Abstract
Agents that stimulate human pancreatic beta cell proliferation are needed to improve diabetes mellitus treatment. Recently, a small molecule, WS6, was observed to stimulate human beta cell proliferation. However, little is known about its other effects on human islets. To better understand the role of WS6 as a possible beta cell regenerative therapy, we carried out in-depth phenotypic analysis of WS6-treated human islets, exploring its effects on non-beta cell proliferation, beta cell differentiation, and islet cell viability. WS6 not only stimulated beta cell proliferation in cultured human islets (in agreement with previous reports), but also human alpha cell proliferation, indicating that WS6 is not a beta cell-specific mitogen. WS6 did not change the proportion of insulin-positive beta cells or the expression of beta cell-specific transcription factors, suggesting that WS6 does not alter beta cell differentiation, and WS6 had no effect on human islet cell apoptosis or viability. In conclusion, WS6 stimulates proliferation of both human beta and alpha cells while maintaining cellular viability and the beta cell differentiated phenotype. These findings expand the literature on WS6 and support the suggestion that WS6 may help increase human islet mass needed for successful treatment of diabetes.
Keywords: Human islet, Differentiation, Alpha cell
Methods to replace pancreatic beta cells for the treatment of diabetes, both type 1 and type 2, are substantially hindered by the quiescent nature of these cells. Unlike rodent pancreatic beta cells, which can be stimulated to proliferate through a number of means including mitogen exposure, human beta cells have shown a resistance to most methods attempted to force proliferation [1, 2]. Adenoviral vectors used to overexpress components of the cell cycle machinery in the human beta cell, in particular CDK6, cyclin D1, and E2F3, have resulted in beta cell proliferation [3, 4]. However, attempts to stimulate human beta cell proliferation with exogenous mitogens have yielded minimal results. Parathyroid hormone-related peptide (PTHrp) and Nodal have been shown to stimulate modest human beta cell proliferation; however, substantial proliferation of these cells in response to exogenous mitogens remains an unmet goal [5, 6]. The identification of proteins or small molecules that can directly stimulate human beta cell proliferation would be advantageous to allow endogenous replication of existing beta cells in individuals with diabetes, but also to expand beta cell mass in vitro prior to islet transplantation.
Recently, WS6, a small molecule identified through a high-throughput screen, was determined to be a human beta cell mitogen [7]. WS6 stimulated human beta cell proliferation both in dissociated human islets as well as whole human islets. The IκB kinase pathway and the Erb3 binding protein-1 (EBP1) appeared to be involved in the mechanism of action of WS6. In vivo studies of WS6 in RIP-DTA mice revealed improvement in blood glucose levels, an increase in beta cell proliferation, but no difference in overall beta cell mass. However, it is unknown if WS6 stimulates proliferation of other cell types in the islet or if this compound adversely impacts differentiation status or viability of human islet cells.
Compounds that stimulate human beta cell proliferation could, in theory, also stimulate other cell types within the islet to replicate. Many studies focus solely on beta cell proliferation as the end point, but examining the effects of mitogens on other cell types in islets is important given the multicellular makeup of pancreatic islets. Furthermore, confirming that mitogenic compounds are not deleterious to cellular viability is crucial. Finally, determining the effect of mitogens on the differentiation status of beta cells is crucial as loss of beta cell-specific transcription factors has been linked to reduced beta cell function, a factor that may also play a role in the pathogenesis of type 2 diabetes [8–10]. To date, beyond the proliferative effect on beta cells, the effects of WS6 on human islets is otherwise unknown.
In the current study, we sought to understand the effects of WS6 on human islet cell proliferation, viability and beta cell differentiation. Our study confirms the ability of WS6 to stimulate human beta cell proliferation. However, our work reveals that WS6 is not a beta cell-specific mitogen as we have identified this compound to also stimulate alpha cell proliferation. Finally, we have identified that WS6 does not adversely affect human islet cell viability or beta cell differentiation status. Our work advances the knowledge of WS6, one of the only small molecule human beta cell mitogens identified to date.
Materials and Methods
Islet culture
Human islets were obtained from Prodo Laboratories (Irvine, CA) and the National Disease Research Interchange (NDRI, Philadelphia, PA) (Table 1). Full and ethical informed consent, allowing for use of the donor pancreatic islets for research, was obtained at the time of pancreas organ procurement. The islets were washed immediately upon arrival, and re-suspended in CMRL media (5.5 mM glucose, GIBCO) containing 10% fetal bovine serum (FBS), glutamine, and penicillin/streptomycin. Islets were cultured in ultra-low adherence plates (Corning) at a concentration of 0.75 to 1 islet equivalent (IEQ) per μL of media. Islets were cultured at 37° C, 5% CO2 and allowed to recover overnight. The next day, WS6 1.0 μM (Sigma) or DMSO was added to the wells. Media and WS6/DMSO were replaced after 48 hours.
Table 1.
Donor demographics for human islets used in the current study
| Gender | Ethnicity | Age | BMI | Cause of death |
|---|---|---|---|---|
| Male | Caucasian | 36 | 33.8 | Gun shot wound |
| Male | Caucasian | 29 | 23.5 | Head trauma |
| Female | Caucasian | 38 | 33.1 | Seizure |
| Male | Asian | 30 | 32.9 | Myocardial infarction |
| Male | Caucasian | 46 | 29.7 | CVA |
| Female | Black | 43 | 28.7 | Gun shot wound |
| Female | Caucasian | 29 | 23.5 | CVA |
| Male | Hispanic | 20 | 21.3 | Head trauma |
| Female | NativeAmerican | 48 | 31.2 | CVA |
(BMI = body mass index, CVA = Cerebrovascular accident)
Flow cytometry
Human islets were harvested from culture, washed with PBS, and incubated with 0.25% Trypsin (GIBCO) at 37° C with gentle agitation for 5 to 10 minutes. The islets were then gently dispersed by pipetting with a 1000 μL pipette. Trypsinization was stopped with CMRL media + 10% FBS. The dispersed cells were filtered with a 40 μm cell strainer. The resultant single-cell suspension was incubated in Live-Dead Fixable Stain (Invitrogen), fixed and permeabilized (Fix/Perm, BD Biosciences), and incubated with a fluorochrome-conjugated antibody (allophycocyanin-Anti-insulin, R&D) for 30 minutes. The cells were washed and subsequently analyzed on a Becton Dickinson FACStarPlus flow cytometer at the University of Nebraska Medical Center Flow Cytometry Core Facility. Data analysis was performed utilizing FlowJo software (Tree Star, Inc).
Immunofluorescence
At the end of the culture period, islets were harvested, washed with PBS, and fixed in 4% paraformaldehyde (PFA) for 24 hours. PFA was removed and the islets were embedded in 1.5% agarose, subsequently embedded in paraffin, and sections made at 4 μm. After deparaffinization and rehydration, antigen retrieval was performed by microwaving slides in sodium citrate (0.01 M, pH 6.0). Sections were blocked with 5% donkey serum for one hour and incubated in primary antibody overnight at 4° C. Secondary antibodies were applied for 1 hour and nuclei were subsequently stained with DAPI (VECTASHIELD, Vector Laboratories). Slides were visualized with an Axio Imager (Carl Zeiss, Oberkochen, Germany). Overlay and cell counting were performed with ImageJ software (available at http://rsb.info.nih.gov/ij). Primary antibodies utilized in this study included: Guinea Pig anti-insulin, mouse anti-Ki67 (Dako), mouse anti-glucagon (Sigma), rabbit anti-Ki67, goat anti-PDX1 (Abcam), and goat anti-NKX6.1 (R&D). Secondary antibodies included: donkey Alexa Fluor-488 and -594 conjugates (Life Technologies and Jackson ImmunoResearch).
Western blot
Islets were stored at −80° C at the end of the culture period. For protein lysate extraction, islets were thawed and lysed in cell lysis buffer for 30 minutes. The protein lysate was centrifuged at 20000 g for 20 minutes to pellet residual cellular materials. A standard Bradford method (BioRad) was used to determine protein concentrations. Proteins were separated on a 4–20% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were blocked with 5% milk for 30 minutes and primary antibodies then added for overnight incubation at 4° C. Membranes were washed and HRP-conjugated secondary antibodies (Jackson ImmunoResearch) were added for one hour. After wash, membranes were developed with ECL (Thermo Scientific). Primary antibodies utilized included: goat anti-NKX6.1 (R&D), rabbit anti-GAPDH, rabbit anti-PDX1, mouse anti-Insulin, and rabbit anti-NeuroD1 (Cell Signaling).
TUNEL staining
Slides were prepared from paraffin-embedded islets and TUNEL assay performed using a fluorescein In Situ Cell Death Kit (Roche), per the manufacturer’s instructions. Imaging and counting were performed with ImageJ software.
Statistical analysis
Values are presented as mean ± standard error of the mean (SEM). A two-tailed student’s t-test and one sample t-test were used to compare between treatment groups. A P-value <0.05 was considered significant.
Results
WS6 stimulates substantial proliferation of human islet cells
WS6 has been shown to stimulate proliferation of human beta cells [7]. We first strove to confirm the proliferative capacity of WS6 on human islet cells. As noted in Fig. 1A, WS6 stimulated a seven-fold increase of general cellular proliferation in human islets. This confirmed the earlier report that WS6 is a pro-proliferative factor for human islet cells. Given the substantial number of cells proliferating and the rates of WS6-induced, beta cell specific proliferation previously reported, we felt that other islet cell types may also be stimulated to proliferate in response to WS6 treatment.
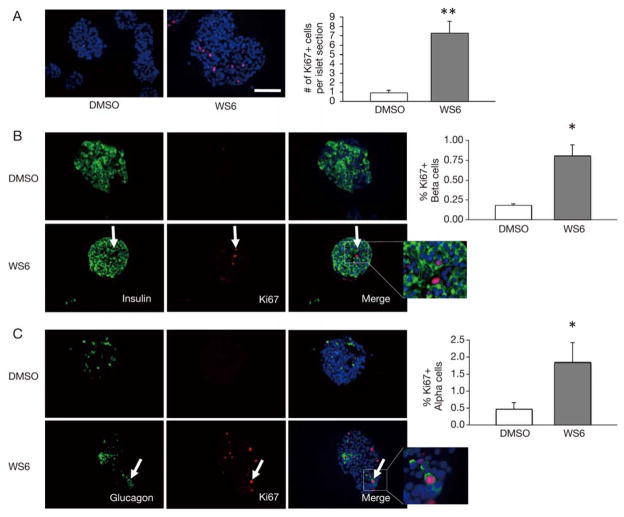
Fig. 1.
WS6 stimulates proliferation of both human pancreatic beta and alpha cells. Immunofluorescent analysis of human islets exposed to WS6 1.0 μM or DMSO (control) for 96 hours.
A. Ki67 (red) staining of human islets with quantification of total Ki67 cells per islet. B. Insulin (green) and Ki67 (red) staining of human islets with quantification of Ki67+ beta cells. White arrow shows Insulin+/Ki67+ double-positive cell.
C. Glucagon (green) and Ki67 (red) staining of human islets with quantification of Ki67+ alpha cells. White arrow shows Glucagon+/Ki67+ double-positive cell. (DAPI staining in blue. n = 4 from four different donors. Bars indicate mean ± SEM. Scale bar = 50 μm. *p < 0.05, **p < 0.01)
WS6 stimulates proliferation of human beta cells and alpha cells
Next, we sought to investigate whether, in our hands, WS6 could stimulate human beta cell proliferation, as reported by Shen et al [7]. Intact human islets cultured in the presence of 1.0 μM WS6 for four days did show a significant, four-fold increase in beta cell proliferation as noted by incorporation of Ki67 (p < 0.05, Fig. 1B). However, a significant number of the proliferating cells were non-beta cells, confirming our assumption that WS6 is not a beta cell-specific mitogen.
Glucagon-producing alpha cells of the pancreas are, like beta cells, a quiescent cell population that has been shown to proliferate in response to certain stimuli [11–13]. Given the pronounced cellular proliferation we observed with WS6 administration in both beta cells and non-beta cells, we hypothesized that alpha cells would also undergo increased proliferation in response to WS6. As shown in Fig. 1C, alpha cell proliferation, as measured by Ki67 expression, was significantly enhanced with WS6 compared to control. Similar to beta cells, alpha cell proliferation was increased four-fold in response to WS6 compared to control (p < 0.05). This finding suggests that WS6 is a non-specific endocrine cell mitogen.
WS6 does not affect differentiation status of human beta cells
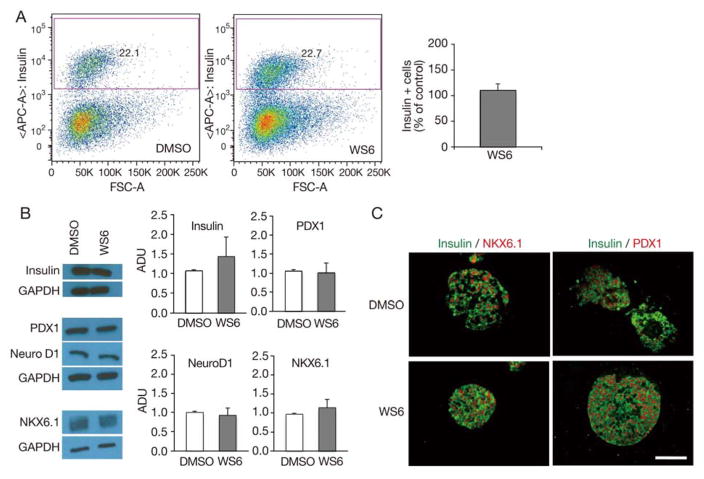
Beta cell proliferation is thought to occur by the direct replication of existing beta cells [14]. The exact mechanism whereby previously quiescent beta cells are stimulated to replicate is not well understood [1]. Studies utilizing cell lines have suggested that immature beta cells may be more prone to replication than mature beta cells [15]. With the knowledge that WS6 stimulated beta cell proliferation, we sought to investigate the effect of WS6 on beta cell maturity. By flow cytometry, WS6 did not alter the proportion of insulin positive cells in human islets compared to control, suggesting no overt impact on absolute beta cell number (Fig. 2A). Similarly, the mean fluorescence intensity (MFI) of insulin positive beta cells was no different in WS6 treated islets compared to control (MFI ratio of WS6 to DMSO = 0.78 ± 0.12, p = 0.22) suggesting WS6 did not alter insulin expression in beta cells. Confirmation of similar insulin expression in WS6-treated islets compared to control islets was shown by western blot (Fig. 2B).
Fig. 2.
WS6 does not impact human beta cell number or beta cell differentiation. Analysis of human islets exposed to WS6 1.0 μM or DMSO (control) for 96 hours.
A. Flow cytometry analysis. Gating strategy for insulin positive cells (left) and quantification of the proportion of insulin positive cells (right) (n = 3 from three different donors, p = NS). B. Western blot analysis of human insulin and beta cell transcription factors, with representative images (left) and quantification (right, n = 3 to 4 from four different donors, p = NS for all). C. Immunofluorescent analysis showing similar NKX6.1 and PDX1 expression in DMSO and WS6 treated islets (ADU = Arbitrary density units. Bars indicate mean ± SEM. Scale bar = 50 μm). Representative images from Figs. 2A and 1B come from different donors.
However, even with intact beta cell insulin expression, in certain models, insulin-positive beta cells can lose expression of other beta cell transcription factors with subsequent reduction in beta cell function [8, 16, 17]. With this information in mind, we compared the levels of beta cell specific transcription factors after exposure of human islets cells to WS6, or vehicle control. We observed that WS6 treatment did not affect expression of PDX1, NeuroD1, or NKX6.1 in isolated human islets (Fig. 2B). By immunofluorescence, control and WS6-treated islets both showed only rare examples of insulin-positive beta cells lacking expression of NKX6.1 or PDX1 (Fig. 2C). These results indicate that WS6 does not overtly affect the differentiation status of human beta cells.
WS6 does not affect viability of human islet cells
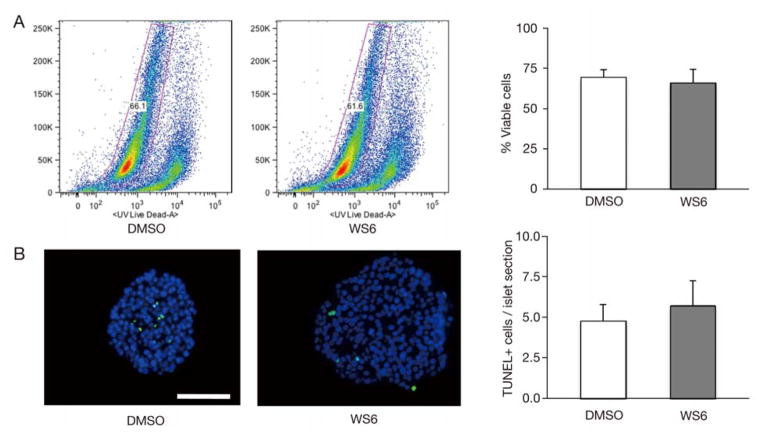
Though WS6 is a human endocrine cell mitogen, we next sought to ensure this compound did not adversely affect viability or stimulate apoptosis. We first quantitated cellular viability by flow cytometry utilizing a Live/Dead stain. The viability in WS6 treated human islet cells was no different than DMSO treated islet cells (Fig. 3A). We then measured the number of cells undergoing apoptosis by TUNEL staining and again found that WS6 did not stimulate increased levels of apoptosis, compared to controls (Fig. 3B). These results confirm that WS6, at the dose and duration used in this study, does not adversely affect human islet cell viability.
Fig. 3.
WS6 does not adversely affect human islet viability. Analysis of human islets exposed to WS6 1.0 μM or DMSO (control) for 96 hours.
A. Viability of human islet cells as assayed by flow cytometry Live/Dead assay. Gating strategy (left) and quantification of viable cells (right, n = 3 from 3 different donors, p = NS.) B. Analysis of apoptosis by immunofluorescent TUNEL assay. Left - Arrows indicate TUNEL+ cells (Green = TUNEL staining, Blue = DAPI). Right – Quantification of TUNEL+ cells per islet (n = 3 from 3 different donors, p = NS). (Bars indicate mean ± SEM. Scale bar = 50 μm)
discussion
Identifying methods that stimulate proliferation of functional human beta cells for the treatment of diabetes remains an unmet need. Customized human islet and beta cell culture methods have been developed to promote chemical library screens in an attempt to identify small molecules that may be efficacious in stimulating beta cell proliferation [18–21]. Small molecules would be ideal as these molecules may be developed into drug therapy without the issues inherent in the synthesis and delivery of peptide molecules. WS6 has recently been identified to stimulate human beta cell proliferation in vitro, both in dispersed and whole human islets [7]. This small molecule was also shown to improve blood glucose levels in a mouse model of diabetes. Due to its importance as a potential diabetes therapy, we sought to test the effects of WS6 on human islet cell proliferation, differentiation, and viability. Our results have revealed that WS6 is a not a beta cell-type specific mitogen in human islets, as it also stimulates proliferation of alpha cells. We have also shown that WS6 does not appear to induce significant dedifferentiation of human beta cells, as noted by proportion of insulin-positive cells per islet, and expression of insulin and key beta cell transcription factors.
Documentation of the non-beta cell activity of beta cell mitogens is crucial to understanding the over-all effects these mitogens may have on islet structure and function. Though islet alpha cell-to-beta cell ratios clearly vary amongst individuals, significant alpha cell proliferation may alter islet function, as is seen with animal models of type 2 diabetes [22, 23]. Furthermore, hyperglucagonemia plays a role in hyperglycemia development in type 1 and type 2 diabetes [24–26]. Therefore, excess alpha cell proliferation compared to beta cell proliferation in response to a mitogenic signal may be deleterious. In our hands, WS6 stimulated similar rates of proliferation of both alpha and beta cells suggesting this compound should not significantly disturb the intrinsic beta cell-to-alpha cell ratio. Though WS6 appears to be a non-specific endocrine cell mitogen in human islets, the effect of WS6 on delta and PP cells is unknown and requires additional investigation, as does the potential effects on proliferation of endothelial cells within the islet.
The maturity state of beta cells after exposure to a pro-proliferative factor is another important consideration. Some level of dedifferentiation may be a requirement for beta cell proliferation, as noted in human in vitro studies and some models of in vivo rodent beta cell proliferation [27, 28]. If dedifferentiation is required for beta cell proliferation, re-differentiation of these expanded cells into functional beta cells is crucial for appropriate function as immature beta cells have a reduced capacity for glucose-stimulated insulin secretion [15, 29, 30]. Importantly, WS6 does not appear to cause a significant reduction in the expression of beta cell transcription factors in human islets. Further studies are needed to investigate whether WS6 has any effect on islet endocrine cell function as noted by glucose-stimulated insulin secretion (GSIS) and glucagon stimulation assays.
Finally, we have shown that WS6 does not adversely affect beta cell viability or stimulate beta cell apoptosis in standard culturing conditions. This is vital knowledge when considering any potential pro-proliferative agent. Whether WS6 can potentially impart a protective role against beta cell toxins (e.g. fatty acids, hyperglycemia, cytokines) in the setting of diabetes is unknown. This point should be studied further to determine if this compound might provide additional protective beta cell benefits in the form of an anti-apoptotic compound.
In conclusion, we have shown WS6 to be a non-specific mitogen for human islet cells, stimulating not only beta cell but also alpha cell proliferation, while maintaining the differentiated beta cell phenotype. Our study expands the current knowledge of the effects of WS6 on human islets and re-affirms the potential this agent may have for use in human beta cell regenerative protocols. Our study also emphasizes the importance of studying other cell types, cellular differentiation, and viability in the islet when investigating potential beta cell mitogens.
Acknowledgments
Funding
This work was supported by the Kieckhefer Foundation (N.E.S.).
Footnotes
declaration of Interest
B.P.B, N.M.G, S.U.M, and N.E.S. declare no conflicts of interest.
References
- 1.Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, et al. Human beta-cell proliferation and intracellular signaling part 2: Still driving in the dark without a road map. Diabetes. 2014;63:819–831. doi: 10.2337/db13-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiao Y, Le Lay J, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human beta-cell replication in the transplant setting. Diabetes. 2014;63:1283–1288. doi: 10.2337/db13-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiaschi-Taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-Castellano I, et al. Induction of human beta-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes. 2010;59:1926–1936. doi: 10.2337/db09-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rady B, Chen Y, Vaca P, Wang Q, Wang Y, et al. Overexpression of E2F3 promotes proliferation of functional human beta cells without induction of apoptosis. Cell Cycle. 2013;12:2691–2702. doi: 10.4161/cc.25834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guthalu Kondegowda N, Joshi-Gokhale S, Harb G, Williams K, Zhang XY, et al. Parathyroid hormone-related protein enhances human beta-cell proliferation and function with associated induction of cyclin-dependent kinase 2 and cyclin E expression. Diabetes. 2010;59:3131–3138. doi: 10.2337/db09-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boerner BP, George NM, Targy NM, Sarvetnick NE. TGF-beta superfamily member nodal stimulates human beta-cell proliferation while maintaining cellular viability. Endocrinology. 2013;154:4099–4112. doi: 10.1210/en.2013-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen W, Tremblay MS, Deshmukh VA, Wang W, Filippi CM, et al. Small-molecule inducer of beta cell proliferation identified by high-throughput screening. J Am Chem Soc. 2013;135:1669–1672. doi: 10.1021/ja309304m. [DOI] [PubMed] [Google Scholar]
- 8.Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 2013;4:1262–1275. doi: 10.1016/j.celrep.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo S, Dai C, Guo M, Taylor B, Harmon JS, et al. Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci USA. 2008;105:13163–13168. doi: 10.1073/pnas.0801059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Kim W, Chen Z, Shin YK, Carlson OD, et al. Insulin and glucagon regulate pancreatic alpha-cell proliferation. PLoS One. 2011;6:e16096. doi: 10.1371/journal.pone.0016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes HL, Moss LG, Schisler JC, Haldeman JM, Zhang Z, et al. Pdx-1 activates islet alpha- and beta-cell proliferation via a mechanism regulated by transient receptor potential cation channels 3 and 6 and extracellular signal-regulated kinases 1 and 2. Mol Cell Biol. 2013;33:4017–4029. doi: 10.1128/MCB.00469-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 15.Szabat M, Johnson JD, Piret JM. Reciprocal modulation of adult beta cell maturity by activin A and follistatin. Diabetologia. 2010;53:1680–1689. doi: 10.1007/s00125-010-1758-0. [DOI] [PubMed] [Google Scholar]
- 16.Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, et al. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 17.Schaffer AE, Taylor BL, Benthuysen JR, Liu J, Thorel F, et al. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic beta cell identity. PLoS Genet. 2013;9:e1003274. doi: 10.1371/journal.pgen.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walpita D, Hasaka T, Spoonamore J, Vetere A, Takane KK, et al. A human islet cell culture system for high-throughput screening. J Biomol Screen. 2012;17:509–518. doi: 10.1177/1087057111430253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Walker JR, Wang X, Tremblay MS, Lee JW, et al. Identification of small-molecule inducers of pancreatic beta-cell expansion. Proc Natl Acad Sci USA. 2009;106:1427–1432. doi: 10.1073/pnas.0811848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Annes JP, Ryu JH, Lam K, Carolan PJ, Utz K, et al. Adenosine kinase inhibition selectively promotes rodent and porcine islet beta-cell replication. Proc Natl Acad Sci USA. 2012;109:3915–3920. doi: 10.1073/pnas.1201149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vetere A, Wagner BK. Chemical methods to induce beta-cell proliferation. Int J Endocrinol. 2012;2012:925143. doi: 10.1155/2012/925143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54:1720–1725. doi: 10.1007/s00125-011-2118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guardado-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick EJ, et al. Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci USA. 2009;106:13992–13997. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unger RH, Aguilar-Parada E, Muller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49:837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherwin RS, Fisher M, Hendler R, Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med. 1976;294:455–461. doi: 10.1056/NEJM197602262940901. [DOI] [PubMed] [Google Scholar]
- 26.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 27.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57:1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- 28.El-Gohary Y, Tulachan S, Wiersch J, Guo P, Welsh C, et al. A smad signaling network regulates islet cell proliferation. Diabetes. 2014;63:224–236. doi: 10.2337/db13-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, et al. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest. 2004;114:828–836. doi: 10.1172/JCI21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu C, Stein GH, Pan N, Goebbels S, Hornberg H, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 2010;11:298–310. doi: 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]