Abstract
Background
Frailty is common in older age, and is associated with important adverse health outcomes including increased risk of disability and long-term care admission.
Objectives
To evaluate whether home-based exercise interventions improve outcomes for frail older people.
Data sources
We searched systematically for randomised controlled trials (RCTs) and cluster RCTs, with literature searching to February 2010.
Study selection
All trials that evaluated home-based exercise interventions for frail older people were eligible. Primary outcomes were mobility, quality of life and daily living activities. Secondary outcomes included long-term care admission and hospitalisation.
Results
Six RCTs involving 987 participants met the inclusion criteria. Four trials were considered of high quality. One high quality trial reported improved disability in those with moderate but not severe frailty. Meta-analysis of long-term care admission rates identified a trend towards reduced risk. Inconsistent effects on other primary and secondary outcomes were reported in the other studies.
Conclusions
There is preliminary evidence that home-based exercise interventions may improve disability in older people with moderate, but not severe, frailty. There is considerable uncertainty regarding effects on important outcomes including quality of life and long-term care admission. Home-based exercises are a potentially simple, safe and widely applicable intervention to prevent dependency decline for frail older people.
Keywords: Frailty, exercise, home-based, community, systematic review
Introduction
Frailty is a common and important syndrome that is increasingly prevalent with advancing age. Whilst there is no internationally agreed definition of frailty, there is a consensus that frailty develops as a consequence of a decline in multiple physiological systems, particularly the neuromuscular, neuroendocrine and immunological systems (1). This decline results in a vulnerability to a sudden change in health status that can be triggered by relatively minor stressor events (2). The resulting frailty phenotype includes: weight loss, exhaustion, low energy expenditure, slow gait speed and loss of muscle mass and strength (sarcopenia) (3). The Fried investigators recorded a frailty prevalence rate of 6.9% in a cohort of 5201 men and women aged 65 years or more, rising to 25.7% in those aged 85 years and over (3).
Frailty is self-perpetuating; its development results in a spiral of decline that leads to worsening frailty and increased risk of adverse consequences that have substantial health and socioeconomic cost including disability, admission to hospital or long-term care, and death (3, 4). Any attenuation of the prevalence or severity of frailty is therefore likely to improve the health and well-being of the individual, their families and society.
Sarcopenia, one of the key components of frailty, is a potential target for frailty prevention interventions that incorporate exercise to increase muscle mass and strength. Falls prevention and pulmonary rehabilitation interventions, which include exercise components, have previously been demonstrated to be effective at improving important outcomes and are part of routine care (5, 6). A proportion of older people who receive these interventions are likely to be frail, but improved outcomes related specifically to frailty are unclear.
A recent systematic review concluded that exercise interventions, particularly those involving strength and balance training, can be successful at improving muscle strength and functional abilities in long-term care residents; a group of older people who are very likely to be frail (7). However, the large majority of older people in the US live at home (8). Exercise interventions for older people living at home can be delivered either individually in their homes, or elsewhere as a group activity. A 2005 Cochrane review concluded that both home-based and group-based exercise interventions are associated with improved outcomes for patients receiving cardiac rehabilitation, but that home-based interventions may be associated with improved adherence (9). In order to explore the available evidence for frail older people, a systematic review was undertaken on the effectiveness of home-based exercise interventions.
Methods
A copy of the full review protocol is available on request from the corresponding author.
Search strategy
We searched systematically for all randomised controlled trials (RCTs) and cluster RCTs that evaluated home-based exercise interventions for frail older people. A search strategy was developed for Medline, with appropriate amendments for AMED, CINAHL, Cochrane Library, EMBASE, PSYCHINFO and PedRO, with literature searching to February 2010. A full copy of the search strategy is available in Appendix 1.
Eligibility criteria
The initial search criteria were deliberately broad and all studies that recruited a cohort of older people (defined for this review as an average age in the study cohort of age 70 years or older) were initially considered for inclusion. The individual study description, selection criteria and reported cohort baseline characteristics were then carefully examined by two independent assessors with expertise in both the assessment of frail older people and frailty indices to determine whether the study populations were frail. Studies were considered as frail only if they selected participants or stratified results using an operationalized definition of frailty or if one or more of the five frailty criteria (weight loss, exhaustion, low energy expenditure, slow gait speed or muscle weakness) were identified.
Studies in which the target population were selected on the basis of the presence of a specific medical condition (e.g. chronic obstructive pulmonary disease, heart failure, cognitive impairment, etc), and studies conducted in care home facilities, were excluded.
Types of outcomes
Our primary outcome measures for this review were measures of mobility (e.g. the Timed Up and Go Test (10)), health-related quality of life indices (e.g. EuroQol Group 5-Dimension Self-Report Questionnaire (EQ5D) (11)) and measures of activities of daily living (ADL, e.g. Barthel index (12)). Secondary outcomes measures were muscle strength, balance, depression, bone strength and adverse outcomes including falls and admission to hospital or long-term care.
Types of interventions
For this review, exercise is defined as an activity requiring physical effort that is intended to improve or maintain fitness. Studies in which the intervention included a mix of home-based and group based exercise were only included if the home-based component formed the greater proportion of the intervention. Entirely group-based exercise interventions were not considered for this review.
The evidence base for falls prevention interventions is already well established and a recent systematic review concluded that the Otago Exercise Programme (OEP), a home-based falls prevention intervention, was effective at reducing falls and mortality (5, 13). However, the evidence base from the falls prevention literature is not necessarily generalisable to frail older people. Falls prevention interventions are usually targeted at older people who are living independently or with few restrictions in ADL. Strengthening exercises in falls prevention interventions are often of moderate-to-high intensity and are usually performed standing with weights or therabands to provide resistance. Balance exercises incorporate dynamic movement and may be of greater risk for frail older people. Additionally the majority of falls prevention interventions include a substantial aerobic component that usually comprises moderate intensity walking/cycling/aerobics for 20-30 minutes, 2-3 days a week. Furthermore, the duration of falls prevention exercise sessions are frequently for between 30 minutes and 90 minutes and this is not necessarily appropriate for frail older people considering the low energy expenditure and fatiguability that characterize frailty. For these reasons, trials in which the intervention had been delivered as the main component of a falls prevention package were also excluded from this systematic review.
Study selection
All titles and abstracts were screened for eligibility by two independent reviewers and any disagreements settled by a third reviewer. Full texts of eligible studies were obtained and reference lists were reviewed for further eligible studies. Two reviewers extracted data using Revman 5.0 software. One reviewer evaluated each study for risk of methodological bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (14). Studies were assessed for allocation sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting and other potential sources of bias. For each of these domains a judgement of adequate, partially adequate or inadequate was recorded in order to determine the risk of methodological bias for individual studies. The assessment of bias risk was to inform a sensitivity analysis whereby greatest emphasis was given to the studies that were at lowest risk of methodological bias. Only studies considered to be at low risk of methodological bias would be considered for meta-analysis. If data available precluded meta-analysis then a narrative synthesis was planned.
Results
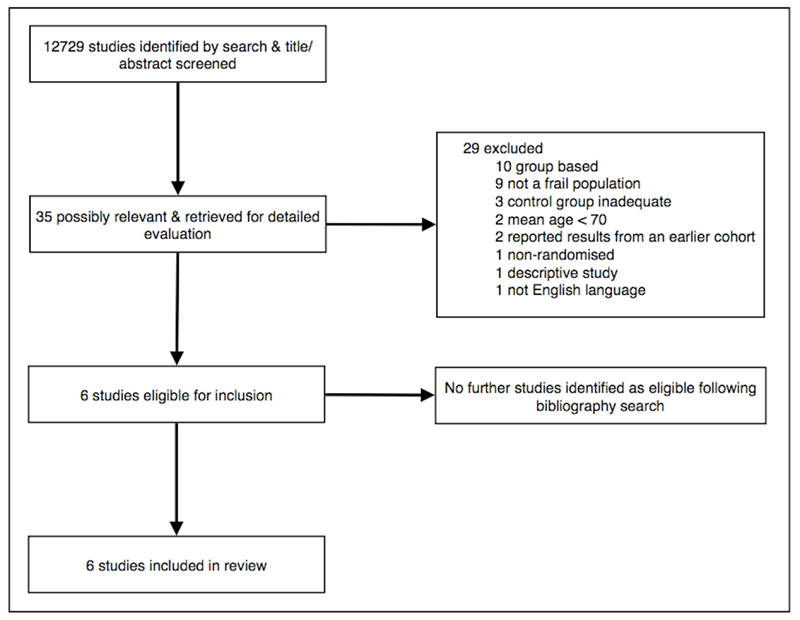
The review process is summarized in figure 1 using the PRISMA guidelines (15).
Figure 1.
PRISMA flow diagram
Study characteristics
Six RCTs involving 987 participants met the review inclusion criteria (16–21) and are summarized in table 1.
Table 1.
Summary table of study characteristics, intervention details, main findings and risk of methodological bias. Key: SD, Standard Deviation; ADL, Activities of Daily Living; NA, not available
| Study | Year | Country | Sample size |
Mean age (SD) |
Study participants | Baseline characteristics |
Intervention | Delivery | Treatment Frequency/ Duration |
Completion (Adherence) rate (%) |
Main Findings | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chandler (16) | 1998 | USA | 100 | 78 (8) | Aged over 64 years and meeting an operationalised, non-validated definition of frailty - inability to descend the stairs without holding the bannister. Participants then further stratified on the basis of whether they were able to rise from a chair without using their arms | 50% male, 50% female 57% were unable to do stairs but able to do chair rise 43% were unable to do either stairs or chair rise | Lower body progressive resistance exercises | Home visits from physiotherapist for each session | 3 times per week for 10 weeks | 87 (NA) | Strength improved. Greater improvement in more frail group. | High |
| Gill (17) | 2002 | USA | 188 | 83 (5) | Aged 75 years and over and meeting an operationalised, non-validated definition of frailty - greater than 10 seconds to perform a rapid gait test or unable to stand from a seated position with arms folded. ‘Moderate’ frailty if one of two criteria present, ‘severe’ frailty if both criteria present | 20% male, 80% female 47% living alone Mean of 2.1 chronic conditions 62% moderate frailty, 38% severe frailty | Complex, individualized progamme of occupational intervention with progressive resistance, balance and range of motion exercises | Home visits from physiotherapist; average of 16 visits over the 6 month period | Once a day, 3 times per week for 6 months | 65 (78) | Improvement in ADL at 6 months. No improvement at 3 months. No improvement in most frail group | Low |
| Luukinen (18) | 2006 | Finland | 486 | 88 (3) | Aged 85 years and over with at least one risk factor for ADL disability or history of recurrent falls | 21% male, 79% female 31% had severe mobility restriction, 25% had slow walking speed, 20% had severe restriction in ADL score, 19% had poor self-related health Mean of 6.5 (SD 4) medications used | Complex individualised exercise and occupational intervention including a combination of; walking, group exercises, home-based resistance exercises and self care exercises | Unclear | Home-based resistance exercises 3 times a day for 18 months | 59 (NA) | Improvement in mobility score. No effect on ADL disability, hospitalisation or long-term care admission. | Low |
| McMurdo (19) | 1995 | UK | 86 | 82 | Aged 75 years and over, living in sheltered housing with limited mobility requiring the use of a walking aid and ADL dependence requiring home help at least once a week | 12% male, 88% female Median Barthel score 19 (range 14-20) | Whole body resistance exercises and range of motion exercises | Exercise cards with diagrams and written explanations. Physiotherapist visit every 3-4 weeks | Once a day for 6 months | 73 (NA) | Trend towards improved mobility. No effect on strength, physical condition. | Low |
| Rosie (20) | 2007 | New Zealand | 66 | 85 (4) | Aged 80 years and over, able to walk 4m with or without a mobility aid, sedentary and mobility-limited. Participants were considered to have a mobility limitation if they were limited ‘a lot’ in vigorous activity and in one or more activity on the Short-Form 36 Health Survey (SF-36) Physical Functioning Scale (PF-10) | 29% male, 71% female 59.1% limited ‘a lot’ in 5 or more domains of the PF-10 63.6% had no falls in the preceding 12 months | Repeat sit-to-stands using a GrandStand system | One home visit from study researcher followed by one weekly telephone call thereafter | Once a day for 6 weeks | 88 (66) | No effect on mobility, ADL or balance | Low |
| Vestergaard (21) | 2007 | Denmark | 61 | 81 (3) | Aged 75 years and over in receipt of home care who were housebound but able to get out of a chair and bed | Intervention group took mean of 19.3 seconds (SD 11.6) to complete 5 repetitions of a chair rise, control group took mean of 16.4 seconds (SD 5.3) | Flexibility and balance exercises, whole body resistance exercises and aerobic exercises | Exercise video & booklet. One home visit from exercise instructor followed by bi-weekly telephone calls thereafter | 3 times a week for 5 months | 83 (89) | Improvement in quality of life. No effect on strength, mobility, physical performance or balance | Moderate |
The median age was 83 years (range 78 - 88) and the majority of participants were female (median 79% female, range 50 - 88%). Three trials were conducted in Western Europe (18, 19, 21), two in the USA (16, 17) and one in New Zealand (20). A median of 71% (range 8 - 88%) of older people living at home were eligible for trial inclusion and, of those who were eligible, a median of 75% (range 17 - 87%) were recruited. The wide range of values reflects the use of different eligibility criteria and different methods of recruitment in the studies.
Two trials used an operationalised, non-validated frailty model to select and stratify participants (16, 17). Four trials did not use an operationalised frailty model to select participants but reported inclusion criteria or baseline characteristics that identified slow walking speed (18, 19, 21) or physical exhaustion (20) and were therefore considered by consensus to be frail.
All trials assessed participants at the end of the intervention. Median duration of follow-up was six months (range six weeks - 18 months). Two studies included follow-up of 12 months or more (17, 18).
Methodological quality & study power
Four trials were assessed as low risk of methodological bias (17–20), one at moderate risk (21) and one at high risk of bias (16). Although the majority of trials were single (assessor) blind, one was unblinded (21). Methods of randomisation were generally well described but an adequate description of the method of allocation concealment was provided in only two studies (19, 20). Only three trials performed an a priori power calculation (18–20). Three of the trails recruited less than 100 subjects; only two recruited more than 200 subjects.
Exercise interventions, completion & adherence
One intervention included a single component of progressive resistance exercise (16). Two combined progressive resistance exercises with one or more additional components of balance, walking or range of motion exercises (19, 21). Two interventions were complex interventions combining multiple exercise components with an occupational intervention (17, 18). One study used an electronic device that counted the number of sit-to-stands (GrandStand™ system) (20).
Modal treatment frequency was three times per week (range 3 - 21 sessions per week). Modal treatment duration was 6 months (mean 28 weeks, range 6 weeks - 18 months).
Information regarding the percentage of participants who completed the exercise intervention through to follow-up was available in all six studies. Completion rates were generally high (median 83%, range 65 - 88%); interventions of shorter duration generally recorded higher completion rates. Rates of adherence to the exercise intervention, measured as the number of individual exercise sessions undertaken as a proportion of the total possible, were recorded in three studies (17, 20, 21). Various methods were used to define acceptable adherence and rates were generally high (median 78%, range 66 - 89%).
Analysis of primary outcome data
Meta-analysis of primary outcome data from the studies at low risk of methodological bias was precluded by the absence of consistent reporting of data required for calculation and pooling of standardized mean differences (SMDs) for these continuous outcomes. Therefore, a narrative synthesis of the available evidence from all studies is provided that describes the direction and size of effect, its consistency across studies and the overall strength of the evidence. A narrative description of the evidence from the studies at low risk of methodological bias is also provided.
Analysis of secondary outcome data
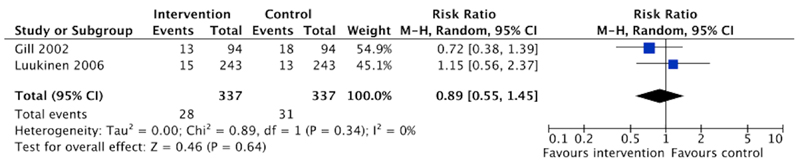
Meta-analysis of long-term care admission data was possible, using dichotomous data from two trials at low risk of methodological bias (17, 18). These data were pooled for meta-analysis using random effects Mantel-Haenszel modeling (Revman 5.0 software) and are presented as risk ratios in a forest plot (figure 2). It was not possible to pool continuous outcome data for the other secondary outcomes due to the data limitations described above and a narrative synthesis is presented.
Figure 2.
Forest plot presenting individual and pooled risk of long-term care admission from two trials at low risk of methodological bias.
Primary outcomes
Effects on mobility
Four trials reported an outcome measure relating to mobility, using various measures of gait speed (18–21). Improved gait speed was reported in one trial (18), a trend towards improved gait speed was reported in one further trial (19) and gait speed did not improve in two (20, 21).
Effects of health-related quality of life
One trial reported an improvement in quality of life, measured using the EQ5D (21). The other trials did not record quality of life measurements.
Effects on activities of daily living
Measures of Activities of Daily Living (ADL) were reported in four trials (17–20). Improvements in ADL were reported in one trial (17), no improvements in ADL were reported in the other three trials (18–20).
Secondary outcomes
The meta-analysis of long-term care admission data is presented in figure 2. A non-significant trend towards reduced long-term care admission is observed (pooled risk ratio 0.89, 95% confidence interval 0.55-1.45).
Three trials measured muscle strength using upper and lower body strength (16, 21) or grip strength (19). One trial reported improved lower body strength (16). There was no improvement in either upper or lower body strength in one trial (21). No improvement in grip strength was recorded in the study that measured this outcome (19). No improvement in general physical performance was reported in one trial (21).
Improved balance was reported in one trial (18) but there was no effect on balance in three trials (19–21). There was no effect on depression (18), bone density or flexibility (19). Hospitalization rates were not reported in any trials.
Adverse outcomes
Between group differences in adverse outcomes were reported in only two trials (17, 18). Increased angina diagnoses were recorded in one trial (17) but no differences in fractures, musculoskeletal pain or death were reported (17, 18).
Trials at low risk of methodological bias
There were four high quality trials at low risk of methodological bias (17–20). One trial selected and stratified participants using an operationalised, but non-validated, measure of frailty (17). Participants were considered for inclusion and defined as being frail if they took >10 seconds to walk three metres, or if they were unable to stand from seated with both arms folded. Participants with one of the two criteria were defined as being moderately frail; participants with both criteria were defined as severely frail. This relatively large trial (n=188) investigated the effects of a six month complex individualised exercise and occupational intervention and reported an improvement in disability score at seven months for people with moderate frailty (17). This improvement was maintained at 12 months follow-up. There was no effect for people with severe frailty.
Although the other three trials at low risk of methodological bias recruited frail older people they did not use an operationalised measure of frailty to stratify results. One large trial (n=486) that investigated a complex individualized exercise intervention reported improved mobility and balance but no effect on ADL (18). One smaller trial (n=86) of a six month intervention in a cohort living in sheltered housing reported a trend towards improved mobility but no effect on ADL, grip strength or balance (19).
One small trial (n=66) of a six week intervention reported no effects on mobility, ADL, grip strength or balance (20).
None of the four high quality trials reported overall effects on the quality of life of participants.
Discussion
This systematic review summarizes the evidence from research trials that recruited 987 participants. Strengths of the review include a robust search strategy and rigorous review procedures that included a detailed assessment of risk of methodological bias using well recognized methods. A potential weakness of the review is that, although a consensus decision was reached regarding whether individual trials included frail older people on the basis of the frailty phenotype, only one high quality trial both selected participants and stratified results using an operationalized measure of frailty.
Included trials were generally of high methodological quality. However, individual sample sizes were frequently small and a priori power calculations were not routinely completed, giving rise to the possibility of Type II statistical error due to small sample size. Limitations of data analysis and reporting precluded meta-analysis of primary outcome data, which could otherwise have pooled statistical power. Guidelines for developing RCTs aimed at preventing functional decline and disability in frail older people are available (22) and reference to these guidelines will help in the development of future RCTs. Standardization of outcome measures and reporting will further aid the future synthesis of evidence for meta-analysis.
One high quality trial used an operationalized, non-validated measure of frailty to both select and stratify participants. This trial reported an improved disability score in people with moderate frailty and this was maintained at 12 months. No improvement was reported for people with severe frailty. Other higher quality trials reported inconsistent effects on mobility and disability. None of the four high quality trials reported effects on quality of life.
Meta-analysis of data from two trials at low risk of methodological bias demonstrated a non-significant trend towards reduced long-term care admission. The relatively low rates of long-term care admission in these two trials and wide confidence intervals identify a requirement for future long-term trials that are adequately powered to detect a significant difference in this important outcome.
Generally high rates of completion and exercise adherence suggest that home-based exercise interventions are acceptable and feasible for frail older people. This supports the similar finding from the earlier systematic review of exercise interventions for older people living in care homes which also identified high rates of intervention completion and adherence (7).
Conclusion
There is preliminary evidence from one high quality trial that selected and stratified participants using an operationalised measure of frailty to suggest that home-based exercise interventions may be effective at improving disability in community-dwelling older people with moderate, but not severe, frailty. Operationalised measures of frailty were not used to stratify participants in the other high quality trials and inconsistent effects of exercise interventions on outcomes including mobility and disability were reported. There is significant uncertainty regarding the effects of home-based exercise interventions on important outcomes including quality of life and long-term care admission for the frail elderly.
Home-based exercises are a potentially simple, safe and widely applicable intervention to prevent dependency decline for frail older people. Adequately powered RCTs that use validated measures to select and stratify frail older people, and that incorporate long-term follow-up of important outcome measures including mobility, disability, quality of life and long-term care admission, will help address the uncertainties that we have identified in this review.
Funding
There were no external sources of funding for this review.
Acknowledgements
The lead author is supported by a Dunhill Medical Trust Research Fellowship, which is gratefully acknowledged. We would also like to thank Rosemary Campbell-Blair from the University of Leeds library for her help in developing the search strategy.
Appendix 1 - Full search strategy
Trials were identified by searching Medline 1950-Jan week 3 2010, AMED 2010, CINAHL 1981 to Jan2010, Cochrane Library Issue 1 2010,EMBASE 2010, PSYCINFO 1806-Jan week 4 2010 and PedRO to Jan2010. We did confine our search to English language publications.
The Cochrane Highly Sensitive Search Strategy for identifying randomised Medline (Higgins, 2008 http://www.cochrane-handbook.org/) was combine following search terms to identify RCTs in Medline. The Medline search s was adapted for use in the other databases searched.
MEDLINE strategy:
early ambulation/ or exercise therapy/ or muscle stretching exercises/ o resistance training/ or occupational therapy/
physical therapy modalities/ or musculoskeletal manipulations/
“Physical Therapy (Specialty)”/
Exercise Movement Techniques/
Exercise/
“Physical Education and Training”/
Physical Fitness/
“Recovery of Function”/
Physical Stimulation/
Health Promotion/
rehabilitation/
walking/
locomotion/
(rehabilitat$ or exercise$ or physiotherap$ or keep fit).tw.
(physical adj3 (therap$ or education or train$ or stimulat$ or fitness or activit$ or function)).tw.
((exercise or movement or occupational) adj3 (therap$ or train$ or treatment or program$)).tw.
((strength$ or aerobic or resistance) adj3 activit$).tw.
(improve$ adj3 (function or mobil$ or recover$)).tw.
((fitness or health) adj3 promotion).tw.
((endurance or balance or strength or flexibility or resistance) adj3 training).tw.
walk$.tw.
or/1-21
exp Aged/
(elder$ or older or oldest or old age or senior$ or geriatr$ or gerontol$ or aging or ageing or late life).tw.
-
25
Geriatric assessment/
or/23-25
-
27
(community adj3 (live or living or dwell$ or based)).tw.
(independen$ adj3 (live or living or dwell$ or based)).tw.
(sheltered adj (hous$ or accomm$ or home$ or living)).tw.
((home or communit$) adj5 (caring or care$)).tw.
(community adj (nurs$ or matron$)).tw.
(housebound or house-bound or home-bound or homebound or home-based or homebased).tw.
Homebound Persons/
“Home Care Services”/
independent living/
activities of daily living/
or/27-36
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
38 or 39 or 40 or 41 or 42 or 43 or 44 or 45
humans.sh.
46 and 47
26 and 37
22 and 49
48 and 50
Footnotes
Contributors
All authors were involved in development of the search strategy and systematic review protocol. AC and SB independently selected studies for inclusion and AF resolved any disagreements. AC and JY identified trials that included frail older people. AC and SB extracted data from the included trials and AC assessed trials for methodological bias. All authors wrote the article and all authors have approved the final version. AC is guarantor for the article.
Conflict of interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) AC is principal investigator (PI) for the Home-based Older People's Exercise (HOPE) trial, a pilot randomised controlled trial to investigate the effects of an exercise intervention for frail older people, funded by the Dunhill Medical Trust; JY is head of the Academic Unit of Elderly Care and Rehabilitation, University of Leeds, which is the host institution for the HOPE trial SB, AF and SI declare that they have no conflicts of interest.
Ethical approval
Not required.
Data sharing
No additional data available
Contributor Information
Dr Andrew P Clegg, Academic Unit of Elderly Care & Rehabilitation, Bradford Institute for Health Research, Temple Bank House, Bradford Teaching Hospitals NHS Foundation Trust, Duckworth Lane, Bradford, BD9 6RJ, UK.
Dr Sally E Barber, Academic Unit of Elderly Care & Rehabilitation, Bradford Institute for Health Research, Temple Bank House, Bradford Teaching Hospitals NHS Foundation Trust, Duckworth Lane, Bradford, BD9 6RJ, UK.
Professor John B Young, Academic Unit of Elderly Care & Rehabilitation, Bradford Institute for Health Research, Temple Bank House, Bradford Teaching Hospitals NHS Foundation Trust, Duckworth Lane, Bradford, BD9 6RJ, UK.
Professor Anne Forster, Academic Unit of Elderly Care & Rehabilitation, Bradford Institute for Health Research, Temple Bank House, Bradford Teaching Hospitals NHS Foundation Trust, Duckworth Lane, Bradford, BD9 6RJ, UK.
Professor Steve J Iliffe, Department of Primary Care and Population Health, University College London, Gower Street, London, WC1E 6BT, UK.
References
- 1.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 2.Strandberg TE, Pitkala KH. Frailty in elderly people. Lancet. 2007;369(9570):1328–9. doi: 10.1016/S0140-6736(07)60613-8. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009(2):CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006(4):CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Forster A, Lambley R, Young JB. Is physical rehabilitation for older people in long-term care effective? Findings from a systematic review. Age Ageing. 2010;39(2):169–75. doi: 10.1093/ageing/afp247. [DOI] [PubMed] [Google Scholar]
- 8.Census 2000 brief. US Census Bureau; Oct, 2001. The 65 years and over population: 2000. Available from: http://www.census.gov/prod/2001pubs/c2kbr01-10.pdf. [Google Scholar]
- 9.Ashworth NL, Chad KE, Harrison EL, et al. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005(1):CD004017. doi: 10.1002/14651858.CD004017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 11.EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 12.Collin C, Wade DT, Davies S, et al. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61–3. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 13.Thomas S, Mackintosh S, Halbert J. Does the ‘Otago exercise programme’ reduce mortality and falls in older adults?: a systematic review and meta-analysis. Age Ageing. 2010;39(6):681–7. doi: 10.1093/ageing/afq102. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J, Altman D. Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2009 Available from: http://www.cochrane-handbook.org/
- 15.Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandler JM, Duncan PW, Kochersberger G et al. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Archives of Physical Medicine and Rehabilitation. 1998;79(1):24–30. doi: 10.1016/s0003-9993(98)90202-7. [DOI] [PubMed] [Google Scholar]
- 17.Gill TM, Baker DI, Gottschalk M et al. A program to prevent functional decline in physically frail, elderly persons who live at home. The New England Journal of Medicine. 2002;347(14):1068–74\. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 18.Luukinen H, Lehtola S, Jokelainen J, et al. Prevention of disability by exercise among the elderly: a population-based, randomized, controlled trial. Scand J Prim Health Care. 2006;24(4):199–205. doi: 10.1080/02813430600958476. [DOI] [PubMed] [Google Scholar]
- 19.McMurdo ME, Johnstone R. A randomized controlled trial of a home exercise programme for elderly people with poor mobility. Age and Ageing. 1995;24(5):425–8\. doi: 10.1093/ageing/24.5.425. [DOI] [PubMed] [Google Scholar]
- 20.Rosie J, Taylor D. Sit-to-stand as home exercise for mobility-limited adults over 80 years of age - GrandStand SystemTM may keep you standing? Age and Ageing. 2007;36(5):555–62. doi: 10.1093/ageing/afm093. [DOI] [PubMed] [Google Scholar]
- 21.Vestergaard S, Kronborg C, Puggaard L. Home-based video exercise intervention for community-dwelling frail older women: a randomized controlled trial. Aging Clin Exp Res. 2008;20(5):479–86. doi: 10.1007/BF03325155. [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52(4):625–34. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]