Abstract
Two-pore channels are members of the voltage-gated ion channel superfamily. They localise to the endolysosomal system and are likely targets for the Ca2+ mobilising messenger NAADP. In this brief review, we relate mutagenesis of the TPC pore to a recently published homology model and discuss how pore mutants are informing us of TPC function. Molecular physiology of these ubiquitous proteins is thus emerging.
Keywords: TPC1, TPC2, homology modelling, site-directed mutagenesis, NAADP, voltage-gated ion channel, structure-function
Introduction
As a major intracellular signalling ion, Ca2+ controls a vast array of cellular processes, from fertilisation to cell death (Berridge et al., 2000). To encode a specific physiological response from such an omnipotent ion, cells vary both the location and timing of the Ca2+ signals. One mechanism for creating these complex signals is via the functional- and potentially physical- interaction of canonical ER Ca2+ stores with the acidic organelles of the endolysosomal system (Kilpatrick et al., 2013; Penny et al., 2014, 2015). Like the ER, endosomes and lysosomes are packed with Ca2+ (Christensen et al., 2002; Patel and Muallem, 2011). The second messenger NAADP can release Ca2+ directly from acidic organelles, which can then initiate further release from the ER, likely via Ca2+-induced Ca2+-release (Cancela et al., 1999; Churchill et al., 2002; Galione, 2014; Lee, 2003). This variety of Ca2+ mobilising mechanisms enables cells to generate physiologically relevant Ca2+ signals with spatial and temporal complexity. In 2009, three independent groups converged on the two-pore channels (TPCs) as the mediators of NAADP-induced Ca2+ release (Brailoiu et al., 2009; Calcraft et al., 2009; Zong et al., 2009). Subsequent work from many independent groups supports the central tenet that TPCs localise to the endolysosomal system, potentiate NAADP-evoked Ca2+ release when overexpressed and are required for endogenous NAADP signalling (reviewed in (Galione, 2014; Grimm et al., 2012; Hooper and Patel, 2012)). NAADP-regulated channel activity and Ca2+ permeability can be demonstrated electro-physiologically, and thus supports this view (Brailoiu et al., 2010; Pitt et al., 2010; Rybalchenko et al., 2012; Schieder et al., 2010; Yamaguchi et al., 2011). However, a 2012 study indicated that TPCs might be both Na+ selective and insensitive to NAADP, instead being activated by PI(3,5)P2 (Wang et al., 2012). More recent studies, which used similar techniques, additional knockout models and pharmacology, support many of the original contentions (Grimm et al., 2014; Jha et al., 2014; Rahman et al., 2014; Ruas et al., 2015). This has led to a refined model where TPCs are both Na+ and Ca2+-permeable, and are co-regulated by NAADP and PI(3,5)P2 (Patel, 2015).
TPC molecular architecture
TPCs are members of the voltage-gated ion channel superfamily, sharing significant topological and structural homology with TRP channels and voltage-gated K+, Na+ and Ca2+ channels (referred to as Kv, Nav and Cav channels respectively) (Clapham and Garbers, 2005). In humans, there are two TPC isoforms (TPC1 and TPC2), both of which localise to the endolysosomal system, with TPC2 predominantly lysosomal and TPC1 more broadly dispersed (Galione, 2014; Grimm et al., 2012; Hooper and Patel, 2012). A third isoform (unsurprisingly termed TPC3) is a pseudogene that underwent a striking lineage-specific loss in rodents and certain primate lineages, including humans (Cai and Patel, 2010), suggesting some strong negative selection pressure(s) within select populations.
TPCs, as with other voltage-gated ion channels, are thought adopt a pseudotetrameric structure, whereby four domains congregate to form a central pore that passes ions (Yu et al., 2005). These four domains can be formed from four individual monomers, as with Kv and TRP channels, or from the folding of one, four-domain polypeptide chain, as with Nav and Cav channels. TPCs are however unique because they create a pseudotetramer from the likely dimerisation of two, two-domain monomers (Churamani et al., 2012; Hooper et al., 2011; Rietdorf et al., 2011). Each individual domain of the pseudotetramer consists of 6 transmembrane helices. The first four transmembrane helices (S1-S4) create the voltage sensor domain, responsible for sensing and transducing voltage changes in Kv, Nav and Cav channels. The voltage sensor domains sit peripherally in the channel architecture, and are connected to the central pore domain by a helical S4-S5 linker. The pore domains (S5-S6) consist of two transmembrane helices linked together by a re-entrant pore loop that contains two short ‘pore helices’. When assembled into the pseudotetramer, the re-entrant loops create an ion-conducting pore bounded by a ring of amino acid residues called the selectivity filter, which controls the identity of the permeating ions. This overall architecture is demonstrated by the crystal structures of Kv channels (Doyle et al., 1998; Long et al., 2005).
Whilst direct structural data on the TPCs is limited, fluorescence protease protection assays, and mapping of antibody epitopes and N-glycosylation sites have confirmed the overall topology of individual TPC monomers (Hooper et al., 2011). They contain 12 transmembrane helices that form two distinct domains, each capable of independent membrane insertion (Churamani et al., 2012). The crystal and cryoEM structures of various Nav channels have been recently resolved (McCusker et al., 2012; Payandeh et al., 2011; Shaya et al., 2013; Tsai et al., 2013; Zhang et al., 2012), and significant sequence homology with the TPCs has enabled the building of a structural homology model (Fig. 1) (Rahman et al., 2014). Molecular docking analyses with this model identified interactions between Cav antagonists and the pore domain, thus explaining early studies showing block of endogenous NAADP responses by these drugs (Genazzani et al., 1996, 1997). Such findings were confirmed for recombinant TPC1 (Rahman et al., 2014). Additional “wet” and “dry” interactions with Nav antagonists, possibly through a common ancestral binding site, raise the possibility that the structural attributes that underlie channel blockade in four-domain channels were present in an ancient two-domain precursor. Importantly, the ability of structurally distinct drugs to inhibit NAADP action correlated with their predicted interaction with the pore, validating the veracity of the model for probing TPC functionality (Rahman et al., 2014). Furthermore, Sakurai et al. showed that blockade of TPCs by Cav antagonists, including the novel and potent inhibitor tetrandrine, could block Ebola infection, highlighting the importance of defining the pharmacology of TPCs (Sakurai et al., 2015). In this review we consider TPCs from a structural standpoint and attempt to relate mutagenesis of the pore region with recent modelling studies in the context of TPC functionality.
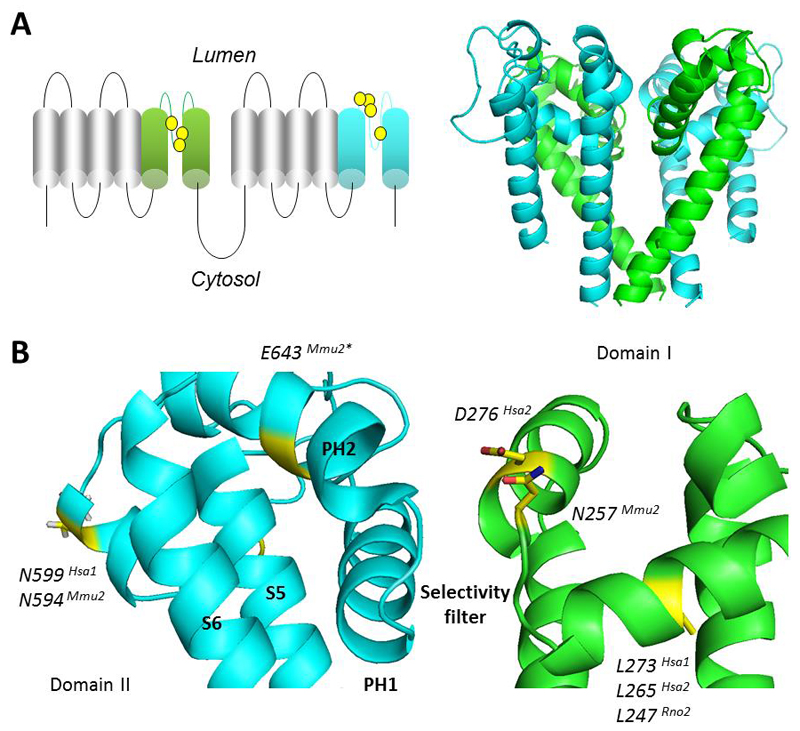
Figure 1. Structural model of the TPC pore.
A, Schematic of the TPC (left) highlighting the pore regions in domain I (green) and domain II (cyan) and residues that have been subjected to site-directed mutagenesis (yellow). Structural model of the sea urchin TPC1 pore region (right) showing the pseudo-tetrameric arrangement of the domains. B, Zoomed view of the pore. Only one of each domain is shown for clarity. Residues (yellow) and side chains in sea urchin TPC1 corresponding to those targeted in other species and isoforms (denoted by the superscripts) were identified by the sequence alignment in Fig. 2. *E643 in mouse TPC2 is not conserved in sea urchin TPC1. Model is adapted from (Rahman et al., 2014).
Pore-based Mutants
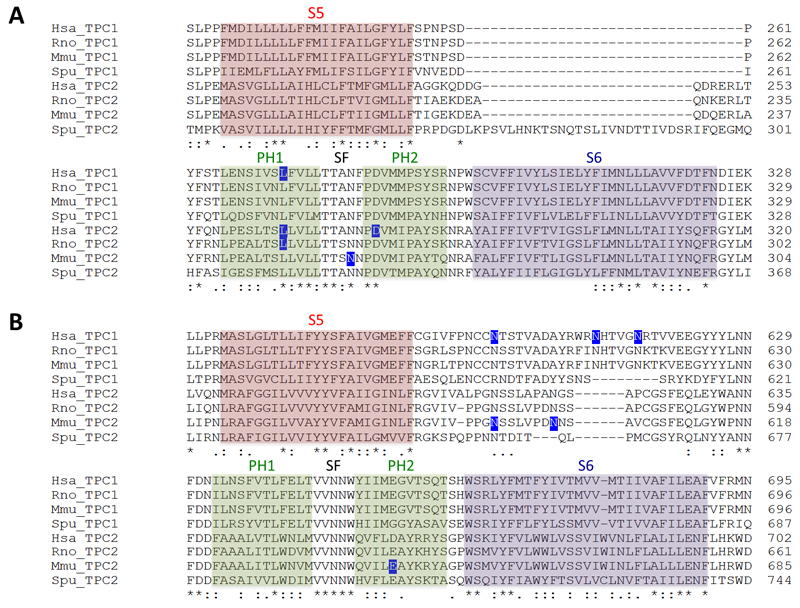
The first mutagenesis carried out on human TPCs was the substitution of leucine 273 of TPC1 for proline (Brailoiu et al., 2009). L273 is highly conserved across TPC isoforms and across species (Fig. 2). As shown in Fig. 1, it maps to the re-entrant pore loop of the first domain of TPC1. Mutation to proline in this spatially-restricted part of the channel is probably not subtle as it is predicted to introduce a substantial kink into the carbonyl backbone. However, subtlety was not the aim in this early study. This mutation blocked NAADP-induced Ca2+ release (Brailoiu et al., 2009) and channel activity (Rybalchenko et al., 2012). Importantly, it also acted in a dominant negative manner, ablating endogenous NAADP responses presumably because the incorporation of at least one inactive subunit in the dimer leads to a dysfunctional channel (Brailoiu et al., 2009). These data provided strong evidence that ion flux through TPC1 is essential for NAADP-induced Ca2+ signalling.
Figure 2. Protein sequence of the TPC pore.
Multiple sequence alignment of the pore region of TPCs in domain I (A) and domain II (B). Residues that have been subjected to site-directed mutagenesis are highlighted in blue. Abbreviations: human (Homo sapiens, Hsa), rat (Rattus norvegicus, Rno), mouse (Mus musculus, Mmu) and sea urchin (Strongylocentrotus purpuratus, Spu). Alignments were performed using ClustalOmega and the following accession numbers: AAI50204 (Hsa TPC1); AAH63008.1 (Hsa TPC2); NP_647548.2 (Rno TPC1); XP_006230846.1 (Rno TPC2); AAH58951.1 (Mmu TPC1); NP_666318.2 (Mmu TPC2); NP_001138446.1 (Spu TPC1); NP_001138448.1 (Spu TPC2). Structural features are highlighted based on alignment with structurally resolved Nav channels, as discussed previously (Rahman et al., 2014). PH refers to the pore helices, SF to the selectivity filter.
These findings were subsequently extended to the homologous leucine in human TPC2 (L265), where the equivalent mutation again ablated NAADP-induced Ca2+signalling and channel activity in a dominant negative fashion (Brailoiu et al., 2010). This mutant has been used to highlight the role of TPC2 action in downstream physiological processes. For example, overexpression of TPC2 L265P prevents autophagic dysfunction by NAADP (Pereira et al., 2011) and Ebola virus entry (Sakurai et al., 2015). TPC2 activity has also been linked to pigmentation defects in Xenopus oocytes (Lin-Moshier et al., 2014). Oocytes are heavily pigmented under normal conditions, however overexpression of human TPC2 creates a pigmentation defect that gives them a ‘balding’ appearance. This is correlated with the aggregation of intracellular, TPC2-positive vesicles. Overexpression of either the L265P mutant or TPC1 had no effect on pigmentation or vesicle aggregation, demonstrating that TPC2 activity can specifically drive a pigmentation defect (Lin-Moshier et al., 2014). Furthermore, HeLa cells overexpressing rat TPC2 display aberrant vesicle morphology that is associated with autophagy defects, whereas expressing the equivalent rat TPC2 mutant (L247P) does not (Lu et al., 2013). Thus, this set of pore-blocking proline mutants, which are predicted to drastically alter the morphology of the pore, have been used to uncover the signalling role and physiological functions of TPCs.
Wang and colleagues have also identified a pore-dead mutant (Wang et al., 2012). These authors targeted a highly conserved aspartate at position 276 in human TPC2 (Fig. 2), substituting this negatively charged residue for a positively charged lysine. The resulting charge-reversal mutant D276K did not support currents in response to PI(3,5)P2. D276 is predicted to sit at the very top of the narrow selectivity filter (Fig. 1). We speculate that adding positive charge here might repel any incoming cations. Interestingly, overexpression of the wild type channel, but not the D276K pore-dead mutant, increased the size of TPC2-positive vesicles (Wang et al., 2012). These results strongly agree with the observations of increased vesicle size in TPC2-overexpressing frog oocytes and HeLa and HEK cell lines (Lin-Moshier et al., 2014; Lu et al., 2013; Ruas et al., 2010). Taken together, these results suggest that TPC2 currents might be involved in vesicular fusion events.
Schieder and colleagues had success with making a functional pore mutant (Schieder et al., 2010). Canonically, voltage-gated Ca2+ channels possess a ring of negatively charged residues lining the ionic pore (Yu et al., 2005). This configuration confers Ca2+ selectivity in Cav channels and some TRP channels, including TRPV5 and TRPV6 (Voets et al., 2004). The authors found that wild-type mouse TPC2 was >1000x selective for Ca2+ over K+ (Schieder et al., 2010), although Na+ permeability was not tested at the time. By using sequence alignments with TRPV5 and TRPV6, they identified a conserved glutamate residue within the second domain of mouse TPC2 (Fig. 2). Mutating this residue to remove the charge (E643A), reduced Ca2+/K+ selectivity to ~8x. Nonetheless, the channel was still functional. This mutant has been used in a more recent paper to assess the specificity of TPC knockout. Ruas et al. found that NAADP-mediated Ca2+ signals were ablated in embryonic fibroblasts of a double knockout TPC1/TPC2 mouse, but that these responses could be rescued by transfection with the wild-type TPCs (Ruas et al., 2015). However, transfection with the functional but non-selective E643A TPC2 mutant failed to recapitulate the NAADP sensitivity, indicating that TPCs must be sufficiently permeable and/or selective to maintain NAADP responses.
When aligned with structurally characterised Nav channels rather than with TRP channels, E643 in mouse TPC2 appears outside the selectivity filter (Fig. 1). Instead, this residue projects away from the mouth of the channel. The E643A mutant might therefore reduce Ca2+ selectivity through gross conformational changes in pore architecture, as opposed to a compromised ability to directly coordinate ions. Notably, this residue is not completely conserved across isoforms and species (Fig. 2).
Using the Nav-based TPC model, Rahman et al recently suggested a role for highly conserved asparagine residues in coordinating ions within the putative selectivity filter of the TPC pore (Rahman et al., 2014) (Fig. 2). Asparagine residues feature within the selectivity filter of NMDA receptors (Burnashev et al., 1992). Notably, these ionotropic glutamate receptors are both Ca2+ and Na+ permeable (similar to TPCs), whereas other glutamate receptors that lack asparagine residues are less Ca2+ permeable (Burnashev et al., 1992). Schieder et al mutated asparagine 257 within domain I of mouse TPC2 (Schieder et al., 2010). Mutation of this residue to alanine (N257A) ablated NAADP-induced channel activity, and prevented rescue of NAADP-induced Ca2+ signalling in fibroblasts from TPC double knockout mice (Ruas et al., 2015). Based on Rahman et al.'s alignment with structurally-resolved Nav channels, N257 is predicted to lie directly within the selectivity filter and is thus well positioned for ion discrimination (Fig. 1). Because alanine is a neutral amino acid, lack of activity of the N257A mutant might result from the inability to co-ordinate permeant ions. More conservative substitutions, such as a glutamine, could yield further insight into TPC ion selectivity.
Finally, Hooper et al identified that human TPC1 was N-glycosylated at three asparagine residues in the second pore domain (N599, N611 and N616) (Hooper et al., 2011). Whilst mutating these residues to glutamine had no observable effects on localisation, the non-glycosylated triple TPC1 mutant displayed exaggerated Ca2+ responses to NAADP. Zong and colleagues also identified two N-glycosylated arginine residues in mouse TPC2 (N594 and N601), although their functional role was not assessed (Zong et al., 2009). The TPC glycosylation sites all map to the turret loops in between S5 and the re-entrant pore loops of domain II (Fig. 1). The first of the glycosylated residues is reasonably well conserved between TPC1 and TPC2, and across species (Fig. 2). Interestingly, it lies approximately at the plane of the membrane in the TPC1 homology model (Rahman et al., 2014), although it should be noted that this region was modelled de novo due to lack of a suitable template. Nevertheless negatively charged oligosaccharide chains attached here may interact with the lysosomal glycocalyx and/or impede approach of cations such as Ca2+, thus accounting for enhanced activity upon deglycosylation.
Conclusions and outlook
Until recently, it has been difficult to understand how the structural changes in TPC mutants manifest their functional effects. The recently published structural homology model of a TPC pore provides a reasonable basis from which to speculate about these effects (Rahman et al., 2014). A summary of pore mutants is provided in Table 1.
Table 1.
Summary of TPC pore mutations.
| TPC1 | |||
|---|---|---|---|
| Human | L273P | Pore-blocking | (Brailoiu et al., 2009; Rybalchenko et al., 2012) |
| N599Q/N611Q/N616Q | Removes glycosylation; Pore-activating | (Hooper et al., 2011) | |
| TPC2 | |||
| Human | L265P | Pore-blocking | (Brailoiu et al., 2010; Lin-Moshier et al., 2014)(Pereira et al., 2011)(Sakurai et al., 2015). |
| D276K | Pore-blocking | (Wang et al., 2012) | |
| Mouse | N257A | Pore-blocking | (Ruas et al., 2015; Schieder et al., 2010) |
| N594Q/N601Q | Removes glycosylation | (Zong et al., 2009) | |
| E643A | Reduces Ca2+/K+ selectivity | (Schieder et al., 2010)(Ruas et al., 2015). | |
| Rat | L247P | Pore-blocking | (Lu et al., 2013) |
As discussed, many of the pore mutants are inactive. This is presumably because the structure and electrostatics within the targeted regions region are finely tuned and therefore somewhat inflexible. At a functional level, mutants have provided insight into the signalling roles of TPCs, such as the crucial requirement of TPCs for NAADP action and in physiological functions such as membrane traffic (Brailoiu et al., 2009, 2010; Lin-Moshier et al., 2014; Lu et al., 2013; Marchant and Patel, 2015; Wang et al., 2012). Notably, emerging evidence indicates that the latter might be relevant to pathologies such as Parkinson's Disease (Hockey et al., 2015), non-alcoholic fatty liver disease (Grimm et al., 2014) and Ebola infection (Sakurai et al., 2015).
But of course a homology model is just that- a model- and is therefore no substitute for a physical structure of TPCs. Lack of structural information is a major gap in our knowledge at present. Nevertheless, modelling has provided insight into pharmacology of TPCs, and predicts a number of putative drug binding positions ripe for future mutagenesis (Rahman et al., 2014). This is now particularly relevant given the emergence of TPCs as potential therapeutic targets (Hockey et al., 2015; Sakurai et al., 2015). Modelling also predicts a possible configuration for the selectivity filter, which could instruct mutagenic strategies to further clarify TPC selectivity – an area of contention (Marchant and Patel, 2013; Morgan and Galione, 2014). Not discussed here are other molecular manipulations that do not fall within the pore region of TPCs such as those within the N-terminus and voltage sensor (Brailoiu et al., 2010; Cang et al., 2014; Churamani et al., 2013; Lin-Moshier et al., 2014). Correlating these with structure predictions of the full length channel may yield further insight into the workings of TPCs.
Although there is much we do not know about TPCs, mutagenesis has been one of the most important tools for understanding TPC function thus far, and will continue to be for some time to come, particularly if interpreted within a structural context.
Acknowledgements
This work was supported by a Biotechnology and Biological Sciences Research Council studentship BB/J014567/1 (to C.J.P.).
Abbreviations
- TPC
two-pore channel
- NAADP
nicotinic acid adenine dinucleotide phosphate
- Nav
voltage-gated sodium channel
- Kv
voltage-gated potassium channel
- Cav
voltage-gated calcium channel
- TRP
transient receptor potential
- TRPV
TRP vanilloid
- ER
endoplasmic reticulum
- PI(3,5)P2
phosphatidylinositol 3,5-bisphosphate
- SF
selectivity filter
- PH
pore helix
Footnotes
Conflict of Interest:
The authors declare no competing financial or ethical interests.
References
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J Biol Chem. 2010;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Schoepfer R, Monyer H, Ruppersberg JP, Günther W, Seeburg PH, Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257:1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Cai X, Patel S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol Biol Evol. 2010;27:2352–2359. doi: 10.1093/molbev/msq122. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang K-T, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela JM, Churchill GC, Galione A. Coordination of agonist-Induced Ca2+ signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- Cang C, Bekele B, Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat Chem Biol. 2014:1–8. doi: 10.1038/nchembio.1522. [DOI] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- Churamani D, Hooper R, Brailoiu E, Patel S. Domain assembly of NAADP-gated two-pore channels. Biochem J. 2012;441:317–323. doi: 10.1042/BJ20111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churamani D, Hooper R, Rahman T, Brailoiu E, Patel S. The N-terminal region of two-pore channel 1 regulates trafficking and activation by NAADP. Biochem J. 2013;453:147–151. doi: 10.1042/BJ20130474. [DOI] [PubMed] [Google Scholar]
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Garbers DL. International Union of Pharmacology. L. Nomenclature and structure-function relationships of CatSper and two-pore channels. Pharmacol Rev. 2005;57:451–454. doi: 10.1124/pr.57.4.7. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science (80-. ) 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Galione A. A primer of NAADP-mediated Ca(2+) signalling: From sea urchin eggs to mammalian cells. Cell Calcium. 2014 doi: 10.1016/j.ceca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Empson RM, Galione A. Unique inactivation properties of NAADP-sensitive Ca2+ Release. J Biol Chem. 1996;271:11599–11602. doi: 10.1074/jbc.271.20.11599. [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Mezna M, Dickey DM, Michelangeli F, Walseth TF, Galione A. Pharmacological properties of the Ca2+ -release mechanism sensitive to NAADP in the sea urchin egg. Br J Pharmacol. 1997;121:1489–1495. doi: 10.1038/sj.bjp.0701295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Hassan S, Wahl-Schott C, Biel M. Role of TRPML and two-pore channels in endolysosomal cation homeostasis. J Pharmacol Exp Ther. 2012;342:236–244. doi: 10.1124/jpet.112.192880. [DOI] [PubMed] [Google Scholar]
- Grimm C, Holdt LM, Chen C-C, Hassan S, Müller C, Jörs S, Cuny H, Kissing S, Schröder B, Butz E, et al. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat Commun. 2014;5:4699. doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci. 2015;128:232–238. doi: 10.1242/jcs.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper R, Patel S. NAADP on target. Adv Exp Med Biol. 2012;740:325–347. doi: 10.1007/978-94-007-2888-2_14. [DOI] [PubMed] [Google Scholar]
- Hooper R, Churamani D, Brailoiu E, Taylor CW, Patel S. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J Biol Chem. 2011;286:9141–9149. doi: 10.1074/jbc.M110.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, Ahuja M, Patel S, Brailoiu E, Muallem S. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3, 5)P2 and multiple protein kinases. EMBO J. 2014;33:501–511. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J Cell Sci. 2013;126:60–66. doi: 10.1242/jcs.118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC. Calcium signaling: NAADP ascends as a new messenger. Curr Biol. 2003;13:R186–R188. doi: 10.1016/s0960-9822(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Lin-Moshier Y, Keebler MV, Hooper R, Boulware MJ, Liu X, Churamani D, Abood ME, Walseth TF, Brailoiu E, Patel S, et al. The Two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc Natl Acad Sci U S A. 2014;111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Campbell ED, MacKinnon R. Crystal structure of a mammalian voltage-dependent Shaker mamily K+ channel. Science (80-. ) 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hao BX, Graeff R, Wong CWM, Wu WT, Yue J. Two pore channel 2 (TPC2) inhibits autophagosomal-lysosomal fusion by alkalinizing lysosomal pH. J Biol Chem. 2013;288:24247–24263. doi: 10.1074/jbc.M113.484253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Marchant JS, Patel S. Questioning regulation of two-pore channels by NAADP. Messenger. 2013;2:113–119. doi: 10.1166/msr.2013.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant JS, Patel S. Two-pore channels at the intersection of endolysosomal membrane traffic. Biochem Soc Trans. 2015 doi: 10.1042/BST20140303. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker EC, Bagnéris C, Naylor CE, Cole AR, D’Avanzo N, Nichols CG, Wallace B. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat Commun. 2012;3:1102. doi: 10.1038/ncomms2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. Two-pore channels (TPCs): Current controversies. BioEssays. 2014;36:173–183. doi: 10.1002/bies.201300118. [DOI] [PubMed] [Google Scholar]
- Patel S, Muallem S. Acidic Ca2+ stores come to the fore. Cell Calcium. 2011;50:109–112. doi: 10.1016/j.ceca.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall Wa. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny CJ, Kilpatrick BS, Han JM, Sneyd J, Patel S. A computational model of lysosome-ER Ca2+ microdomains. J Cell Sci. 2014;44:1–10. doi: 10.1242/jcs.149047. [DOI] [PubMed] [Google Scholar]
- Penny CJ, Kilpatrick BS, Eden ER, Patel S. Coupling acidic organelles with the ER through Ca2+ microdomains at membrane contact sites. Cell Calcium. 2015 doi: 10.1016/j.ceca.2015.03.006. In Press. [DOI] [PubMed] [Google Scholar]
- Pereira GJS, Hirata H, Fimia GM, do Carmo LG, Bincoletto C, Han SW, Stilhano RS, Ureshino RP, Bloor-Young D, Churchill G, et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J Biol Chem. 2011;286:27875–27881. doi: 10.1074/jbc.C110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, et al. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+ J Biol Chem. 2010;285:35039–35046. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci Signal. 2014;7:1–11. doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietdorf K, Funnell TM, Ruas M, Heinemann J, Parrington J, Galione A. Two-pore channels form homo- and heterodimers. J Biol Chem. 2011;286:37058–37062. doi: 10.1074/jbc.C111.289835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, et al. Report Purified TPC Isoforms Form NAADP Receptors with Distinct Roles for Ca 2 + Signaling and Endolysosomal Trafficking. Curr Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Davis LC, Chen C, Morgan AJ, Chuang K, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, et al. Expression of Ca 2 + -permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015 doi: 10.15252/embj.201490009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Ahuja M, Coblentz J, Churamani D, Patel S, Kiselyov K, Muallem S. Membrane potential regulates nicotinic acid adenine dinucleotide phosphate (NAADP) dependence of the pH- and Ca2+-sensitive organellar two-pore channel TPC1. J Biol Chem. 2012;287:20407–20416. doi: 10.1074/jbc.M112.359612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Kolokoltsov AA, Chen C, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-schott C, Biel M, Davey RA. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science (80-. ) 2015;347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieder M, Rötzer K, Brüggemann A, Biel M, Wahl-Schott CA. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J Biol Chem. 2010;285:21219–21222. doi: 10.1074/jbc.C110.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaya D, Findeisen F, Abderemane-Ali F, Arrigoni C, Wong S, Nurva SR, Loussouarn G, Minor DL. Structure of a Prokaryotic Sodium Channel Pore Reveals Essential Gating Elements and an Outer Ion Binding Site Common to Eukaryotic Channels. J Mol Biol. 2013 doi: 10.1016/j.jmb.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CJ, Tani K, Irie K, Hiroaki Y, Shimomura T, McMillan DG, Cook GM, Schertler GFX, Fujiyoshi Y, Li XD. Two alternative conformations of a voltage-gated sodium channel. J Mol Biol. 2013;425:4074–4088. doi: 10.1016/j.jmb.2013.06.036. [DOI] [PubMed] [Google Scholar]
- Voets T, Janssens A, Droogmans G, Nilius B. Outer Pore Architecture of a Ca2+-selective TRP Channel. J Biol Chem. 2004;279:15223–15230. doi: 10.1074/jbc.M312076200. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong X-P, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Jha A, Li Q, Soyombo AA, Dickinson GD, Churamani D, Brailoiu E, Patel S, Muallem S. Transient receptor potential mucolipin 1 (TRPML1) and two-pore channels are functionally independent organellar ion channels. J Biol Chem. 2011;286:22934–22942. doi: 10.1074/jbc.M110.210930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of Molecular Relationships in the Voltage-Gated Ion Channel Superfamily. Pharmacol Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ren W, DeCaen PG, Yan C, Tao X, Tang L, Wang J, Hasegawa K, Kumasaka T, He J, et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486:130–134. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott CA. The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]