Abstract
Background
Many patients who receive maintenance hemodialysis experience poor sleep. Uncontrolled studies suggest frequent hemodialysis improves sleep quality, which is a strong motivation for some patients to undertake the treatment. We studied the effects of frequent in-center (‘daily’) and nocturnal home hemodialysis on self-reported sleep quality in two randomized trials.
Methods
Participants were randomly assigned to frequent (six times per week) or conventional (three times per week) hemodialysis in the Frequent Hemodialysis Network Daily (n = 245) and Nocturnal (n = 87) Trials. We used the Medical Outcomes Study Sleep Problems Index II (SPI II), a validated and reliable instrument in patients with end-stage renal disease, to measure self-reported sleep quality. The SPI II is scored from 0–100, with a higher value indicating poorer quality of sleep. A mean relative decline in SPI II would suggest improved sleep quality. The primary sleep outcome was the change in the SPI II score over 12 months.
Results
In the Daily Trial, after adjustment for baseline SPI II, subjects randomized to frequent as compared with conventional in-center hemodialysis experienced a 4.2 [95% confidence interval (CI) 0.4–8.0] point adjusted mean relative decline in SPI II at 4 months and a 2.6 (95% CI −2.3–7.5) point adjusted mean relative decline at 12 months. In the Nocturnal Trial, subjects randomized to frequent nocturnal as compared with conventional home hemodialysis experienced 2.9 (95% CI −3.4–9.3) and 4.5 (95% CI −3.2–12.2) point mean relative declines at Months 4 and 12, respectively.
Conclusions
Although a possible benefit of frequent in-center hemodialysis was observed at 4 months, neither frequent in-center hemodialysis nor home nocturnal hemodialysis demonstrated significant improvements in self-reported sleep quality compared with conventional hemodialysis at 12 months.
Keywords: daily hemodialysis, frequent hemodialysis, nocturnal hemodialysis, patient-reported outcome, sleep quality
INTRODUCTION
Patients on maintenance dialysis suffer from a variety of distressing symptoms associated with impaired health-related quality of life (HRQOL). Seventy-one percent of patients experience fatigue/tiredness and 44% sleep poorly according to a meta-analysis of symptoms in end-stage renal disease (ESRD) [1]. Sleep problems and diminished sleep quality have been associated with depression, anxiety, hospitalizations, chronic medical conditions, decrements in HRQOL and mortality in patients treated with hemodialysis [2–5]. In our previous work, we demonstrated that there were marked decrements in sleep quality among patients at the time of enrolling in the Frequent Hemodialysis Network (FHN) Trials compared with sleep quality in the general population [4]. Further, impaired sleep quality scores were associated with decrements in physical and mental well-being [4]. The importance of sleep quality to patients undergoing hemodialysis was highlighted by a study assessing patient preferences for more frequent dialysis. This study found that 57% of patients would be willing to undergo more frequent in-center hemodialysis to improve sleep compared with 19% who would undergo more frequent dialysis for up to 3 years of additional life [6].
Since many patients on thrice-weekly hemodialysis sleep poorly, a better correction of uremia using more frequent hemodialysis might improve sleep quality. Indeed, in an uncontrolled trial, Hanly et al. showed that nocturnal hemodialysis significantly improved signs and symptoms of sleep apnea [7, 8]. However, frequent hemodialysis can be time-consuming and burdensome, and might be expected to adversely affect sleep quality in some settings. For instance, nocturnal hemodialysis could decrease the quality of sleep if the machine alarms regularly or if a patient must remain still to avoid line compression or strain.
We designed the FHN clinical trials to test whether more frequent hemodialysis would yield clinically important effects on a large group of intermediate outcomes, separated into nine domains [9, 10]. In addition, the FHN clinical trials tested the hypothesis that more frequent HD improves patient-centered outcomes in patients undergoing dialysis, including quality of sleep. We hypothesized that frequent hemodialysis, as compared with conventional hemodialysis, would improve self-reported sleep quality in both the Daily and Nocturnal Trials. We also explored whether frequent dialysis influenced sleep behaviors such as duration of sleep, napping and snoring.
MATERIALS AND METHODS
Study design
The FHN Daily Trial was a multicenter, prospective, randomized, parallel-group trial of frequent (six times per week) as compared with conventional (three times per week) in-center hemodialysis [10]. The FHN Nocturnal Trial was a similarly designed trial comparing the effects of frequent nocturnal (six times per week) with conventional (three times per week) hemodialysis [11]. The majority of patients in the Nocturnal Trial received conventional hemodialysis at home. Detailed descriptions of the trial designs including randomization, specific inclusion and exclusion criteria and data collection procedures are described elsewhere [12].
Study population
Patients treated with maintenance hemodialysis who achieved mean equilibrated Kt/Vurea > 1.0 for the last two baseline hemodialysis sessions and weighed >30 kg were eligible for inclusion. Major exclusion criteria included age <13 (Daily) or <18 (Nocturnal) years, residual kidney function >3 mL/min/35 L (Daily) or mean of creatinine and urea clearance >10 mL/min/1.73 m2 (Nocturnal), life expectancy <6 months, medical need for hemodialysis more than three times per week, history of poor adherence to hemodialysis, medical conditions preventing cardiac magnetic resonance imaging, inability to communicate in English or Spanish and anticipated kidney transplant or relocation within 14 months. Informed consent was obtained from each subject. The study was approved by the Institutional Review Board at each participating study site [11].
Intervention, control and adherence
After randomization in the Daily Trial, subjects who were assigned to hemodialysis six times per week (n = 125) had a target equilibrated Kt/Vn (where Vn = 3.271 × V2/3) of 0.9 provided that the length of the session was between 1.5 and 2.75 h. Subjects who were assigned to thrice-weekly hemodialysis (n = 120) continued their usual hemodialysis prescriptions, which included a minimum target equilibrated Kt/Vurea [the ratio of the equilibrated urea clearance during each dialysis session (Kt) to the patient's volume of urea distribution (V)] of 1.1 and a session length of 2.5–4.0 h.
After randomization in the Nocturnal Trial, subjects were assigned to either three times per week (n = 42) hemodialysis to a prescribed standard Kt/Vurea of >2.0 and a session length of ≥2.5 h or six times per week (n = 45) hemodialysis to a standard Kt/Vurea of ≥4.0 or ≥6 h per session. For both trials we calculated adherence as the ratio of dialysis sessions attended to dialysis sessions prescribed, by month.
Primary sleep outcome
The primary sleep outcome for the FHN trial was the change on the Medical Outcomes Study Sleep Problems Index II (SPI II) score over 12 months [4]. The Medical Outcomes Study (MOS) Sleep Problems Index (SPI) is a 12-item measure that includes items on sleep initiation and maintenance, sleep adequacy, daytime somnolence and respiratory disturbances. Participants are instructed to relate responses to sleep habits over the previous month [13]. Nine of the 12 items of the SPI are summed to obtain an overall SPI II score that has been shown to have good internal consistency and reliability, discriminative validity and responsiveness to interventions [13, 14] and has previously been used in the hemodialysis population [15, 16]. The other three items were examined separately. The question regarding hours slept each night is filled in and the question regarding sleep latency is scored on a 1–5 scale. The remaining answers are scored on a 1–6 scale, and inventory items correspond to a specific category of sleep quality and/or problem. The SPI II is scored from 0 to 100, with a higher value indicating poorer quality of sleep. We previously published the distribution and correlates of the SPI II score at the time subjects were enrolled in the FHN Trials [4].
Descriptive variables
We collected data on demographic characteristics at baseline. We obtained clinical data and laboratory test results at baseline and serially over the course of the study. We performed standardized assessments of coexisting conditions using a modified version of the Charlson Comorbidity Index [17] supplemented with additional items from the Index of Coexistent Diseases [18]. Covariates included demographics (age, sex, race/ethnicity), clinical characteristics (ESRD vintage, comorbidities including diabetes mellitus and cerebrovascular disease), laboratory parameters (serum creatinine, hemoglobin and phosphate concentrations) and medications (opioids, antidepressants, benzodiazepines). Depressive symptoms were assessed by the Beck Depression Inventory (BDI). We defined clinically important depressive symptoms as a BDI score >15, in accordance with our previous studies in the ESRD population [19]. Laboratory variables were measured predialysis at laboratories affiliated with each clinical center and, if more than one baseline value was collected, the first value was used.
Statistical analysis
We analyzed each of the two trials (Daily and Nocturnal) separately. The baseline characteristics of the two trials were examined according to intervention status (frequent versus conventional) and characterized using mean (± standard deviation), median (10th percentile, 90th percentile) or frequency (%), as appropriate.
We compared between-group mean changes in scores from baseline to Month 12 for the MOS SPI II using linear mixed effects models with an unstructured covariance matrix incorporating baseline, 4-month and 12-month scores for each measure. In accordance with prespecified analysis plans, we adjusted for the baseline score in both trials and for the clinical center in the Daily Trial [20]. We performed secondary analyses of the same relative changes after the dichotomized sleep score (a cutoff of SPI II >47 for clinically important symptoms). A score >47 was used based on a literature review of several different symptomatic medical populations [21, 22]. Results from these analyses were expressed as percentage change from baseline.
Using the continuous SPI II score, we performed prespecified subgroup analyses according to age, sex, race and depression. The primary assessment of treatment interactions with quantitative subgroup factors was based on a test for linear interaction that treated the subgroup factor as a continuous variable. Estimated treatment effects are also provided for the subgroups defined by the above cutoffs for descriptive purposes. In the Daily Trial, we present P-values for the interactions without adjustment for multiple comparisons. Due to its limited sample size, we considered subgroup analyses in the Nocturnal Trial in an exploratory fashion without significance testing.
We examined snoring and napping frequency subscales of the MOS Sleep Problems Index using ordinal logistic regression allowing for random intercepts [23]. These models were run without covariate adjustment. We also tested the appropriateness of the proportional odds assumption using a log-likelihood test and found insufficient evidence to reject. We performed all analyses according to the intention-to-treat principle and interpreted two-tailed P-values <0.05 as statistically significant (including tests for interaction). We used SAS software, version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
Study subjects
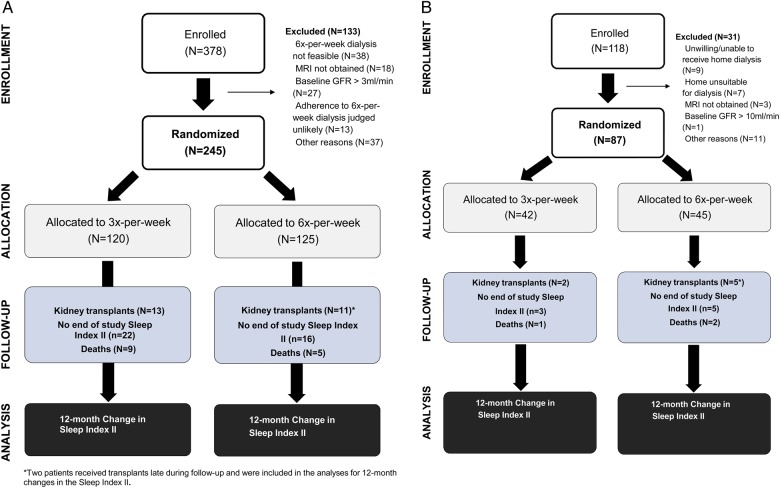
Figure 1A and B shows the number of subjects enrolled and randomized into the Daily and Nocturnal Trials, respectively. The number with measurements of sleep problem symptoms is presented, as well as reasons for missing data (such as death or transplantation). Follow-up SPI II scores were available for 81% of eligible study participants in the Daily Trial and 90% of participants in the Nocturnal Trial.
FIGURE 1:
(A) Flow diagram for the Daily Trial showing the number of subjects enrolled and assigned to each study arm (intervention/control) and the number of subjects analyzed with baseline and 12-month ascertainment of each mental health and depression, including reasons for dropout. (B) Flow diagram for the Nocturnal Trial showing the number of subjects enrolled and assigned to each study arm (intervention/control) and the number of subjects analyzed with baseline and 12-month ascertainment of mental health and depression, including reasons for dropout.
Demographic characteristics
Table 1 shows the baseline characteristics of subjects in the two trials stratified by treatment status (frequent versus conventional hemodialysis). The study population was diverse with respect to age, sex, race/ethnicity, primary cause of kidney disease, coexisting conditions and ESRD vintage. There were no statistically or clinically significant differences in baseline characteristics between the frequent and conventional groups in either trial.
Table 1.
Baseline characteristics of study subjects
| Variables | Daily Trial |
Nocturnal Trial |
||||||
|---|---|---|---|---|---|---|---|---|
| N | All (N = 245) | 3 times (N = 120) | 6 times (N = 125) | N | All (N = 87) | 3 times (N = 42) | 6 times (N = 45) | |
| Age (years) | 245 | 50.4 ± 13.9 | 52.0 ± 14.1 | 48.9 ± 13.6 | 87 | 52.8 ± 13.6 | 54.0 ± 12.9 | 51.7 ± 14.4 |
| Male | 245 | 151 (61.6) | 73 (60.8) | 78 (62.4) | 87 | 57 (65.5) | 28 (66.7) | 29 (64.4) |
| Race/ethnicity | 245 | 87 | ||||||
| Black/African American/African | 102 (41.6) | 53 (44.2) | 49 (39.2) | 23 (26.4) | 11 (26.2) | 12 (26.7) | ||
| White/Caucasian, non-Hispanic | 89 (36.3) | 46 (38.3) | 43 (34.4) | 48 (55.2) | 21 (50.0) | 27 (60.0) | ||
| Other/mixed | 54 (22.1) | 21 (17.5) | 33 (27.4) | 16 (18.3) | 10 (23.8) | 6 (13.3) | ||
| Primary language English | 245 | 196 (80.0) | 101 (84.2) | 95 (76) | 87 | 77 (88.5) | 36 (85.7) | 41 (91.1) |
| ESRD vintage (years) | 245 | 3.64 (0.63, 14.26) | 3.40 (0.58, 12.94) | 3.85 (0.69, 17.31) | 87 | 0.91 (0.09, 11.48) | 0.53 (0.10, 6.00) | 1.32 (0.09, 12.55) |
| Education | 242 | 86 | ||||||
| <High school graduate | 51 (21.1) | 21 (17.6) | 30 (24.4) | 13 (15.1) | 5 (11.9) | 8 (18.2) | ||
| High school graduate | 58 (24.0) | 32 (26.9) | 26 (21.1) | 21 (24.4) | 9 (21.4) | 12 (27.3) | ||
| Post high school | 133 (55.0) | 66 (55.5) | 67 (54.5) | 52 (60.5) | 28 (66.7) | 24 (54.5) | ||
| Diabetes | 245 | 100 (40.8) | 50 (41.7) | 50 (40.0) | 87 | 37 (42.5) | 18 (42.9) | 19 (42.2) |
| Current or former smoking | 245 | 91 (37.1) | 45 (37.5) | 46 (36.8) | 87 | 43 (49.4) | 25 (59.5) | 18 (40.0) |
| Stroke | 245 | 18 (7.3) | 9 (7.5) | 9 (7.2) | 87 | 2 (2.3) | 1 (2.4) | 1 (2.2) |
| Myocardial infarction | 245 | 27 (11.0) | 16 (13.3) | 11 (8.8) | 87 | 9 (10.3) | 4 (9.5) | 5 (11.1) |
| Charlson comorbidity index | 245 | 1.82 ± 1.95 | 1.88 ± 2.03 | 1.76 ± 1.89 | 87 | 1.72 ± 1.75 | 1.88 ± 1.93 | 1.58 ± 1.57 |
| Beck Depression Inventory | 240 | 10 (3, 24) | 10 (3, 24) | 11 (3, 26) | 87 | 10 (3, 26) | 10 (4, 26) | 10 (3, 19) |
| Benzodiazepines | 245 | 31 (12.6) | 13 (10.8) | 18 (14.4) | 87 | 15 (17.2) | 7 (16.7) | 8 (17.8) |
| Antidepressants | 245 | 35 (14.3) | 15 (12.5) | 20 (16.0) | 87 | 22 (25.3) | 10 (23.8) | 12 (26.7) |
| Opioids | 245 | 42 (17.1) | 19 (15.8) | 23 (18.4) | 87 | 18 (20.7) | 7 (16.7) | 11 (24.4) |
| Predialysis SBP (mmHg) | 245 | 146 ± 18 | 146 ± 18 | 147 ± 18 | 87 | 149 ± 18 | 153 ± 22 | 145 ± 13 |
| Predialysis DBP (mmHg) | 245 | 80 ± 12 | 78 ± 12 | 81 ± 11 | 87 | 81 ± 12 | 83 ± 13 | 80 ± 11 |
| Weekly standard Kt/V | 245 | 2.52 ± 0.35 | 2.53 ± 0.39 | 2.50 ± 0.31 | 84 | 2.34 ± 0.31 | 2.34 ± 0.34 | 2.35 ± 0.28 |
| Hemoglobin (g/dL) | 244 | 11.9 ± 1.3 | 12.0 ± 1.2 | 11.9 ± 1.3 | 87 | 11.8 ± 1.1 | 11.9 ± 1.1 | 11.6 ± 1.1 |
| Phosphate (mg/dL) | 245 | 5.78 ± 1.64 | 5.64 ± 1.53 | 5.91 ± 1.73 | 87 | 5.80 ± 1.61 | 5.77 ± 1.65 | 5.82 ± 1.59 |
| Albumin (g/dL) | 245 | 3.94 ± 0.42 | 3.94 ± 0.46 | 3.94 ± 0.37 | 87 | 3.91 ± 0.49 | 3.92 ± 0.51 | 3.90 ± 0.48 |
Results are shown as mean ± standard deviation, median (10th, 90th percentiles range), or frequency (%), as appropriate. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Selected treatment parameters, including dialysis metrics and medications known to affect the central nervous system, are shown in Table 2 for the Daily and Nocturnal Trials. In the Daily Trial, there were no between-group differences in the proportion of subjects using benzodiazepines, antidepressants, or opioids at baseline or at 12 months. In the Nocturnal Trial, there were no differences in the use of antidepressants or opioids, but benzodiazepines were more widely used at the 12-month follow-up in the group randomized to frequent nocturnal hemodialysis.
Table 2.
Selected baseline and end-of-study characteristics
| Characteristic | Daily Trial |
Nocturnal Trial |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
12-month |
Baseline |
12-month |
|||||
| 3×/week (N = 120) | 6×/week (N = 125) | 3×/week (N = 120) | 6×/week (N = 125) | 3×/week (N = 42) | 6×/week (N = 45) | 3×/week (N = 42) | 6×/week (N = 45) | |
| Weekly standard Kt/Vurea | 2.49 ± 0.38 | 2.49 ± 0.27 | 2.47 ± 0.27 | 3.49 ± 0.63 | 2.35 ± 0.35 | 2.33 ± 0.30 | 2.61 ± 0.44 | 4.47 ± 1.60 |
| Ultrafiltration rate (mL/min) | 14.8 ± 4.0 | 15.0 ± 5.5 | 14.5 ± 4.3 | 13.9 ± 4.7 | 10.9 ± 6.2 | 10.1 ± 6.4 | 10.4 ± 3.8 | 6.0 ± 3.6 |
| Benzodiazepines, n (%) | 10 (13) | 14 (14) | 13 (14) | 16 (16) | 7 (18) | 5 (14) | 6 (15) | 10 (27) |
| Antidepressants, n (%) | 11 (12) | 15 (15) | 14 (15) | 15 (15) | 9 (23) | 10 (26) | 10 (27) | 9 (24) |
| Opioids, n (%) | 15 (16) | 17 (17) | 21 (23) | 22 (21) | 7 (18) | 11 (30) | 14 (36) | 14 (38) |
End-of-study sample sizes range from 92 to 104 in the daily study and 37 to 40 in the nocturnal study.
Quality of sleep
Daily Trial
In the Daily Trial, after adjustment for baseline SPI II and clinical center, subjects randomized to frequent as compared with conventional in-center hemodialysis experienced a 4.2 (95% CI 0.4–8.0, P = 0.03) relative mean decline in SPI II at 4 months and a −2.6 (95% CI −7.5–2.3, P = 0.36) decline at 12 months (Table 3). There were no differences in the proportion of patients with poor SPI II scores at 4 or 12 months (Figure 2A). There were no differences observed in self-reported hours of sleep at 4 or 12 months (Table 3). There were no differences observed in self-reported snoring or number of naps at 4 or 12 months (Supplementary data, Appendix). The effects of frequent hemodialysis on quality of sleep did not significantly differ according to age, sex, race/ethnicity, ESRD vintage, current or former smoking or score on the BDI.
Table 3.
Daily Trial: comparison of changes for frequent (six times per week) versus conventional (three times per week) in-center hemodialysis
| Variable | Trt | Observed dataa (mean ± SD) |
Adjusted means and treatment effectsb (± SE or 95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 4 | Month 12 | Month 4 |
Month 12 |
||||
| Change from baseline | Treatment comparison (6× versus 3×) | Change from baseline | Treatment comparison (6× versus 3×) | |||||
| SPI II | 3× | 34.7 ± 19.4 | 36.4 ± 19.0 | 34.0 ± 22.8 | 0.4 ± 1.5 | −4.2c (−8.0 to −0.4) |
−1.2 ± 1.9 | −2.6 (−7.5–2.3) |
| 6× | 35.2 ± 20.5 | 30.6 ± 19.1 | 30.9 ± 22.2 | −3.8 ± 1.4 | −3.8 ± 1.8 | |||
| Hours of sleep | 3× | 5.98 ± 1.44 | 5.93 ± 1.72 | 5.89 ± 1.77 | −0.00 ± 0.14 | +0.02 (−0.35–0.39) |
−0.05 ± 0.16 | 0.02 (−0.39–0.43) |
| 6× | 5.94 ± 1.82 | 6.16 ± 1.64 | 6.04 ± 1.77 | 0.02 ± 0.14 | −0.02 ± 0.15 | |||
SE, standard error; Trt, treatment frequency.
aStatistics apply to subjects with measures at all three time points.
bValues adjusted for clinical center and baseline score.
cP < 0.05.
FIGURE 2:
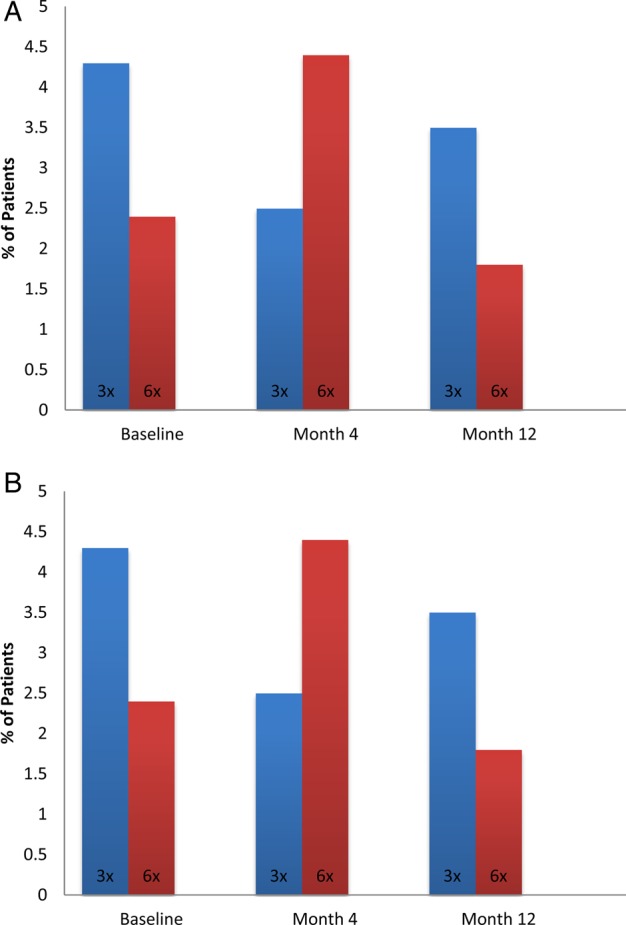
(A) Percent of participants in the Daily Trial with SPI II score >47 by time and treatment. (B) Percent of participants in the Nocturnal Trial with SPI II score >47 by time and treatment.
Nocturnal Trial
In the Nocturnal Trial, subjects randomized to frequent as compared with conventional home hemodialysis experienced 2.9 (95% CI −3.4–9.3, P = 0.30) and 4.5 (95% CI −3.2–12.2, P = 0.25) relative mean declines at Months 4 and 12, respectively (Table 4). There were no differences in the proportion of patients with poor SPI II scores at 4 or 12 months (Figure 2B). There was no difference in self-reported hours of sleep at 4 or 12 months (Table 4). There were also no differences observed in self-reported snoring or number of naps at 4 or 12 months (Supplementary data, Appendix).
Table 4.
Nocturnal Trial: comparison of changes for frequent (six times per week) versus conventional (three times per week) in-center hemodialysis
| Variable | Trt | Observed dataa (mean ± SD) |
Adjusted means and treatment effectsb (± SE or 95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 4 | Month 12 | Month 4 |
Month 12 |
||||
| Change from baseline | Treatment comparison (6× versus 3×) | Change from baseline | Treatment comparison (6× versus 3×) | |||||
| SPI II | 3× | 32.0 ± 18.4 | 33.3 ± 19.1 | 33.0 ± 23.1 | 1.7 ± 2.4 | −2.9 (−9.3–3.4) |
+1.2 ± 2.8 | −4.5 (−12.2–3.2) |
| 6× | 33.8 ± 17.4 | 30.9 ± 16.3 | 29.8 ± 17.7 | −1.3 ± 2.3 | −3.3 ± 2.8 | |||
| Hours of sleep | 3× | 6.37 ± 1.45 | 6.72 ± 1.83 | 6.24 ± 1.55 | 0.25 ± 0.22 | −0.13 (−0.75–0.49) |
−0.16 ± 0.19 | 0.43 (−0.09–0.96) |
| 6× | 6.51 ± 1.43 | 6.59 ± 1.70 | 6.80 ± 1.71 | 0.12 ± 0.23 | +0.27 ± 0.19 | |||
SE, standard error; Trt, treatment frequency.
aStatistics apply to subjects with measures at all three time points.
bValues adjusted for baseline score.
DISCUSSION
The baseline sleep quality scores from the FHN Trials hemodialysis population demonstrate a substantial burden of poor sleep quality. Short sleep, frequent naps and snoring were also reported at high rates in this population. Frequent in-center hemodialysis as compared with conventional in-center hemodialysis did not produce a statistically significant improvement in sleep quality as assessed by the prespecified primary sleep outcome (the SPI II) after 12 months of treatment. There was a significant, but small, benefit of frequent in-center hemodialysis at 4 months. Due in part to the small sample size in the Nocturnal Trial, we were unable to conclude whether frequent nocturnal hemodialysis yielded a benefit or detriment in self-reported sleep quality.
In our previous work, we examined the distribution and correlates of the FHN baseline sleep quality scores. In this work we demonstrated that sleep quality was substantially impaired. The average sleep quality score from the FHN Trial is 10 points higher than the general population scores. Further, the lowest quartile of sleep quality scores was comparable to a symptomatic fibromyalgia or restless legs syndrome population. The sleep quality scores were strongly correlated with quality of life and time to recovery. Since the publication of this work describing baseline scores, the FREEDOM Study published their findings demonstrating a baseline score of 42 (22). The FHN Trial baseline scores were nearly seven points lower (better sleep quality). The difference in baseline scores between the FHN Trials and the FREEDOM Study could be attributed to the different study populations, different study design and different approaches to collection of self-reported data.
More frequent hemodialysis was associated with improvements in symptoms of restless legs and sleep quality in an observational study of 235 subjects undergoing frequent hemodialysis [15]. This study reported a significant within-patient decline in the MOS-SLEEP of 7 points at the 12-month follow-up. This change observed in the FREEDOM Study was larger in magnitude than the change observed in the FHN Trials. The location of daily hemodialysis (home versus in-center), session length and dose of dialysis in FREEDOM differed from that delivered in the FHN Daily Trial. There are also a number of biases found in descriptive studies that can lead to inflated estimates of effects [24]. A randomized trial of nocturnal hemodialysis conducted in Alberta, Canada, demonstrated no significant effect on sleep quality, although the sample size was considerably smaller than in FHN [25].
The observation of a significant 4.2 point improvement in the frequent in-center arm at 4 months should be judged in light of the modest effect size, and that it was not sustained. The effect was approximately half of the effect observed when using gabapentin in patients with neuropathy [26]. The effect of frequent in-center hemodialysis on self-reported sleep quality may have been attenuated by the presence of multiple sleep disorders such as sleep apnea and restless legs syndrome affecting this population. Conversely, it may have been accentuated by the fact that the trial was not blinded.
There was no significant effect of frequent nocturnal hemodialysis on self-reported sleep quality. These findings are consistent with the Alberta trial, which examined self-reported sleep in patients receiving nocturnal hemodialysis. This contrasts with the work of Hanly et al. [7, 8]. It is thought that nocturnal hemodialysis could partially correct sleep apnea in patients on nocturnal hemodialysis due to the increased solute clearance and reduced interdialytic change in volume in the upper airway [7, 27]. However, correlations between the severity of sleep apnea and subjective reports of sleep quality have been relatively weak in both the general population and among patients with ESRD [28]. In the London Daily/Nocturnal Hemodialysis Study, one of the patients doing home nocturnal hemodialysis discontinued due to poor sleep quality [29]. Anecdotal evidence suggests that patients undergoing frequent nocturnal hemodialysis may have difficulties with sleep.
Unlike prior reports examining the effects of frequent hemodialysis on sleep parameters, the FHN trials were relatively large in size, conducted at multiple sites across the USA and Canada, long in duration and, importantly, randomized. We achieved relatively large group separation by the number of sessions per week and solute clearance when considering weekly standard Kt/Vurea. We deployed trained interviewers who were blinded to subjects' group assignment. Adherence to the intervention was excellent, and few subjects were lost to follow-up. However, there are several important limitations. First, difficulty in recruitment reduced the sample size of the Nocturnal Trial, which precluded detection of small but potentially meaningful effects of frequent nocturnal hemodialysis on quality of sleep. Both the Daily and Nocturnal Trials were challenging to recruit participants, perhaps somewhat limiting the generalizability of the trial findings [9, 30]. Second, neither subjects nor their care providers were blinded, given the stark differences in the interventions. Third, we used patient-reported data to measure quality of sleep in the FHN trials. We considered employing polysomnography before and after the intervention—either in all subjects or a subcohort—but abandoned our plans given resource constraints and the complexities of conducting such studies within free-standing dialysis facilities or in subjects' homes. Fourth, there was no systematic assessment of restless legs syndrome in the FHN Trials. As discussed above, observational data demonstrate an association between more frequent dialysis and improvement in the symptoms of restless legs and this work is unable to directly address this finding.
Improving the sleep quality of patients on hemodialysis should be considered a key patient-centered goal of therapy, given the high prevalence and substantial burden of sleep complaints in this population. While frequent hemodialysis might ameliorate signs and symptoms of sleep apnea, in balance it may not yield a net benefit in overall sleep quality. Very large clinical trials would be required to demonstrate an effect (benefit or harm) of frequent hemodialysis on mortality or major health events. As such, the potential beneficial and detrimental effects of frequent hemodialysis on sleep and other elements of daily living should be balanced individually when considering candidates for this modality.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract format. None of the authors declared conflicts of interest regarding this article.
Supplementary Material
ACKNOWLEDGEMENTS
Members of the FHN Trial Group are Achinger S., Anderson S., Appel L., Apruzzes R., Atwal J., Augustine B., Ayus J., Bardsley J., Bay W., Beach S., Beck G., Bharti B., Briggs J., Bullas R., Burkart J., Burrowes J., Cabezon E., Callegari J., Carter M., Champagne J., Chan C., Chan W., Chang J., Chertow G., Cheung A., Copland M., Coplon N., Coppley A., Daugirdas J., Dellagrottaglie S., Depner T., Derse A., Dominguez A., Doss S., Eggers P., Eknoyan G., Escalada R., Fensterer A., Finkelstein F., Fofie Y., Franzwa B., Frome R., Fu Z., Garg A., Gassman J., Gayda P., Geller N., Geronemus R., Goodman W., Gorodetskaya I., Gotch F., Greene T., Greenwood R., Grimm R., Gutierrez M., Hall Y., Handelman G., Henderson L., Hernandez A., Higgins H., Hilkin A., Hostetter T., Hoy C., Humphreys M., Hunsicker L., James S., Kariisa M., Kaufman A., Kaufman T., Kaysen G., Ke S., Keene R., Kimmel P., Kliger A., Kotanko P., Kramer C., Kuhlmann M., Kwan S., Kwok S., Lacson E., Larive B., Leavell E., Lemus D., Levin A., Levin N., Li M., Lilli K., Lindsay R., Lockridge R., Luan J., MacKrell J., Manaster R., Mandaci O., Mathew R., Mauck V., Mazzorato A., McCulloch C., McGrath-Chong M., McLeroy S., Mehta R., Meisels I., Miller B., Mohr P., Moossavi S., Nabali A., Narva A., Nissenson A., Ornt D., Painter P., Pepas J., Peterson C., Pierratos A., Pipkin M., Prichard S., Rajagopalan S., Ramos R., Rashid M., Rastogi A., Regozo K., Riley J., Rivas M., Rocco M., Rodriquez R., Roecker E., Roger D., Rogers J., Salusky I., Sanz G., Sanz J., Schiller-Moran B., Schlarb J., Schuessler R., Schulman G., Schweitzer S., Sergeyeva O., Shah S., Sherer S., Sika M., Sioson L., Skelton R., Smith M., Snell C., Somers D., Sonico J., Spanner E., Star R., Steigerwald D., Stokes J., Suri R., Suter M., Tamura M., Tarallo M., Tichy M., Ting G., Tran T., Ulloa D., Unruh M., Vassalotti J., Wallace W., Waterman E., Wei J., Weiss B., West J., Wiggins K., and Winchester J. This work was supported by the NIH, National Institute of Diabetes and Digestive and Kidney Diseases, the Center for Medicare and Medical Services and the NIH Research Foundation. Contributors to the NIH Foundation in support of the FHN trials included Amgen, Baxter, and Dialysis Clinics. Additional support was provided by DaVita, Dialysis Clinics Inc, Fresenius Medical Care, Renal Advantage, RRI, and Satellite Healthcare.
Contributor Information
Collaborators: for the FHN Trial Group, S. Achinger, S. Anderson, L. Appel, R. Apruzzes, J. Atwal, B. Augustine, J. Ayus, J. Bardsley, W. Bay, S. Beach, G. Beck, B. Bharti, J. Briggs, R. Bullas, J. Burkart, J. Burrowes, E. Cabezon, J. Callegari, M. Carter, J. Champagne, C. Chan, W. Chan, J. Chang, G. Chertow, A. Cheung, M. Copland, N. Coplon, A. Coppley, J. Daugirdas, S. Dellagrottaglie, T. Depner, A. Derse, A. Dominguez, S. Doss, P. Eggers, G. Eknoyan, R. Escalada, A. Fensterer, F. Finkelstein, Y. Fofie, B. Franzwa, R. Frome, Z. Fu, A. Garg, J. Gassman, P. Gayda, N. Geller, R. Geronemus, W. Goodman, I. Gorodetskaya, F. Gotch, T. Greene, R. Greenwood, R. Grimm, M. Gutierrez, Y. Hall, G. Handelman, L. Henderson, A. Hernandez, H. Higgins, A. Hilkin, T. Hostetter, C. Hoy, M. Humphreys, L. Hunsicker, S. James, M. Kariisa, A. Kaufman, T. Kaufman, G. Kaysen, S. Ke, R. Keene, P. Kimmel, A. Kliger, P. Kotanko, C. Kramer, M. Kuhlmann, S. Kwan, S. Kwok, E. Lacson, B. Larive, E. Leavell, D. Lemus, A. Levin, N. Levin, M. Li, K. Lilli, R. Lindsay, R. Lockridge, J. Luan, J. MacKrell, R. Manaster, O. Mandaci, R. Mathew, V. Mauck, A. Mazzorato, C. McCulloch, M. McGrath-Chong, S. McLeroy, R. Mehta, I. Meisels, B. Miller, P. Mohr, S. Moossavi, A. Nabali, A. Narva, A. Nissenson, D. Ornt, P. Painter, J. Pepas, C. Peterson, A. Pierratos, M. Pipkin, S. Prichard, S. Rajagopalan, R. Ramos, M. Rashid, A. Rastogi, K. Regozo, J. Riley, M. Rivas, M. Rocco, R. Rodriquez, E. Roecker, D. Roger, J. Rogers, I. Salusky, G. Sanz, J. Sanz, B. Schiller-Moran, J. Schlarb, R. Schuessler, G. Schulman, S. Schweitzer, O. Sergeyeva, S. Shah, S. Sherer, M. Sika, L. Sioson, R. Skelton, M. Smith, C. Snell, D. Somers, J. Sonico, E. Spanner, R. Star, D. Steigerwald, J. Stokes, R. Suri, M. Suter, M. Tamura, M. Tarallo, M. Tichy, G. Ting, T. Tran, D. Ulloa, M. Unruh, J. Vassalotti, W. Wallace, E. Waterman, J. Wei, B. Weiss, J. West, K. Wiggins, and J. Winchester
REFERENCES
- 1.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis 2007; 14: 82–99 [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez L, Tighiouart H, Scott T et al. . Association of sleep disturbances with cognitive impairment and depression in maintenance hemodialysis patients. J Nephrol 2013; 26: 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedayati SS, Bosworth HB, Briley LP et al. . Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int 2008; 74: 930–936 [DOI] [PubMed] [Google Scholar]
- 4.Unruh M, Kurella Tamura M, Larive B et al. . Impact of sleep quality on cardiovascular outcomes in hemodialysis patients: results from the frequent hemodialysis network study. Am J Nephrol 2011; 33: 398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unruh ML, Buysse DJ, Dew MA et al. . Sleep quality and its correlates in the first year of dialysis. Clin J Am Soc Nephrol 2006; 1: 802–810 [DOI] [PubMed] [Google Scholar]
- 6.Ramkumar N, Beddhu S, Eggers P et al. . Patient preferences for in-center intense hemodialysis. Hemodial Int 2005; 9: 281–295 [DOI] [PubMed] [Google Scholar]
- 7.Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med 2001; 344: 102–107 [DOI] [PubMed] [Google Scholar]
- 8.Chan CT, Hanly P, Gabor J et al. . Impact of nocturnal hemodialysis on the variability of heart rate and duration of hypoxemia during sleep. Kidney Int 2004; 65: 661–665 [DOI] [PubMed] [Google Scholar]
- 9.Rocco MV, Lockridge RS Jr, Beck GJ et al. . The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 2011; 80: 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group FHNT, Chertow GM, Levin NW et al. . In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363: 2287–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocco MV, Larive B, Eggers PW et al. . Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. Am J Kidney Dis 2011; 57: 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suri RS, Garg AX, Chertow GM et al. . Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int 2007; 71: 349–359 [DOI] [PubMed] [Google Scholar]
- 13.Hays RD, Martin SA, Sesti AM et al. . Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med 2005; 6: 41–44 [DOI] [PubMed] [Google Scholar]
- 14.Viala-Danten M, Martin S, Guillemin I et al. . Evaluation of the reliability and validity of the Medical Outcomes Study sleep scale in patients with painful diabetic peripheral neuropathy during an international clinical trial. Health Qual Life Outcomes 2008; 6: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaber BL, Schiller B, Burkart JM et al. . Impact of short daily hemodialysis on restless legs symptoms and sleep disturbances. Clin J Am Soc Nephrol 2011; 6: 1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unruh ML, Hartunian MG, Chapman MM et al. . Sleep quality and clinical correlates in patients on maintenance dialysis. Clin Nephrol 2003; 59: 280–288 [DOI] [PubMed] [Google Scholar]
- 17.Hemmelgarn BR, Manns BJ, Quan H et al. . Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 2003; 42: 125–132 [DOI] [PubMed] [Google Scholar]
- 18.Miskulin DC, Athienites NV, Yan G et al. . Comorbidity assessment using the Index of Coexistent Diseases in a multicenter clinical trial. Kidney Int 2001; 60: 1498–1510 [DOI] [PubMed] [Google Scholar]
- 19.Unruh ML, Larive B, Chertow GM et al. . Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials. Am J Kidney Dis 2013; 61: 748–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer, 2000 [Google Scholar]
- 21.van Seventer R, Bach FW, Toth CC et al. . Pregabalin in the treatment of post-traumatic peripheral neuropathic pain: a randomized double-blind trial. Eur J Neurol 2010; 17: 1082–1089 [DOI] [PubMed] [Google Scholar]
- 22.Colangelo KJ, Pope JE, Peschken C. The minimally important difference for patient reported outcomes in systemic lupus erythematosus including the HAQ-DI, pain, fatigue, and SF-36. J Rheumatol 2009; 36: 2231–2237 [DOI] [PubMed] [Google Scholar]
- 23.Agresti A. Analysis of Ordinal Categorical Data, 2nd edn New York, NY: John Wiley & Sons, 2010 [Google Scholar]
- 24.Abdel-Kader K, Unruh ML. Benefits of short daily home hemodialysis in the FREEDOM Study: is it about person, place, time, or treatment? Kidney Int 2012; 82: 511–513 [DOI] [PubMed] [Google Scholar]
- 25.Culleton BF, Walsh M, Klarenbach SW et al. . Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007; 298: 1291–1299 [DOI] [PubMed] [Google Scholar]
- 26.Arnold LM, Goldenberg DL, Stanford SB et al. . Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum 2007; 56: 1336–1344 [DOI] [PubMed] [Google Scholar]
- 27.Beecroft JM, Hoffstein V, Pierratos A et al. . Nocturnal haemodialysis increases pharyngeal size in patients with sleep apnoea and end-stage renal disease. Nephrol Dial Transplant 2008; 23: 673–679 [DOI] [PubMed] [Google Scholar]
- 28.Unruh ML, Sanders MH, Redline S et al. . Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the sleep heart health study. Am J Kidney Dis 2008; 52: 305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindsay RM. The London, Ontario, Daily/Nocturnal Hemodialysis Study. Semin Dial 2004; 17: 85–91 [DOI] [PubMed] [Google Scholar]
- 30.Sergeyeva O, Gorodetskaya I, Ramos R et al. . Challenges to enrollment and randomization of the Frequent Hemodialysis Network (FHN) Daily Trial. J Nephrol 2012; 25: 302–309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.