Abstract
Laboratory-induced stress produces elevations in cortisol and deficits in memory, especially when stress is induced immediately before retrieval of emotionally valent stimuli. Sex and sex steroids appear to influence these stress-induced outcomes, though no study has directly compared the effects of laboratory-induced stress on cortisol and emotional retrieval across the menstrual cycle. We examined the effect of psychosocial stress on cortisol responsivity and emotional retrieval in women tested during either the follicular phase (low estradiol and progesterone) or the luteal phase (higher estradiol and progesterone). Forty women (50%White; age 18–40 years) participated in the study; 20 completed the task during the luteal phase and 20 during the follicular phase. Psychosocial stress was induced with the Trier Social Stress Test (TSST). On the day before the TSST, participants learned two lists of word pairs to 100% criterion. The next day, participants recalled one list after the control condition and the other after the TSST. Women in the follicular phase, but not the luteal phase, demonstrated a significant cortisol response to the TSST. There was a stress-induced decrease in emotional retrieval following the TSST, but this effect was not modified by menstrual phase. However, regression and correlational analyses showed that individual differences in stress-induced cortisol levels were associated with impaired emotional retrieval in the follicular phase only. The present findings indicate that cortisol responsivity and the impairing effects of cortisol on emotional memory are lower when levels of estradiol and progesterone are high compared to when levels are low.
Keywords: Estradiol, Cognition, Stress, Cortisol, Memory, Emotion
Introduction
Important insights into the hormonal mechanisms underlying sex differences in the risk of anxiety and affective disorders can be gained by studying the interplay between natural fluctuations in endogenous sex steroid hormones and hypothalamic–pituitary–adrenal (HPA) axis function. In humans, a commonly used paradigm to induce stress and cortisol release in the laboratory is the Trier Social Stress Test (TSST), a test of social evaluative threat (Kirschbaum et al., 1993). In the TSST, participants first prepare and give a speech, most commonly one related to their personal qualifications for a job, and then they perform a mental arithmetic task in front of evaluators who maintain neutral expressions throughout the test session (Kirschbaum et al., 1993). The validity of the TSST as a laboratory stressor was demonstrated in a meta-analysis which found that social evaluative threat combined with uncontrollability produces the largest cortisol increases in the laboratory (Dickerson and Kemeny, 2004). In general men show elevated cortisol responses to the TSST compared to women (Kudielka and Kirschbaum, 2005), but not all studies find a sex difference (Kudielka et al., 2004). Menstrual cycle effects on stress responsivity have also been reported and compared to findings in men, but results have been somewhat inconsistent (Kirschbaum et al., 1999; Schoofs and Wolf, 2009). For example, in an early study, women tested in the luteal phase of the menstrual cycle (higher estradiol and progesterone), showed TSST-induced elevations in cortisol that were similar in magnitude to men and higher than both women in the follicular phase (low estradiol and progesterone) and women using oral contraceptives (low endogenous estradiol and progesterone; high exogenous levels of estrogens and progestogens) (Kirschbaum et al., 1999). Conversely, a second study found that women tested in the luteal phase had a smaller increase in cortisol response to the TSST compared to men (Schoofs and Wolf, 2009).
In humans, the TSST is often used to evaluate the effects of stress and elevations in endogenous cortisol on memory, and findings from these studies are generally similar to those from studies using pharmacological intervention with exogenous glucocorticoids such as hydrocortisone (Wolf, 2009). The effects of elevations in endogenous or exogenous glucocorticoids on memory are complex and depend on many factors, including the valence of stimuli, and the timing of exposure to the stressor or glucocorticoid in relation to encoding, consolidation, and retrieval processes (Het et al., 2005; Wolf, 2009). A reliable deficit in memory retrieval is observed when participants learn a list of words under normal conditions, and then several hours to days later are exposed to the TSST and are immediately asked to retrieve the words (Het et al., 2005). Similarly, glucocorticoid administration before memory retrieval produces a reliable, impairing effect in rodents (Roozendaal, 2002) and humans (de Quervain et al., 2000). The TSST and cortisone administration differentially impair retrieval of emotional stimuli compared to neutral stimuli (Kuhlmann et al., 2005a, 2005b; Wolf, 2009). Mirroring the cortisol response, the extent to which the TSST induces impairments in emotional retrieval differs between the sexes, with greater impairing effects in men compared to women (Wolf et al., 2001). Menstrual cycle studies suggest that ovarian steroid hormones might protect against stress-induced impairments in emotional retrieval (Schoofs and Wolf, 2009). In contrast to men, no impairing effect of stress or cortisol on emotional retrieval was observed in women tested during the luteal phase of the menstrual cycle when levels of estradiol and progesterone are high (Schoofs and Wolf, 2009). A direct comparison of women in the follicular and luteal phases of the menstrual cycle is needed to better characterize how hormonal variations across the menstrual cycle affects cortisol responsivity and emotional retrieval. If high levels of estradiol and progesterone during the luteal phase are associated with low cortisol responses and better emotional retrieval, then low levels of these ovarian hormones during the follicular phase might be associated with elevated cortisol and greater impairments in emotional retrieval.
In the present study, we examined cortisol responsivity and emotional retrieval following the TSST in women tested at different phases of the menstrual cycle. The first aim was to determine whether the effect of the TSST on cortisol levels is smaller during the luteal phase of the menstrual cycle compared to the follicular phase. The second aim was to determine whether the effect of TSST on emotional retrieval is also smaller in the luteal phase compared to the follicular phase. Lastly, we examined whether the association between cortisol and emotional retrieval was stronger in the follicular phase compared to the luteal phase.
Material and methods
Participants
Participants were recruited from the University of Illinois at Chicago (UIC) and the surrounding community via advertisements on campus and websites. Inclusionary criteria were: 18 to 40 years of age; English as a first language; and regular menstrual cycles defined as 25+/−5 days in length for the prior three months. Exclusionary criteria included: use in the prior six months of medications or botanicals influencing the central nervous system (e.g., antidepressants, Ginkgo biloba), glucocorticoids, or oral contraceptives; current smoking; phobia of math or phobia of public speaking; history of depression, psychiatric illness, serious medical illness (e.g., HIV or cancer), traumatic brain injury or loss of consciousness greater than 30 min, or drug or alcohol dependency or abuse; sensory impairment that would interfere with testing; current pregnancy; child birth or lactation in the previous 6 months; and BMI >30. Participants received compensation for their time and travel.
General procedures
Participants were first screened by phone for general inclusion criteria. Women were then randomized to be tested on two consecutive days during either the follicular or luteal phase of the menstrual cycle as follows. If randomized to the luteal phase group, they visited UIC for an initial visit, and they completed informed consent as well as the Mini-Screen, a 21-item self-report measure used to rule out psychiatric diagnoses (Sheehan et al., 1998). They also were given an at-home ovulation kit (Clearblue Easy ovulation tests, Unipath Diagnostics, Inc.) and were instructed to use the kit and to contact the study coordinator on the day that the test indicated they were ovulating. Their first test session was then scheduled 8 to 10 days later. Alternatively, if they were randomized to the follicular phase group, they were instructed to contact the coordinator on the day they began menstruating and were scheduled for their first test session two to four days later after the anticipated start of their menstrual cycle (the first day of bleeding). Study coordinators contacted the participants the day before testing to confirm menstrual phase. Women randomized to the follicular phase completed informed consent and the Mini-Screen at their first visit. The two consecutive test sessions for all participants were scheduled between 1:00 pm and 5:00 pm due to the minimal variation in cortisol during that time of day.
During the first of the two consecutive test sessions, participants met one-on-one with an examiner who was blinded to menstrual status. Blood was drawn at the beginning of the session for hormone assays to confirm menstrual phase. A key procedure at the end of the test session was the acquisition phase of the Emotional Paired Associates test, where participants learned two lists of word pairs to 100% criterion (see below). A neuropsychological test battery comprised of measures of memory, psychomotor speed, attention, language, and spatial abilities was also administered (data not reported here). Participants also completed a series of questionnaires measuring self-reported mood and anxiety, menstrual distress, and estimated IQ for use as covariates, if group differences were observed. During the second test session, retrieval of the emotional word pairs learned 24 h earlier was tested and stress-related outcomes were obtained after the TSST and a non-stress control condition. The non-stress control condition involved completing questionnaires relating to mood, symptoms and lifestyle habits. Stress-related outcomes included self-reported anxiety and stress (State-Trait Anxiety Scale, Visual Analog Scale), and cortisol levels. Measures of heart rate and heart rate variability were also obtained throughout the second session for some women but are not reported due to missing data (n= 12 missing).
Emotional paired associates
The primary behavioral outcome was the Emotional Paired Associates Test. During the first test session, participants learned two matched lists (Lists A and B) of 15 emotional word pairs to 100% criterion. There were five negative (e.g., “suffocate-loneliness”), five positive (e.g., “champion-laughter”), and five neutral (e.g., “item-passage”) pairs. The two word lists were balanced on overall ratings of valence, arousal, word frequency, and word length based on Affective Norms for English Words (Bradley and Lang, 1999). The word pairs in each list were presented in a random order on each learning trial. The examiner read the list aloud at a rate of one pair every 3 s. After each presentation, the examiner prompted the participant with the first member of each pair, and asked the participant to recall the corresponding word. The prompts were also randomly ordered and differed for each recall trial. The examiner responded “that's correct” if the response was correct. If the participant did not respond within 5 s or responded incorrectly, the examiner provided the correct answer by saying, “No, ___ goes with ___.” After the first recall trial, the examiner only prompted the participant to recall pairs that she had not successfully recalled. The reading/prompting/learning procedure was repeated until all pairs were correctly recalled once. The list was taught to 100% criterion to ensure equivalent learning across groups, avoid floor effects at recall, and ensure that any errors in recall on the next day were not due to unsuccessful encoding. After both lists were learned to criterion, recall was again measured once for each list. Participants were instructed to try to remember the word pairs because they would be asked about them again. At Session 2, 24–28 h after they learned the two lists to 100% criterion, participants were asked to recall the word pairs of List A (or B) after the control condition and List B (or A) after the stressor condition. The presentation of lists was counter-balanced across conditions (TSST or control) and groups (follicular, luteal). For each list recall, participants were prompted with the first member of each pair and asked to recall the corresponding word. Participants received 1 point for each pair recalled (e.g., “What word goes with ‘toxic’?”).
Trier Social Stress Test
Each participant was told that she would take the role of a job applicant for her “ideal job position” and would need to give a speech introducing herself and convincing managers that she is the ideal applicant for a vacant position. She was given 10 min to prepare a 5-minute speech and was told that the speech would be recorded and given in front of three experts in the assessment of nonverbal behavior, who were confederate lab personnel. One expert served as the “Chair”, and delivered instructions to the participant. After the preparation period, the participant gave her speech to the “experts.” Those who finished in less than 5 min were told sternly to continue the speech for the remainder of the 5 min. After the 5-minute speech, the Chair instructed the participant to serially subtract the number 13 from 1687 as quickly and accurately as possible. If she made an error, she was instructed to start again from 1687. After 5 min of serial subtractions, the task was stopped. A 20-minute non-stressful control condition was employed in this study in which participants completed a series of questionnaires relating to mood, symptoms and lifestyle habits. The control condition always preceded the stress condition. A slight modification was made to the standard TSST protocol, and likewise implemented in the control condition. Both conditions were interrupted at 11, 14, and 16 min into the condition to complete a 1-minute cognitive flexibility task at each interruption (data not shown) (Alexander et al., 2007). At the end of the test session, the examiner debriefed the participant and told her that no analysis of her nonverbal behavior would be performed.
Subjective stress and anxiety
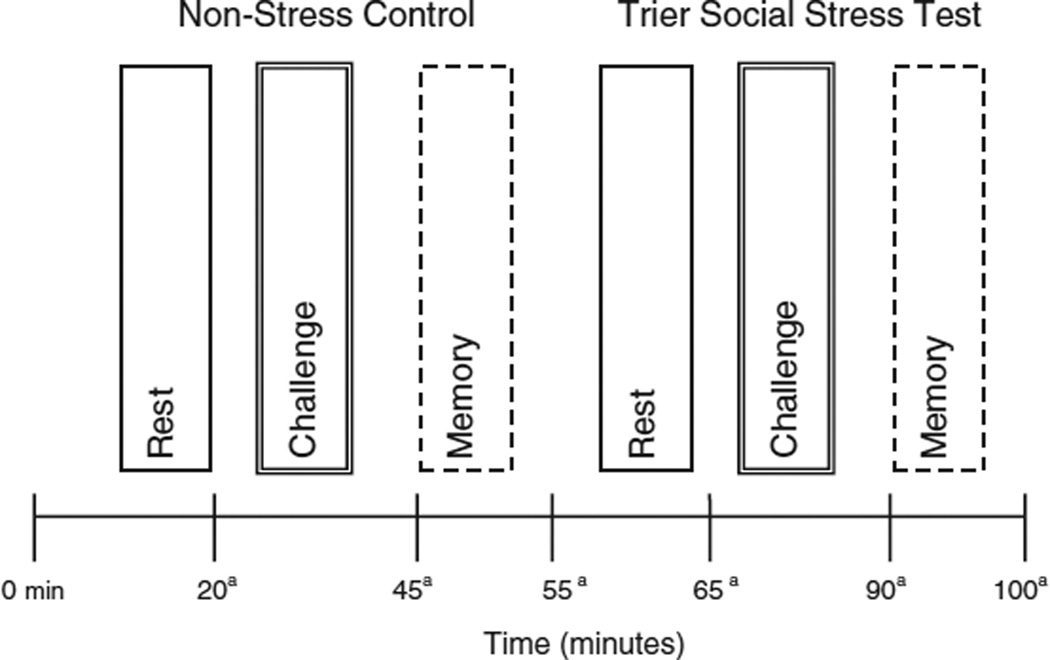
Select measures were obtained at specific time points before, during, and after the TSST and control conditions to measure subjective stress (See Fig. 1). At six timepoints concurrent with saliva sampling, participants completed a two-item Visual Analog Scale (VAS) measuring how “anxious” and “stressed” they felt on a 10-cm line (maximum distress rating = 80 cm). They also completed the State-Trait Anxiety Inventory: Short Form (STAI-6), a 6-item questionnaire assessing the extent to which one feels calm, tense, upset, relaxed, content, and worried (Marteau and Bekker, 1992). Ratings were made on a 4-point Likert scale ranging from “not at all” to “very much so.”
Fig. 1.
Experimental design. Notes: Condition (Non-Stress versus Stress) and Time (Rest, Challenge, Memory) were within-subject factors and menstrual phase (Follicular versus Luteal) was a between-subjects factor. aSalivary cortisol and self-reported anxiety and stress measures were obtained.
Salivary cortisol assays
To minimize the influence of extra-test factors on cortisol levels, on the day before the TSST, participants were given instructions to refrain from caffeine and physical exertion for 3 h prior to their appointment, eat a light lunch low in fat and protein, and refrain from all eating and drinking for 1 h before their appointment. During the TSST and control conditions, we collected six saliva samples that corresponded to the rest, challenge, and memory phases of each of the two conditions (stress, non-stress control) (see Fig. 1). Participants were asked to spit into the tube using a straw until at least 1.0 mL of saliva was collected. Samples were collected in plain Nalgene tubes with no preservatives and were placed into refrigerators set at −80 °C. Unbound cortisol was measured with a commercially available enzyme immunoassay (EIA) (Diagnostic Systems Laboratory., Webster, TX). Each of the samples for each participant was run on the same assay plate. Assay sensitivity for cortisol was 0.011 µg/d. At high (4.09 mg/dL), medium (1.41 mg/dL) and low (0.47 mg/dL) concentrations of cortisol, the intra-assay coefficients of variation (CV) were 1.9%, 2.8% and, 4.8% respectively. The inter-assay CVs for high (4.12 mg/dL), medium (1.51 mg/dL) and low (50 mg/dL) concentrations of cortisol were 3.8%, 2.8% and 7.2% respectively.
Sex hormone assays
Blood samples were collected into sterile uncoated blood collection tubes by a registered phlebotomist in the UIC Medical Center. Samples were centrifuged and aliquoted for analysis of estradiol, progesterone, and testosterone at Quest Diagnostics (Wood Dale, IL). Serum estradiol was measured using a chemiluminescent immunoassay (Siemens Centaur E2-6 III). Estradiol assay sensitivity was 7 pg/mL. At high, medium, and low concentrations of estradiol, intra-assay coefficient of variation (CV) was determined to be 8%, 8%, and 10% respectively. Serum progesterone was measured with a chemiluminescent immunoassay (Siemens Centaur Progesterone). Progesterone assay sensitivity was determined to be 0.5 ng/mL. At high, medium, and low concentrations of progesterone, intra-assay CV was determined to be 5%, 5%, and 12% respectively. Serum testosterone was measured with a chemiluminescent immunoassay (Siemens Centaur Testosterone). Testosterone assay sensitivity was determined to be 20 ng/mL. At high, medium, and low concentrations of testosterone, intra-assay CV was 8%, 9%, and 15% respectively.
Questionnaires to assess potential confounds
The Center for Epidemiological Studies Depression Scale (CES-D) is a self-administered questionnaire measuring how often (“rarely” to “most of the time”) participants experienced depressive symptoms (e.g., feeling sad, lonely) in the past week. The Beck Anxiety Inventory (BAI) is a 21-item, self-report measure where participants rate on a 4-point scale how much they have been bothered by each symptom (e.g., nervous, shaky) over the prior week. The Perceived Stress Scale (PSS) is a 10-item questionnaire measuring on a 5-point Likert scale the degree to which situations in one's life over the past month are perceived as stressful (e.g., unpredictable, uncontrollable). On the Positive and Negative Affect Schedule (PANAS) participants rate on a 5-point scale the extent to which they have experienced ten pleasant mood states and ten unpleasant mood states during the previous week. The Brief Fear of Negative Evaluation (BFNE) is a 12-item self-report measure where participants rate on a 5-point scale, the degree to which they experience apprehension at the prospect of being evaluated negatively (e.g., “I am afraid that others will not approve of me”). The Menstrual Distress Questionnaire (MDQ) assesses 47 premenstrual symptoms grouped into eight scales including pain, water retention, negative affect, autonomic reaction, impaired concentration, behavioral change, arousal, and control (Moos et al., 1969). The National Adult Reading Test (NART; Revised Version, NART-R) is an untimed test for estimating premorbid levels of intelligence based on the ability to correctly pronounce 61 words with atypical pronunciation (Nelson and Willison, 1991). A score of 100 is average.
Statistical analysis
Differences between groups (follicular, luteal) in demographic characteristics, self-reported mood and anxiety symptoms, and hormone levels were examined using independent t-tests for continuous variables and Chi-square tests for categorical variables.
For the first aim addressing cortisol levels, a mixed factorial analysis of variance (ANOVA) was conducted in which Condition (non-stress control, TSST) and Time (rest, challenge, memory) were the within-subject variables and Group (follicular, luteal) was the between-subject variable. Support for our primary hypothesis would be evident in a significant Condition by Group interaction where the difference in cortisol levels between the TSST and control conditions was greater in the follicular phase than in the luteal phase. To allow for comparisons with the previously published study of women in the luteal phase (Schoofs and Wolf, 2009), a responder analysis was also conducted; participants with an increase in cortisol >2.5 nmol/L from the control challenge to the post-TSST challenge were categorized as “responders” and otherwise were categorized as “non-responders”. Group differences in responder rates were analyzed using Chi-Square. Six women were missing cortisol levels (5 follicular, 1 luteal) due to a malfunctioning freezer. To confirm that women found the TSST to be stressful, the independent and interactive effects of Condition and Menstrual Phase on subjective ratings of stress and anxiety were also examined in a mixed factorial ANOVA.
For the second aim regarding emotional retrieval, a mixed factorial ANOVA was conducted with Condition (non-stress control, TSST) and Valence (Negative, Positive, Neutral words) as the within-subject variables and Group (follicular, luteal) as the between-subject variable. Support for our hypothesis would be evident in a significant Condition × Phase interaction where the difference in retrieval between the TSST and stress control condition would be greater in the follicular phase compared to the luteal phase. All follow-up tests were computed using the appropriate error term from the primary mixed-factorial analysis. Greenhouse-Geisser corrected p-values were used to control for family-wise error.
Lastly, to determine whether the association between cortisol and emotional memory differed by menstrual phase, we conducted a multivariable linear regression analyses. Specifically, we examined whether the negative effect of stress on emotional memory (performance during TSST minus control) was related to increases in cortisol (cortisol during TSST minus control) after controlling for increases in self-reported anxiety (STAI during TSST minus control). To allow for comparisons with previous work (Schoofs and Wolf, 2009), we also examined these correlations within phase. Significance was defined as p < 0.05 (two-sided). Only significant effects are reported, unless otherwise noted.
Results
Forty naturally-cycling women between 18 and 40 years of age participated in the study. Twenty were randomized to complete the task during the luteal phase and 20 during the follicular phase. There were no group differences in any demographic or psychological outcomes (See Table 1). The overall mean age was 27 years, education was 16 years, estimated IQ was 107, and body mass index (BMI) was 25. Mean scores on the psychological outcomes (e.g., depression, anxiety) were within normal limits. Half of the women were white and 30% were African American. Levels of estradiol and progesterone were significantly higher in the luteal group compared to the follicular group, p < 0.001. There was no significant effect of menstrual cycle phase on the rate of learning of the word lists (all ps > .39); on average it took about 4.20 trials to learn List A and about 4.37 trials to learn List B. Similarly, there was no significant Phase by Order interaction, p = 0.75. Therefore, neither learning rate nor order was controlled in subsequent analyses.
Table 1.
Participant characteristics by menstrual cycle phase.
| Variables | Menstrual cycle phase | p-Value | |
|---|---|---|---|
| Follicular (n = 20) M (SD) |
Luteal (n = 20) M (SD) |
||
| Demographics | |||
| Age | 25.60 (5.39) | 28.05 (5.83) | 0.18 |
| Years of education | 15.90 (1.45) | 16.30 (2.15) | 0.49 |
| Full scale IQ | 107.13 (7.66) | 106.82 (7.96) | 0.90 |
| Body mass index | 24.61 (4.46) | 25.14 (5.61) | 0.74 |
| Race (%) | |||
| White (non-Hispanic) | 10 (50) | 10 (50) | 1.00 |
| African-American (non-Hispanic) | 6 (30) | 6 (30) | |
| Other | 4 (20) | 4 (20) | |
| Self-report questionnaires | |||
| CES-D (range: 0–60) | 7.95 (6.27) | 8.70 (7.21) | 0.73 |
| Beck Anxiety Inventory (range: 0–63) | 4.60 (3.73) | 5.22 (6.21) | 0.70 |
| Perceived Stress Scale (range: 0–40) | 11.80 (6.35) | 12.70 (6.23) | 0.65 |
| PANAS (range: 1–5) | |||
| Positive | 3.65 (0.91) | 3.59 (0.70) | 0.82 |
| Negative | 1.65 (0.66) | 1.79 (0.67) | 0.52 |
| BFNE (range: 12–60) | 30.55 (7.13) | 34.20 (9.06) | 0.16 |
| MDQ (range: 0–225) | 19.25 (5.90) | 23.10 (19.23) | 0.40 |
| Hormone levels/factors | |||
| Estradiol (pg/mL) | 37.75 (18.35) | 132.70 (30.94) | <0.001 |
| Progesterone (ng/mL) | 1.02 (0.42) | 14.33 (6.08) | <0.001 |
| Testosterone (ng/dL) | 24.60 (11.25) | 19.40 (6.86) | 0.09 |
Note. Full Scale IQ is an estimate derived from the NART-R.
CESD = Center for Epidemiologic Studies Depression Scale. PANAS = Positive and Negative Affect Schedule. BFNE = Brief Fear of Negative Evaluation Scale. MDQ = Menstrual Distress Questionnaire.
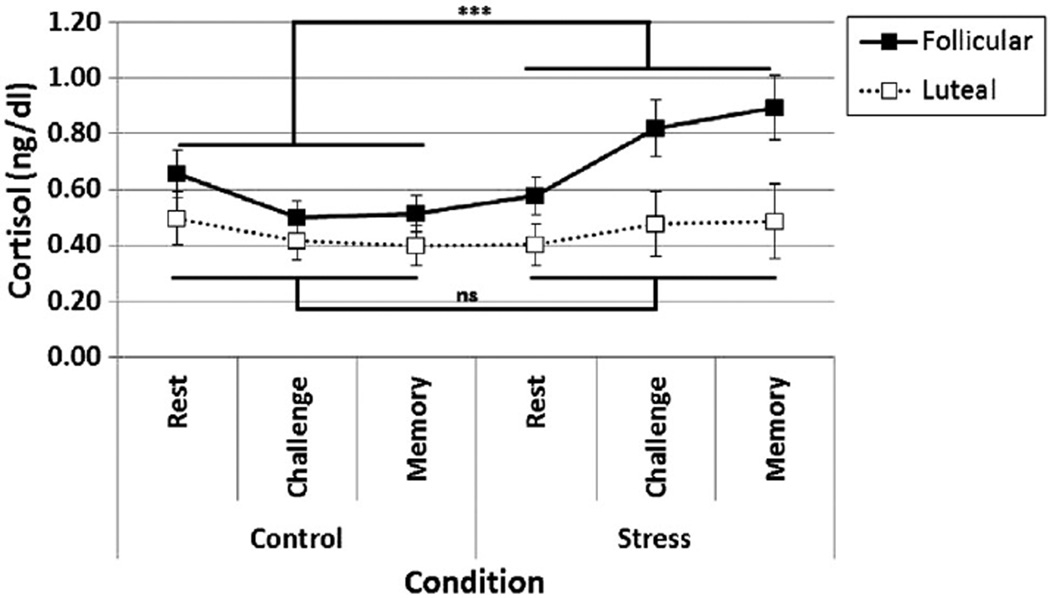
We first examined the independent and interactive effects of stress condition and menstrual phase on cortisol levels (See Fig. 2). As expected, there was a main effect of Condition with cortisol levels being higher in the TSST (M = 0.61, SE = 0.06) versus the non-stress control condition (M = 0.49, SE = 0.05), F(1, 32) = 11.12, p = 0.002. The test of the primary hypothesis—the two-way Condition × Phase interaction was also significant, F(1, 32) = 7.87, p = 0.008. The Follicular group showed a significant increase in cortisol levels from the non-stress control to the TSST condition, F(1, 32) = 5.61, p = 0.02, whereas the Luteal group did not show a significant increase in cortisol levels across conditions, F(1, 32) = 0.05, p = 0.81. Additionally, the Condition × Time interaction was significant indicating that the change in cortisol levels over time (i.e., between the rest, challenge, and memory phases) differed by Condition, F(2, 64) = 14.54, p < 0.001. During the TSST condition, cortisol levels were higher during challenge and memory phase compared to rest (ps < 0.05). Conversely, in the non-stress control condition, cortisol levels were higher during rest compared to challenge and memory (ps < 0.05). Although there were no other significant effects, there was a trend for a three-way Phase × Condition × Time interaction, F(2, 64) = 2.79, p = 0.09. To allow comparisons to previous work, we also conducted a responder analysis where a stress-related increase of 2.5 nmol/L defines a responder (see Statistical analysis section) (Schoofs and Wolf, 2009). A significant Phase effect in cortisol response to the stressor was found, where 73% of women in the follicular group were cortisol responders, compared to only 32% of women in the luteal group, χ2 (df = 1, 34) = 8.59, p = 0.016.
Fig. 2.
Women in the follicular phase, but not luteal phase showed a significant cortisol response to the Trier Social Stress Test. Notes. p < 0.001. There was a significant main effect of stress Condition, F(1, 32) = 11.12, p = 0.002, a significant interaction between Condition and Phase: F(1, 32) = 7.87, p = 0.008, and a significant interaction between Condition and Time: F(2, 64) = 14.54, p < 0.001.
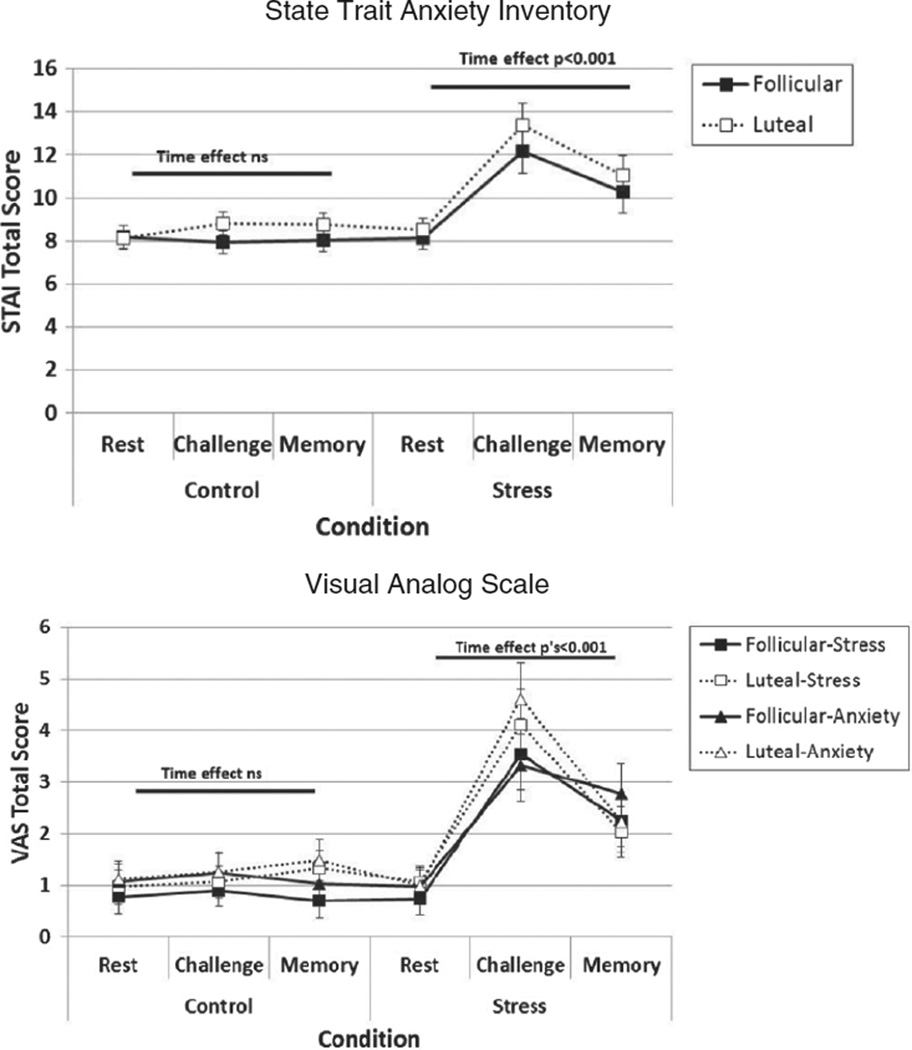
We next examined subjective ratings of stress and anxiety on three outcome measures during the TSST and non-stress control conditions (See Fig. 3). Similar patterns of results were evident on all three outcomes; ratings differed by Condition and Time, and were influenced by the interaction of Condition and Time, but not by Menstrual Phase. On the STAI, subjective ratings of anxiety were higher during the stress (M = 10.58, SE = 0.51) compared to the control condition (M = 8.31, SE = 0.34), F(1, 38) = 41.31, p < 0.001. Ratings also differed over time, F(2, 76) = 25.05, p < 0.001, and the magnitude of change over time differed by condition, Condition × Time: F(2, 76) = 24.89, p < 0.001. Specifically, subjective ratings of anxiety differed significantly over time during the stress condition, F(2, 76) = 84.74, p < 0.001, but not during the non-stress control condition, F(2, 76) = 0.20, ns. Anxiety ratings increased from rest to the challenge time point, F(1, 76) = 110.01, p < 0.001, and decreased from challenge to the memory timepoint, F(1, 76) = 25.09, p < 0.001. There was no effect of menstrual phase on anxiety ratings, nor did phase interact with condition or time on this outcome. Similarly, on the VAS measure of anxiety, there was a significant main effect of Condition, F(1, 38) = 24.20, p < 0.001 and Time, F(2, 76) = 23.00, p < 0.001, and a significant Condition × Time interaction, F(2, 76) = 16.81, p < 0.001, but no effect of menstrual phase. On the VAS measures of stress, there was also a significant main effect of Condition, F(1, 38) = 36.20, p < 0.001, and Time F(2, 76) = 30.25, p < 0.001, and a significant Condition × Time interaction, F(2, 76) = 16.69, p < 0.001, but no effect of menstrual phase.
Fig. 3.
Subjective anxiety and stress measured with the State-Trait Anxiety Inventory and Visual Analog Scales increase during the TSST but are not influenced by menstrual phase. Notes. Significant main effects of Condition and Time (i.e., rest, challenge, memory) and a significant Condition × Time interaction were evident on each measure. See text for details.
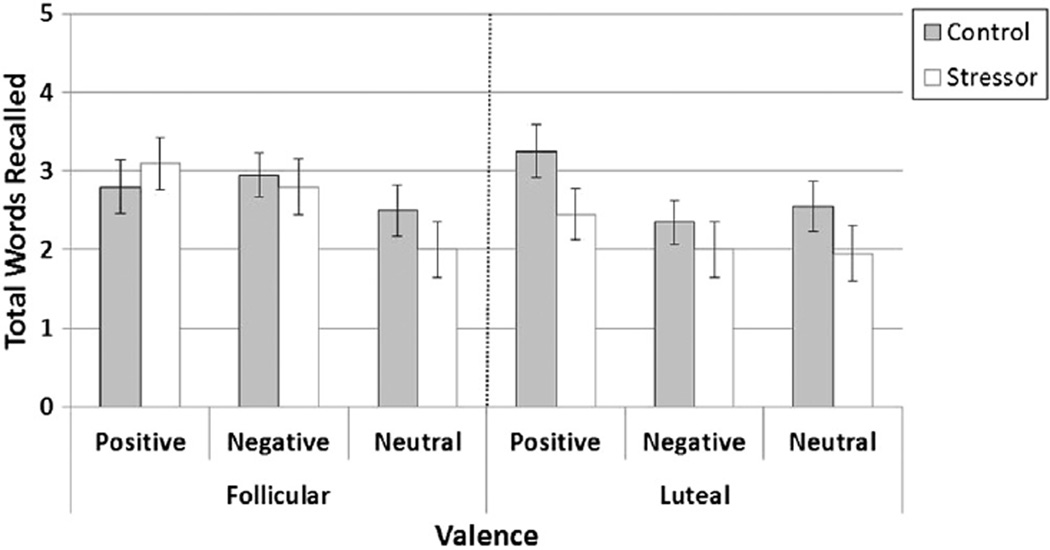
For the second aim, we examined emotional memory during the TSST and non-stress control condition (see Fig. 4). Overall, there was a main effect of Condition with worse memory during the TSST (M = 2.38, SE = 0.21) compared with the non-stress control condition (M = 2.73, SE = 0.17), F(1, 38) = 4.29, p = 0.045. There was also a main effect of Valence, F(2, 76) = 8.24, p = 0.001. This valence effect was driven by better memory for positive words (M = 2.90, SE = 0.20) compared to neutral words (M = 2.25, SE = 0.20), F(1, 76) = 7.82, p < 0.05. Memory for negative words (M = 2.52, SE = 0.19) did not differ from memory for positive or neutral words, F(1, 76) = 2.60, ns and F(1, 76) = 1.40, ns, respectively. Unexpectedly, there was no support for a significant interaction between Condition and Phase, F(1, 38) = 1.91, p = 0.17. However, there was a trend for a Valence × Phase interaction, F(2, 76) = 2.77, p = 0.07. That interaction was driven by significantly better memory for negative words in the follicular group (M = 2.87, SE = 0.27) compared to the luteal group (M = 2.17, SE = 0.27), F(1, 76) = 4.54, p < 0.01. Luteal and follicular groups did not differ in memory for positive or neutral words, F(1, 76) = 0.09 and F(1, 76) = 0, respectively.
Fig. 4.
Performance on the emotional retrieval task during stress and non-stress control conditions for women in the follicular phase versus luteal phase of the menstrual cycle. Notes. Significant overall Valence effect: F(2, 76) = 8.24, p = 0.001, due to better recall for positive words compared to neutral words. Significant overall Condition effect: F(1, 38) = 4.29, p = 0.045, due to lower retrieval following TSST compared to non-stress control condition. Trend for a Valence × Phase interaction: F(2, 76) = 2.77, p = 0.07. Follicular women recalled more negative words than did luteal women, F(1, 76) = 4.54, p < 0.01, but did not differ in recall of positive, F(1, 76) = 0.09, ns or neutral words, F(1, 76) = 0, ns.
We next examined whether the association between stress-induced increases in cortisol levels and stress-induced decrements in emotional retrieval differed by menstrual phase. Regression analyses of outcomes in the follicular group showed that the negative effect of stress on overall memory performance was associated with increases in cortisol (β = −0.37, p = 0.04) but not increases in self-reported anxiety on the STAI (β = −0.09, p = 0.59). As shown in Table 2, performance on the emotion memory task was significantly associated with cortisol levels in the follicular but not the luteal phase, and these correlations were significant for neutral, positive and negative words. Individual differences in estradiol and progesterone levels within each menstrual phase were unrelated to memory performance.
Table 2.
Bivariate associations between emotional memory and cortisol levels during the stress condition.
| Recalled words post stressor |
Follicular | Luteal | ||||
|---|---|---|---|---|---|---|
| Rest | Challenge | Memory | Rest | Challenge | Memory | |
| Total words | −0.57* | −0.67** | −0.50T | −0.15 | −0.26 | −0.22 |
| Negative words | −0.52* | −0.55* | −0.37 | −0.17 | −0.09 | −0.04 |
| Positive words | −0.51T | −0.72** | −0.66** | 0.01 | −0.21 | −0.20 |
| Neutral words | −0.53* | −0.58* | −0.36 | −0.18 | −0.23 | −0.28 |
Data are not adjusted for any other factor.
p < 0.01.
p < 0.05.
p > 0.05 and p < 0.10.
Discussion
The present findings indicate that menstrual phase influences cortisol levels following a laboratory stressor, but does not influence subjective ratings of anxiety or stress. Specifically, cortisol responses to the TSST were lower in women tested during the luteal phase when levels of the ovarian hormones estradiol and progesterone are high compared to a group of women tested in the follicular phase when hormone levels are low. This conclusion was supported by an analysis of cortisol levels measured continuously, where there was a significant Condition by Phase interaction, and by a categorical analysis of cortisol responders, where 73% of women in the follicular group were responders compared to 32% of women in the luteal group. Retrieval performance in the emotional memory test was significantly worse following the TSST compared to the non-stress control, confirming that stress impairs emotional retrieval. However, this effect did not differ by menstrual phase. While menstrual cycle did not generally influence emotional retrieval following the TSST, individual differences in cortisol responses to the TSST were associated with impaired retrieval only during the follicular phase. Specifically, in a regression analysis, increases in cortisol levels (but not anxiety levels) were associated with stress-induced decreases in emotional recall during the follicular phase only. Similarly, absolute levels of cortisol correlated with memory performance only during the follicular phase.
In addressing our first aim, we found that cortisol responses to a social stressor were lower in the luteal phase compared to the follicular phase. An early study by Kirschbaum and colleagues using similar definitions for menstrual phase found the opposite pattern of findings with the TSST; cortisol levels, also measured with salivary free cortisol, were lower in the follicular phase (n = 19) compared to luteal phase (n = 21), and levels during the luteal phase were similar to those of men (n = 20) (Kirschbaum et al., 1999). Cold pressor-induced stress also led to greater increases in cortisol during the luteal phase (i.e., Days 18–24) compared to the follicular phase (Andreano et al., 2008). In contrast, a more recent study found that women tested in the luteal phase (n = 36) showed a smaller increase in cortisol to the TSST compared to men (n = 19) (Kuhlmann et al., 2005b; Schoofs and Wolf, 2009). We did not find a significant increase in cortisol between non-stress and stress conditions during the luteal phase, only in the follicular phase. There are some notable differences between that recent study and the present one. We tested women in the afternoon whereas they tested women in late morning. In their study the control and stress conditions were conducted on different days and counterbalanced, while in the present study the two conditions were on the same day and non-stress always preceded stress. In their study, a list of words was learned 24 h prior to retrieval testing, but was not learned to criterion. In the present study, women learned a list of word pairs to 100% criterion and associative memory was tested 24 h later.
In addressing our second aim, we found that overall menstrual cycle did not significantly affect emotional retrieval following a stressor. However, the TSST did not produce a cortisol response in all women; 73% of women showed a cortisol response during the follicular phase compared with 32% during the luteal phase. Thus in addressing our third aim – examination of individual differences in cortisol response with regression and correlational analyses – we found that increases in cortisol were significantly associated with decreases in emotional retrieval during the follicular phase only. The finding that there were no significant correlations during the luteal phase is not surprising given that the cortisol response during that phase was considerably attenuated. Generally then, these findings indicate that cortisol responsivity is heightened in the follicular phase and that heightened cortisol levels during that phase negatively relate to memory performance. Conversely, cortisol responsivity is dampened during the luteal phase to a level at which small individual differences in cortisol levels are not related to emotional retrieval. Although ours is the first study to directly compare the effects of the TSST on emotional retrieval in women during the follicular versus luteal phase, a previous investigation examined the effects of hydrocortisone administration on emotional retrieval during the same two menstrual phases (Kuhlmann and Wolf, 2005). In that study, women in the follicular (n = 13) and women in the luteal phase (n = 14) showed decreased retrieval following cortisone administration. Unlike the present study, however, cortisone administration led to similar increases (15-fold) in cortisol in during both menstrual phases.
Some studies have examined whether cortisol responder status (responder versus non-responder) moderates the effects of the TSST on emotional retrieval. For example, Buchanan found that men and women showing a cortisol response experienced decreased retrieval whereas those not showing a cortisol response showed enhanced retrieval (Buchanan and Tranel, 2008). In contrast, in their study of women tested in the luteal phase, Schoofs and Wolf found that memory retrieval was preserved even in women who were categorized as responders (Schoofs and Wolf, 2009). Interestingly, 50% of the women tested in the luteal phase were cortisol responders in his study compared to only 32% of women in the luteal phase in the present study. We had insufficient power to formally test whether those 32% of women showed preserved retrieval or not. However, like Schoofs and Wolf, our correlational analyses showed no relationship between stress-induced increases in cortisol and stress-induced decreases in memory in the luteal phase (Schoofs and Wolf, 2009) — we only found such relationships during the follicular phase. Their interpretation was that glucocorticoid sensitivity is reduced during the luteal phase, a finding that is consistent with the observation that dexamethasone-induced suppression of plasma cortisol was lower in the luteal phase of the menstrual cycle compared to the follicular phase (Altemus et al., 1997). Our correlation findings would support this interpretation since there was a significant stress-induced increase in cortisol in the luteal phase (albeit significantly lower than in the follicular phase), but no correlation was observed between cortisol levels and retrieval impairment. On the other hand, our findings are broadly in alignment with Buchanan (Buchanan and Tranel, 2008) as we found more responders in the follicular phase than during the luteal phase, and only during the follicular phase did cortisol levels correlate with retrieval impairment.
The ethnic composition of the Kirschbaum study (Kirschbaum et al., 1999), the Schoofs and Wolf study (Schoofs and Wolf, 2009) and the Buchanan study (Buchanan and Tranel, 2008) was not specified, but our sample was 50% white and 50% minority. In the present study, we screened out women with a reported history of mood and anxiety disorders, and questionnaires indicated that mood and anxiety levels were within normal limits. However, in other studies of healthy women from our lab, we have found a very high prevalence of emotional neglect and abuse, despite no self-reported history of depressive or anxiety disorders. Therefore, we cannot rule out the possible influence of an undocumented history of trauma on the present findings. Individuals with a history of emotional trauma show blunted cortisol responses to the TSST (Carpenter et al., 2007). Ongoing studies in our laboratory are examining whether the effects of early life trauma on cortisol responsivity vary by hormonal status.
The mechanisms underlying stress-induced impairments in memory retrieval are beginning to be elucidated. In humans, administration of propranolol, a centrally acting β-adrenoceptor antagonist, prevents cortisone-induced impairments in retrieval of emotional words (de Quervain et al., 2007). Similarly, propranolol blocks the amnestic effects of the TSST on retrieval of emotional words in men (Schwabe et al., 2009). Studies in male rats indicate that cortisone-induced impairments in retrieval are mediated, at least in part, by noradrenergic activity in the hippocampus and basolateral amygdala (Roozendaal et al., 2004a, 2004b). Neuroimaging studies in humans demonstrate that during emotional retrieval, the hippocampus and amygdala are active, functionally connected, and regulated by the orbitofrontal cortex (Smith et al., 2006). Cortisone administration before retrieval decreased regional cerebral blood flow in the right posterior medial temporal lobe in men, particularly in the parahippocampal gyrus (de Quervain et al., 2003). Neuroimaging studies in women are needed to elucidate how ovarian steroid hormones influence the brain circuitry underlying the impairing effects of stress on memory retrieval. Studies in premenopausal women suggest that estrogen may modulate changes in brain circuitry underlying processing of emotionally valent items (Amin et al., 2006; Goldstein et al., 2005; Protopopescu et al., 2005). Goldstein and colleagues studied responses to negative valence/high arousal versus neutral valence/low arousal images in premenopausal women at two phases of the menstrual cycle — early follicular (onset of the menstrual cycle: low estrogen, low progesterone) and late follicular (mid-cycle: high estrogen, low progesterone). Greater activation in the early follicular phase was evident in the amygdala, paraventricular and ventromedial hypothalamus, hippocampus, orbitofrontal cortex, anterior cingulate, and peripenduncular nucleus. The authors attributed this pattern of effects to estradiol and indicated that estradiol assays were needed to definitively demonstrate this relationship (Goldstein et al., 2005). This pattern of results raises the possibility that high levels of estradiol during the luteal phase might alter amygdala and hippocampal function, at least in part through noradrenergic-dependent mechanisms, and thereby mitigate against deleterious effects of stress on emotional retrieval.
The study has strengths and limitations. Due to a malfunctioning freezer, cortisol levels were missing on six women, including one woman in the luteal group and five women in the follicular group. Despite this loss, we were able to detect significant menstrual cycle effects both in our primary analysis of continuous cortisol outcomes and in our secondary analysis of categorical outcomes (i.e., responders versus non-responders). Like other studies that have examined cortisol effects in the follicular versus luteal phase (Andreano et al., 2008; Kirschbaum et al., 1999; Kuhlmann and Wolf, 2005), we used a between-subjects design. Although a within-subject design allows for greater control over individual differences in cortisol responsivity, a notable advantage of the between-subjects design is that cortisol responsivity to the TSST decreases significantly on the second administration (Kirschbaum et al., 1995). We consistently administered the non-stress control condition before the TSST in the same day rather than on separate days to control for the main factor of interest, ovarian hormone status during tightly controlled menstrual cycle phases. It is possible that poorer emotional retrieval after stress compared to after the non-stress condition is due not to the stress but rather to fatigue, lack of motivation, tiredness or other factors. However, the fact that there was a significant negative correlation between cortisol and emotional memory performance during the follicular phase, when most women showed a cortisol response, suggests that it was the stress and not those other factors that accounted for the impaired emotional retrieval after stress. Future studies could include a group that received two non-stress conditions only to more definitely rule out this alternative hypothesis.
Conclusions
The present findings indicate that menstrual phase influences cortisol responsivity to a laboratory stressor, but does not influence subjective ratings of anxiety or stress. Specifically, cortisol responsivity is lower in the luteal phase when levels of estradiol and progesterone are high compared to the follicular phase when levels are low. Furthermore, it is only when ovarian hormone levels are low that cortisol levels are associated with impaired retrieval. These findings suggest a possible protective effect of ovarian hormones against the impairing effects of cortisol on memory, or given previous findings (Schoofs and Wolf, 2009), they may reflect reduced glucocorticoid sensitivity during the luteal phase.
Acknowledgments
Dr. Rubin's effort was supported by grant number 1K01MH098798-01 from the National Institute of Mental Health (NIMH) and by grant number K12HD055892 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institutes of Health Office of Research on Women's Health (ORWH).
Contributor Information
Pauline M. Maki, Email: pmaki@psych.uic.edu.
Kristen L. Mordecai, Email: k_mordecai@hotmail.com.
Leah H. Rubin, Email: lrubin@psych.uic.edu.
Erin Sundermann, Email: erin.sundermann@einstein.yu.edu.
Antonia Savarese, Email: asavarese@psych.uic.edu.
Erin Eatough, Email: erin.m.eatough@gmail.com.
Lauren Drogos, Email: ldrogos@gmail.com.
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J. Cogn. Neurosci. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Altemus M, Redwine L, Leong YM, Yoshikawa T, Yehuda R, Detera-Wadleigh S, Murphy DL. Reduced sensitivity to glucocorticoid feedback and reduced glucocorticoid receptor mRNA expression in the luteal phase of the menstrual cycle. Neuropsychopharmacology. 1997;17:100–109. doi: 10.1016/S0893-133X(97)00039-0. [DOI] [PubMed] [Google Scholar]
- Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage. 2006;32:457–464. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33:874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): instruction manual and affective ratings. Tech. Rep. C-1. 1999
- Buchanan TW, Tranel D. Stress and emotional memory retrieval: effects of sex and cortisol response. Neurobiol. Learn. Mem. 2008;89:134–141. doi: 10.1016/j.nlm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat. Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C. Glucocorticoid-induced impairment of declarative-memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur. J. Neurosci. 2003;17:1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Roozendaal B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am. J. Psychiatry. 2007;164:967–969. doi: 10.1176/ajp.2007.164.6.967. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J. Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom. Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Wolf OT. Cortisol and memory retrieval in women: influence of menstrual cycle and oral contraceptives. Psychopharmacology (Berl) 2005;183:65–71. doi: 10.1007/s00213-005-0143-z. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Kirschbaum C, Wolf OT. Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiol. Learn. Mem. 2005a;83:158–162. doi: 10.1016/j.nlm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. J. Neurosci. 2005b;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br. J. Clin. Psychol. 1992;31(Pt 3):301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- Moos RH, Kopell BS, Melges FT, Yalom ID, Lunde DT, Clayton RB, Hamburg DA. Fluctuations in symptoms and moods during the menstrual cycle. J. Psychosom. Res. 1969;13:37–44. doi: 10.1016/0022-3999(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Nelson H, Willison J. The Revised National Adult Reading Test — Test Manual. Windsor, UK: NFER-Nelson; 1991. [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, Silbersweig D, Stern E. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, de Quervain DJ, Schelling G, McGaugh JL. A systemically administered beta-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol. Learn. Mem. 2004a;81:150–154. doi: 10.1016/j.nlm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J. Neurosci. 2004b;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs D, Wolf OT. Stress and memory retrieval in women: no strong impairing effect during the luteal phase. Behav. Neurosci. 2009;123:547–554. doi: 10.1037/a0015625. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Romer S, Richter S, Dockendorf S, Bilak B, Schachinger H. Stress effects on declarative memory retrieval are blocked by a beta-adrenoceptor antagonist in humans. Psychoneuroendocrinology. 2009;34:446–454. doi: 10.1016/j.psyneuen.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. (quiz 34–57) [PubMed] [Google Scholar]
- Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala– hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Stress and memory in humans: twelve years of progress? Brain Res. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]