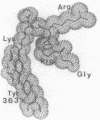
Abstract
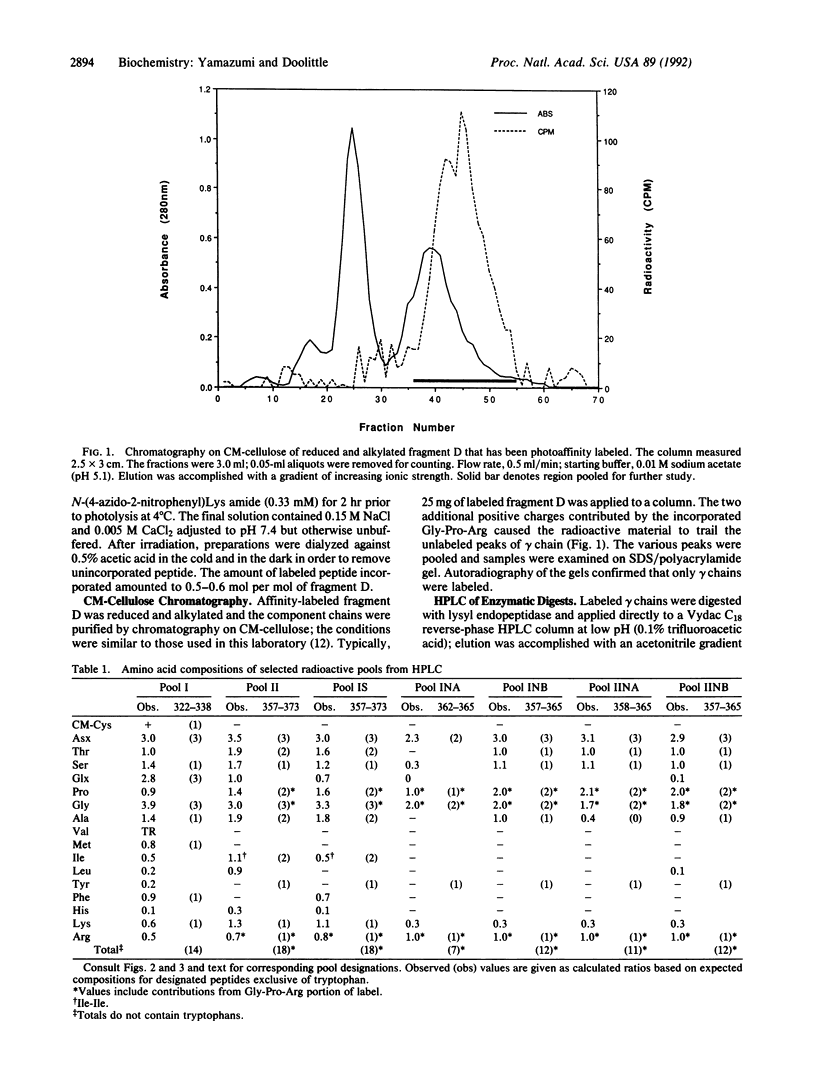
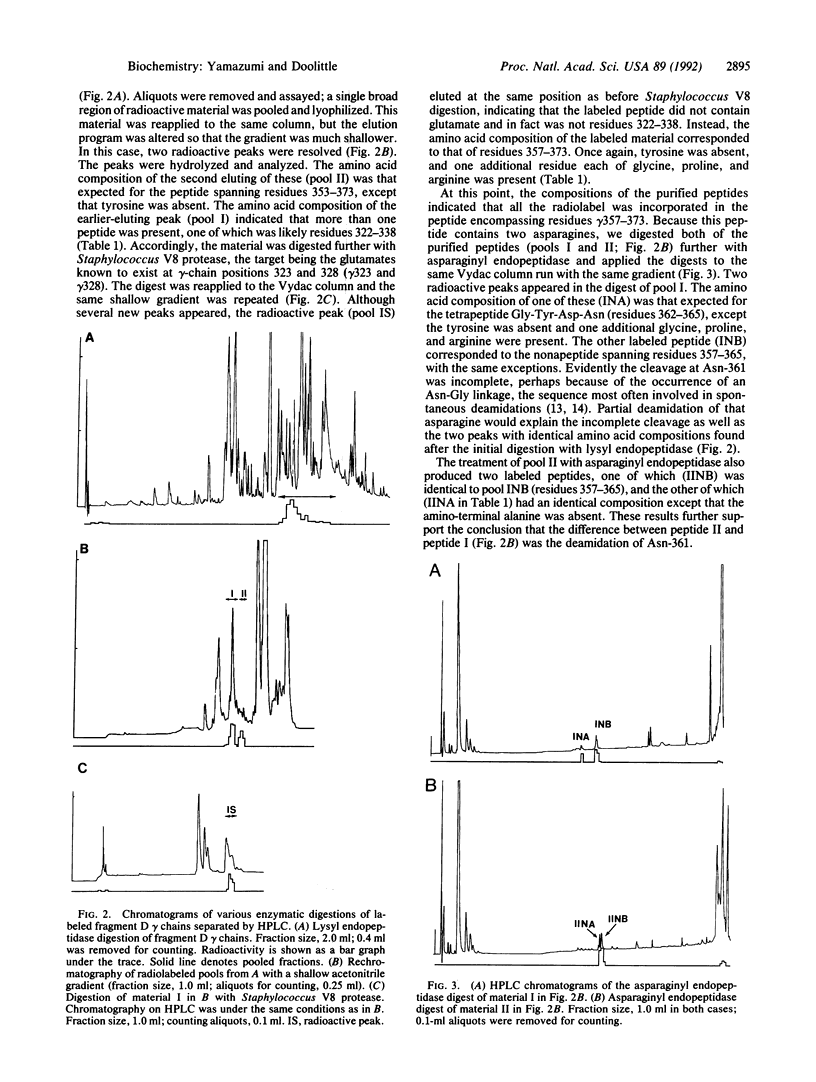
Fragment D prepared from human fibrinogen was labeled specifically by photoactivation of the peptide [14C]Gly-Pro-Arg-N-(4-azido-2-nitrophenyl)Lys amide. The preparation was freed of excess labeling reagents and then reduced and alkylated. The component alpha, beta, and gamma chains were purified by chromatography on carboxymethylcellulose and the radioactivity was found to be restricted to the gamma chain. Isolated gamma chains were digested with various endopeptidases, both alone and in tandem, and the products were fractionated by gradient HPLC. The amino acid compositions of all labeled peptides led to the conclusion that the modification occurs exclusively on gamma-chain Tyr-363.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohonus V. L., Doolittle R. F., Pontes M., Strong D. D. Complementary DNA sequence of lamprey fibrinogen beta chain. Biochemistry. 1986 Oct 21;25(21):6512–6516. doi: 10.1021/bi00369a026. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Cottrell B. A., Riley M. Amino acid compositions of the subunit chains of lamprey fibrinogen. Evolutionary significance of some structural anomalies. Biochim Biophys Acta. 1976 Dec 22;453(2):439–452. doi: 10.1016/0005-2795(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- Gráf L., Bajusz S., Patthy A., Barát E., Cseh G. Revised amide location for porcine and human adrenocorticotropic hormone. Acta Biochim Biophys Acad Sci Hung. 1971;6(4):415–418. [PubMed] [Google Scholar]
- Horwitz B. H., Váradi A., Scheraga H. A. Localization of a fibrin gamma-chain polymerization site within segment Thr-374 to Glu-396 of human fibrinogen. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5980–5984. doi: 10.1073/pnas.81.19.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman J., Haverkate F., Briët E., Lord S. T. A congenitally abnormal fibrinogen (Vlissingen) with a 6-base deletion in the gamma-chain gene, causing defective calcium binding and impaired fibrin polymerization. J Biol Chem. 1991 Jul 15;266(20):13456–13461. [PubMed] [Google Scholar]
- Kuyas C., Haeberli A., Walder P., Straub P. W. Isolation of human fibrinogen and its derivatives by affinity chromatography on Gly-Pro-Arg-Pro-Lys-Fractogel. Thromb Haemost. 1990 Jun 28;63(3):439–444. [PubMed] [Google Scholar]
- Laudano A. P., Doolittle R. F. Studies on synthetic peptides that bind to fibrinogen and prevent fibrin polymerization. Structural requirements, number of binding sites, and species differences. Biochemistry. 1980 Mar 4;19(5):1013–1019. doi: 10.1021/bi00546a028. [DOI] [PubMed] [Google Scholar]
- Laudano A. P., Doolittle R. F. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3085–3089. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottspeich F., Henschen A. Amino acid sequence of human fibrin. Preliminary note on the completion of the gamma-chain sequence. Hoppe Seylers Z Physiol Chem. 1977 Jul;358(7):935–938. [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z. Localization of a fibrin polymerization site. J Biol Chem. 1981 Apr 10;256(7):3544–3549. [PubMed] [Google Scholar]
- Shimizu A., Nagel G. M., Doolittle R. F. Photoaffinity labeling of the primary fibrin polymerization site: isolation and characterization of a labeled cyanogen bromide fragment corresponding to gamma-chain residues 337-379. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2888–2892. doi: 10.1073/pnas.89.7.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C., Thompson E., Panico M., Etienne T., Morris H. R., Lane D. A. Characterization of peptides cleaved by plasmin from the C-terminal polymerization domain of human fibrinogen. J Biol Chem. 1985 Oct 25;260(24):13095–13101. [PubMed] [Google Scholar]
- Staros J. V., Wright R. W., Swingle D. M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986 Jul;156(1):220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- Váradi A., Scheraga H. A. Localization of segments essential for polymerization and for calcium binding in the gamma-chain of human fibrinogen. Biochemistry. 1986 Feb 11;25(3):519–528. doi: 10.1021/bi00351a001. [DOI] [PubMed] [Google Scholar]
- Wright H. T. Sequence and structure determinants of the nonenzymatic deamidation of asparagine and glutamine residues in proteins. Protein Eng. 1991 Feb;4(3):283–294. doi: 10.1093/protein/4.3.283. [DOI] [PubMed] [Google Scholar]