Abstract
Aims
To determine whether self-regulation can be studied successfully in a rodent model and whether persistent facial pain influences self-regulatory behavior.
Methods
Thirty male Sprague-Dawley rats, divided into two groups, (1) chronic constriction injury of the infraorbital nerve (CCI-ION) and (2) naïve, were used in a two-part behavioral paradigm of self-regulation. This paradigm consisted of both a cued go/no-go task (part one) and a persistence trial (part two). All animals were acclimated and trained for a period of 4 weeks prior to the experimental manipulation and then tested for a total of 5 weeks following experimental manipulation. Results were analyzed with t tests, one-way analysis of variance, and two-way, repeated measures analysis of variance.
Results
CCI-ION surgery induced significant mechanical hypersensitivity of the ipsilateral whisker pad that began 3 weeks postsurgery and persisted through the duration of the experiment (P < .001). At weeks 4 and 5 post–experimental manipulation, naïve animals demonstrated a significant decrease in lever presses during the persistence task (P < .05) compared to baseline, whereas CCI-ION animals did not (P = .55).
Conclusion
These results suggest that persistent pain influences behavioral regulation and that animals experiencing persistent pain may have difficulty adapting to environmental demands.
Keywords: chronic constrictive injury, infraorbital nerve, learning, orofacial pain, self-regulation
Persistent pain is a major health problem and is a primary reason that many people seek health care services.1,2 Persistent pain conditions are challenging to live with and are often associated with high levels of stress for the individual.3,4 These conditions are characterized by complex interactions between cognitive, emotional, and physiologic disturbances that bring about significant coping challenges for patients. Each of these disturbances requires that individuals exert effort toward regulating their behavior in multiple domains while enduring and subsequently managing the persistent pain condition.4 The successful management of persistent pain mediated by the trigeminal nerve is the goal of orofacial pain patients and clinicians alike.
It is not surprising that persistent orofacial pain taxes personal resources and has been associated with self-regulatory deficits in patients. Self-regulation involves the capacity to exert control over cognition, emotions, and behaviors.5-7 It is defined as one's ability to alter his/her own responses by overriding one response in favor of a less common but more desired response.4,8 Self-regulation is also related to executive functioning, including the ability to make choices. Research in human populations indicates that performing an initial self-regulatory task may cause fatigue that results in poorer subsequent performance on executive tasks.9,10 Thus, self-regulatory fatigue and executive capacity covary inversely in a way that can lead to a potential downward spiral where repeated responses to demands leads to self-regulatory fatigue, which in turn reduces executive cognitive resources.4 This pattern increases the difficulty for an individual to meet further demands such as those associated with persistent intractable pain in structures mediated by the trigeminal nerve.
Although self-regulation has been implicated as important in the management of persistent pain conditions, research outcomes show that persistent pain itself can interfere with the ability to self-regulate.11 In the one human study on pain and self-regulation found in the literature, patients with persistent pain conditions, ie, fibromyalgia and temporomandibular disorders, had less capacity to persist on a persistence task following an initial self-regulation task than persons without persistent pain.11 These findings suggest that the presence of persistent pain leaves one vulnerable to self-regulatory fatigue because of the significant demands required for coping with persistent pain.
In the past, many researchers have argued that self-regulation occurs only in humans; however, a recent animal study has shown that it is possible to examine these effects in other species.12 In that study, dogs in a self-regulatory condition (ie, dogs commanded to sit and stay for a short period of time) performed worse on a persistence task (ie, attempting to retrieve food from a toy) than dogs that had not been required to self-regulate prior to exposure to the persistence task. These findings suggest that self-regulatory capacity may be a finite resource across species and is involved in guiding and directing a broad spectrum of behaviors.
Although past research on self-regulation has been primarily conducted with human participants,13-15 it would be beneficial to study the effects of persistent pain on self-regulation in a rodent model. A rodent model of persistent pain allows for greater experimental control and fewer threats to internal validity. For example, in human populations with persistent pain, both physical and psychological comorbid conditions are common.16,17 Studying persistent pain conditions with animals in a controlled setting allows investigators to limit the variables affecting the relationship between persistent pain and self-regulation. Additionally, animal models allow for investigation of physiological pathways responsible for self-regulatory deficits as well as possible pharmacological treatments.
The aims of the current study were to determine (1) whether self-regulation can be studied successfully in a rodent model and (2) whether persistent facial pain influences self-regulatory behavior. It was hypothesized that animals experiencing persistent neuropathic pain would perform more poorly on a persistence task that immediately followed an initial self-regulatory depletion task than naïve animals that were not experiencing pain. Specifically, it was expected that as a result of increased fatigue following the self-regulation task, animals that underwent surgery to induce a chronic constriction injury of the infraorbital nerve (CCI-ION) would press a lever during the persistence task for a shorter period of time as well as a fewer number of times than naïve animals.
Materials and Methods
Animals
Thirty male Sprague-Dawley rats were used in the study (200–300 g; Harlan, Indianapolis, IN, USA) and were randomly divided between the experimental (ie, CCI-ION) and naïve groups. Animals were singly housed and maintained under a reverse 12:12 light:dark cycle (lights on at 7:00 pm). All training and experimental procedures took place during the middle of the dark phase of the light:dark cycle. A low-soy-content diet (Harlan Teklab 8626) was provided. Adequate measures were taken to minimize pain or discomfort in this study. Experiments were carried out in accordance with the Guidelines of the US National Institutes of Health regarding the care and use of animals for experimental procedures. Experiments were approved by the Institutional Animal Care and Use Committee at the University of Kentucky. All animals were housed in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International and US Department of Agriculture.
Surgical Model
Rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) and the head of the rat was fixed in a stereotaxic frame. Surgery was performed under direct visual control with the use of a Zeiss operating microscope (6 to 40×). Surgical ligation of the ION was completed using previously established procedures.18-20 First, lidocaine (2%. 0.1 mL) was injected subcutaneously at the site of surgical incision. A midline scalp incision was then made, exposing the skull and nasal bone. The edge of the orbit, formed by the maxillary, frontal, lacrimal, and zygomatic bones, was dissected free. The ION was separated from other structures at its most rostral end of the orbital cavity, just outside the infraorbital foramen.
In order to ligate the ION, a suture was looped over a small neural hook (2 mm) instrument with a blunt tip inserted and gently pulled under the nerve. Two chromic gut (5-0) ligatures were loosely tied (with about 2 mm spacing) around the nerve. To obtain the desired degree of constriction, the ligations reduced the diameter of the nerve by a just noticeable amount but did not interrupt the epineural circulation.21 Blood circulation through epineural vessels was visually observed in each animal with a ligature. The scalp incision was closed using polydioxanone absorbable suture (PDS II) and the wound treated with triple antibiotic ointment (polymycin B sulfate, bacitracin zinc, and neomycinpramoxine HCI) and 2% lidocaine.
All animals in the CCI-ION group underwent ligation of their left-side ION; the right-side ION remained untouched. Naïve animals (ie, animals that did not undergo any type of anesthesia or surgery) were used as the control group rather than sham animals, due to the possibility of damaging the nerve and surrounding tissue during sham surgery, and thus producing some degree of pain. Indeed, a pilot study found mild mechanical hypersensitivity following sham surgical procedures. This extra precaution was taken due to the experimental goal of studying animals displaying pain-related behaviors versus naïve animals. Animals were allowed 7 days to recover from surgery with food and water available ad libitum.
Behavioral Measure
To ensure the effectiveness of the CCI-ION model of persistent pain, von Frey filaments were used to assess mechanical hypersensitivity on the whisker pad under non- or minimal-restraint conditions.22 Animals were habituated to stand against the experimenter's hand wearing a regular leather work glove. Each animal was handled and habituated to the experimental procedure for 30 minutes on two occasions during the week prior to the first baseline trial (ie, week −3, Fig 1). Additionally, animals were habituated on each trial day for a period of 15 minutes prior to testing.
Fig 1.
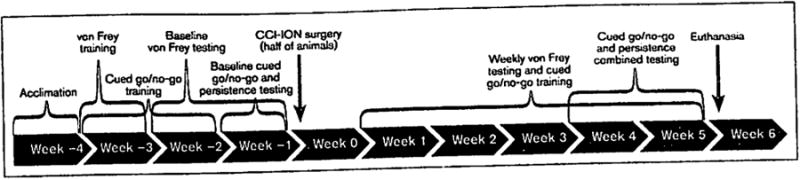
Time course of experimental data collection. CCI-ION = chronic constriction injury of the infraorbital nerve.
Mechanical hypersensitivity was measured with eight von Frey fibers (0.4, 0.6, 1, 2, 4, 6, 8, 15 g; Stoelting) by the modified up-and-down method with a default maximal 50% withdrawal threshold at a gram force of 18.72 g.23-25 Mechanical stimuli were applied within the ION innervation region, near the whisker pad centers but avoiding contact with the animals' whiskers, and both ipsilateral and contralateral to the surgery site. Responses to von Frey filaments applied to the rat whisker pad were analyzed with a statistical equation to determine the threshold required for 50% head withdrawals. Each filament was applied five times at intervals of a few seconds. If head withdrawal was observed at least three times after probing with a filament, the rat was considered responsive to that filament. Whenever a positive response to a stimulus occurred, the next smaller von Frey filament was applied. Otherwise, the next higher filament was applied. Behavioral changes to mechanical stimuli were tested once a week for 2 weeks (weeks −2 and −1) to obtain baseline measures and then once a week during the 5 weeks after animals in the CCI-ION condition underwent surgery (ie, weeks 1 through 5) (see the full timeline of study procedures provided in Fig 1).
Self-Regulation Model
The self-regulation model was based on models used in previous human studies. In the majority of human experiments, self-regulatory fatigue is studied by exposing participants to two tasks. The first task requires participants to complete an activity that involves self-regulation. For example, participants are asked to watch a video recording while ignoring words that are flashing on the bottom of the screen. The second task exposes participants to an unsolvable anagram or other impossible activity and measures the amount of time they are willing to persist in the activity.
The current study modified this basic design to examine self-regulation in rats. The tests were performed in standard operant chambers with fixed levers (model ENV-022, Med Associates) and used the automated MedPC IV Software System (Med Associates). Animals were exposed to two tasks, an initial activity requiring self-regulation and a subsequent impossible task that was used to measure persistence. The self-regulation portion of the experiment consisted of a cued go/no-go task. During this task, animals were placed into a test chamber containing a fixed lever for a period of 24 minutes and received a food reward for every four lever presses they made (ie, animals were rewarded on 4:1 fixed ratio schedule). However, animals were only rewarded for pressing the lever when a cue light was illuminated (the cue light cycled on and off every 3 minutes, beginning with a light “on” cycle). The second portion of the experiment began immediately following the initial self-regulation task (immediately following a light “off” cycle). During this persistence task, the cue light remained illuminated for a period of 10 minutes, but animals were not rewarded for any lever presses. Thus, the total time animals remained in the chamber to complete both the self-regulation and the persistence tasks was 34 minutes. The time duration of these tasks was selected based on pilot data that determined animals would not become satiated during the total 34-minute task and would continue pressing the lever at a consistent pace to receive food rewards.
Data automatically recorded by MedPC software included number of lever presses during the initial 24-minute task (differentiating between lever presses when the cue light was on and off, or rewarded and non-rewarded lever presses, respectively), number of lever presses during the subsequent 10-minute persistence task, and the time of each animal's last lever press during the 10-minute persistence task. The time of each animal's last lever press during the 10-minute persistence task was used to calculate the total time each animal spent trying to obtain a food reward. For example, an animal that last pressed the lever 8 minutes into the persistence task was said to have spent 8 minutes persisting in trying to obtain a food reward.
Training and Experimental Procedure
To train the rats to receive a food reward by pressing a lever on a 4:1 fixed ratio schedule only when a cue light was illuminated, training and shaping techniques adapted from a previous study were used.26 Animals were restricted to 10 g of food on days immediately preceding training or trial tasks. Animals were always allowed access to water ad libitum, and were allowed access to food ad libitum on all days not preceding trials or training tasks. Animal body weights were recorded daily to ensure proper health during food restriction. None of the animals experienced a 10% or greater decrease in body weight within any 7-day period or throughout the study period.
All animals underwent 2 weeks of training (weeks −3 and −2) and 1 week of baseline testing (week −1) at the onset of the study. During the 2 weeks of training, animals were trained first to press a lever to receive a food pellet reward on a 4:1 fixed ratio schedule (meaning animals were rewarded for every four lever presses made) and were then taught to press for a reward only when a cue light was illuminated. During the week of baseline testing (ie, week −1), animals underwent 2 training days and 2 baseline trial days (eg, alternating as follows: training, baseline trial, training, baseline trial). On trial days during the baseline week, animals were placed into the test chamber for the 24-minute cued go/no-go task followed immediately by the 10-minute persistence task (during which the cue light remained illuminated but animals did not receive food rewards regardless of lever presses). On training days, animals were placed into the test chamber for only the 24-minute cued go/no-go task.
Animals in the CCI-ION group were allowed to recover for 7 days following surgery and neither group of animals was tested during this time period. Following this week during which animals were not tested (week 0), all animals underwent one training task as described above weekly for 5 weeks to maintain performance in the cued go/no-go task (weeks 1 through 5). Animals were not tested with the combination cued go/no-go and persistence tasks until animals that underwent surgery (ie, animals in the CCI-ION group) developed stable mechanical hypersensitivity. Thus, all animals were again tested in the combined tasks as previously described (undergoing one training and one trial task per week) during weeks 4 and 5. Upon completion of testing, rats were anesthetized by intraperitoneal injection of sodium pentobarbital (150 mg/kg; euthanasia occurred during week 6).
Statistical Analyses
All statistical analyses were completed with IBM SPSS Statistics (Version 20 for Windows). Outliers, defined as animals having a score greater than two standard deviations away from the overall mean on any analysis (ie, lever presses in the cued go/no-go task when the light was on or off, lever presses in the persistence task, or time spent in the persistence task), were identified and excluded. Statistical analyses were conducted on mechanical hypersensitivity as measured by von Frey fibers and performance in the initial self-regulation and subsequent persistence tasks. Analyses of performance in the self-regulation task and performance in the persistence task were completed using focused contrasts and two-way, repeated measures analysis of variance (ANOVA), measuring both within and between subject variables, which were used to measure the main effects of group (ie, CCI-ION versus naïve animals) and trial (ie, time; baseline measurements taken with 2 weeks of training versus trial measurements taken with 7 to 8 weeks of training) as well as the interaction between group and time. Where appropriate, initial analyses were followed up with post-hoc contrasts. One-way ANOVA was used to evaluate groups for baseline differences and to evaluate von Frey test results (ie, mechanical hypersensitivity). Analyses of percent change across time points were completed for each individual animal but were not significant and are therefore not discussed. For all analyses, P < .05 was considered significant.
Results
Identification of Outliers
The experiment was initiated with 30 male Sprague-Dawley rats, 13 in the naïve group and 17 in the CCI-ION surgery group. All animals were tested on the whisker pad with von Frey fibers for evidence of mechanical hypersensitivity. The highest obtainable von Frey value was a force of 18.72 g (ie, the maximum 50% withdrawal threshold as determined by statistical analyses); and all animals during baseline trials, as well as animals in the naïve group for the duration of the study, remained constant at this value. Four animals in the CCI-ION group were excluded from all behavioral analyses because their ipsilateral von Frey values remained equal to a force of 18.72 g postsurgery, indicating that these animals did not experience mechanical hypersensitivity. Thus, in the present study, CCI-ION surgery produced mechanical hypersensitivity of the ipsilateral whisker pad in 76% of rats (eg, 13 of the 17 animals that underwent surgery developed mechanical hypersensitivity) by week 3 postsurgery. Statistical analyses were completed comparing naïve animals, CCI-ION animals that developed hypersensitivity, and CCI-ION animals that did not develop hypersensitivity. These analyses were consistent with the results obtained when comparing naïve and CCI-ION animals that developed hypersensitivity, in that animals that underwent CCI-ION surgery and did not develop hypersensitivity behaved in a manner similar to naïve animals; these results are therefore not discussed below. Five animals (2 naïve animals and 3 CCI-ION animals) were identified as outliers (ie, they had a score that was greater than two standard deviations away from the mean on at least one behavioral measure) and were excluded from all analyses. Thus, a total of 21 animals (11 naïve, 10 CCI-ION) were available for analysis (Fig 2).
Fig 2.
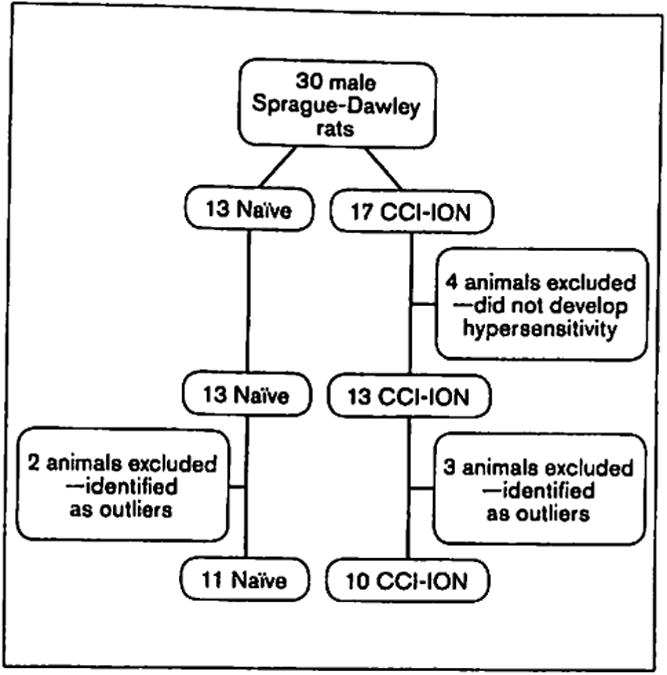
Experimental design detailing each group of animals, including animals that did not develop hypersensitivity and outliers. CCI-ION = chronic constriction injury of the infraorbital nerve.
Mechanical Hypersensitivity
Animals in the CCI-ION group experienced a significant decrease on the ipsilateral side in 50% withdrawal threshold in weeks 3, 4, and 5 postsurgery compared with baseline measurements, indicating mechanical hypersensitivity, whereas naïve animals remained at baseline values throughout the duration of the experiment. Mechanical hypersensitivity of the ipsilateral side was evident for animals in the CCI-ION condition on days 21, 28, and 35 postsurgery (day 21, F[1,19] = 24.60, P < .001; day 28, F[1,19] = 3777.19, P < .001; day 35, F[1,19] = 1365.54, P < .001; Fig 3), whereas before day 14 the effect was not observed (day 7, F[1,19] = 2.15, P = .16; day 14, F[1,19] = 1.91, P = .18). All contralateral von Frey values for CCI-ION animals remained constant at the maximum 50% withdrawal threshold of 18.72 g. For full statistical results see Table 1.
Fig 3.
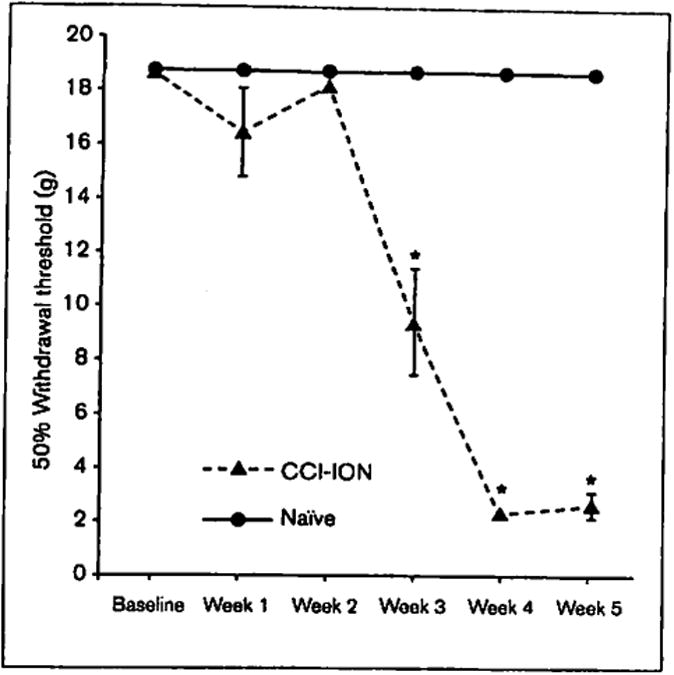
Ipsilateral mechanical hypersensitivity induced by chronic constriction injury of the infraorbital nerve (CCI-ION) on days 21, 28, and 35 postsurgery (P < .001). n = 21 (11 naïve, 10 CCI-ION). *P < .001.
Table 1. von Frey Testing Analyses and Descriptive Statistics.
| Von Frcy value, g (mean ± SD) | CCI-ION | Naïve | |
|---|---|---|---|
| Baseline† | 18.72 ± 0 | 18.72 ± 0 | |
| Week 1 | 16.50 ± 5.04 | 18.72 ± 0 | |
| Week 2 | 18.26 ± 1.10 | 18.72 ± 0 | |
| Week 3 | 9.48 ± 6.19 | 18.72 ± 0 | |
| Week 4 | 2.51 ± 0.88 | 18.72 ± 0 | |
| Week 5 | 2.67 ± 1.44 | 18.72 ± 0 | |
| One-way ANOVA | df | F value | P value |
|
| |||
| Week 1 | 1,19 | 2.15 | .16 (ns) |
| Week 2 | 1,19 | 1.91 | .18 (ns) |
| Week 3 | 1,19 | 24.60 | < .001 *** |
| Week 4 | 1,19 | 3777.19 | < .001 *** |
| Week 5 | 1,19 | 1365.54 | < .001 *** |
Significant at P < .05.
Significant at P < .0.
Significant at P < .001.
Average of weeks −2 and −1.
CCI-ION = chronic constriction injury of the infraorbital nerve; n = 21 (11 naïve, 10 CCI-ION); ipsilateral values; ANOVA = analysis of variance; ns = not significant.
Self-Regulation Task
Performance in the self-regulation task was analyzed by comparing the number of rewarded (ie, cue light on) and non-rewarded (ie, cue light off) lever presses and the ratio of rewarded to total lever presses made during the initial self-regulation task. Results for the self-regulation task are shown in Table 2. For non-rewarded lever presses, a significant effect of group (F[1,19] = 0.71, P =.41) was not observed. There was a significant effect of time, such that animals in both groups produced significantly fewer non-rewarded lever presses in trials conducted after 7 to 8 weeks of training than they did in baseline trials after 2 weeks of training (F[1,19] = 13.38, P < .01). Figure 4a demonstrates that the interaction of group by time approached significance (F[1,19] = 3.22, P = .09), suggesting that naïve animals had a greater decrease in non-rewarded lever presses than did animals that underwent CCI-ION surgery. However, the analysis of non-rewarded lever presses was the only analysis completed in which a significant difference was found between groups at the presurgery baseline trial (P = .05), so these results should be interpreted with caution. Similar analyses performed on rewarded lever presses indicated that there was no significant difference between the two groups on number of rewarded lever presses made during the self-regulation task.
Table 2. Self-Regulation Task Analyses and Descriptive Statistics.
| Task analysis (mean ± SD) | CCI-ION | Naïve | |
|---|---|---|---|
| Rewarded lever presses–baseline | 190.45 ± 71.70 | 185.32 ± 36.37 | |
| Rewarded lever presses–trial | 187.55 ± 63.50 | 182.73 ± 43.00 | |
| Non-rewarded lever presses–baseline | 40.20 ± 10.33 | 52.36 ± 15.43 | |
| Non-rewarded lever presses–trial | 31.85 ± 19.21 | 27.91 ± 14.54 | |
| Rewarded/total ratio–baseline | 0.82 ± 0.06 | 0.78 ± 0.07 | |
| Rewarded/total ratio–trial | 0.86 ± 0.07 | 0.86 ± 0.06 | |
| Two-way ANOVA | df | F value | P value |
|
| |||
| Rewarded presses | |||
| Group | 1,19 | 0.05 | .83 (ns) |
| Trial/Time | 1,19 | 0.15 | .71 (ns) |
| Interaction (Group × Time) | 1,19 | 0.00 | .98 (ns) |
| Non-rewarded presses | |||
| Group | 1,19 | 0.71 | .41 (ns) |
| Trial/Time | 1,19 | 13.38 | < .01** |
| Interaction (Group × Time) | 1,19 | 3.22 | .09 (ns) |
| Rewarded/Total ratio | |||
| Group | 1,19 | 0.47 | .50 (ns) |
| Trial/Time | 1,19 | 14.80 | < .01** |
| Interaction (Group × Time) | 1,19 | 2.12 | .16 (ns) |
Significant at P < .05.
Significant at P < .01.
Significant at P < .001.
CCI-ION = chronic constriction injury of the infraorbital nerve; n = 21 (11 naïve, 10 CCI-ION); ANOVA = analysis of variance: ns = not significant.
Fig 4.
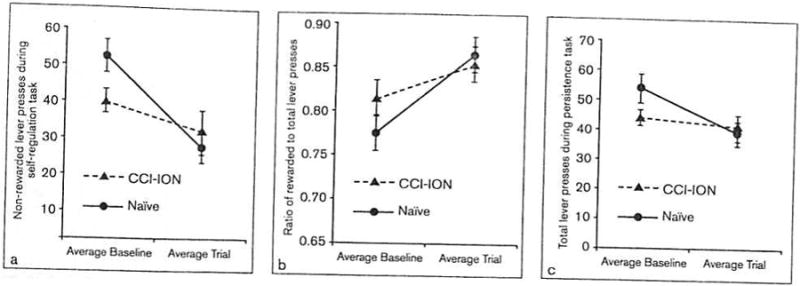
Behavior in self-regulation and persistence tasks compared across time points and groups; n = 21 (11 naïve, 10 chronic constriction injury of the infraorbital nerve [CCI-ION]). (a) Non-rewarded lever presses during self-regulation task. Both groups made significantly fewer non-rowarded lever presses (lever presses when the cue light was off) during the self-regulation task in postsurgery trials than in presurgery baseline trials (P < .01). Naïve animals had an even greater decrease in non-rewarded lever presses than did animals that underwent CCI-ION (P = .09). (b) Ratio of rewarded to total lever presses during self-regulation task. Both groups had a significant increase in their ratio of rewarded to total lever presses during the self-regulation task in postsurgery trials as compared to presurgery baseline trials (P < .01). (c) Total lever presses during persistence task. Naïve animals had a greater decrease in lever presses made during the persistence task in postsurgery trials (P < .05) than did animals that underwent CCI-ION (P = .55).
In the analysis of the ratio of rewarded to total lever presses in the self-regulation task, significant effects of group (F[1,19] = 0.47, P = .50) and the interaction of group by time (F[1,19] = 2.12, P = .16) were not observed. However, a significant effect of time was observed, such that animals in both groups had a significantly higher ratio of rewarded to total lever presses in trials conducted after 7 to 8 weeks of training than they did in baseline trials (F[1,19] = 14.80, P < .01; Fig 4b).
Persistence Task
To evaluate the a priori hypothesis, performance in the persistence task was analyzed by comparing the total number of lever presses made as well as the total time persisted (calculated using the time of the last lever press) across time-points for each group during the 10-minute persistence task. Results for the persistence task are shown in Table 3. In the persistence task, the number of lever presses made by animals in the naïve group decreased significantly in later trials as compared to baseline trials (t[10] = 2.64, P < .05; Fig 4c), which is suggestive of adaptive behavior. In contrast, animals in the CCI-ION group consistently pressed the lever about the same number of times in both the baseline trials and the later trials after 7 to 8 weeks of training (t[9] = 0.63, P = .55). Follow-up analyses indicated no significant effect of group (F[1,19] = 0.82, P = .38) or interaction of group by time (F[1,19] = 2.20, P = .16) for number of lever presses made during the persistence task. However, the time effect was significant, indicating that all animals made fewer lever presses in later trials than in baseline trials (F[1,19] = 5.47, P < .05). Similar analyses performed on the time persisted indicate that there was no significant difference between the two groups.
Table 3. Persistence Task Analyses and Descriptive Statistics.
| Persistence task analysis (mean ± SD) | CCI-ION | Naïve | |
|---|---|---|---|
| Total time (s)–baseline | 433.90 ± 130.52 | 508.68 ± 83.19 | |
| Total time (s)–trial | 445.50 ± 110.93 | 449.68 ± 99.37 | |
| Total lever presses–baseline | 44.35 ± 8.38 | 54.14 ± 16.19 | |
| Total lever presses–trial | 41.00 ± 14.13 | 39.18 ± 13.69 | |
| df | F value | P value | |
|
| |||
| Total presses | |||
| t test | |||
| CCI-ION (baseline vs trial) | 9 | 0.63 | .55 (ns) |
| Naïve (baseline vs trial) | 10 | 2.64 | < .05* |
| Two-way ANOVA | |||
| Group | 1,19 | 0.82 | .38 (ns) |
| Trial/Time | 1,19 | 5.47 | < .05* |
| Interaction (Group × Time) | 1,19 | 2.20 | .16 (ns) |
| Time persisted | |||
| t test | |||
| CCI-ION (baseline vs trial) | 9 | −0.26 | .80 (ns) |
| Naïve (baseline vs trial) | 10 | 1.37 | .20 (ns) |
| Two-way ANOVA | |||
| Group | 1,19 | 1.28 | .27 (ns) |
| Trial/Time | 1,19 | 0.59 | .45 (ns) |
| Interaction (Group × Time) | 1,19 | 1.30 | .27 (ns) |
Significant at P < .05.
Significant at P < .01.
Significant at P < .001.
CCI-ION = chronic constriction injury of the infraorbital nerve: n = 21 (11 naïve, 10 CCI-ION); ANOVA = analysis of variance; ns = not significant.
Discussion
The results of this study help to expand scientific knowledge of self-regulation and the models that may be used to study self-regulation. Also, the findings provide important insight into the associations between persistent pain and self-regulatory behavior. Here the influence of neuropathic pain on a persistence task that immediately followed an initial self-regulatory depletion task was tested using a CCI-ION pain model in the rat. In this model, CCI-ION animals persisted in lever pressing during a persistence task and completed more non-rewarded lever presses than naïve animals, indicating that animals experiencing persistent neuropathic pain were unable to respond appropriately to environmental demands. The findings support the premise that self-regulation can be studied in rodents and provides three potential benefits. First, a rodent model allows for greater experimental control than human studies. Second, physiological mechanisms underlying self-regulatory processes can be examined using this model. Third, this model would allow for preclinical trials of novel treatments for both persistent pain and self-regulatory induced fatigue associated with persistent pain.
The present experiment was successful in using the CCI-ION model of persistent neuropathic pain as a manipulation suitable to study the effects of pain on subsequent behavioral tasks, and this effect was robust enough to influence subsequent behavior in animals that were differentiated by mechanical hypersensitivity. It was expected that animals in the CCI-ION group would continue pressing a lever during the persistence task for a shorter period of time and for fewer times than naïve animals due to higher levels of fatigue following the self-regulation task. This hypothesis was not supported. There was, however, a significant difference between groups (ie, CCI-ION vs naïve) in the number of lever presses made during the persistence task, with CCI-ION animals continuing to press the lever at a consistent rate throughout trials and naïve animals experiencing a decrease in lever presses from baseline to later trials; however, this effect was in the direction opposite of the original hypothesis.
There are several possible explanations that could account for the consistent lever pressing by the CCI-ION animals during the persistence task as compared to the reduction in lever pressing over the duration of the experiment demonstrated in naïve animals. First, it is possible that the difference seen may be related to the animals' capacity to learn. The persistence task used in the study is essentially an extinction trial, during which animals are no longer rewarded for previously rewarded behavior. Typically, animals learn that they are no longer being rewarded and adjust their behavior by ceasing to press the lever. The findings suggest that naïve animals behaved in this manner, and continued to improve over the duration of the experiment (ie, made fewer lever presses each time they were exposed to the persistence task). However, animals with mechanical hypersensitivity did not show this improvement and continued to press the lever about the same number of times as during the baseline trials. Thus, the animals experiencing mechanical hypersensitivity may have been less able to learn that they were no longer being rewarded and did not adjust their behavior accordingly. Another possible explanation for these results (other than a deficit in continued learning) is that the animals with mechanical hypersensitivity were experiencing a deficit in self-regulation or a combination of deficits in learning and self-regulation. Self-regulation is very closely related to executive functioning, so much so that a deficit in one domain can lead to further deficits in the other.9 Therefore, an alternative explanation is that animals with mechanical hypersensitivity were able to learn but not able to regulate their behavior to reflect this learning. A third explanation is that animals with mechanical hypersensitivity experienced a deficit in self-regulation that in turn caused fatigue for continued learning. These hypotheses are supported by previous studies that demonstrate a deficit in decision-making behavior in both humans and rats experiencing pain.27-30 This effect is hypothesized to be controlled at least partially by media! prefrontal cortex deactivation, which is caused by hyperactivity in the amygdala.31 Other studies have also supported the importance of the amygdala and other structures for the cognitive deficits associated with pain.28,32,33
Previous human behavioral studies in which participants persisted on tasks in order to please the experimenter have been interpreted as demonstrating the importance of socialization processes in guiding behavior.34-36 Animals, lacking this form of socialized behavior in response to a laboratory task, are not likely to have the drive to persist simply to please the experimenter. Thus, it is reasonable to conclude that the naïve animals in this study were responding in an adaptive way (ie, naïve animals did not persist in the task when they learned they were no longer being rewarded), whereas animals with mechanical hypersensitivity did not respond in this adaptive way. This finding suggests that non-human animal research provides a major benefit for studying these phenomena by eliminating the drive to behave in a socially favorable way that may confound research with humans.
There are other interpretations of these data. For example, the observed perseveration of animals with mechanical hypersensitivity may be due to an increase in impulsive actions during the task. Previous research has demonstrated this effect in humans with fibromyalgia who experienced difficulty with response inhibition.37 Alternatively, other research has shown that analgesia may be associated with repeated muscle activity such that animals with mechanical hypersensitivity may be obtaining relief by the distraction of repeatedly pressing the lever.38 Finally, it is possible that animals with mechanical hypersensitivity lack the cognitive resources to attend to stimuli and learn correct responses; previous research has shown an association between pain conditions and decreased ability to attend.39 In summary, it is not entirely clear which explanation is most plausible for the outcomes observed. However, it is plausible that persistent mechanical hypersensitivity in this CCI-ION neuropathic pain model may interfere with learning and behavior in a significant way. Further studies are needed to clarify the processes by which neuropathic pain influences learning and behavior, and this model appears to provide a logical approach for observing these phenomena.
The present study is not without its limitations. First, a significant baseline difference was observed between groups in non-rewarded lever presses made during the self-regulation task. This finding indicates that trial results of non-rewarded lever presses made during the self-regulation task should be interpreted with caution, as these differences may be due to differences between groups and not due to pain. However, this was the only behavioral analysis to demonstrate a baseline difference, and no baseline differences were found for other major parts of the findings, such as lever presses made during the persistence task. A second limitation is that the control group in the current experiment did not undergo any surgical manipulation and thus it is possible that surgery itself, and not pain, could have influenced the animal behavior. The decision to use a completely naïve control group was made due to results of a pilot study that found mild mechanical hypersensitivity following sham surgical procedures. Future studies should further explore the difference between CCI-ION surgery, sham surgical procedures, and naïve animals. This research could provide an interesting look at the difference in self-regulatory behavior caused by different pain situations. Another possible limitation of the study is the fact that the rodent model of self-regulation was developed based on self-regulation protocols used with other species (ie, humans and dogs) and may not be the most effective approach for studying this phenomenon in rodent behavior.
In summary, data from the current study in the rodent may be interpreted as demonstrating that persistent neuropathic pain alters subsequent behaviors when preceded by a task that engages self-regulatory capacity. In addition, persistent pain states lead to marked changes in behavior and these changes can be especially noted when self-regulatory capacity is depleted. Thus, these findings suggest the importance of further explorations of the effects of persistent neuropathic pain on behaviors that are influenced by self-regulatory mechanisms.
Acknowledgments
This research was supported by NIH/NIDCR grant 2P20RR020145-06.
Footnotes
The authors report no conflicts of interest related to this study.
Contributor Information
Tracey C. Kniffin, Department of Psychology, University of Kentucky, Lexington, Kentucky, USA.
Robert J. Danaher, Department of Oral Health Practice, University of Kentucky, Lexington, Kentucky, USA.
Karin N. Westlund, Department of Physiology, University of Kentucky, Lexington, Kentucky, USA.
Fei Ma, Department of Physiology, University of Kentucky, Lexington, Kentucky, USA.
Craig S. Miller, Department of Oral Health Practice, University of Kentucky, Lexington, Kentucky, USA.
Charles R. Carlson, Department of Psychology, University of Kentucky, Lexington, Kentucky, USA.
References
- 1.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: A World Health Organization study in primary care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 2.Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001-02. Vital Health Stat. 2006;13:1–66. [PubMed] [Google Scholar]
- 3.Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7:544–555. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- 4.Solberg Nes L, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain: A review. Ann Behav Med. 2009;37:173–183. doi: 10.1007/s12160-009-9096-5. [DOI] [PubMed] [Google Scholar]
- 5.Baumeister RF. The self. In: Gilbert DT, Fiske ST, Lindzey G, editors. The Handbook of Social Psychology. NewYork, NY: McGraw-Hill; 1998. p. 1085. [Google Scholar]
- 6.Carver CS, Scheier MF. On the Self-Regulation of Behavior. New York: Cambridge University Press; 2001. [Google Scholar]
- 7.Higgins ET. The “self digest”: Self-knowledge serving self-regulatory functions. J Pers Soc Psychol. 1996;71:1062–1083. doi: 10.1037//0022-3514.71.6.1062. [DOI] [PubMed] [Google Scholar]
- 8.Baumeister RF, Schmeichel BJ, Vohs KD. Self-regulation and the executive function: The self as controlling agent. In: Kruglanski AW, Higgins ET, editors. Social Psychology: Handbook of Basic Principles. New York: Guilford; 2007. pp. 516–539. [Google Scholar]
- 9.Schmeichel BJ. Attention control, memory updating, and emotion regulation temporarily reduce the capacity for executive control. J Exp Psychol Gen. 2007;136:241–255. doi: 10.1037/0096-3445.136.2.241. [DOI] [PubMed] [Google Scholar]
- 10.Schmeichel BJ, Vohs KD, Baumeister RF. Intellectual performance and ego depletion: Role of the self in logical reasoning and other information processing. J Pers Soc Psychol. 2003;85:33–46. doi: 10.1037/0022-3514.85.1.33. [DOI] [PubMed] [Google Scholar]
- 11.Solberg Nes L, Carlson CR, Crofford LJ, de Leeuw R, Segerstrom SC. Self-regulatory deficits in fibromyalgia and temporomandibular disorders. Pain. 2010;151:37–44. doi: 10.1016/j.pain.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Miller HC, Pattison KF, DeWall CN, Rayburn-Reeves R, Zentall TR. Self-control without a “self”?: Common self-control processes in humans and dogs. Psychological science. 2010;21:534–538. doi: 10.1177/0956797610364968. [DOI] [PubMed] [Google Scholar]
- 13.Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? J Pers Soc Psychol. 1998;74:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- 14.Gailliot MT, Baumeister RF, DeWall CN, et al. Self-control relies on glucose as a limited energy source: Willpower is more than a metaphor. J Pers Soc Psychol. 2007;92:325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- 15.Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol Sci. 2007;18:275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- 16.Burris JL, Evans DR, Carlson CR. Psychological correlates of medical comorbidities in patients with temporomandibular disorders. J Am Dent Assoc. 2010;141:22–31. doi: 10.14219/jada.archive.2010.0017. [DOI] [PubMed] [Google Scholar]
- 17.Dominick CH, Blyth FM, Nicholas MK. Unpacking the burden: Understanding the relationships between chronic pain and comorbidity in the general population. Pain. 2012;153:293–304. doi: 10.1016/j.pain.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Gregg JM. A surgical approach to the ophthalmic-maxillary nerve trunks in the rat. J Dent Res. 1973;52:392. doi: 10.1177/00220345730520024001. [DOI] [PubMed] [Google Scholar]
- 19.Jacquin MF, Zeigler HP. Trigeminal orosensation and ingestive behavior in the rat. Behav Neurosci. 1983;97:62–97. doi: 10.1037//0735-7044.97.1.62. [DOI] [PubMed] [Google Scholar]
- 20.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 22.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 23.Ma F, Zhang L, Westlund KN. Trigeminal nerve injury ErbB3/ErbB2 promotes mechanical hypersensitivity. Anesthesiology. 2012;117:381–388. doi: 10.1097/ALN.0b013e3182604b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 25.Ma F, Zhang L, Lyons D, Wesllund KN. Orofacial neuropathic pain mouse model induced by Trigeminal Inflammatory Compression (TIC) of the infraorbital nerve. Mol Brain. 2012 Dec 28;5:44. doi: 10.1186/1756-6606-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thut PD, Hermanstyne TO, Flake NM, Gold MS. An operant conditioning model to assess changes in feeding behavior associated with temporomandibular joint inflammation in the rat. J Orofac Pain. 2007;21:7–18. [PubMed] [Google Scholar]
- 27.Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients aro impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. J Neurophysiol. 2011;106:960–973. doi: 10.1152/jn.00762.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pais-Vieira M, Aguiar P, Lima D, Galhardo V. Inflammatory pain disrupts the orbitofrontal neuronal activity and risk-assessment performance in a rodont decision-making task. Pain. 2012;153:1625–1635. doi: 10.1016/j.pain.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience. 2009;161:671–679. doi: 10.1016/j.neuroscience.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Ji G, Sun H, Fu Y, et al. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci. 2010;30:5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: Chronic pain hurts the brain, disrupting tho default-mode network dynamics. J Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal R. Experimenter effects in behavioral research. East Norwalk, CT: Appleton-Century-Crofts; 1966. [Google Scholar]
- 35.Rosenthal R, Kohn P, Greenfield PM, Carota N. Data desirability, experimenter expectancy, and the results of psychological research. J Pers Soc Psychol. 1966;3:20–27. doi: 10.1037/h0022604. [DOI] [PubMed] [Google Scholar]
- 36.Orne MT. On the social psychology of the psychological experiment: With particular reference to demand characteristics and their implications. Am Psychol. 1962;17:776–783. [Google Scholar]
- 37.Glass JM, Williams DA, Fernandez-Sanchez ML, et al. Executive function in chronic pain patients and healthy controls: Different cortical activation during response inhibition in fibromyalgia. J Pain. 2011;12:1219–1229. doi: 10.1016/j.jpain.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nijs J, Daenen L, Cras P, Struyf F, Roussel N, Oostendorp RA. Nociception affects motor output: A review on sensory-motor interaction with focus on clinical implications. Clin J Pain. 2012;28:175–181. doi: 10.1097/AJP.0b013e318225daf3. [DOI] [PubMed] [Google Scholar]
- 39.Eccleston C, Crombez G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]


