and increased Pittsburgh Compound B Positron Emission Tomography beta-amyloid load [46]. Many of these characteristics are indicative of and consistent with the preclinical stages of AD. In addition, SCD has been associated with AD-like pathology in autopsy studies [47], [48].
In sum, this study shows that a report of memory decline in old age is partly related to ongoing (past and future) memory decline, and is not merely a depressive interpretation of normal age-related cognitive loss. Furthermore, our data suggest that at the group level, subjects begin to report incident SCD after their memory starts to deviate from normal. This would be consistent with the “self-experience of decline” hypothesis of SCD in the context of preclinical AD.
Research in context.
-
1.
Systematic review: Subjective cognitive decline (SCD) in memory appears to be a risk factor for future cognitive decline and dementia. The association with trajectories of decline in individual cognitive domains and potentially SCD-preceding cognitive decline are not well studied. The authors investigated a large epidemiological cohort from Germany to address these questions.
-
2.
Interpretation: Subjective decline in memory and related concerns (SCD+C) are related to subtle decline in memory performance. They are risk factors for future decline in memory functions. Before the onset of SCD, memory decline already occurred.
-
3.
Future directions: The study further supports the concept of SCD as a risk factor for cognitive decline and an indicator of very first impairment of cognitive performance in elderly. These results can aid in designing future prevention trials.
Acknowledgments
Members of the AgeCoDe Study Group:
Principal Investigators*: Wolfgang Maier, Martin Scherer
Heinz-Harald Abholz, Christian Brettschneider, Cadja Bachmann, Horst Bickel, Wolfgang Blank, Hendrik van den Bussche, Sandra Eifflaender-Gorfer, Marion Eisele, Annette Ernst, Angela Fuchs, Kathrin Heser, Frank Jessen, Hanna Kaduszkiewicz, Teresa Kaufeler, Mirjam Köhler, Hans-Helmut König, Alexander Koppara, Carolin Lange, Tobias Luck, Melanie Luppa, Manfred Mayer, Edelgard Mösch, Julia Olbrich, Michael Pentzek, Jana Prokein, Anna Schumacher, Steffi Riedel-Heller, Janine Stein, Susanne Steinmann, Franziska Tebarth, Michael Wagner, Klaus Weckbecker, Dagmar Weeg, Jochen Werle, Siegfried Weyerer, Birgitt Wiese, Steffen Wolfsgruber, and Thomas Zimmermann.
*Hendrik van den Bussche (2002–2011)
We want to thank all participants and their general practitioners for their collaboration. Special thanks to Luca Kleineidam, Christopher Hautmann, and Moritz Daerr for their support.
GPs participating at the time of follow-up visits:
Bonn: Claudia Adrian, Hanna Liese, Inge Bürfent, Johann von Aswege, Wolf-Dietrich Honig, Peter Gülle, Heribert Schützendorf, Elisabeth Benz, Annemarie Straimer, Arndt Uhlenbrock, Klaus-Michael Werner, Maria Göbel-Schlatholt, Hans-Jürgen Kaschell, Klaus Weckbecker, Theodor Alfen, Markus Stahlschmidt, Klaus Fischer, Wolf-Rüdiger Weisbach, Martin Tschoke, Jürgen Dorn, Helmut Menke, Erik Sievert, Ulrich Kröckert, Gabriele Salingré, Christian Mörchen, Peter Raab, Angela Baszenski, Clärli Loth, Christian Knaak, Peter Hötte, Jörg Pieper, Dirk Wassermann, Hans Josef Leyendecker, Gerhard Gohde, Barbara Simons, Achim Brünger, Uwe Petersen, Heike Wahl, Rainer Tewes, Doris Junghans-Kullmann, Angela Grimm-Kraft, Harald Bohnau, Ursula Pinsdorf, Thomas Busch, Gisela Keller, Susanne Fuchs-Römer, and Wolfgang Beisel.
Düsseldorf: Birgitt Richter-Polynice, Florinela Cupsa, Roland Matthias Unkelbach, Gerhard Schiller, Barbara Damanakis, Michael Frenkel, Klaus-Wolfgang Ebeling, Pauline Berger, Kurt Gillhausen, Uwe Hellmessen, Helga Hümmerich, Hans-Christian Heede, Boguslaw- Marian Kormann, Wolfgang Josef Peters, Ulrich Schott, Dirk Matzies, Andre Schumacher, Tim Oliver Flettner, Winfried Thraen, Harald Siegmund, Claus Levacher, Tim Blankenstein, Eliane Lamborelle, Ralf Hollstein, Edna Hoffmann, Ingeborg Ghane, Regine Claß, Stefan-Wolfgang Meier, Leo W. Moers, Udo Wundram, Klaus Schmitt, Rastin Missghian, Karin Spallek, and Christiane Schlösser.
Hamburg: Kathrin Groß, Winfried Bouché, Ursula Linn, Gundula Bormann, Gerhard Schulze, Klaus Stelter, Heike Gatermann, Doris Fischer-Radizi, Otto-Peter Witt, Stefanie Kavka, Günther Klötzl, Karl-Christian Münter, Michael Baumhöfener, Maren Oberländer, Cornelia Schiewe, Jörg Hufnagel, Anne-Marei Kressel, Michael Kebschull, Christine Wagner, Fridolin Burkhardt, Martina Hase, Matthias Büttner, Karl-Heinz Houcken, Christiane Zebidi, Johann Bröhan, Christiane Russ, Frank Bethge, Gisela Rughase-Block, Margret Lorenzen, Arne Elsen, Lerke Stiller, Angelika Giovanopoulos, Daniela Korte, Ursula Jedicke, Rosemarie Müller-Mette, Andrea Richter, Sanna Rauhala-Parrey, Constantin Zoras, Gabriele Pfeil-Woltmann, Annett Knöppel-Frenz, Martin Kaiser, Johannes Bruns, Joachim Homann, Georg Gorgon, Niklas Middendorf, Kay Menschke, Hans Heiner Stöver, Hans H. Bayer, Rüdiger Quandt, Gisela Rughase-Block, Hans-Michael Köllner, Enno Strohbehn, ThomasHaller, Nadine Jesse, Martin Domsch, and Marcus Dahlke.
Leipzig: Thomas Lipp, Ina Lipp, Martina Amm, Horst Bauer, Gabriele Rauchmaul, Hans Jochen Ebert, Angelika Gabriel-Müller, Hans-Christian Taut, Hella Voß, Ute Mühlmann, Holger Schmidt, Gabi Müller, Eva Hager, Bettina Tunze, Barbara Bräutigam, Thomas Paschke, Heinz-Michael Assmann, Ina Schmalbruch, Gunter Kässner, Iris Pförtzsch, Brigitte Ernst-Brennecke, Uwe Rahnefeld, Petra Striegler, Marga Gierth, Anselm Krügel, Margret Boehm, Dagmar Harnisch, Simone Kornisch-Koch, Birgit Höne, Lutz Schönherr, Frank Hambsch, Katrin Meitsch, Britta Krägelin-Nobahar, Cornelia Herzig, Astrid Georgi, Erhard Schwarzmann, Gerd Schinagl, Ulrike Pehnke, Mohammed Dayab, Sabine Müller, Jörg-Friedrich Onnasch, Michael Brosig, Dorothea Frydetzki, Uwe Abschke, Volkmar Sperling, Ulrich Gläser, Frank Lebuser, and Detlef Hagert.
Mannheim: Gerhard Arnold, Viet-Harold Bauer, Hartwig Becker, Hermine Becker, Werner Besier, Hanna Böttcher-Schmidt, Susanne Füllgraf-Horst, Enikö Göry, Hartmut Grella, Hans Heinrich Grimm, Petra Heck, Werner Hemler, Eric Henn, Violetta Löb, Grid Maaßen-Kalweit, Manfred Mayer, Hubertus Mühlig, Arndt Müller, Gerhard Orlovius, Helmut Perleberg, Brigitte Radon, Helmut Renz, Carsten Rieder, Michael Rosen, Georg Scheer, Michael Schilp, Angela Schmid, Matthias Schneider, Christian Schneider, Rüdiger Stahl, Christian Uhle, Jürgen Wachter, Necla Weihs, Brigitte Weingärtner, Monika Werner, Hans- Georg Willhauck, Eberhard Wochele, and Bernhard Wolfram.
München: Andreas Hofmann, Eugen Allwein, Helmut Ruile, Andreas Koeppel, Peter Dick, Karl-Friedrich Holtz, Gabriel Schmidt, Lutz-Ingo Fischer, Johann Thaller, Guntram Bloß, Franz Kreuzer, Günther Holthausen, Karl Ludwig Maier, Walter Krebs, Christoph Mohr, Heinz Koschine, Richard Ellersdorfer, Michael Speth, Maria Kleinhans, Panagiota Koutsouva-Sack, Gabriele Staudinger, Johann Eiber, Stephan Thiel, Cornelia Gold, Andrea Nalbach, Kai Reichert, Markus Rückgauer, Martin Neef, Viktor Fleischmann, Natalija Mayer, Andreas Spiegl, Fritz Renner, Eva Weishappel-Ketisch, Thomas Kochems, Hartmut Hunger, Marianne Hofbeck, Alfred Neumeier, Elfriede Goldhofer, Thomas Bommer, Reinhold Vollmuth, Klaus Lanzinger, Simone Bustami-Löber, Ramona Pauli, Jutta Lindner, Gerlinde Brandt, Otto Hohentanner, Rosita Urban-Hüttner, Peter Porz, Bernd Zimmerhackl, Barbara Naumann, Margarete Vach, Alexander Hallwachs, Claudia Haseke, Andreas Ploch, Paula Bürkle-Grasse, Monika Swobodnik, Corina Tröger, Detlev Jost, Roman Steinhuber, Renate Narr, Gabriele Nehmann-Hörwick, Christiane Eder, Helmut Pillin, Frank Loth, Beate Rücker, Nicola Fritz, Michael Rafferzeder, and Dietmar Zirpel.
GPs who participated at baseline:
Bonn: Heinz-Peter Romberg, Hanna Liese, Inge Bürfent, Johann von Aswege, Wolf-Dietrich Honig, Peter Gülle, Heribert Schützendorf, Manfred Marx, Annemarie Straimer, Arndt Uhlenbrock, Klaus-Michael Werner, Maria Göbel-Schlatholt, Eberhard Prechtel, Hans-Jürgen Kaschell, Klaus Weckbecker, Theodor Alfen, Jörg Eimers-Kleene, Klaus Fischer, Wolf-Rüdiger Weisbach, and Martin Tschoke.
Düsseldorf: Birgitt Richter-Polynice, Michael Fliedner, Binjamin Hodgson, Florinela Cupsa, Werner Hamkens, Roland Matthias Unkelbach, Gerhard Schiller, Barbara Damanakis, Angela Ackermann, Michael Frenkel, Klaus-Wolfgang Ebeling, Bernhard Hoff, Michael Kirsch, Vladimir Miasnikov, Pauline Berger, Kurt Gillhausen, Uwe Hellmessen, Helga Hümmerich, Hans-Christian Heede, Boguslaw-Marian Kormann, Dieter Lüttringhaus, Wolfgang Josef Peters, Ulrich Schott, Dirk Matzies, Andre Schumacher, Tim Oliver Flettner, Winfried Thraen, Clemens Wirtz, and Harald Siegmund.
Hamburg: Kathrin Groß, Bernd-Uwe Krug, Petra Hütter, Dietrich Lau, Gundula Bormann, Ursula Schröder-Höch, Wolfgang Herzog, Klaus Weidner, Doris Fischer-Radizi, Otto-Peter Witt, Stefanie Kavka, Günther Klötzl, Ljudmila Titova, and Andrea Moritz.
Leipzig: Thomas Lipp, Ina Lipp, Martina Amm, Horst Bauer, Gabriele Rauchmaul, Hans Jochen Ebert, Angelika Gabriel-Müller, Hans-Christian Taut, Hella Voß, Ute Mühlmann, Holger Schmidt, Gabi Müller, Eva Hager, Bettina Tunze, Barbara Bräutigam, Sabine Ziehbold, Thomas Paschke, Heinz-Michael Assmann, Ina Schmalbruch, Gunter Kässner.
Mannheim: Gerhard Arnold, Viet-Harold Bauer, Werner Besier, Hanna Böttcher-Schmidt, Hartmut Grella, Ingrid Ludwig, Manfred Mayer, Arndt Müller, Adolf Noky, Gerhard Orlovius, Helmut Perleberg, Carsten Rieder, Michael Rosen, Georg Scheer, Michael Schilp, Gerhard Kunzendorf, Matthias Schneider, Jürgen Wachter, Brigitte Weingärtner, and Hans- Georg Willhauck.
München: Helga Herbst, Andreas Hofmann, Eugen Allwein, Helmut Ruile, Andreas Koeppel, Peter Friedrich, Hans-Georg Kirchner, Elke Kirchner, Luitpold Knauer, Peter Dick, Karl-Friedrich Holtz, Elmar Schmid, Gabriel Schmidt, Lutz-Ingo Fischer, Johann Thaller, Guntram Bloß, Franz Kreuzer, Ulf Kahmann, Günther Holthausen, Karl Ludwig Maier, Walter Krebs, Christoph Mohr, Heinz Koschine, Richard Ellersdorfer, and Michael Speth.
GPs who participates in the study previously:
Bonn: Heinz-Peter Romberg, Eberhard Prechtel, Manfred Marx, Jörg Eimers-Kleene, Paul Reich, Eberhard Stahl, Reinhold Lunow, Klaus Undritz, Bernd Voss, Achim Spreer, Oliver Brenig, Bernhard G. Müller, Ralf Eich, Angelika Vossel, Dieter Leggewie, Angelika Schmidt, Nahid Aghdai-Heuser, Lutz Witten, and Michael Igel.
Düsseldorf: Michael Fliedner, Benjamin Hodgson, Werner Hamkens, Angela Ackermann, Bernhard Hoff, Michael Kirsch, Vladimir Miasnikov, Dieter Lüttringhaus, Clemens Wirtz, Rolf Opitz, Jürgen Bausch, Dirk Mecking, Friederike Ganßauge, Elmar Peters, and Alfons Wester.
Hamburg: Werner Petersen, Martin Daase, Martin Rüsing, Christoph von Sethe, Wilmhard Borngräber, Brigitte Colling-Pook, Ullrich Weidner, Peter Rieger, Lutz Witte, Hans-Wilhelm Busch, Jürgen Unger, Angela Preis, Michael Mann, Ernst Haeberle, Horst Köhler, Ruth Schäfer, Helmut Sliwiok, Volker L. Brühl, Hans-Heiner Stöver, Harald Deest, Margret Ackermann-Körner, Dieter Reinstorff, Christamaria Schlüter, Henrik Heinrichs, Ole Dankwarth, Michael Böse, Ulricke Ryll, Reinhard Bauer, Dieter Möltgen, Sven Schnakenbeck, Karin Beckmann, Annegret Callsen, Ewa Schiewe, Holger Gehm, Volker Lambert, Karin Hinkel-Reineke, Carl-Otto Stolzenbach, Peter Berdin, and Friedhelm Windler.
Leipzig: Sabine Ziehbold, Sabine Weidnitzer, Erika Rosenkranz, Norbert Letzien, Doris Klossek, Martin Liebsch, Andrea Zwicker, Ulrike Hantel, Monika Pilz, Volker Kirschner, Rainer Arnold, and Ulrich Poser.
Mannheim: Wolfgang Barthel, Fritz Blechinger, Marcus Fähnle, Reiner Walter Fritz, Susanne Jünemann, Gabriele Kirsch, Jürgen Kulinna, Gerhard Kunzendorf, Andreas Legner-Görke, Christa Lehr, Wolfgang Meer, Adolf Noky, Christina Panzer, Achim Raabe, Helga Schmidt-Back, Ralf Schürmann, Hans-Günter Stieglitz, and Marie-Luise von der Heide.
München: Helga Herbst, Peter Friedrich, Hans-Georg Kirchner, Elke Kirchner, Luitpold Knauer, Elmar Schmid, Ulf Kahmann, Jörg Kastner, Ulrike Janssen, Albert Standl, Clemens Göttl, Marianne Franze, Gerhard Moser, Almut Blümm, Petra Weber, Wolfgang Poetsch, Heinrich Puppe, Thomas Bommer, Gerd Specht, Leonard Badmann, May Leveringhaus, Michael Posern, Andreas Ploch, Ralph Potkowski, Christiane Eder, Michael Schwandner, Rudolf Weigert, and Christoph Huber.
Footnotes
Conflict of interest disclosure: The authors report no competing interests.
Funding: This study is part of the German Research Network on Dementia (KND) and the German Research Network on Degenerative Dementia (KNDD), and was funded by the German Federal Ministry of Education and Research (grants KND: 01GI0102, 01GI0420, 01GI0422, 01GI0423, 01GI0429, 01GI0431, 01GI0433, 01GI0434; grants KNDD: 01GI0710, 01GI0711, 01GI0712, 01GI0713, 01GI0714, 01GI0715, 01GI0716).
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2015.02.005.
Supplementary data
Effects of Covariates on the cognitive development in episodic memory, word fluency, working memory and Mini-Mental State Examination (MMSE).
Supplementary 2.
Trajectories of observed means and standard errors for controls (CO), SCD without concerns (SCD-C) and SCD with concerns (SCD+C).
Supplementary 3.
Trajectories of observed means and standard errors for stable controls (CO), converters into SCD without concerns (SCD-C) and converters into SCD with concerns (SCD+C).
References
- 1.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmand B., Jonker C., Hooijer C., Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46:121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- 3.Geerlings M.I., Jonker C., Bouter L.M., Adèr H.J., Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. Am J Psychiatry. 1999;156:531–537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- 4.Rabin L.A., Wang C., Katz M.J., Derby C.A., Buschke H., Lipton R.B. Predicting Alzheimer's disease: neuropsychological tests, self-reports, and informant reports of cognitive difficulties. J Am Geriatr Soc. 2012;60:1128–1134. doi: 10.1111/j.1532-5415.2012.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen F. Prediction of dementia by subjective memory impairment effects of severity and temporal association with cognitive impairment dementia and subjective memory impairment. Arch Gen Psychiatry. 2010;67:414. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 6.Jorm A.F., Christensen H., Korten A.E., Henderson A.S., Jacomb P.A., Mackinnon A. Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychol Med. 1997;27:91–98. doi: 10.1017/s0033291796003923. [DOI] [PubMed] [Google Scholar]
- 7.Bolla K.I., Lindgren K.N., Bonaccorsy C., Bleecker M.L. Memory complaints in older adults: fact or fiction? Arch Neurol. 1991;48:61–64. doi: 10.1001/archneur.1991.00530130069022. [DOI] [PubMed] [Google Scholar]
- 8.Mewton L., Sachdev P., Anderson T., Sunderland M., Andrews G. Demographic, clinical, and lifestyle correlates of subjective memory complaints in the Australian population. Am J Geriatr Psychiatry. 2014;22:1222–1232. doi: 10.1016/j.jagp.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Jessen F., Wiese B., Cvetanovska G., Fuchs A., Kaduszkiewicz H., Koelsch H. Patterns of subjective memory impairment in the elderly: association with memory performance. Psychol Med. 2007;37:1753–1762. doi: 10.1017/S0033291707001122. [DOI] [PubMed] [Google Scholar]
- 10.Gagnon M., Dartigues J.F., Mazaux J., Dequae L., Letenneur L., Giroire J.M. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID Research Program. Neuroepidemiology. 1994;13:145–154. doi: 10.1159/000110373. [DOI] [PubMed] [Google Scholar]
- 11.Jonker C., Launer L.J., Hooijer C., Lindeboom J. Memory complaints and memory impairment in older individuals. J Am Geriatr Soc. 1996;44:44–49. doi: 10.1111/j.1532-5415.1996.tb05636.x. [DOI] [PubMed] [Google Scholar]
- 12.Dufouil C., Fuhrer R., Alperovitch A. Subjective cognitive complaints and cognitive decline: consequence or predictor? The epidemiology of vascular aging study. J Am Geriatr Soc. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 13.Blazer D.G., Hays J.C., Fillenbaum G.G., Gold D.T. Memory complaint as a predictor of cognitive decline A comparison of African American and White elders. J Aging Health. 1997;9:171–184. doi: 10.1177/089826439700900202. [DOI] [PubMed] [Google Scholar]
- 14.Mol M.E., van Boxtel M.P., Willems D., Jolles J. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht Aging Study. Int J Geriatr Psychiatry. 2006;21:432–441. doi: 10.1002/gps.1487. [DOI] [PubMed] [Google Scholar]
- 15.Jorm A.F., Christensen H., Korten A.E., Jacomb P.A., Henderson A.S. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7-8 years. Psychol Med. 2001;31:441–449. [PubMed] [Google Scholar]
- 16.Dik M.G., Jonker C., Comijs H.C., Bouter L.M., Twisk J.W., van Kamp G.J. Memory complaints and APOE-4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57:2217–2222. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- 17.Hohman T.J., Beason-Held L.L., Lamar M., Resnick S.M. Subjective cognitive complaints and longitudinal changes in memory and brain function. Neuropsychology. 2011;25:125–130. doi: 10.1037/a0020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 19.Zaudig M., Mittelhammer J., Hiller W., Pauls A., Thora C., Morinigo A. SIDAM–A structured interview for the diagnosis of dementia of the Alzheimer type, multi-infarct dementia and dementias of other aetiology according to ICD-10 and DSM-III-R. Psychol Med. 1991;21:225–236. doi: 10.1017/s0033291700014811. [DOI] [PubMed] [Google Scholar]
- 20.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 22.König W LPM. MüllerWA Comparative analysis of the development and structure of educational systems: methodological foundations and the construction of a Comparative Education Scale.
- 23.Reisberg B., Ferris S.H., de Leon M.J., Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 24.Blessed G., Tomlinson B.E., Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 25.Luck T., Zaudig M., Wiese B., Riedel-Heller S.G. SIDAM: Alters- und bildungsspezifische Normen des kognitiven Leistungsteiles nach der neuen CASMIN-Bildungsklassifikation. Zeitschrift für Gerontopsychologie & -psychiatrie. 2007;20:31–38. [Google Scholar]
- 26.Muthén L.K., Muthén B.O. Seventh Edition. Muthén & Muthén; Los Angeles, CA: 1998. Mplus User's Guide. [Google Scholar]
- 27.Duncan T.E., Duncan S.C., Strycker L.A. Erlbaum; Mahwah, NJ: 2006. An introduction to latent variable growth curve modeling concepts, issues, and applications. [Google Scholar]
- 28.McArdle J.J. Longitudinal models of growth and survival applied to the early detection of Alzheimer's disease. J Geriatr Psychiatry Neurol. 2005;18:234–241. doi: 10.1177/0891988705281879. [DOI] [PubMed] [Google Scholar]
- 29.Browne M.W., Cudeck R., Bollen K.A., Long J.S. Alternative ways of assessing model fit. Sage Focus Editions. 1993;154:136. [Google Scholar]
- 30.Bentler P.M. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 31.Bollen K.A., Long J.S. vol. 154. SAGE Publications; Newbury Park: 1993. (Testing structural equation models). [Google Scholar]
- 32.Byrne B.M. L. Erlbaum Associates; Mahwah, N.J: 1998. Structural equation modeling with LISREL, PRELIS, and SIMPLIS: Basic concepts, applications, and programming. [Google Scholar]
- 33.McArdle J., Grimm K. Five steps in latent curve and latent change score modeling with longitudinal data. In: van Montfort K., Oud J.H., Satorra A., editors. Longitudinal research with latent variables. Springer; Berlin Heidelberg: 2010. pp. 245–273. [Google Scholar]
- 34.Nesselroade J.R. Temporal selection and factor invariance in the study of development and change. Life-span development and behavior. 1983;5:59–87. [Google Scholar]
- 35.Little T.D. Oxford University Press; New York: 2013. The Oxford handbook of quantitative methods. ©. [Google Scholar]
- 36.Caselli R.J., Chen K., Locke D.E., Lee W., Roontiva A., Bandy D. Subjective cognitive decline: self and informant comparisons. Alzheimers Dement. 2014;10:93–98. doi: 10.1016/j.jalz.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomeni R., Simeoni M., Zvartau-Hind M., Irizarry M.C., Austin D., Gold M. Modeling Alzheimer's disease progression using the disease system analysis approach. Alzheimers Dement. 2012;8:39–50. doi: 10.1016/j.jalz.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Royall D.R., Palmer R., Chiodo L.K., Polk M.J. Depressive symptoms predict longitudinal change in executive control but not memory. Int J Geriatr Psychiatry. 2012;27:89–96. doi: 10.1002/gps.2697. [DOI] [PubMed] [Google Scholar]
- 39.Amieva H., Le Goff M., Millet X., Orgogozo J.M., Pérès K., Barberger-Gateau P. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 40.Stewart R., Godin O., Crivello F., Maillard P., Mazoyer B., Tzourio C. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br J Psychiatry. 2011;198:199–205. doi: 10.1192/bjp.bp.110.078683. [DOI] [PubMed] [Google Scholar]
- 41.Mathews M., Abner E., Caban-Holt A., Kryscio R., Schmitt F. CERAD practice effects and attrition bias in a dementia prevention trial. Int Psychogeriatr. 2013;25:1115–1123. doi: 10.1017/S1041610213000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zehnder A.E., Bläsi S., Berres M., Spiegel R., Monsch A.U. Lack of practice effects on neuropsychological tests as early cognitive markers of Alzheimer disease? Am J Alzheimers Dis Other Demen. 2007;22:416–426. doi: 10.1177/1533317507302448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jessen F., Feyen L., Freymann K., Tepest R., Maier W., Heun R. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Engvig A., Fjell A.M., Westlye L.T., Skaane N.V., Sundseth Ø., Walhovd K.B. Hippocampal subfield volumes correlate with memory training benefit in subjective memory impairment. Neuroimage. 2012;61:188–194. doi: 10.1016/j.neuroimage.2012.02.072. [DOI] [PubMed] [Google Scholar]
- 45.Scheef L., Spottke A., Daerr M., Joe A., Striepens N., Kolsch H. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 46.Amariglio R.E., Becker J.A., Carmasin J., Wadsworth L.P., Lorius N., Sullivan C. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kryscio R.J., Abner E.L., Lin Y., Cooper G.E., Fardo D.W., Jicha G.A. Adjusting for mortality when identifying risk factors for transitions to mild cognitive impairment and dementia. J Alzheimers Dis. 2013;35:823–832. doi: 10.3233/JAD-122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kryscio R.J., Abner E.L., Cooper G.E., Fardo D.W., Jicha G.A., Nelson P.T. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83:1359–1365. doi: 10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of Covariates on the cognitive development in episodic memory, word fluency, working memory and Mini-Mental State Examination (MMSE).