Abstract
Purpose
This first-in-human phase I trial assessed the safety, tolerability, and preliminary anti-tumor activity of apitolisib (GDC-0980), a dual inhibitor of class I phosphatidylinositol-3-(PI3K) and mammalian target of rapamycin (mTOR) kinases.
Experimental Design
Once-daily (QD) oral apitolisib was administered to patients with solid tumors for days 1-21 or 1-28 of 28-day cycles. Pharmacokinetic and pharmacodynamic parameters were assessed.
Results
Overall, 120 patients were treated at doses between 2-70 mg. The commonest ≥G3 toxicities related to apitolisib at the recommended phase 2 dose (RP2D) at 40mg QD included hyperglycemia (18%), rash (14%), liver dysfunction (12%), diarrhea (10%), pneumonitis (8%), mucosal inflammation (6%), and fatigue (4%). Dose-limiting toxicities (one patient each) were G4 fasting hyperglycemia at 40 mg (21/28-schedule), and G3 maculopapular rash and G3 fasting hyperglycemia at 70 mg (21/28-schedule). The pharmacokinetic profile was dose-proportional. Phosphorylated serine-473 AKT levels were suppressed by ≥90% in platelet-rich plasma within 4 hours at the maximum tolerated dose (50 mg). Pharmacodynamic decreases in FDG-PET uptake of >25% occurred in 66% (21/32) of patients dosed at 40 mg QD. Evidence of single agent activity included ten RECIST partial responses (confirmed for peritoneal mesothelioma, PIK3CA mutant head- and-neck cancer, and three pleural mesotheliomas).
Conclusion
Apitolisib exhibited dose-proportional pharmacokinetics with target modulation at doses ≥16 mg. The RP2D was 40 mg QD 28/28-schedule; severe on-target toxicities were apparent at ≥40 mg, particularly pneumonitis. Apitolisib was reasonably tolerated at 30 mg, the selected dose for pleural mesothelioma patients given limited respiratory reserve. Modest but durable anti-tumor activity was demonstrated.
Keywords: Apitolisib, PI3K, mTOR, clinical trial, dual kinase inhibitor
INTRODUCTION
The phosphatidylinositol-3-kinase (PI3K), protein kinase B (AKT), and mammalian target of rapamycin (mTOR) signaling network promotes cell growth, survival, metabolism, and motility, but becomes a critical oncogenic driver under aberrant conditions that control the tumor microenvironment and angiogenesis (1, 2). The PI3K-AKT-mTOR axis is the most frequently deregulated signaling pathway in human cancers (1). PI3Kα has high rates of activating mutations associated with tumor formation (3-5) in squamous cell head and neck cancer (SCCHN), hepatocellular, breast, colon, and ovarian carcinomas (6, 7). Other causes of pathway hyperactivation include loss of function of the tumor suppressor PTEN (8, 9), gain of function mutations in AKT (10, 11) and PDK1 (12), or up-regulation of receptor tyrosine kinases (2).
Compelling evidence for targeting PI3K-AKT-mTOR has led to the design and evaluation of multiple pan- and isoform-specific PI3K, AKT, and mTOR kinase inhibitors (13). Outside of breast and renal cell cancers, mTOR inhibitors demonstrate modest single agent activity (1, 13). The lack of cancer cell death is probably attributable to feedback loops, cross-talk, and selection of compensatory pathways (14-16). A particular flaw of solitary mTORC1 inhibition is mTORC2-mediated AKT phosphorylation on serine-473, which has reportedly promoted resistance to rapalogs (17). Together, these data provide a strong rationale for targeting the PI3K-AKT-mTOR axis at three crucial nodes: PI3K, mTORC1, and mTORC2 (1). This could potentially increase therapeutic efficacy through heightened pathway blockade as well as circumvent feedback loops, compensatory pathway activation, and mTORC2-mediated AKT hyperactivation. Phase I studies have been reported with other dual PI3K/mTOR inhibitors including BEZ-235 (18), SF1126 (19), BGT226 (20), XL765 (21), and PF-04691502 (22), although the majority of these studies confirmed limited single agent anti-tumor efficacy.
Apitolisib is an orally bioavailable, potent, dual catalytic site inhibitor of PI3K and mTOR, with a half maximum inhibitory concentration (IC50) for class I kinases PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ, of 5, 27, 14, and 7 nmol/L respectively, and with an inhibition constant (Ki) of 17.3 nmol/L for mTOR kinase (23). Tumor growth inhibition has been demonstrated in multiple xenograft cancer models with PI3K-mutant, PTEN-null, and KRAS-mutant cell lines (23, 24). We investigated apitolisib in solid tumors and hematological malignancies for its safety, tolerability, dose-limiting toxicities (DLTs), maximum tolerated dose (MTD), pharmacokinetic (PK) and pharmacodynamic (PD) profiles, and anti-tumor efficacy.
PATIENTS AND METHODS
This international phase I trial of apitolisib was conducted in two stages. Stage 1 involved dose escalation to estimate the MTD, and stage 2 was an expanded cohort at the recommended phase 2 dose (RP2D). Patients were enrolled at the Royal Marsden Hospital NHS Foundation Trust (Sutton, UK), Dana-Farber Cancer Institute (Boston, MA, USA), Sarah Cannon Research Institute/Tennessee Oncology (Nashville, TN, USA), University Of Chicago (Chicago, Il, USA), and Memorial Sloan Kettering Cancer Center (New York, NY, USA). This study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice, and was approved by each center’s Regulatory and Ethics Committees. All participants provided written informed consent. This study was registered at clinicaltrials.gov with the identifier, NCT00854152.
Patients
Eligible patients had advanced solid malignancies, non-Hodgkin’s lymphoma (NHL), or multiple myeloma with no standard treatments available, age ≥18 years with a life expectancy ≥12 weeks, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Participation required solid tumors to be assessable by radiological methods; adequate hematological, renal, and liver function with normal glycosylated hemoglobin (HbA1C) levels and serum glucose of ≤120 mg/dL; with cessation of prior anti-cancer treatments or radiotherapy at least 3 weeks before cycle 1 day 1. Use of proton pump inhibitors (PPIs), antacids, and H2 blockers were excluded within 12 hours of apitolisib dosing, unless patients were in the PPI cohort.
Patients were excluded if there were unresolved toxicities from preceding therapies assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) (25), were pregnant or breastfeeding, or had uncontrolled medical disorders (diabetes requiring medication or grade ≥2 dyslipidemia). Stage 2 excluded former treatment with PI3K or mTOR inhibitors.
Participants enrolled had any histological subtype, although enrichment for PIK3CA mutation carriers in stage 2 was prospectively undertaken to determine if dual PI3K/mTOR inhibition by apitolisib promoted anti-tumor efficacy in this subgroup. Hence, this expansion cohort enrolled patients with solid tumors or NHL that harbored a PIK3CA hotspot mutation. SCCHN also qualified if it was positive for human papillomavirus (HPV), in view of the high prevalence of PIK3CA mutations in these tumors (26). A malignant pleural mesothelioma (MPM) expansion cohort was also undertaken in stage 2 due to clinical activity observed in stage 1. An additional cohort evaluated the interaction of apitolisib with the PPI, rabeprazole.
Study Design and treatment
This was an open-label, multi-center phase I study utilizing a modified 3+3 design (27). During dose escalation, 100% dose increments were implemented until a drug-related toxicity of grade ≥2 was observed. Thereafter, dose increases were limited to ≤50%.
Patients in stage 1 received oral apitolisib on day 1 and then once daily (QD) on day 8-28 of a 35-day cycle. The single dose, 7-day run-in established the terminal elimination half-life. Subsequent cycles were 28-days, with QD dosing on day 1-21 (21/28 schedule). The starting dose of 2 mg daily was 1/10th of the projected severe toxic dose in pre-clinical toxicology studies. The 21/28 schedule was selected to allow a 7 day drug-free period to aid recovery from acute toxicities and maximize the administered dose of apitolisib. A continuous QD dosing 28/28 schedule was implemented to further explore safety and pharmacodynamics at the MTD in stage 1.
Definitions of DLTs and MTD
The primary outcome measures were the occurrence of DLTs assessed using CTCAE (version 3.0) (25) and defined as the following treatment-related adverse events (AEs) occurring within the first cycle: grade 4 thrombocytopenia, grade 4 neutropenia lasting ≥5 days or in association with fever, fasting grade 4 or sustained (≥24 hours) or symptomatic grade 3 hyperglycemia, grade 4 hypercholesterolemia or triglyceridemia for 2 weeks despite lipid-lowering agent, grade 3 liver dysfunction, grade ≥2 decrease in diffusion capacity of carbon monoxide (DLCO) as well as any grade ≥3 non-hematological AE with the exception of alopecia or poorly managed grade 3 nausea, vomiting or diarrhea.
In the event of one DLT, the cohort was expanded to 6 evaluable patients; if 2 of the 6 patients had a DLT in the first cycle, dose escalation was terminated. The MTD was defined as the highest dose at which DLT was experienced by <33% patients (DLT ≤1/6 patients). The MTD was considered the RP2D unless a lower dose was identified with adequate biological activity and drug exposure. Once the MTD was established, a 28/28 dosing schedule was prospectively pursued with the RP2D dose level being expanded to 14 patients to better estimate DLT rate and evaluate tolerability. Stage 2 investigated the safety and tolerability in a larger cohort at the RP2D on the 28/28 dosing schedule. Intra-patient dose escalation in stage 1 was permitted to the dose below that which was being tested. Participants experiencing unacceptable toxicities were discontinued from the study. A treatment break of up to 28 days was permitted for treatment-related or other significant AEs, until the toxicity resolved to baseline; then, up to two apitolisib dose reductions were allowed to manage side effects. Study treatment continued until disease progression, unacceptable toxicities, dose interruption of more than 28 days or consent withdrawal. In stage 1, patients considered unevaluable for DLT were replaced by an additional patient if they missed ≥3 or 4 apitolisib doses on the 21/28 or 28/28 day schedule, respectively, for reasons other than DLT.
Safety and Efficacy
Patients were assessed at baseline and on days 1, 2, 3, 8, 15, 22, and 29 during cycle 1; days 1, 8, 15, and 22 during cycle 2; and days 1 and 15 of subsequent cycles. Each assessment consisted of medical history, documentation of performance status, physical examination and laboratory evaluations (full blood count, clotting, serum biochemistry, glucose, urinalysis) and an electrocardiogram. AEs and laboratory measures were graded with CTCAE (version 3.0) (25) and causality ascribed. Tumor response was assessed after the first and second cycle and every two cycles subsequently by response evaluation criteria in solid tumors (RECIST) (v. 1.0) (28) or modified RECIST (29) for MPM patients as part of central review by an independent radiologist.
Pharmacokinetics
In stage 1, PK sampling was done in the first cycle on day 1 (pre-dose and 0.5, 1, 2, 3, 4, and 8 h post-dose), on days 2 and 3 (post-dose), on day 8 (pre-dose and 2 h post-dose), on day 15 (pre-dose and 0.5, 1, 2, 3, 4, and 8 h post-dose), and on days 22 and 29 (pre-dose and 2 h post-dose); and on the first day of each cycle thereafter. In the stage 2 PPI cohort, patients received apitolisib on day 1 followed by rabeprazole (20 mg) on days 4-7 of apitolisib, then co-administered with rabeprazole on day 8. PK sampling was done pre- and post-dose as outlined for stage 1, with the exception that the multiple sampling on day 15 (stage 1) was performed on day 8 for the PPI cohort. No PK samples were collected for the other cohorts of stage 2. Plasma and urine apitolisib concentrations were quantified by liquid chromatography with tandem mass spectrometry assay (30).
Pharmacodynamics
Blood samples for surrogate PD markers to evaluate platelet-rich plasma (PRP) were collected pre-dose and 1, 3, 8, and 24 hours post-dose on day 1; pre-dose on days 8, 15 and 22; and 1 hour post-dose on day 15. Total AKT and phospho-AKT (pAKT) (S473) in PRP were measured by a Meso Scale Discovery (MSD) assay; the percent change in pAKT relative to baseline was calculated. Where feasible, patients underwent tumor biopsies during pretreatment and 1 to 4 hours after dosing on cycle 1 day 15.
The PI3K pathway plays a role in glucose homeostasis and has demonstrated an impact on the Glut1 transporter (31). Thus, changes in tumor glucose uptake as measured by fluorodeoxyglucose positron emission tomography (FDG-PET) imaging may be used as a PD marker for PI3K and mTOR inhibition, and to define the optimal dosing in preclinical models (32). FDG-PET/CT scans were acquired at baseline and 1-4 hours after dosing with apitolisib on days 22 and 55, and were centrally assessed using the European Organization for Research and Treatment of Cancer (EORTC) criteria.
Pathway alteration biomarkers
Mandatory archival formalin-fixed paraffin embedded (FFPE) tumor tissue was collected prior to starting the study. PTEN expression was performed by immunohistochemistry. DNA was extracted for PIK3CA, NRAS, KRAS, BRAF, EGFR, and AKT1 mutational analysis using DxS allele-specific PCR, qRT-PCR assays, or Sanger Sequencing (33-35). The assays targeted eight single nucleotide mutations in four hotspots within the PIK3CA gene found to be common in human cancer and transformed cells in vitro (C420R, E542K, E545K, E545G, E545A, H1047R, H1047L, and H1047Y). Sanger sequencing to cover additional mutations was applied for patients with evidence of tumor response and no hotspot mutations.
Statistical analysis
Analyses were descriptive and all safety and anti-tumor activity data included patients who received at least one dose of apitolisib. PK analyses included treated patients who had available PK data. Biomarkers assessed in this study were exploratory. The data cut off was 28 Jan 2015; Statistical Analysis System (SAS v9.2), and the software R (https://www.r-project.org) (v3.1.1), were used for data analyses.
RESULTS
Patient characteristics
Between 16 March, 2009, and 15 January, 2014, 120 patients were enrolled and treated; 56 in stage 1, and 64 in stage 2 (Supplementary Figure 1). The baseline demographics and disease characteristics are shown in Table 1.
Table 1.
Demographics and baseline patient characteristics
| Stage 1 (n=56) | Stage 2 (n=64) | All (N=120) | |
|---|---|---|---|
| Sex | |||
| Female | 21 (37.5%) | 31 (48.4%) | 52 (43.3%) |
| Age | |||
| Mean (SD) | 56.8 (10.8) | 59.2 (12.4) | 58.1 (11.7) |
| Median | 58.0 | 58.5 | 58.0 |
| Minimum-Maximum | 35-84 | 25-83 | 25-84 |
| Performance status | |||
| 0 | 36 (64.3%) | 30 (46.9%) | 66 (55.0%) |
| 1 | 20 (35.7%) | 34 (53.1%) | 54 (45.0%) |
| Cancer type | |||
| Malignant Pleural Mesothelioma (MPM) | 6 (10.7%) | 27 (42.2%) | 33 (27.5%) |
| Peritoneal Mesothelioma | 2 (3.6%) | 1 (1.6%) | 3 (2.5%) |
| Head and Neck (SCCHN) | 0 (0%) | 15 (23.4%) | 15 (12.5%) |
| Colorectal | 8 (14.3%) | 4 (6.2%) | 12 (10%) |
| GIST | 10 (17.9%) | 1 (1.6%) | 11 (9.2%) |
| Sarcoma | 8 (14.3%) | 3 (4.7%) | 11 (9.2%) |
| Breast | 3 (5.4%) | 6 (9.4%) | 9 (7.5%) |
| Non-small cell Lung | 1 (1.8%) | 3 (4.7%) | 4 (3.3%) |
| Ovarian | 2 (3.6%) | 1 (1.6%) | 3 (2.5%) |
| Esophageal | 1 (1.8%) | 1 (1.6%) | 2 (1.7%) |
| Other | 15 (26.8%) | 2 (3.1%) | 17 (14.1%) |
| Number of prior systemic therapies | |||
| Mean (SD) | 3.7 (2.8) | 2.8 (2.0) | 3.2 (2.4) |
| Median | 3.0 | 2.0 | 3.0 |
| Range | 0-13 | 0-10 | 0-13 |
| Months from primary diagnosis | |||
| Mean (SD) | 55.4 (42.3) | 37.6 (34.9) | 45.9 (39.4) |
| Median | 42.2 | 24.2 | 31.9 |
| Range | 6-210 | 5-156 | 5-210 |
DLTs and RP2D
Eight dose levels between 2 and 70 mg/day on the 21/28-day schedule were evaluated in stage 1. One DLT of grade 4 hyperglycemia was recorded at 40 mg QD. Two DLTs, grade 3 maculopapular rash and grade 3 hyperglycemia, were recorded at 70 mg QD (Table 2). The MTD (DLT <33.3%) was 50 mg QD on the 21/28-day dosing schedule. At the continuous dosing schedule (28/28) the maximum administered dose (MAD) was 50 mg QD; the MTD was not reached for this dosing schedule. AEs that occurred outside the DLT window and influenced dose escalation and RP2D decisions included 1 patient with grade 5 colitis at 50 mg QD on the 21/28 day dosing schedule and 8 patients with pneumonitis, including 3 severe cases (1 grade 3 at 70 mg QD 21/28 schedule; 1 grade 3 pneumonitis and 1 grade 5 pneumonia/pneumonitis at 40 mg QD 28/28 schedule). Generally, pneumonitis fully resolved with apitolisib discontinuation, antibiotics, and high dose steroids; however, steroids were not administered to the latter patient with extensive bulky MPM. Based on these events, the RP2D was 40 mg QD on the 28/28-day schedule except for MPM patients. The exposure-response relationship between AE and apitolisib [maximum concentration (Cmax) and area under the curve (AUC)] was used to project the probability of AE ≥ grade 1; this analysis suggested a low probability of pneumonitis at 30 and 40 mg QD (manuscript in preparation). However, because patients with MPM have potentially limited pulmonary reserve and may have increased susceptibility to severe clinical course of pneumonitis, 30 mg QD was the recommended dose for MPM.
Table 2.
Dose-limiting toxicities and dose modifications during dose-escalation (stage 1).
| Dose | N | DLTs | Significant AEs either reaching DLT criteria outside first cycle or necessitating dose reductions/discontinuations# | ||
|---|---|---|---|---|---|
|
|
|||||
| Events (n) | Description | Events (n) | Reason | ||
| 2 mg 21/28 d | 3 | 0 | - | 0 | - |
| 4 mg 21/28 d | 3 | 0 | - | 0 | - |
| 8 mg 21/28 d | 3 | 0 | - | 0 | - |
| 16 mg 21/28 d | 3 | 0 | - | 0 | - |
| 32 mg 21/28 d | 7 | 0 | 2 | G3 Fatigue G3 Hepatic encephalopathy# |
|
| 40 mg 21/28 d | 4 | 1 | G4 Hyperglycemia | 1 | G4 Hyperglycemia# |
| 40 mg 28/28 d | 14 | 0 | 6 | G3 Liver dysfunction (n=2) G3 Pneumonitis# G3 Rash (n=2); 1# G5 Pneumonitis# |
|
| 50 mg 21/28 d | 6 | 0 | 3 | G3 Intestinal fistula# G3 Rectal hemorrhage# G5 Colitis# |
|
| 50 mg 28/28 d | 5 | 0 | - | 2 | G3 Liver dysfunction (n=2) |
| 70 mg 21/28 d | 8 | 2 | G3 Rash G3 Hyperglycemia |
5 | G3 Hyperglycemia# G3 Mucosal inflammation (n=2); 1# G3 Pneumonitis# G3 Rash# |
| All | 56 | 3 | Hyperglycemia (n=2) Rash |
19 | Colitis Fatigue Hepatic encephalopathy Hyperglycemia (n=2) Intestinal fistula Liver dysfunction (n=4) Mucosal inflammation (n=2) Pneumonitis (n=3) Rash (n=3) Rectal hemorrhage |
Safety and tolerability
In stage 2, 64 patients on a 28/28 continuous schedule received either 40 mg QD (37 patients) or 30 mg QD (27 patients with MPM). Safety analyses described (Table 3) included all stage 2 patients and 14 stage 1 patients treated at 40mg QD 28/28 (n=51). The most common treatment related ≥ grade 3 AEs at the R2PD 40mg QD were hyperglycemia (18%), rash (14%), liver dysfunction [aspartate aminotransferase (AST), alanine aminotransferase (ALT), or liver function test (LFT) increase] (12%), diarrhea (10%), pneumonitis (8%), and mucosal inflammation (6%). AEs were generally of mild to moderate severity (Table 3) and manageable with standard treatments. Hyperglycemia was usually asymptomatic and responded to metformin administration. Dermatological toxicity involved a maculopapular or pruritic rash; mainstay of treatment was topical emollients and steroids, although 4 patients (5.1%) in stage 2 (2 at 40 mg and 2 at 30 mg QD) required apitolisib treatment discontinuation for rash. Other AEs that led to dose reductions in more than one patient included diarrhea (n=2), mucosal inflammation (n=2), and pneumonitis (n=2); Supplementary Table 1.
Table 3.
Treatment related AE occurring in ≥10% of patients on study
| 30mg 28/28d (n=27) | 40mg 28/28d (n=51) | All patients in study (n=120) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| ≤G2 | G3 | ≥G4 | ≤G2 | G3 | ≥G4 | ≤G2 | G3 | ≥G4 | |
| Patients | |||||||||
| Patients with any AE related to apitolisib | 12 (44%) | 14 (52%) | 1 (4%) | 20 (39%) | 26 (51%) | 5 (10%) | 60 (50%) | 51 (42%) | 8 (7%) |
| Events | |||||||||
| Fatigue | 15 (56%) | 4 (15%) | 0 | 34 (67%) | 2 (4%) | 0 | 73 (61%) | 7 (6%) | 0 |
| Diarrhea | 16 (59%) | 2 (7%) | 0 | 23 (45%) | 5 (10%) | 0 | 60 (50%) | 11 (9%) | 0 |
| Rasha | 12 (44%) | 5 (19%) | 0 | 20 (39%) | 7 (14%) | 0 | 47 (39%) | 14 (12%) | 0 |
| Nausea | 11 (41%) | 1 (4%) | 0 | 22 (43%) | 1 (2%) | 0 | 48 (40%) | 2 (2%) | 0 |
| Decreased appetite | 13 (48%) | 0 | 0 | 11 (22%) | 0 | 0 | 42 (35%) | 0 | 0 |
| Mucosal inflammation | 3 (11%) | 0 | 0 | 15 (29%) | 3 (6%) | 0 | 27 (23%) | 4 (3%) | 0 |
| Hyperglycemia | 0 | 1 (4%) | 1 (4%) | 5 (10%) | 9 (18%) | 0 | 9 (8%) | 18 (15%) | 2 (2%) |
| Vomiting | 5 (19%) | 0 | 0 | 10 (20%) | 1 (2%) | 0 | 24 (20%) | 1 (1%) | 0 |
| Pruritus | 2 (7%) | 0 | 0 | 13 (25%) | 1 (2%) | 0 | 22 (18%) | 1 (1%) | 0 |
| Dry skin | 7 (26%) | 0 | 0 | 7 (14%) | 1 (2%) | 0 | 16 (13%) | 1 (1%) | 0 |
| Pneumonitis | 3 (11%) | 1 (4%) | 0 | 1 (2%) | 2 (4%) | 2 (4%) | 8 (7%) | 4 (3%) | 2 (2%) |
| Liver dysfunctionb | 1 (4%) | 1 (4%) | 0 | 2 (4%) | 6 (12%) | 0 | 4 (3%) | 9 (8%) | 0 |
Rash includes maculopapular, pruritic, macular, erythematous, and generalized rashes.
Liver dysfunction includes abnormal liver function tests (LFTs) namely raised transaminases, gamma-glutamyltransferase alanine and aspartate aminotransferases.
There were 4 treatment related deaths on study. One in a metastatic colorectal patient treated at 50 mg QD (21/28 schedule) was due to drug-induced colitis complicated by septicemia. The patient was hospitalized with severe diarrhea on study day 43, treated with high dose steroids for drug-induced colitis, and antibiotics for a grade 4 bronchopneumonia. The course was complicated by resection for a bowel perforation; the patient died from septicemia on study day 68. Three deaths due to pulmonary events occurred at 40 mg QD (28/28 schedule). One patient with extensive MPM who was treated with prior radiotherapy and chemotherapy was diagnosed with pneumonia on study day 57. Despite treatment with antibiotics, the patient died on study day 61 from probable pneumonitis; the patient did not receive steroids. The second patient with gastrointestinal stromal tumor was hospitalized on study day 164 with confusion, and rapidly developed respiratory failure secondary to Pneumocystis jivorecii pneumonia that was complicated by rapid disease progression and multi-organ failure, and died on study day 172. The third patient with colorectal carcinoma developed significant respiratory distress on study day 92 with CT findings consistent with severe pulmonary edema or acute respiratory distress syndrome. Despite aggressive treatment including steroids and antibiotics, the status deteriorated with the patient dying on study day 96 due to pneumonitis.
Pharmacokinetics
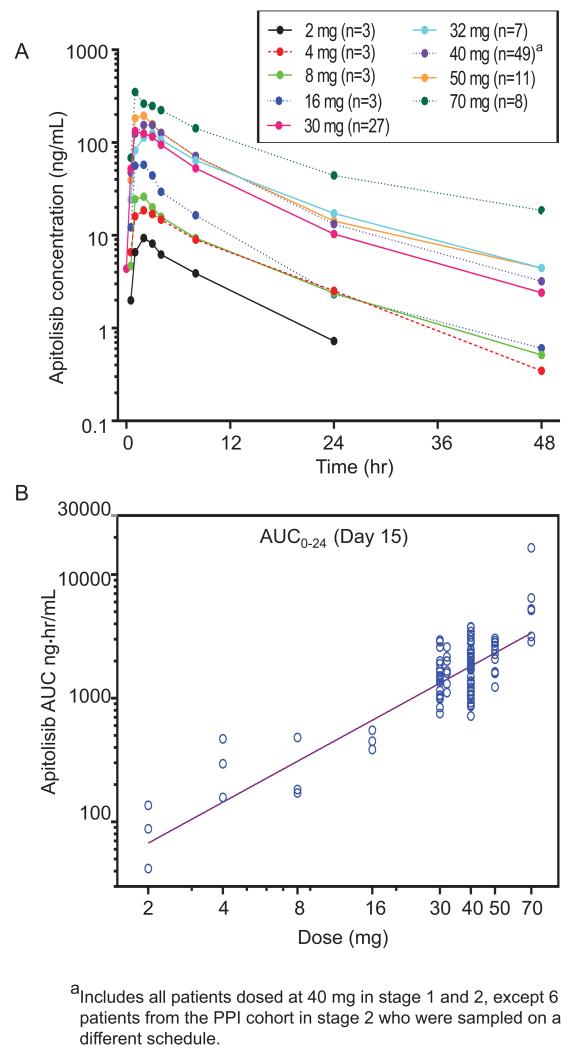
Drug exposure measured as area under the plasma concentration-time curve (AUC) was dose proportional (Figure 1). There were no dose dependent trends in normalized AUC0-24h or maximum concentration, and there was moderate inter-patient variability (manuscript in preparation). Apitolisib was rapidly absorbed; mean plasma concentration peaked 1-2 h after dose with a mean terminal elimination half-life of 11.3 h (range 3.26–45.4 h; Figure 1, Supplementary Table 2). Generally, apitolisib accumulation was very low (less than 1.2), and time to maximal concentration (Tmax) did not change on repeated doses (Supplementary Table 2). Since apitolisib is a weakly basic molecule that displays pH-dependent drug solubility in vitro, PPI use was excluded in all patients except 6 patients in the PPI cohort with rabeprazole 20 mg daily dosing for 5 continuous days. The PPI has no significant impact on apitolisib PK (data not shown) which is consistent with data from a healthy volunteer study where subjects were dosed with a single dose of apitolisib at 10 mg (36).
Figure 1.
Single dose apitolisib pharmacokinetic profile on day 15 for all dose levels. (A) Mean plasma concentration versus time profile for all dose levels. (B) Area under the curve (AUC) versus dose.
Pharmacodynamics
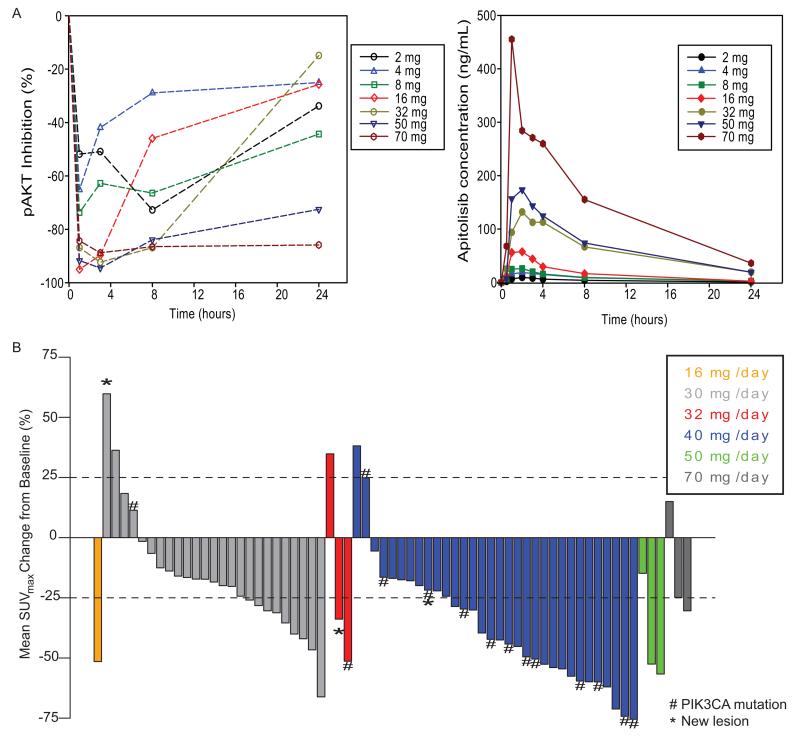
PD functional studies showed >90% suppression of the surrogate biomarker, platelet pAKT, at apitolisib doses ≥16 mg (Figure 2a), which supported the RP2D of 40 mg QD and 30 mg QD in MPM. The RP2D in MPM is further backed up by anti-tumor activity at this dose range. Few on-treatment tumor samples were undertaken thereby preventing detailed analysis. Radiological PD biomarker studies demonstrated decreased FDG-PET tumor uptake in the majority of patients following apitolisib treatment ≥16 mg (Figure 2b); three-quarters of patients (9/12) with PIK3CA mutations treated at 40 mg apitolisib QD displayed more than a 25% mean decrease in maximum standardized uptake (SUVmax), among 10 out of 14 (71%) PIK3CA mutation patients who responded from all dose cohorts.
Figure 2.
Pharmacodynamic analyses: (A) Percentage change in levels of phosphorylated serine-473 AKT (pAKT) in platelet-rich plasma and plasma concentration of apitolisib for up to 24 hours post administration of apitolisib on day 1. (B) Best overall FDG-PET response in all patients at all dose levels.
Furthermore, a comparison of PET responses between patients with steady-state AUC0-24h higher than the median exposure for the RP2D of 40 mg QD to patients below the median (Supplementary Figure 2a) demonstrated that a greater mean reduction in FDG uptake may still be seen with higher exposures (−34% with high vs −18% low exposure compared to median, p=0.028), although there are relatively few data points at low exposures as almost all FDG-PET data were acquired at 30 mg QD or higher.
Treatment-related hyperglycemia may potentially confound FDG-PET results, as FDG is in direct competition with increased blood glucose for tumor uptake. Hence, correlating alterations in pre-scan fasting blood glucose values for each patient relative to baseline values (<187 mg/dl) during the PET procedure are displayed in Supplementary Figure 2b. When exposure level and change in glucose were analyzed as variables in a linear regression model for FDG uptake, the exposure level was still a significant predictor of uptake (p=0.041), along with glucose change (p=0.021). This simple model does not take into account the dependence of glucose change on exposure, but still shows that while reduction in FDG uptake is correlated with an increase in pre-scan fasting blood glucose, other factors such as changes in cell proliferation and necrosis are also driving the FDG-PET response.
Anti-tumor activity
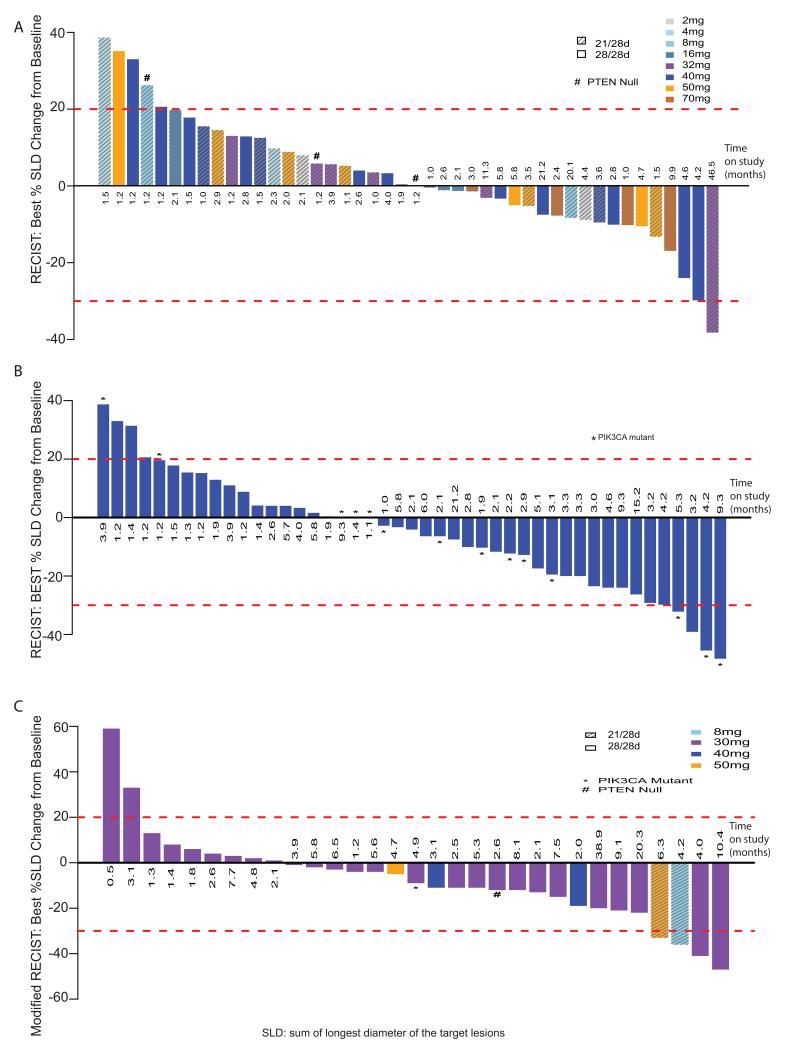
In stage 1, among 56 patients, there were 4 partial responses (PR) that included peritoneal mesothelioma (n=1), MPM (n=2), and adrenocortical tumor (n=1). Of these, 2 were confirmed responses for 1 peritoneal and 1 pleural mesothelioma patients at 32 mg QD 21/28 schedule and 50 mg QD 21/28, respectively. Stable disease (SD) was seen in 77% of patients and approximately half of the 44 patients with RECIST evaluable target lesions and post-baseline assessments had demonstrable tumor shrinkage (Figure 3a and Supplementary Table 3). In stage 1 patients with tumor shrinkage, no PIK3CA mutations or loss of PTEN were identified, although the adrenocortical tumor patient with unconfirmed PR had decreased PTEN levels. Prolonged stable disease of over 6 months was seen in 7 patients (7/56; 13%), with 3 patients (5%) continuing on study for over 12 months; including a patient with PEComa who had previously progressed on a rapalog. Interestingly, evidence of anti-tumor activity was apparent in mesothelioma with 2 PRs in MPM patients by modified RECIST; one partial responder at 8mg QD had a non-hotspot PIK3CA mutation (R88Q) detected on sequencing (Supplementary Table 4).
Figure 3.
Waterfall plots demonstrating maximal RECIST response to apitolisib compared to baseline in patients in (A) Stage 1 dosed at 2-70 mg apitolisib evaluated by RECIST (excluding MPM patients), (B) Stage 1 and 2 dosed at 40 mg continuous dosing schedule, and (C) Malignant pleural mesothelioma patients in stage 1 and stage 2 at 30 mg apitolisib intermittent or continuous dosing schedule evaluated by modified RECIST.
In stage 2, 64 patients were enrolled on the 28/28-day schedule: 37 patients at 40 mg and 27 patients at 30 mg. At the RP2D of 40 mg 28/28 schedule, 49 patients, including stage 1 expansion (n=12) and stage 2 (n=37), were RECIST evaluable with 45 patients who had post-baseline measurements (Figures 3b,c and Supplementary Table 3). Five PRs included SCCHN (n=3), clear cell ovarian (n=1), and adrenocortical tumor (n=1). One PR was confirmed for an E542K SCCHN PIK3CA mutant. Two additional patients (one SCCHN and one ovarian cancer) with unconfirmed PRs also had an E545K PIK3CA mutation (Figure 3b). The aforementioned patient with metastatic clear cell ovarian cancer who had received two prior chemotherapy regimens remained on apitolisib for 282 days with maximal tumor shrinkage of −48.3% change from baseline. The patient came off study due to a mixed response of the non-evaluable pelvic disease. Interestingly, the partial response seen in the target disease of lung and common iliac lymphadenopathy remains stable almost 3 years later (Supplementary Figure 3). In addition to the five PRs, stable disease with tumor regression was apparent in 20 patients dosed at 40 mg 28/28 (Figure 3b). Median time on study was 2.9 months (range 1-21.2) (Supplementary Table 3). Of the 14 evaluable patients with PIK3CA mutations, there were 3 PRs (1 confirmed), 8 SD, and 3 PD as best radiological responses (Figure 3b, Supplementary Table 3).
Of the 27 MPM patients treated at 30 mg 28/28 schedule, 26 were evaluable by modified RECIST independent review. Median time on study was 4 months (range 0.5-38.9) with 8 patients (29.6%) on study for more than 6 months, including 2 patients (7.4%) for more than 12 months (Figure 3c, Supplementary Table 3). SD was the overall best response in 20 patients (74.1%). There were two confirmed PRs, however, the molecular characteristics were unable to be confirmed in these patients. Only 2 patients of this cohort had molecular abnormalities detected, one PTEN loss and one PIK3CA mutation (E545A) (Supplementary Table 3) both with SD as their best response.
DISCUSSION
This first-in-human phase I study demonstrated the feasibility of dual inhibition of PI3K and mTOR with apitolisib in patients with solid tumors. Apitolisib was reasonably tolerated at lower biologically active doses and exhibited dose-proportional PK with a mean terminal half-life of 11.3 h favoring once daily dosing. The drug showed increased toxicity and 4 fatalities at doses ≥40 mg. Rash and hyperglycemia were dose limiting at 70 mg QD 21/28 but manageable with supportive treatment and dose reductions at the RP2D. The rash was maculopapular or pruritic, not blistering or acneiform, and therefore consistent with previous reports with other PI3K, AKT, and mTOR inhibitors, suggesting a mechanism-based toxic effect (13). The hyperglycemia, another hallmark of PI3K inhibition (2), required administration of metformin and subcutaneous insulin but fully reversed on cessation of apitolisib. A total of 14 pneumonitis cases were reported on study, six of which were severe: four grade 3 events (at 70 mg 21/28, 30 mg 28/28, and 2 patients at 40 mg 28/28) and two grade 5 events at 40 mg 28/28. There were no clear baseline predisposing factors in this small diverse group. Baseline lung function and radiology did not suggest any underlying pulmonary inflammation nor was there any relationship with prior radiotherapy. The pneumonitis appeared to be dose-dependent occurring primarily at doses at or exceeding 40 mg. The rate of severe pneumonitis (grade ≥3) with apitolisib was 8%, which is higher than reported for PI3K and dual mTOR inhibitors (18-21, 37). This is presumably related to mTOR inhibition, correlates with the frequency seen with rapalogs (38), and suggests increased mTOR pathway blockade compared to published dual PI3K/mTOR inhibitors. Given these toxicities and one grade 5 colitis on the 50 mg 28/28 schedule, the RP2D was 40 mg continuous QD oral dosing on a 28/28 schedule. The 30 mg continuous schedule was selected for MPM patients given their potential for increased respiratory toxicities. The most common drug-related toxicity for apitolisib at the RP2D was hyperglycemia at higher than published rates, possibly suggesting more of an on-target effect. Rash, liver dysfunction, and diarrhea were at similar rates to published data for pan PI3K inhibitors (21, 37, 39). The mTOR effects of pneumonitis, hyperlipidemia, and mucositis were more prominent than published dual PI3K/mTOR inhibitors, suggesting possibly increased pathway inhibition.
Apitolisib exhibited a narrow therapeutic window; anti-tumor activity was apparent from 8 mg, although severe toxicity including deaths occurred at doses at or exceeding 40 mg. There was, however, a good correlation between PK and PD with robust evidence of target modulation. Most of the toxicities appeared to be on-target; therefore, studying intermittent schedules could prove beneficial. Pulsatile dosing has been investigated with weekly schedules for mTOR inhibitors; weekly dosing of apitolisib has also been explored in another study (ClinicalTrials.gov NCT00854126).
Pharmacodynamic evidence of target modulation was confirmed at doses of apitolisib exceeding 16 mg on intermittent schedule. Significant reductions in PI3K inhibition of ≥90% were apparent in PRP at doses ≥16 mg. Changes in FDG-PET tumor uptake confirmed inhibition in more than half the patients treated with apitolisib at ≥30 mg QD. These changes were seen regardless of the molecular background of the tumor. Significant reductions in SUVmax of >25% were recorded in three quarters of PIK3CA mutant patients and 66% of all patients treated at the RP2D of 40 mg QD. The reduction of FDG-PET uptake as an indication of target inhibition is supportive of the RP2D.
The 28/28-day schedule was selected for stage 2 to enable maximal pathway suppression, as tolerability was equivalent to 21/28 dosing. The selection of 40 mg 28/28 days as the RP2D was based on the optimal risk-benefit for phase 2 studies. The 40 mg 28/28 dosage is high enough within the therapeutic window to ensure significant target modulation; evidenced by sustained >90% pAKT inhibition and significant reduction in FDG-PET uptake in two thirds of patients. The respiratory toxicities were felt to be manageable by prompt diagnosis and active management with early apitolisib interruption and subsequent dose reduction. Our safety concerns regarding the potential for severe apitolisib-induced lung toxicity favored a more conservative dose of 30 mg 28/28, well within the active therapeutic range.
The apitolisib partial response rate at the RP2D was 10.2% at 40 mg QD; whilst modest, the response rate was triple the published reports of 0-3% for single agent PI3K inhibitors (37, 39-41). Durable RECIST responses were detected at ≥30 mg. Enrichment for PIK3CA mutations doubled the responses to 18.8%. The biological background appears to define sensitivity, hence, the presence of a PIK3CA mutation alone may not associate with anti-tumor response, as seen with the contrasting response with the single agent BRAF inhibitors in melanoma and colorectal cancers. The identification and clinical validation of predictive biomarkers and development of drug combinations will be crucial to determine the optimal use of to this class of agents. Prolonged disease stabilisation to apitolisib was seen in patients who had progressed on rapalogs, this strengthens the hypothesis that either a direct mTOR kinase inhibitor or a multi-nodal approach may be more effective than an indirect inhibitor of mTOR.
Interestingly, responses in MPM patients were seen at lower doses and the PR rate with apitolisib was higher at 12% compared to other tumor types in this study. Furthermore, this response rate in MPM previously treated with chemotherapy compares favorably to the low response rates reported for commonly used second-line agents such as gemcitabine or vinorelbine (2%) in the same patient population (42). While modest, this is encouraging in a disease entity with an unmet clinical need that is difficult to measure by current radiological approaches. There were some prolonged RECIST responses at 30 mg, with 30% of patients being on study for >6 months. Curiously, two patients with peritoneal mesothelioma but no detectable molecular abnormalities had significant tumor regression and continued on trial for almost 2 years. Analysis of tumor tissue of the 27 MPM treated at the RP2D detected a much lower rate of PTEN loss (4%, 1/24) and PIK3CA mutations (5%, 1/21) compared to previous studies reporting 30–60% (43, 44) and up to 100% (45), respectively. The documented molecular changes did not correlate with anti-tumor activity with most having disease stability as best radiological response. Interestingly, a patient with the PIK3CA R88Q non-hotspot mutation had a partial response at only 8mg 21/28 day schedule. Putative causes for the anti-tumor activity could be stromal response or coexisting unevaluable genomic aberrations, such as neurofibromatosis 2 (NF2) reported to be lost in 40-60% of MPM (46), or BAP1 (47). Further pre-clinical studies are warranted to explain the effect of dual PI3K/mTOR inhibition on mesothelioma. This may help identify patients that are more sensitive to this compound.
Daily administration of apitolisib in this phase I study and two phase 2 trials (ClinicalTrials.gov NCT01455493; NCT01442090) has demonstrated insufficient tolerability and clinical activity for it to succeed as a single agent. Hence, investigation of rational combinations is warranted. Putative options to be explored include weekly paclitaxel in conjunction with brief pulses of apitolisib as a chemo-sensitizer. If synergistic, this could provide a beneficial therapeutic strategy across multiple tumor types.
This study provides preliminary evidence that apitolisib has anti-tumor efficacy. Durable RECIST responses were evident in mesothelioma, some PIK3CA mutant tumors, and SCCHN; the underlying reason for these remains to be elucidated. Further investigation of activity in phase II trials has been conducted in refractory endometrial and renal cell cancers. Next-generation sequencing may be useful to decipher if a particular molecular sub-group benefits most from this agent. Additional early combination clinical trials are essential to fully assess this compound. Notably, pulsatile apitolisib dosing may increase dose density and anti-tumor activity. Thereafter, combination studies with chemotherapies such as paclitaxel or carboplatin may be warranted.
In conclusion, the oral PI3K/mTOR inhibitor, apitolisib, demonstrated favorable pharmacokinetic properties and robust pharmacodynamic substantiation of target modulation. Apitolisib has a narrow therapeutic window; based on the optimal risk-benefit ratio, a dose of 40 mg 28/28 days was the preferred RP2D. Due to the putative increased risk of pulmonary toxicities in mesothelioma patients, 30 mg 28/28 days was pursued. Both doses are biologically active with evidence of durable anti-tumor activity. The modest single agent response rate of 10-19% would necessitate combination with cytotoxics for future development, possibly with higher pulsatile dosing, to putatively increase dose density and efficacy.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Deregulation of the PI3K-PTEN-AKT-mTOR signaling network is a frequent and critical driver of oncogenesis. Successful inhibition of this system is a major objective in cancer drug discovery. Multiple inhibitors have been assessed with modest outcomes owing to feedback loops and pathway cross talk providing the rationale for targeting three crucial nodes (PI3K, mTORC1, and mTORC2). We report on the first-in-human phase I study of apitolisib, a potent oral dual PI3K and TORC 1/2 small molecule inhibitor, in patients with advanced solid tumors. Apitolisib demonstrated favorable pharmacokinetics and evidence of biological activity. Robust pharmacodynamic biomarker assessment confirmed target modulation through significant suppression of AKT and FDG uptake on PET imaging. Single-agent anti-cancer activity was observed in pleural and peritoneal mesothelioma, PIK3CA mutant ovarian and squamous cell head and neck cancers. Cancers driven by the PI3K-PTEN-AKT-mTOR pathway seem to respond to monotherapy, indicating triple effective pathway blockade could be clinically beneficial.
ACKNOWLEDGEMENTS
The authors thank the patients who participated in this study and their families.
GRANT SUPPORT
This study was supported by Genentech, Inc. The Royal Marsden and Institute of Cancer Research Drug Development Unit is supported by an Experimental Cancer Medicine Center grant and the National Institute for Health Research Biomedical Research Center grant. Authors have received funding from the National Cancer Institute, from Cancer Research UK, and from the Medical Research Council.
PRIOR PRESENTATIONS
35th Congress of the European Society for Medical Oncology (ESMO), October 8-12, 2010, Milan; Annual Meeting of the American Society of Clinical Oncology (ASCO), June 3-7, 2011, Chicago; Congress of the International Mesothelioma Interest Group (iMig), 11-14 September, 2012 (Boston); 17th European Cancer Congress (ECCO), 38th Congress of the European Society for Medical Oncology (ESMO), 32nd European Society for Radiotherapy and Oncology Congress (ESTRO) 27 September-1 October, 2013, Amsterdam; 15th World Conference on Lung Cancer, International Association for the Study of Lung Cancer (IASLC), 27-31 October, 2013, Sydney
Footnotes
DISCLOSURES
The following authors have no conflicts of interest to declare: SOD and JCB.
JOL, DA, RFY, JOF, JMS, HK, and JAW are employees of Genentech, Inc., South San Francisco, CA.
The following authors have disclosures:
AJW received research funding from Genentech, Amgen, Novartis, and Sanofi; consulting fees from Daiichi-Sankyo.
MGZ received research funding from.Verastem, Inc.
LMK received research funding from Genentech, Inc.
TYS received honoraria from Merck (MSD), BMS, and Amgen.
HLK received research funding and consulting fees from Genentech, Inc.
MPL received consulting fees from Roche and is member of a study steering committee at Roche.
JDB received consulting fees from Genentech, AstraZeneca, Pfizer, GSK, and Chugai; and is an employee of the Institute of Cancer Research, a not-for-profit organization with a commercial interest in PI3K and AKT inhibitors.
REFERENCES
- 1.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–83. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 4.Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–5. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 5.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102:802–7. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3:761–9. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 8.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 10.Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992;89:9267–71. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–7. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 13.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10:143–53. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 14.Klempner SJ, Myers AP, Cantley LC. What a tangled web we weave: emerging resistance mechanisms to inhibition of the phosphoinositide 3-kinase pathway. Cancer Discov. 2013;3:1345–54. doi: 10.1158/2159-8290.CD-13-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011;17:1116–20. doi: 10.1038/nm.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–19. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 18.Burris H, Rodon J, Sharma S, Herbst R, Tabernero J, Infante J, et al. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28:S3005. [Google Scholar]
- 19.Mahadevan D, Chiorean EG, Harris WB, Von Hoff DD, Stejskal-Barnett A, Qi W, et al. Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer. 2012;48:3319–27. doi: 10.1016/j.ejca.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markman B, Tabernero J, Krop I, Shapiro GI, Siu L, Chen LC, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol. 2012;23:2399–408. doi: 10.1093/annonc/mds011. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulos KP, Tabernero J, Markman B, Patnaik A, Tolcher AW, Baselga J, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245409 (XL765), a novel, orally administered PI3K/mTOR inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2445–56. doi: 10.1158/1078-0432.CCR-13-2403. [DOI] [PubMed] [Google Scholar]
- 22.Britten CD, Adjei AA, Millham R, Houk BE, Borzillo G, Pierce K, et al. Phase I study of PF-04691502, a small-molecule, oral, dual inhibitor of PI3K and mTOR, in patients with advanced cancer. Invest New Drugs. 2014;32:510–7. doi: 10.1007/s10637-013-0062-5. [DOI] [PubMed] [Google Scholar]
- 23.Sutherlin DP, Bao L, Berry M, Castanedo G, Chuckowree I, Dotson J, et al. Discovery of a potent, selective, and orally available class I phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) kinase inhibitor (GDC-0980) for the treatment of cancer. J Med Chem. 2011;54:7579–87. doi: 10.1021/jm2009327. [DOI] [PubMed] [Google Scholar]
- 24.Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–36. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) 2006 http://ctepcancergov/protocoldevelopment/electronic_applications/docs/ctcaev3pdf.
- 26.Sewell A, Brown B, Biktasova A, Mills GB, Lu Y, Tyson DR, et al. Reverse-Phase Protein Array Profiling of Oropharyngeal Cancer and Significance of PIK3CA Mutations in HPV-Associated Head and Neck Cancer. Clin Cancer Res. 2014;20:2300–11. doi: 10.1158/1078-0432.CCR-13-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–47. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–60. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 30.Ding X, Salphati L, Kim A, Morinello E, Wong L, Pang J, et al. Determination of GDC-0980 (apitolisib), a small molecule dual phosphatidylinositide 3-kinase/mammalian target of rapamycin inhibitor in dog plasma by LC-MS/MS to support a GLP toxicology study. Biomed Chromatogr. 2015 doi: 10.1002/bmc.3417. [DOI] [PubMed] [Google Scholar]
- 31.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 32.Lheureux S, Lecerf C, Briand M, Louis MH, Dutoit S, Jebahi A, et al. (18)F-FDG is a surrogate marker of therapy response and tumor recovery after drug withdrawal during treatment with a dual PI3K/mTOR inhibitor in a preclinical model of cisplatin-resistant ovarian cancer. Transl Oncol. 2013;6:586–95. doi: 10.1593/tlo.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleifman EB, Desai R, Spoerke JM, Xiao Y, Wong C, Abbas I, et al. Targeted biomarker profiling of matched primary and metastatic estrogen receptor positive breast cancers. PLoS One. 2014;9:e88401. doi: 10.1371/journal.pone.0088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid AH, Attard G, Brewer D, Miranda S, Riisnaes R, Clark J, et al. Novel, gross chromosomal alterations involving PTEN cooperate with allelic loss in prostate cancer. Mod Pathol. 2012;25:902–10. doi: 10.1038/modpathol.2011.207. [DOI] [PubMed] [Google Scholar]
- 35.Patel R, Tsan A, Tam R, Desai R, Spoerke J, Schoenbrunner N, et al. Mutation scanning using MUT-MAP, a high-throughput, microfluidic chip-based, multi-analyte panel. PLoS One. 2012;7:e51153. doi: 10.1371/journal.pone.0051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smelick G, Dalziel G, Salphati L, Pellett J, Li J, Dean B, et al. An integrated approach to assess the impact of gastric pH on GDC-0941 and GDC-0980 pharmacokinetics. Clin Pharmacol Ther. 2014;95:S104–S10. [Google Scholar]
- 37.Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, et al. First-in-Human Phase I Study of Pictilisib (GDC-0941), a Potent Pan-Class I Phosphatidylinositol-3-Kinase (PI3K) Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015;21:77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willemsen AE, Grutters JC, Gerritsen WR, van Erp NP, van Herpen CM, Tol J. mTOR inhibitor-induced interstitial lung disease in cancer patients: Comprehensive review and a practical management algorithm. International journal of cancer Journal international du cancer. 2015 doi: 10.1002/ijc.29887. [DOI] [PubMed] [Google Scholar]
- 39.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro GI, Rodon J, Bedell C, Kwak EL, Baselga J, Brana I, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:233–45. doi: 10.1158/1078-0432.CCR-13-1777. [DOI] [PubMed] [Google Scholar]
- 41.Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O'Bryant CL, et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–82. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 42.Zauderer MG, Kass SL, Woo K, Sima CS, Ginsberg MS, Krug LM. Vinorelbine and gemcitabine as second- or third-line therapy for malignant pleural mesothelioma. Lung Cancer. 2014;84:271–4. doi: 10.1016/j.lungcan.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal V, Campbell A, Beaumont KL, Cawkwell L, Lind MJ. PTEN protein expression in malignant pleural mesothelioma. Tumour Biol. 2013;34:847–51. doi: 10.1007/s13277-012-0615-9. [DOI] [PubMed] [Google Scholar]
- 44.Opitz I, Soltermann A, Abaecherli M, Hinterberger M, Probst-Hensch N, Stahel R, et al. PTEN expression is a strong predictor of survival in mesothelioma patients. Eur J Cardiothorac Surg. 2008;33:502–6. doi: 10.1016/j.ejcts.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 45.Cedres S, Montero MA, Martinez P, Martinez A, Rodriguez-Freixinos V, Torrejon D, et al. Exploratory analysis of activation of PTEN-PI3K pathway and downstream proteins in malignant pleural mesothelioma (MPM) Lung Cancer. 2012;77:192–8. doi: 10.1016/j.lungcan.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Sekido Y, Pass HI, Bader S, Mew DJ, Christman MF, Gazdar AF, et al. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 1995;55:1227–31. [PubMed] [Google Scholar]
- 47.Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–72. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.