Abstract
Type II diabetes mellitus (DM) increases risk for cognitive decline and is associated with brain atrophy in older demented and non-demented individuals. We investigated (1) the cross-sectional association between fasting blood glucose level and cortical thickness in a sample of largely middle-aged, cognitively normal adults, and (2) whether these associations were modified by genes associated with both lipid processing and dementia. To explore possible modifications by genetic status, we investigated the interaction between blood glucose levels and the apolipoprotein E (APOE) ε4 allele and the translocase of the outer mitochondrial membrane (TOMM) 40 ′523 genotype on cortical thickness. Cortical thickness measures were based on mean thickness in a subset of a priori-selected brain regions hypothesized to be vulnerable to atrophy in Alzheimer's disease (AD) (i.e., ‘AD vulnerable regions’). Participants included 233 cognitively normal subjects in the BIOCARD study who had a measure of fasting blood glucose and cortical thickness measures, quantified by magnetic resonance imaging (MRI) scans. After adjustment for age, sex, race, education, depression, and medical conditions, higher blood glucose was associated with thinner parahippocampal gyri (B = −0.002; 95% CI −0.004, −0.0004) and temporal pole (B = −0.002; 95% CI −0.004, −0.0001), as well as reduced average thickness over AD vulnerable regions (B = −0.001; 95% CI −0.002, −0.0001). There was no evidence for greater cortical thinning in ε4 carriers of the APOE gene or in APOE ε3/3 individuals carrying the TOMM40 VL/VL genotypes. When individuals with glucose levels in the diabetic range (≥126 mg/dL), were excluded from the analysis, the associations between glucose levels and cortical thickness were no longer significant. These findings suggest that glucose levels in the diabetic range are associated with reduced cortical thickness in AD vulnerable regions as early as middle age.
Keywords: Cortical thinning, Alzheimer's disease, APOE, TOMM40, Blood glucose
1. Introduction
Approximately 28.9 million people in the United States have type II diabetes mellitus (DM), a metabolic disorder characterized by high blood sugar levels (hyperglycemia) in the context of insulin resistance. Older adults are disproportionately affected; it is estimated that nearly 30% of people over the age of 65 have DM and approximately one-quarter of DM cases are undiagnosed [2]. Past research has established that DM is a risk factor for dementia [30], with one study estimating that 6–10% of dementia cases are directly attributable to DM [37]. Further, studies have shown that diabetics have reduced mean cortical thickness and reduced thickness in AD-vulnerable brain regions, measured on magnetic resonance imaging (MRI) scans, compared to nondiabetics with a range of clinical diagnoses from normal to dementia [5,8,45,56]. However, past studies have not examined the relationship of glucose levels and cortical thickness in a cognitively normal, primarily middle-aged cohort that is not exclusively a diabetic sample, nor have they examined how genetic factors may impact these associations. To our knowledge, only one study has shown that higher glucose and glycated hemoglobin (HbA1c) levels among middle-aged and older non-demented adults are associated with grey matter atrophy in multiple regions [39]. This study, however, included a small number of patients with a diagnosis of DM, as well as individuals with glucose values in the diabetic range, who may have had undiagnosed diabetes. It therefore remains unclear if associations between glucose levels and cortical thickness only exist for individuals with glucose levels in the diabetic range, or also for individuals with glucose levels in the pre-diabetic and normal range.
Two genes associated with risk for Alzheimer's disease (AD) are of particular relevance to the potential impact of blood glucose on cortical thinning, as they alter lipid and other metabolic pathways [3,33,41]. The apolipoprotein E (APOE) gene is critical for lipid processing and clearance of beta-amyloid (Aβ) from the brain [41]. Compared to the ε2 or ε3 alleles, the ε4 allele is associated with an increased risk of Alzheimer's dementia [19], cognitive decline [6,7], and smaller brain volumes and cortical thinning [18,20]. Further, evidence suggests an interaction between both DM and APOE, such that individuals who have both risk factors have compounded risk for poor cognitive outcomes compared to individuals who have just one of these risk factors [55]. More recently, a poly-T variant rs10524523 or ′523 in intron 6 of the translocase of the outer mitochondrial membrane gene (TOMM) 40 ′523, which has three allelic length variations – short (S), long (L), and very long (VL) – has also been linked with risk of AD dementia. TOMM40 is hypothesized to impact dementia risk through mitochondrial functioning [3,33]. Research has shown that impaired mitochondrial function leads to reduced glucose uptake in older individuals, and can lead to insulin resistance [36,47]), thus perpetuating the cycle linking TOMM40, DM, and dementia. Compared to the TOMM40 short (S) allele, the very long (VL) allele is associated with earlier onset of Alzheimer's dementia [50], smaller brain volumes, and poorer cognitive performance [21].
Despite evidence linking AD-dementia to both TOMM40 and APOE, as well as to DM, prior studies have not directly examined whether these genotypes alter the association between blood glucose levels and structural brain measures. The goals of the current study, therefore, were two-fold. First, extending findings from previous work [9,10], which focused on volumetric measures of medial temporal regions (e.g., hippocampus, amygdala), the present study examined associations between glucose and cortical thickness in AD-vulnerable regions in a sample that was cognitively normal and largely middle aged. Importantly, our analyses took into account cases of possibly undiagnosed DM. Second, this is the first study, to our knowledge, to examine if the relationship between blood glucose levels and cortical thickness is modified by genetic risk factors for AD. We hypothesized that higher baseline blood glucose would be associated with reduced cortical thickness, particularly in brain regions associated with AD-related atrophy, and that this relationship would be stronger among those with an APOE ε4 allele or the TOMM40 ′523 VL/VL genotype.
2. Methods
2.1. BIOCARD study
The data for these analyses come from the BIOCARD Study, which was initiated at the National Institutes of Health (NIH) in 1995 by the Geriatric Psychiatry Branch of the National Institute of Mental Health (PI: Trey Sunderland). It was designed to investigate risk factors for cognitive decline and dementia in a healthy, mainly middle-aged cohort, enriched with those positive for a family history of dementia. The study was discontinued in 2005 for administrative reasons; it was then re-initiated in 2009 at the Johns Hopkins University School of Medicine (PI: Marilyn Albert). To date, approximately 90% of the original participants have been reenrolled [1].
At baseline, participants completed a multi-day evaluation at the Clinical Center of the NIH, which included a clinical and neurological exam, cognitive testing, standard biochemical assays (e.g., potassium, albumin, glucose), and history of current or past medical conditions (e.g., cardiovascular disease, metabolic conditions) and smoking and substance abuse. At enrollment, the majority of participants also underwent an MRI scan of the brain and a lumbar puncture to obtain samples of cerebrospinal fluid (CSF). Annual follow-up exams included a physical and neurological exam and cognitive testing. Details regarding the neuropsychological assessment have been published elsewhere [1]; briefly, the battery includes tests that measure a range of cognitive domains (i.e., memory executive function, language, visuospatial ability, attention, speed of processing, and psychomotor speed). While the study was being conducted at the NIH, participants completed MRI scans and CSF collection every two years.
2.2. Participants
At baseline, participants (n = 349) were primarily middle-aged (mean age = 57.2 years, standard deviation (SD) = 10.3 years). By design, approximately three-quarters of participants had a first-degree relative with dementia. Exclusion criteria included cognitive impairment, and significant medical, psychiatric, or neurological disorders (e.g., severe cardiovascular disease, bipolar disorder, Parkinson's disease, epilepsy, etc.). Informed consent was obtained from all participants.
2.3. Glucose and diabetes measures
Fasting blood glucose was obtained from blood drawn at baseline and at follow-up visits at the NIH Clinical Center. The fasting blood draw and MRI scan (described below) used in the present analyses were performed within a three month period. DM status was ascertained during the clinical exam, during which participants were also asked to report medication use, including DM medication (e.g., Metformin, injectable insulin, etc.). Four participants had a recorded DM diagnosis. An additional 14 participants had blood glucose measures at one or more study visits that were in the diabetic range (≥126 mg/dL) established by the American Diabetes Association [2]). There were 54 individuals with glucose values in the pre-diabetic range (101 to 125 mg/dL).
2.4. Genotyping
APOE genotyping was completed by Athena Diagnostics (Worcester, MA), using restriction endonuclease digestion of polymerase chain reaction (PCR) amplified genomic DNA. For the analyses described below, genotypes were coded dichotomously by APOE ε4 status – those with one or more ε4 alleles (1) and those without an ε4 allele (0).
For TOMM40 genotyping, DNA samples were plated on 96-well plates for long-range PCR and genotyping of the TOMM40-523 poly-T variant, which was performed at Polymorphic DNA Technologies (Alameda, CA, USA); see Roses et al. [50] and Linnertz et al. [40] for further details. Alleles of the TOMM40-523 poly-T variant were classified using the convention established by Roses and colleagues for naming allele lengths: short (S), ≤18 poly-T repeats; long (L) 19–30 repeats; and very long (VL) ≥31 repeats [40,50]. The TOMM40 ′523 analyses were also coded dichotomously – those with the VL/VL genotype (1) and those with the S/S genotype (0) (described in more detail under Statistical Analyses, below).
2.5. MRI parameters and cortical thickness measures
MRI scans were completed on a 1.5 Tesla General Electric scanner using a standard multimodal protocol. The scanning protocol included localizer scans, axial Fast Spin Echo sequence (repetition time (TR) = 4250, echo time (TE) = 108, field of view (FOV) = 512 × 512, thickness/gap = 5.0/0.0 mm, flip angle = 90, 28 slices), axial Flair sequence (TR = 9002, TE = 157.5, FOV = 256 × 256, thickness/gap = 5.0/0.0 mm, flip angle = 90, 28 slices), coronal Spoiled Gradient Echo (SPGR) sequence (TR = 24, TE = 2, FOV = 256 × 256, thickness/gap = 2.0/ 0.0 mm, flip angle = 20, 124 slices), sagittal SPGR sequence (TR = 24, TE = 3, FOV = 256 × 256, thickness/gap 1.5/0.0 mm, flip angle = 45, 124 slices).
Cortical thickness measures were obtained from FreeSurfer (version 5.1), image analysis software that is freely available for download online (http://surfer.nmr.mgh.harvard.edu/). These methods have been detailed elsewhere [11,22–25]. Briefly, cortical thickness is calculated by estimating the distance from the grey/white boundary to the grey/CSF boundary at each vertex on the tessellated surface, utilizing both intensity and continuity information from the entire three dimensional MR volume in segmentation and deformation procedures [22]. These procedures have been validated against histological analysis [49] and manual measurements [38,52], and show good test-retest reliability across scanner manufacturers and across field strengths [32]. Visual inspection of images was carefully carried out by one of the co-authors (CP) to ensure accuracy of the skull strip, white/pial surface generation, and tissue classifications. Manual corrections were performed on scans as necessary.
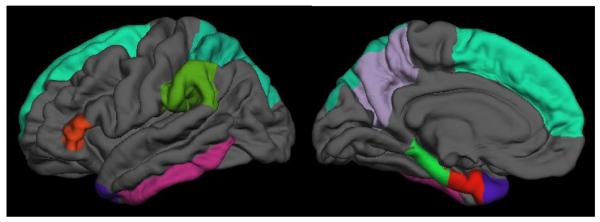
The present analyses focused on cortical thickness measures obtained from nine FreeSurfer-labeled regions of interest (ROI) that were selected to reflect ‘AD vulnerable regions’ previously identified by Dickerson et al.[15,16] as ‘AD signature’ regions (i.e., regions that show greater atrophy in subjects with AD dementia as compared to controls). These 9 regions include the: entorhinal cortex, inferior temporal gyrus, parahippocampal gyrus, pars triangularis, precuneus, superior frontal gyrus, superior parietal gyrus, supramarginal gyrus, and temporal pole, each averaged over the left and right hemispheres (Fig. 1). In the present analyses, we examined each region individually and also created an average of these 9 regions. Cortical thickness measures were available for 240 of the 333 participants with fasting blood glucose data, all of whom were cognitively normal at baseline. The 240 with cortical thickness measures excludes an additional 62 participants, either because coronal SPGRs were not available (n = 22) or FreeSurfer surface reconstructions were unreliable (e.g., motion artifact or low contrast resulted in poor scan quality or regions were missing from surfaces, n = 40). Of those with useable FreeSurfer data, data from an additional 29 were not considered for analysis (n = 22 have not yet re-enrolled or withdrawn from the study and n = 7 had clinical symptom onset at- or prior-to baseline, as per current consensus diagnosis procedures). See Albert et al. [1] for further details regarding the diagnostic procedures in the study.
Fig. 1.
The ‘AD vulnerable’ regions included in analyses based on FreeSurfer cortical labels. Regions are depicted on the left hemisphere pial surface for the lateral (left) and medial (right) views.
2.6. Statistical analyses
Analyses included 233 participants with complete cortical thickness and glucose measures, 227 of whom had APOE genotyping and were not ε2/4 carriers, and 79 of whom were TOMM40 S/S or VL/VL carriers without an ε4 allele. We used chi-square tests and Student's t-tests to compare participant demographic variables by APOE and ′523 genotypes (i.e., ε4-carrier versus non-carrier and S/S versus VL/VL). We then used linear regression models to determine the association between blood glucose level and cortical thickness, both measured at baseline. In secondary analyses, we examined whether blood glucose levels in the pre-diabetic range negatively impact cortical thickness. To do so, we investigated the association between glucose level and average cortical thickness across AD vulnerable regions accounting for hyperglycemia and DM. Specifically, we excluded participants with diagnosed DM (n = 4) and possible DM (one or more glucose measures ≥126 mg/dL (n = 14), and performed separate stratified analyses in those below and in those at and above the pre-diabetic threshold (100 mg/dL) established by the American Diabetes Association [2]. For each of these analyses, we ran two regression models, Model 1 and Model 2. In Model 1, we adjusted for age and sex. In Model 2, we adjusted for the same covariates as in Model 1, as well as race, education, and baseline medical conditions (i.e., cardiovascular disease, hypertension, hypercholesterolemia, history of traumatic brain injury, and depression), because these factors have also been associated with brain atrophy and dementia.
We created interaction terms (cross-products) in order to investigate whether APOE or TOMM40 ′523 modified the association between glucose and cortical thickness. The first term reflected the interaction between glucose level and APOE genotype (ε4 carrier (1) versus non-carrier (0)); the second term reflected the interaction between glucose level and TOMM40 ′523 genotype (VL/VL (1) versus S/S (0)). For the TOMM40 ′523 interaction term, we restricted the sample to only those participants who were APOE ε3 homozygous. We did so because the S allele is associated with poorer cognitive outcomes in APOE ε3 heterozygotes [27–29,44]. This restriction also helped to nullify any effect that the APOE ε2 and ε4 alleles might have had on cortical thickness in TOMM40 analyses [27–29,44]. Additionally, because the L allele is in high linkage disequilibrium with the ε4 allele (i.e., recombination occurs at different frequencies than if there was random assortment), and therefore the alleles' effects are not separable statistically, we compared people with the VL/VL genotype to people with the S/S genotype [29]. The TOMM40 analyses also excluded non-white participants (n = 3), because early evidence suggests that, like APOE, the distribution of TOMM40 alleles and associated risk vary by race and ethnic group. Since these variations are not yet well understood, as they are for APOE, we took a more conservative approach of limiting the race/ethnic composition of our analytic sample [51]. We added the APOE and TOMM40 interaction terms to Models 1 and 2 (described above, containing the main effects under study), and if the interaction coefficient was significant at a level of p < 0.05, we stratified by risk genotype. All analyses were completed using Stata 12.0 [53].
3. Results
3.1. Participant characteristics
Participant characteristics and baseline cognitive performance are shown stratified by APOE ε4 status and TOMM40 status in Table 1. After adjusting for age and education, there was no difference in cognitive performance as a function of APOE ε4 status and TOMM40 status (results not shown). Individuals with an ε4 allele were more likely to have a history of hypercholesterolemia and depression, compared to non-carriers.
Table 1.
Participant characteristics at baseline, n (%) or mean ± standard deviation.
| Total (N = 233) |
APOE ε4 carriers (N = 70) |
APOE ε4 non-carriers (N = 157) |
p | TOMM40 VL/VL (N = 39) |
TOMM40 S/S (N = 40) |
p | |
|---|---|---|---|---|---|---|---|
| Age | 56.4 ± 9.8 | 57.1 ± 8.3 | 56.0 ± 10.5 | 0.44 | 57.0 ± 11.3 | 55.1 ± 10.5 | 0.46 |
| Female | 145 (62) | 43 (61) | 97 (62) | 0.96 | 25 (64) | 24 (60) | 0.71 |
| Caucasian | 230 (99) | 68 (97) | 156 (99) | 0.17 | N/A | N/A | N/A |
| Education | 17.0 ± 2.4 | 17.3 ± 2.2 | 16.9 ± 2.5 | 0.35 | 16.8 ± 2.4 | 16.9 ± 2.3 | 0.95 |
| Baseline glucose (mg/dL) | 98.6 ± 24.1 | 97.1 ± 17.9 | 99.4 ± 26.9 | 0.53 | 100.8 ± 34.5 | 101.4 ± 28.4 | 0.95 |
| Diabetes | 4 (2) | 0 (0) | 4 (3) | 0.17 | 0 (0) | 1 (3) | 0.32 |
| Cardiovascular conditions | 11 (5) | 4 (6) | 6 (4) | 0.56 | 3 (8) | 1 (3) | 0.29 |
| Hypertension | 29 (13) | 12 (17) | 17 (11) | 0.23 | 3 (8) | 3 (8) | 0.97 |
| Hypercholesterolemia | 31 (14) | 17 (24) | 14 (9) | 0.003 | 3 (8) | 4 (10) | 0.72 |
| Traumatic brain injury (TBI) | 4 (2) | 0 (0) | 4 (3) | 0.17 | 1 (3) | 0 (0) | 0.31 |
| Depression, ever | 44 (20) | 19 (27) | 23 (15) | 0.03 | 8 (21) | 6 (15) | 0.51 |
| Depression, within 2 years of baseline | 31 (14) | 15 (21) | 15 (10) | 0.02 | 8 (21) | 4 (10) | 0.21 |
| Logical Memory Immediate | 15.0 ± 3.2 | 15.1 ± 3.2 | 14.9 ± 3.2 | 0.82 | 15.1 ± 3.1 | 15.7 ± 2.4 | 0.46 |
| Logical Memory Delayed | 13.0 ± 3.8 | 13.2 ± 3.6 | 12.9 ± 4.0 | 0.67 | 12.8 ± 3.3 | 14.5 ± 2.9 | 0.09 |
| Logical Memory, % retention | 51.9 ± 15.4 | 52.7 ± 14.4 | 51.6 ± 15.8 | 0.67 | 51.3 ± 13.0 | 58.0 ± 11.6 | 0.09 |
| Paired Associate Immediate | 19.9 ± 3.6 | 20.3 ± 3.7 | 19.7 ± 3.6 | 0.39 | 20.0 ± 3.6 | 20.8 ± 2.8 | 0.48 |
| Paired Associate Delayed | 7.6 ± 0.8 | 7.6 ± 0.8 | 7.6 ± 0.9 | 0.86 | 7.6 ± 0.8 | 7.9 ± 0.5 | 0.16 |
| Rey Figure Recall | 18.9 ± 6.5 | 17.9 ± 6.5 | 19.4 ± 6.4 | 0.11 | 18.1 ± 7.1 | 19.8 ± 6.5 | 0.27 |
| Block Design Subtest | 33.2 ± 9.3 | 32.9 ± 10.2 | 33.3 ± 8.9 | 0.76 | 34.4 ± 8.6 | 33.7 ± 10.3 | 0.75 |
| Boston Naming Test, % correct | 93.6 ± 7.4 | 93.7 ± 5.6 | 93.6 ± 8.1 | 0.95 | 94.0 ± 5.4 | 94.6 ± 6.9 | 0.68 |
| Digit Symbol Test | 54.8 ± 11.0 | 56.8 ± 12.1 | 53.9 ± 10.4 | 0.05 | 54.1 ± 9.4 | 56.4 ± 11.6 | 0.35 |
Values presented as n (%) were compared between groups using chi-square tests; values presented as mean ± standard deviation were compared between groups using t-tests.
Bold numbers indicate significance at p < 0.05 level.
3.2. Blood glucose and cortical thickness
In Model 1, which adjusted for age and sex and included data from all participants, we did not observe an association between blood glucose level and cortical thickness (Table 2). In Model 2, which was adjusted for age, sex, education, race, and medical conditions, higher glucose levels were associated with lower average thickness in AD vulnerable regions (B = −0.001 95% CI −0.002, −0.0001), and in two individual ROIs: parahippocampal gyri (B = −0.002, 95% CI −0.004, −0.0004) and temporal pole (B = −0.002, 95% CI −0.004, −0.0001). In secondary analyses, excluding those with diagnosed and possible DM and stratifying at the pre-diabetic threshold, we did not observe an association between glucose level and average thickness in AD vulnerable regions for either the normal (n = 164; Model 1: B = 0.0003, 95% CI −0.002, 0.003, p = 0.837; Model 2: B = −0.0003, 95% CI −0.003, 0.003, p = 0.841) or pre-diabetic groups (n = 54; Model 1: 0.00006, 95% CI −0.001, 0.001, p = 0.920; Model 2: B = −0.0009, 95% CI −0.003, 0.0008, p = 0.301). With regard to interactions of glucose with APOE ε4 and glucose with TOMM40, there were no significant interactions at the p < 0.05 level.
Table 2.
Association between blood glucose and cortical thickness.
| Model 1 (N = 226) |
Model 2 (N = 209) |
|||
|---|---|---|---|---|
| B (95% CI) | p | B (95% CI) | p | |
| Entorhinal cortex | 0.0001 (−0.002, 0.002) | 0.896 | −0.002 (−0.004, 0.0005) | 0.139 |
| Inferior temporal gyrus | −0.0002 (−0.0009, 0.0005) | 0.622 | 0.00004 (−0.0008, 0.0009) | 0.932 |
| Parahippocampal gyrus | −0.001 (−0.003, 0.0004) | 0.131 | −0.002 (−0.004, −0.0004) | 0.016 |
| Pars triangularis | −0.0007 (−0.001, 0.00003) | 0.061 | −0.0007 (−0.002, 0.0002) | 0.146 |
| Precuneus | −0.00006 (−0.0008, 0.0007) | 0.874 | −0.0003 (−0.001, 0.0005) | 0.508 |
| Superior frontal gyrus | −0.00009 (−0.001, 0.0008) | 0.832 | −0.0006 (−0.002, 0.0004) | 0.246 |
| Superior parietal gyrus | 0.0003 (−0.0004, 0.001) | 0.421 | −0.00005 (−0.0008, 0.0007) | 0.907 |
| Supramarginal gyrus | 0.0003 (−0.0004, 0.001) | 0.424 | −0.0002 (−0.001, 0.0006) | 0.674 |
| Temporal pole | −0.0005 (−0.002, 0.001) | 0.509 | −0.002 (−0.004, −0.0001) | 0.043 |
| Average thickness | −0.0002 (−0.0009, 0.0004) | 0.491 | −0.001 (−0.002, −0.0001) | 0.033 |
Average thickness is over the AD-specified areas. Model 1 adjusted for age and sex. Model 2 adjusted for age, sex, race, education, depression, and medical conditions.
Bold numbers indicate significance at p < 0.05 level.
To investigate whether the observed association was specific to the AD-signature regions, or extended to other regions, we examined the association between glucose and thickness averaged across all 34 regions for which FreeSurfer measures cortical thickness. Glucose was not associated with whole surface thickness in either Model 1 or 2 (results not shown). This suggests that our findings are specific to the AD-vulnerable ROIs.
4. Discussion
We investigated the cross-sectional association between fasting blood glucose level and cortical thickness, and whether this association is modified by APOE and TOMM40 ′523 genotypes, in a healthy, cognitively normal, middle-aged sample. In a model adjusted for age, sex, education, race, depression and medical conditions, we found that higher fasting blood glucose was significantly associated with decreased thickness for the mean of the AD vulnerable regions, as well as decreased thickness in two individual ROIs: the parahippocampal gyrus and temporal pole. These associations were no longer significant in secondary analyses that excluded participants with diagnosed and possible DM, and were stratified by pre-diabetic versus normal status. Further, the association between blood glucose and cortical thickness was not modified by either APOE ε4 or TOMM40 genotype.
These results extend previous findings in several ways. First, the only prior study to report an association between blood glucose levels and cortical thickness in a largely non-diabetic sample [39] included significantly older individuals (mean age = 68) whose cognitive status ranged from normal to mild impairment (Mini-Mental State Examination scores = 23–30). The present study extends the age at which a relationship between blood glucose levels and cortical thickness can be observed in a largely non-diabetic sample and shows that such associations are present even among cognitively normal, middle-aged adults. Second, the study by Leritz et al. [39] included a small number of subjects with diabetes as well as some individuals with glucose levels in the diabetic range. It was unclear, therefore, whether associations between glucose levels and cortical thickness can be observed among individuals with normal or pre-diabetic glucose levels. Our results showed that once individuals with glucose levels in the diabetic range are excluded (independent of an actual diabetes diagnosis), there was no association between glucose and cortical thickness, even for individuals with glucose levels in the pre-diabetic range. While Leritz et al. [39] did not conduct similar analyses, their results also appeared to be driven by individuals with the highest glucose levels. Taken together, this suggests that only glucose levels in the diabetic range negatively impact cortical thickness. However, given that up to one-quarter of diabetes cases among older adults are undiagnosed, our results also highlight the potentially serious effects of untreated diabetes on brain structure and future risk of cognitive impairment. Lastly, the finding that neither TOMM40, nor APOE-ε4 modified the relationship between blood glucose levels and cortical thickness may suggest these two genotypes alter the risk for late onset AD through mechanisms that are largely independent of the mechanism by which diabetes and glucose metabolism modify the risk for AD dementia.
Imaging studies in non-demented older DM patients have suggested that the cortical thinning observed in these patients mirrors the patterns of thinning seen in AD patients independent of white matter hyperintensities or microbleeds [46]. Specifically, in both DM and AD, thinning begins in the temporal, entorhinal, and parietal cortex and then advances to the orbitofrontal areas, and is more advanced in the left hemisphere [45,46,54]. Consistent with these prior results, the current study also found the strongest relationship between glucose levels and cortical thickness in temporal regions. It may be that higher glucose levels lead to grey matter atrophy via cerebral glucose hypometabolism, which leads to neuronal injury, which has been shown to be independent of Aβ accumulation [48]. Although research has shown that cortical thinning occurs in the prodromal stages of several types of dementia (i.e., dementia with Lewy bodies, AD, and vascular dementia) [4,13,14, 35], few studies have investigated cortical thickness in a cognitively normal, healthy sample [17,34,42].
Moreover, studies have found that among diabetics (aged 47–75) with cortical thinning, one year of insulin therapy produces increases in cortical thickness [8]. Our findings raise the possibility that even in undiagnosed DM, glucose levels may have a measureable negative effect on brain integrity in cognitively normal, middle-aged subjects. Thus, targeting modifiable risk factors (e.g., DM) in middle age or later middle age may significantly reduce the risk or delay the onset of dementia [12]. Additionally, DM treatments have been shown to improve cognitive performance and brain function [26]. Identifying individuals at greatest risk for dementia, or in its earliest stages via biomarkers, such as grey matter atrophy, may help to better focus these intervention efforts.
This study has many strengths, including the careful evaluation of cognitively normal individuals, the largely middle-aged cohort, and inclusion of both APOE and TOMM40 genotyping. However, this study also has several limitations. First, we used fasting blood glucose levels, as opposed to HbA1c measures. The latter are a measure of glucose over a period of three months, and therefore are more representative of glucose control. That we found significant associations in the present study suggests that the effect of glucose on grey matter atrophy may be strong, given we observed this association despite using a predictor that may be less stable (i.e., fasting blood glucose). Second, we may have had insufficient power to detect differences in the association between blood glucose and cortical thickness by TOMM40 genotype, as our sample size of VL homozygotes (n = 40) and S homozygotes (n = 41) was limited. Future research should elucidate possible interaction effects in larger samples, and investigate whether other TOMM40 genotypes modify the relationship between glucose or DM and cortical thickness. Third, many participants were missing BMI data at baseline, so we were unable to include it as a covariate in the models. Although BMI has been linked to brain atrophy [31], this association may be through hyperglycemia [43]. Therefore, including BMI as a covariate may have been over-adjustment. Fourth, this study sample was primarily white, had a high mean level of education, and most participants had a first-degree relative with dementia. These characteristics limit the generalizability of these findings to the population as a whole. Finally, because this study was cross-sectional, we cannot infer a causal association between blood glucose and cortical thinning. Because follow-up of the cohort has continued, longitudinal analyses may be feasible at a later date.
4.1. Conclusions
Our results suggest elevated glucose levels may promote cortical thinning. Treatment of modifiable risk factors, such as DM, may be an effective way to slow the progression of cognitive decline. Moving forward, it will be important to better understand whether early evidence of cortical thinning predicts development of MCI and dementia, the role blood glucose plays in this association, and whether blood glucose control may be a means of slowing or preventing cortical thinning and cognitive impairment.
Acknowledgements
Dr. Spira has received grants from the National Institute on Aging, Johns Hopkins University, and the William and Ella Owens Medical Research Foundation. Dr. Roses is the Jefferson-Pilot Professor of Neurosciences and Neurology at Duke University. Dr. Roses is the CEO and sole shareholder of Zinfandel Pharmaceuticals, Inc. (a NC S-Corp). Zinfandel is an alliance with Takeda Pharmaceuticals to manage the TOMMORROW study.
This study was supported in part by grants from the National Institutes of Health (U19-AG03365, P50-AG005146, and T32-AG027668). The BIOCARD Study consists of 7 Cores with the following members: (1) the Administrative Core (Marilyn Albert, Barbara Rodzon); (2) the Clinical Core (Ola Selnes, Marilyn Albert, Anja Soldan, Rebecca Gottesman, Ned Sacktor, Guy McKhann, Scott Turner, Leonie Farrington, Maura Grega, Gay Rudow, Daniel D'Agostino, Scott Rudow); (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Timothy Brown, Hayan Chi, Anthony Kolasny, Kenichi Oishi, Thomas Reigel, Laurent Younes); (4) the Biospecimen Core (Abhay Moghekar, Richard O’Brien, Abby Spangler); (5) the Informatics Core (Roberta Scherer, David Shade, Ann Ervin, Jennifer Jones, Matt Toepfner, Lauren Parlett, April Patterson, Aisha Mohammed); (6) the Biostatistics Core (Mei-Cheng Wang, Qing Cai, Daisy Lu); and (7) the Neuropathology Core (Juan Troncoso, Barbara Crain, Olga Pletnikova, Gay Rudow, and Karen Fisher). The authors are grateful to the members of the BIOCARD Scientific Advisory Board who provide continued oversight and guidance regarding the conduct of the study including: Drs John Cernansky, David Holtzman, David Knopman, Walter Kukull, and John McArdle, and Drs Neil Buckholtz, John Hsiao, Laurie Ryan, and Jovier Evans, who provide oversight on behalf of the National Institute on Aging and the National Institute of Mental Health (NIMH), respectively. The authors thank the members of the BIOCARD Resource Allocation Committee who provide ongoing guidance regarding the use of the biospecimens collected as part of the study, including: Drs Constantine Lyketsos, Carlos Pardo, Gerard Schellenberg, Leslie Shaw, Madhav Thambisetty, and John Trojanowski.
The authors acknowledge the contributions of the Geriatric Psychiatry Branch of the intramural program of NIMH who initiated the study (Principal investigator: Dr. Trey Sunderland). The authors are particularly indebted to Dr. Karen Putnam, who has provided ongoing documentation of the Geriatric Psychiatry Branch study procedures and the data files received from NIMH.
Role of the funder/sponsor: The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflict of interests: Drs. Wennberg, Pettigrew, Soldan, Zipunnikov, Rebok, Lutz, Miller, Thambisetty, and Albert report no conflicts of interest.
References
- [1].Albert M, Soldan A, Gottesman R, McKhann G, Sacktor N, Farrington L, Selnes O. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr. Alzheimer Res. 2014;11(8):773–784. doi: 10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Diabetes Association . National Diabetes Statistics Report, 2014. Centers for Disease Control; Atlanta, GA: 2014. [Google Scholar]
- [3].Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005;58(4):495–505. doi: 10.1002/ana.20624. http://dx.doi.org/10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- [4].Blanc F, Colloby SJ, Philippi N, de Petigny X, Jung B, Demuynck C, Taylor JP. Cortical thickness in dementia with Lewy bodies and Alzheimer's disease: a comparison of prodromal and dementia stages. PLoS One. 2015;10(6):e0127396. doi: 10.1371/journal.pone.0127396. http://dx.doi.org/10.1371/journal.pone.0127396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ. Cerebral cortical thickness in patients with type 2 diabetes. J. Neurol. Sci. 2010;299(1–2):126–130. doi: 10.1016/j.jns.2010.08.048. http://dx.doi.org/10.1016/j.jns.2010.08.048. [DOI] [PubMed] [Google Scholar]
- [6].Caselli RJ, Reiman EM, Locke DE, Hutton ML, Hentz JG, Snyder C. Hoffman, Osborne D. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch. Neurol. 2007;64(9):1306–1311. doi: 10.1001/archneur.64.9.1306. http://dx.doi.org/10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- [7].Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62(11):1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- [8].Chen Z, Sun J, Yang Y, Lou X, Wang Y, Wang Y, Ma L. Cortical thinning in type 2 diabetes mellitus and recovering effects of insulin therapy. J. Clin. Neurosci. 2015;22(2):275–279. doi: 10.1016/j.jocn.2014.07.014. http://dx.doi.org/10.1016/j.jocn.2014.07.014. [DOI] [PubMed] [Google Scholar]
- [9].Cherbuin N, Sachdev P, Anstey KJ. Higher normal fasting plasma glucose is associated with hippocampal atrophy: the PATH Study. Neurology. 2012;79(10):1019–1026. doi: 10.1212/WNL.0b013e31826846de. http://dx.doi.org/10.1212/WNL.0b013e31826846de. [DOI] [PubMed] [Google Scholar]
- [10].Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc. Natl. Acad. Sci. U. S. A. 2003;100(4):2019–2022. doi: 10.1073/pnas.0336073100. http://dx.doi.org/10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. http://dx.doi.org/10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- [12].de Bruijn RF, Bos MJ, Portegies ML, Hofman A, Franco OH, Koudstaal PJ, Ikram MA. The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam Study. BMC Med. 2015;13:132. doi: 10.1186/s12916-015-0377-5. http://dx.doi.org/10.1186/s12916-015-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Desikan RS, Cabral HJ, Fischl B, Guttmann CR, Blacker D, Hyman BT, Killiany RJ. Temporoparietal MR imaging measures of atrophy in subjects with mild cognitive impairment that predict subsequent diagnosis of Alzheimer disease. AJNR Am. J. Neuroradiol. 2009;30(3):532–538. doi: 10.3174/ajnr.A1397. http://dx.doi.org/10.3174/ajnr.A1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Desikan RS, Fischl B, Cabral HJ, Kemper TL, Guttmann CR, Blacker D, Killiany RJ. MRI measures of temporoparietal regions show differential rates of atrophy during prodromal AD. Neurology. 2008;71(11):819–825. doi: 10.1212/01.wnl.0000320055.57329.34. http://dx.doi.org/10.1212/01.wnl.0000320055.57329.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Buckner RL. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. http://dx.doi.org/10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76(16):1395–1402. doi: 10.1212/WNL.0b013e3182166e96. http://dx.doi.org/10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doherty BM, Schultz SA, Oh JM, Koscik RL, Dowling NM, Barnhart TE, Okonkwo OC. Amyloid burden, cortical thickness, and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. Alzheimers Dement. (Amst) 2015;1(2):160–169. doi: 10.1016/j.dadm.2015.01.003. http://dx.doi.org/10.1016/j.dadm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiol. Aging. 2008;29(3):329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. http://dx.doi.org/10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- [19].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- [20].Fennema-Notestine C, Panizzon MS, Thompson WR, Chen CH, Eyler LT, Fischl B, Kremen WS. Presence of ApoE epsilon4 allele associated with thinner frontal cortex in middle age. J. Alzheimers Dis. 2011;26(Suppl. 3):49–60. doi: 10.3233/JAD-2011-0002. http://dx.doi.org/10.3233/jad-2011-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ferencz B, Laukka EJ, Lovden M, Kalpouzos G, Keller L, Graff C, Backman L. The influence of APOE and TOMM40 polymorphisms on hippocampal volume and episodic memory in old age. Front. Hum. Neurosci. 2013;7:198. doi: 10.3389/fnhum.2013.00198. http://dx.doi.org/10.3389/fnhum.2013.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. http://dx.doi.org/10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. http://dx.doi.org/10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- [24].Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Dale AM. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- [26].Freiherr J, Hallschmid M, Frey WH, 2nd, Brunner YF, Chapman CD, Holscher C, Benedict C. Intranasal insulin as a treatment for Alzheimer's disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27(7):505–514. doi: 10.1007/s40263-013-0076-8. http://dx.doi.org/10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer's disease: preliminary findings and future directions. Biochim. Biophys. Acta. 2012;1822(3):416–422. doi: 10.1016/j.bbadis.2011.07.009. http://dx.doi.org/10.1016/j.bbadis.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gold BT, Powell DK, Andersen AH, Smith CD. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer's disease. NeuroImage. 2010;52(4):1487–1494. doi: 10.1016/j.neuroimage.2010.05.036. http://dx.doi.org/10.1016/j.neuroimage.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Greenbaum L, Springer RR, Lutz MW, Heymann A, Lubitz I, Cooper I, Beeri MS. The TOMM40 poly-T rs10524523 variant is associated with cognitive performance among non-demented elderly with type 2 diabetes. Eur. Neuropsychopharmacol. 2014;24(9):1492–1499. doi: 10.1016/j.euroneuro.2014.06.002. http://dx.doi.org/10.1016/j.euroneuro.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J. Diabetes Investig. 2013;4(6):640–650. doi: 10.1111/jdi.12087. http://dx.doi.org/10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E. Relationship between body mass index and brain volume in healthy adults. Int. J. Neurosci. 2008;118(11):1582–1593. doi: 10.1080/00207450701392282. http://dx.doi.org/10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- [32].Han X, Jovicich J, Salat D, Kouwe A. van der, Quinn B, Czanner S, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. http://dx.doi.org/10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- [33].Humphries AD, Streimann IC, Stojanovski D, Johnston AJ, Yano M, Hoogenraad NJ, Ryan MT. Dissection of the mitochondrial import and assembly pathway for human Tom40. J. Biol. Chem. 2005;280(12):11535–11543. doi: 10.1074/jbc.M413816200. http://dx.doi.org/10.1074/jbc.M413816200. [DOI] [PubMed] [Google Scholar]
- [34].Hurtz S, Woo E, Kebets V, Green AE, Zoumalan C, Wang B, Apostolova LG. Age effects on cortical thickness in cognitively normal elderly individuals. Dement. Geriatr. Cogn. Disord. Extra. 2014;4(2):221–227. doi: 10.1159/000362872. http://dx.doi.org/10.1159/000362872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim HJ, Ye BS, Yoon CW, Noh Y, Kim GH, Cho H, Seo SW. Cortical thickness and hippocampal shape in pure vascular mild cognitive impairment and dementia of subcortical type. Eur. J. Neurol. 2014;21(5):744–751. doi: 10.1111/ene.12376. http://dx.doi.org/10.1111/ene.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. http://dx.doi.org/10.1161/circresaha.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kloppenborg RP, van den Berg E, Kappele LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur. J. Pharmacol. 2008;585(1):97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- [38].Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch. Gen. Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. http://dx.doi.org/10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- [39].Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Milberg WP. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. NeuroImage. 2011;54(4):2659–2671. doi: 10.1016/j.neuroimage.2010.10.050. http://dx.doi.org/10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Linnertz C, Saunders AM, Lutz MW, Crenshaw DM, Grossman I, Burns DK, Chiba-Falek O. Characterization of the poly-T variant in the TOMM40 gene in diverse populations. PLoS One. 2012;7(2):e30994. doi: 10.1371/journal.pone.0030994. http://dx.doi.org/10.1371/journal.pone.0030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. http://dx.doi.org/10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Llado-Saz S, Atienza M, Cantero JL. Increased levels of plasma amyloid-beta are related to cortical thinning and cognitive decline in cognitively normal elderly subjects. Neurobiol. Aging. 2015 doi: 10.1016/j.neurobiolaging.2015.06.023. http://dx.doi.org/10.1016/j.neurobiolaging.2015.06.023. [DOI] [PubMed] [Google Scholar]
- [43].Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur. J. Pharmacol. 2008;585(1):119–129. doi: 10.1016/j.ejphar.2008.02.048. http://dx.doi.org/10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lyall DM, Harris SE, Bastin ME, Maniega S. Munoz, Murray C, Lutz MW, Deary IJ. Alzheimer's disease susceptibility genes APOE and TOMM40, and brain white matter integrity in the Lothian Birth Cohort 1936. Neurobiol. Aging. 2014;35(6):1513, e1525–1533. doi: 10.1016/j.neurobiolaging.2014.01.006. http://dx.doi.org/10.1016/j.neurobiolaging.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Moran C, Beare R, Phan TG, Bruce DG, Callisaya ML, Srikanth V. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015 doi: 10.1212/WNL.0000000000001982. http://dx.doi.org/10.1212/wnl.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Srikanth V. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36(12):4036–4042. doi: 10.2337/dc13-0143. http://dx.doi.org/10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. http://dx.doi.org/10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Roberts RO, Knopman DS, Cha RH, Mielke MM, Pankratz VS, Boeve BF, Lowe VJ. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J. Nucl. Med. 2014;55(5):759–764. doi: 10.2967/jnumed.113.132647. http://dx.doi.org/10.2967/jnumed.113.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- [50].Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, Reiman EM. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer's disease. Pharmacogenomics J. 2010;10(5):375–384. doi: 10.1038/tpj.2009.69. http://dx.doi.org/10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Roses AD, Lutz MW, Saunders AM, Goldgaber D, Saul R, Sundseth SS, Welsh-Bohmer KA. African-American TOMM40′523-APOE haplotypes are admixture of West African and Caucasian alleles. Alzheimers Dement. 2014;10(6):592–601. e592. doi: 10.1016/j.jalz.2014.06.009. http://dx.doi.org/10.1016/j.jalz.2014.06.009. [DOI] [PubMed] [Google Scholar]
- [52].Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cereb. Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. http://dx.doi.org/10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- [53].StataCorp . Stata Statistical Software: Release. Vol. 11. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- [54].Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Toga AW. Dynamics of gray matter loss in Alzheimer's disease. J. Neurosci. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer's disease: The counfounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35:152–160. doi: 10.1093/epirev/mxs012. [DOI] [PubMed] [Google Scholar]
- [56].van Velsen EF, Vernooij MW, Vrooman HA, van der Lugt A, Breteler MM, Hofman A, Ikram MA. Brain cortical thickness in the general elderly population: the Rotterdam Scan Study. Neurosci. Lett. 2013;550:189–194. doi: 10.1016/j.neulet.2013.06.063. http://dx.doi.org/10.1016/j.neulet.2013.06.063. [DOI] [PubMed] [Google Scholar]