Abstract
Innovative developments are necessary for treating and defeating cancer, an oftentimes deadly group of diseases characterized by the uncontrolled growth and spread of abnormal cells. Breast cancer (BC) is the second leading cause of cancer-related deaths of women in the USA, and prostate cancer (PC) is the second leading cause of cancer-related deaths of American men. Although some efficacious BC drugs are pharmaceutically marketed, they affect the quality of life for some patients because they are toxic in that their usages have been accompanied by side effects such as stroke, thrombosis, slow heart rate, seizure, increased blood pressure, nausea, emesis, and more. Therefore, there is an urgent need for the discovery of molecular markers for early detection of this disease and discovery of targets for the development of novel, less toxic therapeutics. A botanical plant Vernonia amygdalina has been widely used in Nigerian and other Central and West African cultures for centuries as an herbal medicine. Mounting evidence suggests that treatment with low concentrations of aqueous leaf extracts of the edible Nigerian V. amygdalina plant (Niger-VA) arrests the proliferative activities and induces apoptosis in estrogen receptor-positive, estrogen receptor-negative, and triple-negative human breast cancerous cells and in androgen-independent human PC-3. Also, in athymic mice, Niger-VA potentiates increased efficacies and optimizes treatment outcomes when given as a cotreatment with conventional chemotherapy drugs. Evidence of its noticeable cytostatic activities ranging from changes in DNA synthesis to growth inhibition, mechanisms of inducing apoptosis in different cancer cell lines, and in vivo antitumorigenic activities and chemopreventive efficacy reinforce the idea that Niger-VA deserves increased attention for further development as a phytoceutical, anticancer drug entity. Hence, the present review article highlights impactful published literature on the anticancer effects of Niger-VA in multiple cancerous cell lines and in a nude mouse model, supporting its potential usefulness as a natural product, chemotherapeutic medicine for treatment of both BC and PC.
Keywords: breast cancer, prostate cancer, anticancer agents, antitumorigenic agents, chemopreventive natural products
Introduction
All cancers involve the malfunction of genes controlling cell growth and death. Inherited factors play a larger role in determining risk for a small proportion of cancers (eg, colorectal, breast, and prostate) than for others, with familial cancers arising from the interplay between common gene variations and lifestyle/environmental risk factors. Inherited mutations of the BRCA1 or BRCA2 genes are the reason that breast and ovarian cancers are much more common in some families.1 Yet, most cancers do not result from inherited genes. According to a report from the American Cancer Society (2000), African-American women (AAW) are approximately 33% more likely to die of cancer than white women, and more than twice likely to die of cancer as are women who are Asian Islanders, Native Americans, or Hispanics.1–3 A reported 13.7 million Americans with a history of cancer were fortunately alive on January 1, 2012, yet in 2014, approximately 1,665,540 new cancer cases were reported to cancer registries and approximately 585,720 Americans were expected to die of cancer.4 Lung cancer ranks first in cancer-related deaths, followed by breast cancer (BC) in females, with prostate cancer (PC) ranking as the second leading cancer-related killer of males.4 Death rates for BC have steadily decreased in women since 1989, with the largest decreases in younger women from 2006 to 2010, possibly due to improvements in early detection and treatment.1,4 Still, BC has the highest incidence of all organ sites of cancers in adult women in the USA, and health disparities exist in that there are large differences in BC survival by race. Cancer incidences have declined in some ethnic populations, yet cancer mortality rates continually increase worldwide.4,5 The 5-year survival rate is 90% for white women and 79% for AAW for all BC stages combined.4 Also, disproportionally higher mortality rates and prevalence of triple-negative breast cancer (TNBC) occur in AAW than other women and anticancer treatment agents are far less effective in such tumors. BC treatment may involve surgery, radiation, hormone therapy, immune therapy, targeted therapy, and/or chemotherapy. However, resistance to treatment and harsh side effects associated with BC treatment regimens have caused researchers to search for more natural chemotherapeutic agents to combat the cancer epidemic. A survey by the National Center for Complementary and Integrative Health on complementary and alternative medicine (CAM) use concluded that most people use CAM as a way to increase their quality of life.6 Unfortunately, to date, CAM usage has not harnessed total buy-in from physicians and other caregivers.
Pertaining to PC, in the USA in 2014, there were an estimated 233,000 new cases compared with 116,000 new cases of lung and bronchus cancer, with 29,480 deaths from PC and 86,930 deaths in males from lung and bronchus cancer.4,7 Age, ethnicity, and familial history of PC are the only well-established risk factors and racial and ethnic disparities exist in relation to the disease, evidenced by the following facts. PC occurs more often in African-American men (AAM) than men of other races, and AAM are more likely to be diagnosed at an advanced stage and are twice as likely to die of PC compared with white men. Additionally, AAM and Jamaican men of African descent have the highest PC incidence rates in the world while PC occurs less often in Asian-American and Hispanic/Latino men than in non-Hispanic whites.8 Local PC treatments involve minor or major surgery and radiation therapy, while the systemic treatments are chemotherapy and hormonal therapy. The commonly used chemotherapeutic regimen combines the drug Docetaxel with the corticosteroid prednisone, but this regiment is riddled with harsh side effects. Hormonal therapy aims to block prostate cancerous cells from getting dihydrotestosterone, an active metabolite of testosterone, a hormone required for the growth and spread of most PC cells, but leads to hormone therapy resistance within 2 years unless accompanied with either chemical or surgical castration. Two drugs, finasteride and dutasteride, are approved to treat symptoms associated with benign prostate enlargement, but they cause reduced libido and the risk of erectile dysfunction. Long-term survival data from participants in a finasteride trial reported no effect of the drug on overall survival or survival after the diagnosis of PC. Thus, neither drug is approved for the prevention of PC at this time. In addition to the numerous anti-BC studies we have conducted, two studies have been done in our laboratories indicating that Nigerian Vernonia amygdalina (Niger-VA) shows efficacy toward growth inhibition in PC cells.
Chemoprevention is an active research area, and use of CAM is a novel chemopreventive approach based on plant products, herbs, vegetables, and spices used in folk and traditional medicine.6,9,10 Numerous results from cell culture model experiments show that some herbal products have potential for use as chemopreventive and chemotherapeutic agents for certain types of cancers.11–38 We believe the medicinal value of Niger-VA is substantive because of its efficacy against estrogen receptor-positive (ER+), estrogen receptor-negative (ER−), and triple-negative human breast cancerous cells, animal model mammary tumors, as well as PC cells, a broader range than has been described with other anticancer agents. This article presents a compilation of published data generated in our laboratories as we continue to ascertain the therapeutic value of Niger-VA toward attenuating the increasing worldwide death rates associated with BC and PC.
Complementary and alternative medicine
CAM is defined as a group of diverse medical and health care systems, practices, and products not considered conventional medicine, encompassing both broad types of therapies.6 Typical examples of CAM approaches are herbalism (phytotherapy), meditation, yoga, and diet-based therapies.10 Complementary medicine can also be used together with conventional medicine, and some of these integrative treatment approaches have exhibited synergism in efficacy.6 Within the USA, the reported number of CAM users increased from 38% to over 62% when the definition of CAM was expanded to include the deliberate use of megavitamin therapy and prayer for health reasons.6,9 Efficacy studies on patients' attitudes and behaviors have revealed that the most common reasons identified for trying it was the belief that CAM would improve health when used in combination with conventional medicine, that conventional medicine would not work and/or they would rather try a natural product instead. Data also showed that more than 75% of cancer patients use some form of CAM, mainly to treat chronic or recurring pain.6 A recent patient preference trial proposed a complex nursing care intervention involving CAM therapies and counseling on CAM coupled with chemotherapy, aimed at increasing health-related quality of life in patients undergoing chemotherapy.39 This study is based on how effective nursing care interventions of CAM would be toward orienting patients to choose CAM therapies.39 Data will be collected, and the results analyzed by mid-year 2016.
Physicians often influence patients' beliefs about use of CAM treatments. In a study conducted at Mayo Clinic in Rochester, MN, USA, the link to a Web-based survey was sent to 660 internists to determine conventionally trained physicians' knowledge of medical efficacy, beliefs, intentions, behaviors, and attitudes toward CAM. Of the 233 respondent physicians, 76% had never referred a patient to a CAM practitioner, yet 44% stated that they would refer a patient if a CAM practitioner were available at their institution. Also, 57% thought that incorporating CAM therapies would have a positive effect on patient satisfaction and although most agreed that some CAM therapies hold promise, many did not feel knowledgeable about CAM safety or efficacy. And, 81% expressed their need for more education on CAM modalities before counseling their patients about their use of CAM.40 Findings from these studies raise important issues for medical education and patient care. We provide evidence-based information that may encourage oncologists and other cancer health care providers to recommend CAM to accompany prescribed conventional therapies.
Niger-VA
The therapeutic use of plant products is among the oldest of medical practices, with plants serving as excellent sources for developing safer and more effective drugs. This premise is supported by reports on the inverse relationship between the consumption of fruits and vegetables and the reduction in the risks of cancers of many sites. Known in Africa as bitter leaf, the Nigerian Yoruba name for the plant, V. amygdalina, is “ewuro”, and the Igbo calls it as “onugbu”. The leaves are boiled in soups and are also sold in the market after being shredded, parboiled, and made into fist-sized balls.17 Aqueous extracts of V. amygdalina have been shown to have antibacterial,41,42 amebicidal,43 antioxidant,44,45 hypoglycemic/antidiabetic,46 oxytocic,47 hepatoprotective,48 serum lipid modulatory,49 gastric secretory,50 analgesic,41 and phytotoxic43 efficacies. Also, earlier investigators have shown that purified fractions of the chloroform extract of V. amygdalina elicited anticancer effects in human carcinoma of the nasopharynx.51 However, the anticancer effects of Niger-VA are the focus for this review article. Niger-VA treatment inhibits the proliferation of ER+, ER−, and triple-negative human breast carcinoma cells, as well as induces apoptosis in BC and PC cells with no effect on normal human peripheral blood mononuclear cells. This review could prove to be foundational in support of development of Niger-VA for phytoceutical usage as a viable treatment for BC and PCs.
V. amygdalina has demonstrated growth under a wide range of ecological zones in Africa and produces large masses of forage while remaining drought tolerant.52 There are approximately 1,000 known species of the Vernonia genus, and V. amygdalina Delile (Figure 1) is likely the most used and most documented for its medicinal benefits.53 The bitter taste is due to alkaloids, saponins, tannins, and glycosides that have been shown to provide biological benefits.51 The leaves, harvested for human consumption and washed to get rid of the bitter taste, are often eaten as a vegetable, one known to stimulate the gastrointestinal tract and reduce fever.50 Leaves are also used as a local medicine against schistosome worm transmitting leeches in treating amoebic dysentery,54,55 malaria, some sexually transmitted diseases, wounds, and hepatitis.56 In-depth studies of V. amygdalina (VA) identified nine important minerals in the plant leaves, including zinc, magnesium, iron, copper, and others, as well as condensed tannins and soluble tannins.57–62 Other studies have shown that the antiplasmodial activity of VA extracts may be related to the presence of flavonoids, saponins, alkaloids, terpenes, steroids, coumarins, phenolic acids, lignans, xanthones, and anthraquinones,63,64 and VA has been shown to have high cytotoxic effects on human hepatocellular carcinoma cells and on human urinary bladder carcinoma cells.65
Figure 1.

Vernonia amygdalina Delile (A–C).
Note: Family: Asteraceae; Genus: vernonia; Species: Vernonia amygdalina Delile. Reproduced with permission from © Robert von Blittersdorff (B and C) and © Stefan Dressler (A), www.africanplants.senckenberg.de.96
Preparation of crude extracts
Pesticide-free fresh VA leaves, collected in Benin City, Nigeria, were rinsed with cold water, soaked overnight, and then crushed to a mixture. Following filtering through clean white gauze to remove particulate matter, the mixture was filter sterilized using a 0.45 μm filter unit. It is the resultant crude aqueous leaf extracts of the edible Nigerian VA plant, Niger-VA, that demonstrates anticancer properties when used in low concentrations to treat cancerous cells. Niger-VA, used alone or in combination with known anticancer drugs, is emerging as a very strong candidate chemotherapeutic. The usage of Niger-VA as CAM in the near future is relatively high; however, VA leaves must first be authenticated as a quality control measure.
Preparation of edotides and edoTIDEplus
Reconstituted lyophilized fractions of Niger-VA were separated using preparative reverse-phase chromatography (PRPC), and two samples were selected and subjected to ion exchange chromatography (IEC), yielding ten IEC subfractions from one of them. These ten IEC subfractions were loaded on columns and separated by reverse-phase chromatography (RPC), yielding 35 subfractions for each of the ten IEC subfractions for a total of 350 RPC fractions. Of the 350 RPC subfractions from the ten IEC subfractions (from a single PRPC fraction), three fractions, which are peptides, possessed DNA synthesis inhibitory effects at 100 ng/mL concentrations.38 In the water-soluble extraction method, lyophilized water-soluble protein fractions of Niger-VA separated by PRPC and followed by IEC and RPC yielded edotides. The company EdoBotanics (edobotanics.com) specializes in the production of encapsulated pills containing edotides, and these water-soluble anticancer agents are now US patented.38 They are marketed as the herbal supplement edoTIDEplus, which promotes general health and well-being, breast and prostate health, and immune system health and minimizes harsh side effects.
Fingerprint analysis to study active fractions
Niger-VA authentication and activity determination protocols (Figures 2–4) aimed at improving its medicinal benefits and safety have been developed.66–68 Increases in costs of prescription medications combined with an interest in CAM remedies drive the push to determine the specific ingredient(s) causing a therapeutic effect. Since crude Niger-VA contains a complex mixture of natural compounds, each with a specific action, it is capable of eliciting complex physiological responses alone or in combination with other molecules. Achieving consistent content suggests the need for a detailed knowledge of the mixture components and reproducible strategies for monitoring consistency. Studies have shown that VA contains bioactive principles. These include sesquiterpene lactones, vernodalin, hydroxyvernolide, vernomygdin, and others51 including vernolepin that possesses antiplatelet activity,69 some novel stigmastane-type steroid glycosides vernoniosides A-1, A-2, A-3 and related B-1,11 and vernoniosides D and E.69 However, none of these constituents attribute to VA's antiproliferative activity in breast cancerous cells. Therefore, aqueous and organic extractions were utilized to obtain the fingerprint analysis (Table 1) and to study of active fractions of Niger-VA.66–68 These anticancer components of Niger-VA are highly extractable by polar protic solvents, and further separation efforts to narrow the fractions to only a few of the most active molecules are underway.
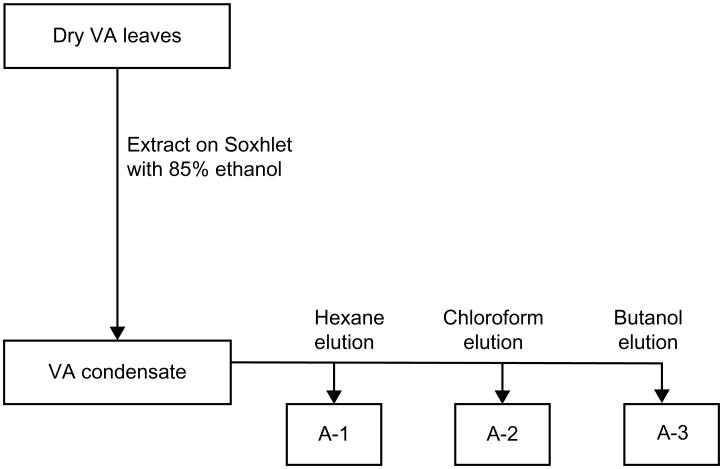
Figure 2.
Flow diagram showing the stages in the organic extraction and separation of fractions A-1, A-2, and A-3 using various solvents.
Abbreviation: VA, Vernonia amygdalina.
Figure 4.
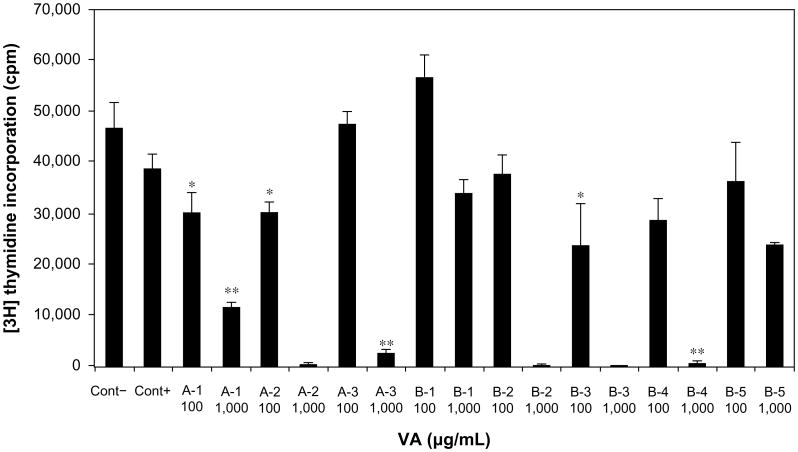
Multiple-solvent fractions of Vernonia amygdalina (VA) extracts inhibited DNA synthesis.
Notes: Cells at the logarithmic growth phase were treated with either 100 or 1,000 μg/mL for 18 hours before the addition of 1 μCi/mL [3H]thymidine for 6 hours. Each data point represents the mean of three independent experiments done in duplicates (N=6). Exposure of cells to multiple-solvent fractions (butanol, chloroform, ethyl acetate, hexane, and methanol) of VA inhibited DNA synthesis in both concentration and solvent-dependent fashion. *Hexane, chloroform, and ethyl acetate extracted fractions (A-1, A-2, and B-3) at 100 μg/mL inhibited DNA synthesis by approximately 30%. **One thousand microgram per milliliter of fractions of hexane, chloroform, butanol, and ethyl acetate (A-1–3, B-2, B-3, and B-4) inhibited DNA synthesis by 76%, 98%, 94%, 98%, 98%, and 96% (P<0.001), respectively [3H]thymidine uptake was determined.
Abbreviations: cpm, counts per minute; Cont, control.
Table 1. RT and heights of two characteristic peaks in HPLC fingerprints of four fraction extracts of a batch of VA.
| VA fraction no | Peak 1 RT (min) | Peak 2 RT (min) | Mean RT (min) | SD RT (min) | Peak 1 height (mAU) | Peak 2 height (mAU) | Mean height (mAU) | SD height (mAU) |
|---|---|---|---|---|---|---|---|---|
| 4 | 2.206 | 2.208 | 2.207 | 0.001 | 5.50 | 14.0 | 9.75 | 6.010 |
| A-2 | 2.210 | 2.213 | 2.2115 | 0.002 | 1.2 | 3.5 | 2.35 | 1.626 |
| B-2 | 2.185 | 2.187 | 2.186 | 0.001 | 6.9 | 25.0 | 15.95 | 12.799 |
| B-3 | 2.150 | 2.152 | 2.151 | 0.001 | 2.25 | 6.7 | 4.475 | 3.147 |
Notes: All fraction peaks expressed at a relatively similar retention time, while higher peak height was expressed in fractions 4 and B-3. Fingerprint experiment was conducted in duplicates, and mean and standard deviations for peak height and retention time computed.
Abbreviations: RT, retention time; VA, Vernonia amygdalina; SD, standard deviation; HPLC, high performance liquid chromatography.
Paclitaxel (Taxol, TAX) is an example of a natural product that has been successful as an anticancer agent. Its discovery was stimulated through an inquiry by the National Cancer Institute (NCI) in 1962 geared toward finding natural products that might cure cancer. It was discovered that TAX is a known antimicrotubule agent.70 Although the drug showed efficacy in restricting tumor cell growth, it presented several difficulties specific to the harvesting and synthesis of the active compound.70 The first company to achieve large-scale production of TAX was Polysciences, Inc. (Warrington, PA, USA), and clinical trials were made possible once a method was derived to extract a precursor of TAX, 10-deacetylbaccatin III, from the common yew relative Taxus baccata, the precursor then converted by chemical synthesis to the trademarked Taxol.70 Currently, a cell culture method developed by Phyton Catalytic, Inc. (Ithaca, NY, USA) is used by Bristol-Myers Squibb (New York, NY, USA) to produce the drug, with lingering challenges associated with formulating a delivery system acceptable for human use. Once the isolated compound in Taxol was combined with the excipient cremophor EL, its use resulted in a range of preclinical toxic effects and high cell turnover in gastrointestinal, lymphatic, and reproductive tissues.70 Niger-VA development is warranted for the management of BC and PC, especially in light of the fact that TAX treatment is toxic and TAX resistance is common, it is minimally effective against TNBC and PC, Niger-VA given alone is more efficacious than TAX treatment alone, and Niger-VA synergizes with TAX for increased inhibition of cancerous cell and tumor growth.
Anticancer activity of Niger-VA
Niger-VA possesses antitumor activity with no deleterious effects in humans, as the leaves are a large part of the diets of Nigerians and people in other West African cultures. In our hands, both Niger-VA and edotides demonstrated anticancer activities. The earliest studies done in our laboratories on the effects of Niger-VA on BC show that low concentrations retard the proliferation of human estrogen receptor-positive MCF-7 cells in vitro in a concentration-dependent fashion.38 Niger-VA showed no significant cytotoxicity effects at a concentration range of 3–25 μg/mL and did not change the viable/nonviable cell ratio, and Niger-VA concentrations higher than 25 μg/mL caused incrementally greater inhibition of cell proliferation. The concentration of Niger-VA required to inhibit the growth of 50% of the cell population, computed using a regression analysis was 5.68±2 μg/mL, which is greater than 1,400 times more efficacious than other plant extracts previously reported.38 Additionally, the observed inhibition of cancer cell growth by Niger-VA was not seen in nonwater-soluble VA extracts.47 Over the past 12 years, we have generated substantial biochemical and biophysical evidence providing clear perspectives on Niger-VA's anticancer actions and supporting its chemotherapeutic potential,38,66–68 summarized in this review.
Mode of action of Niger-VA against BC and PC
The use of Niger-VA has remained limited only to the cultures that use it as a vegetable component in soups or porridges after maceration to remove the bitter taste.47 We argue that development of Niger-VA as a novel therapeutic will provide a less toxic therapy, lead to new interventions and early prevention strategies and impact therapeutic decision making at the point of care for patients with BC and PC. In this article, we review much of the work we have done toward discovery of targets for the development of Niger-VA, a novel, less toxic therapeutics. Before our work, mechanisms of actions mediated by Niger-VA to elicit its anticancer actions were then unknown.
Niger-VA as an inducer of xenobiotic metabolizing enzymes
When focusing on xenobiotic processes, cytochrome P450 comprises a large and diverse superfamily of liver hemoproteins that use a plethora of both exogenous and endogenous compounds as substrates in enzymatic reactions to remove foreign compounds from cells.71 Xenobiotic metabolism is divided into three phases, and in Phase I, enzymes such as cytochrome P450 oxidases introduce reactive or polar groups into xenobiotics. These modified compounds are then conjugated to polar compounds in the Phase II reactions, which are catalyzed by transferase enzymes. Finally, in Phase III, the conjugated xenobiotics may be further processed, before being recognized by efflux transporters and pumped out of cells.71 Many of the natural anticancer agents have been shown to act as monofunctional inducers of metabolic enzymes and dose range optimization studies have been performed.22,29 Data from time- and dose-dependent experiments of MCF-7 cells treated with Niger-VA suggest that Niger-VA acts as a monofunctional inducer, increasing Phase II enzyme expression without affecting Phase I enzymes.71 This pattern is in direct contrast with mostly all other cancer pharmaceuticals today. These data are useful toward further validating Niger-VA as a potential clinically useful natural anticancer agents and provides some support for the concept that modulation in CYP3A4 expression in response to treatment is relevant to prognosis.14,29,72
Niger-VA as an inhibitor of extracellular signal-regulated kinases
Niger-VA has been shown to modulate extracellular signal-related kinases 1 and 2 (ERK 1/2) activities in ER+ MCF-7 cells, thus suggesting a mechanism for its antimitotic actions in breast cancerous cells.72 Before this study was reported the mechanisms by which Niger-VA inhibits cultured MCF-7 cell growth had not been examined. The data reveled that treatment of cells with 10 μg/mL of Niger-VA potently inhibited ERK activities, DNA synthesis (P<0.005), and cell growth (P<0.01) in a concentration-dependent fashion, both in the absence and in the presence of serum.73 The results suggested that Niger-VA exhibits cytostatic action to retard the growth of human BC cells. The ERK signaling pathways may be one or more of the intracellular targets for Niger-VA's antineoplastic actions.73
Membrane disruption and subsequent efflux eliciting Niger-VA's anticancer actions
Niger-VA alters MCF-7 cell membrane permeability and efflux.74,75 Recognizing from the studies of others that membrane disruption and subsequent efflux and apoptosis regulation were mechanisms used by some plant extracts to evoke their anticancer effects, we sought to provide additional insights on Niger-VA's mode of action by evaluating cell membrane permeability and efflux in Niger-VA treated MCF-7 cells. We report that exposure of cells to Niger-VA decreased 3H-thymidine uptake but increased 3H-thymidine release into the medium, suggesting alteration in member permeability of the Niger-VA treated cells.75 Thionins are found in the seeds, stems, roots, and leaves of some plants. These low molecular weight proteins are active components, which elicit a wide range of activities. Investigations by others have indicated that thionins may be responsible for the anticancer activities of the plant extracts they studied.76,77 The thionins are believed to form channels on the cell plasma membrane surface to alter the cell efflux, depolarization, and subsequent cell death.
Antimitotic actions of Niger-VA in ER− breast cancerous cells
Most chemotherapeutic agents are less effective in patients with BC with ER− tumors than those with ER+ ones, and AAW are disproportionately diagnosed with ER− tumors compared to their white counterparts.78,79 It was reported in our laboratories that the Niger-VA inhibited mitosis in ER− human ductal carcinoma, BT-549 cells in a dose-dependent fashion with the 100 μg/mL dose being the most efficacious. Although ER+ cells are more sensitive to Niger-VA than these, we showed that this botanical plant proved to be an effectual treatment in BT-549 cells while these cells are insensitive to TAX.80,81 However, Niger-VA treatment exhibited synergism toward inhibition of cancerous growth when combined with several known efficacious anticancer agents, including TAX, Tamoxifen,82 Doxorubicin, Vincristine, and others dependent on the cell line employed. This suggests that Niger-VA treatment can complement current chemotherapy, a finding that stands to be one of the most impactful regarding Niger-VA's use as a cancer treatment.74,75,80,81,83–93
Niger-VA-induced DNA damage activities
Niger-VA has been shown to protect against hydroperoxidation and elicits an antioxidant effect against acrylonitrile-induced oxidative stress in the DI TNC1 rat astrocyte cell line derived from rat brain. Analysis using the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) assay and the alkaline single-cell gel electrophoresis (Comet) assay method suggested that it is capable of crossing the cell membrane, entering into the extracellular matrix, protecting normal cells from reactive oxygen species and lowering basal level DNA damage.83 However, Niger-VA represents a DNA-damaging agent in cancerous cells. MTT assay analyses indicated that Niger-VA treatment significantly reduced the viability of MCF-7 cells, and data generated from the Comet assay also indicated a slight increase in comet tail-length, tail arm, and tail moment, as well as in percentages of DNA cleavage suggesting minimal Niger-VA-induced DNA damage in MCF-7 cells.84 These findings provide evidence that Niger-VA is protective in normal cells yet elicits activity against BC cells using a mechanism of action, at least in part, through minimal induction of DNA damage and moderate toxicity. The type of cell line does matter. We found that exposure to the ethanolic extracts of VA, not Niger-VA, altered microtubule formation in HeLa cells by targeting alpha tubulin.85
Growth arrest and apoptosis
Studies on cancer treatments reveal that most, if not all, chemotherapeutic agents kill cancer cells through the induction of apoptosis. Treatment of MCF-7 cancer cells yielded early signs of apoptosis resulting from phosphatidylserine externalization as judged using an annexin V-FITC (apoptotic)/propidium iodide (necrotic) kit. The extent of DNA damage increased with increasing doses of Niger-VA. The findings demonstrated that Niger-VA-induced cytotoxicity and apoptosis in MCF-7 cells was due to phosphatidylserine externalization accompanied by secondary necrotic cell death due to loss of membrane integrity.86 Niger-VA potently inhibits MCF-7 and MDA-MB-231 cell growth, as well as DNA synthesis, and induces apoptosis by caspase activation through both extrinsic and intrinsic methods independent of p53.88,90 This time- and dose-dependent Niger-VA-induced apoptosis, analyzed using MTT assays, is accompanied by secondary necrotic cancer cell death, with no effects on normal human peripheral blood mononuclear cells. The underlying mechanism of this growth inhibition involved the stimulation of cell-type specific G1/S phase cell cycle arrest in MCF-7 cells but not in MDA-MB-231 cells. Although the growth arrest was associated with increased levels of p53 and p21, and a concomitant decrease in the levels of cyclin D1 and cyclin E, we exploited the wild-type p53 inhibitor pifithrin-α, to shown that Niger-VA treatment causes cell cycle arrest through a p53-independent pathway.90 Another groundbreaking finding came out of this study. Niger-VA treatment inhibits the expression of ER-alpha (ER-α) and its downstream player, Akt. Since it has been estimated that approximately 70% of diagnosed BCs express ER-α, this highlights the potential clinical significance of Niger-VA.90
Niger-VA's efficacy on PC cells
We have not yet amassed extensive data of the effects of Niger-VA on PC-3 cells. However, we report two compelling in vitro studies done in our laboratories indicating that PC-3 cells are more sensitive to Niger-VA than to TAX.87,89,91 One study showed that TAX-resistant PC-3 cell growth is inhibited by up to 73% by Niger-VA at a concentration of 1 mg/mL. The data suggested that this Niger-VA sensitivity could perhaps be explained by differential regulatory patterns of MAPK, c-Myc, Akt, and Pgp activities/expressions.86,88 In contrast, TAX in comparable concentrations failed to significantly affect cell growth, suggesting TAX resistance.87,89 This study laid the framework for a later study conducted at Jackson State University (Jackson, MS, USA) representing the first report showing the antiproliferative activity of methanol-extracted Cameroon-cultivated VA in PC-3 cell line.91 The proposed mechanism for methanolic VA extract's antiproliferative activity in PC-3 is phosphatidylserine externalization due to oxidative stress and apoptotic caspase-3 activation without inducing DNA fragmentation.91
Most recent in vitro and in vivo preliminary laboratory results with Niger-VA
Since disproportionately higher mortality rates and prevalence of TNBCs occur in AAW than in other women and anticancer treatment agents are far less effective against such tumors, we conducted in vitro and in vivo studies that suggest that Niger-VA alone or in combination with TAX has greater efficacy than TAX alone,92,93 against tumors derived from triple-negative breast cancerous cells in nude mice inoculated subcutaneously, corroborating earlier studies.87–90 Briefly, the effects of TAX alone or VA extract alone or with TAX were evaluated in Hsd:Athymic Nude-Foxn1nu, age 5–6 weeks, female mice inoculated subcutaneously with Hras cells transformed from the HMLE mammary epithelial cell line using the HRASV12 oncogene. Studies revealed that although there was a lag in tumor growth for all treatment groups, the most significant reduction in tumor size was observed in the group of animals that had been pretreated with Niger-VA each day for 1 week prior to inoculation with Hras cells. We then evaluated the effects of injecting 20 mg/kg of Niger-VA each day for 15 days before administering HRAS cells and AAW-derived MDA-MB-468 cells at different sites on the same animal, versus combined treatment with very low doses of Niger-VA and TAX combined (5 mg/kg of each agent administered subcutaneously) toward inhibition of growth of tumors in our nude mice model.93 Niger-VA exhibits chemopreventive effectiveness in vivo, perhaps involving the cytotoxic, genotoxic, and apoptotic mechanisms as disclosed through our in vitro findings.92 A long-term benefit is that Niger-VA could be developed into a new treatment for mammary cancer. The major drawback impeding progress at this time is that this project has been underpowered due to the lack of funding primary deemed not fundable since Niger-VA is an herbal extract, not a purified active agent. This has also been a hindrance to promoting Niger-VA human clinical trials.
Conclusion
Taken together, there is compelling evidence to show that Niger-VA therapy or supplementation with edotides may benefit patients with cancer. However, the challenges are twofold. First, it is very unlikely that a single molecule working alone is responsible for the varied anticancer activities of Niger-VA. Instead, multiple molecules, working alone or in combination with others, are more likely to be responsible for each of these biological activities, giving credence to using Niger-VA alone or in combination with conventional medicine. This holds especially true in cases of ER− cancers that are not responsive to current therapies and certainly with TNBC wherein there are no effective therapies. The second challenge relates to the antagonistic relationships existing between conventional medicine and traditional medicine practitioners. Botanics are a potential source of new therapies for diseases,70,94 and we advocate for the undergirding of Niger-VA by NCI and other entities as a natural phytotherapeutic for treatment of BC and PC, providing a broad scope of its effective use. In summation, the present compilation of studies presents convincing evidence and corroborates previous assertions by earlier investigators that Niger-VA has pronounced anticancer activity against BC and PC and may prevent and/or delay disease promotion and progression without the threat of hazardous side effects. Compressing over a decade's work into the page limits of this review barred inclusion of other contributions that hail the importance of developing natural products and natural products research, termed pharmacognosy in action, in a special volume dedicated to the 50th anniversary of the American Society of Pharmacognosy.95 Although we are still performing preclinical assessment, we believe the efforts of NCI from the 1960s should be re-amped today to develop this natural product, Niger-VA, which might indeed cure cancer.
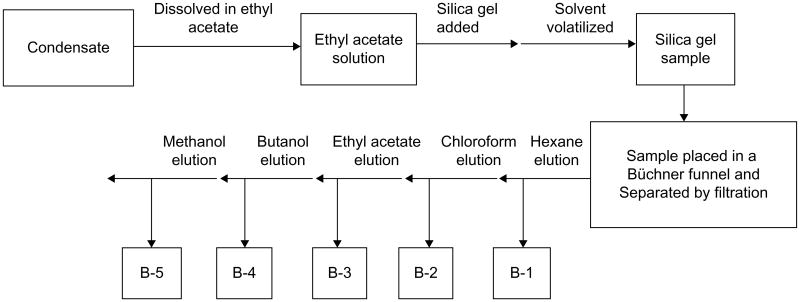
Figure 3.
Flow chart diagram showing stages in the organic extraction and elution of fractions B-1, B-2, B-3, B-4, and B-5 from Vernonia amygdalina condensate using silica gel.
Note: Bioactivity assays reveal that B-2 and B-3 are biologically active.
Acknowledgments
The research reported in this manuscript was supported by grants from the following agencies: US Department of Education, Strengthening Environmental Science Ph D Programs Through Research (grant #P031E090210), National Center for Research Resources (grant #5 G12 RR013459-15), and the National Institutes on Minority Health and Health Disparities (grant #8 G12 MD007581-15) from the National Institutes of Health and Transforming the Climate and Advancing STEM Women at Jackson State University, an HBCU in the South (JSUAdvance) from the National Science Foundation (NSF Award #1008708). Any opinions, findings, and conclusions or recommendations are those of the authors and do not reflect the view of NSF. We acknowledge the photographers Stefan Dressler for the Vernonia amygdalina Delile photo taken in Cameroon and Robert V Blittersdorff for the photos taken in Tanzania, Rukwa, Sumbawanga and Muse.
Footnotes
Disclosure: The authors report no conflicts of interest in this work.
References
- 1.Greenlee RT, Murray T, Bolden S, et al. Cancer statistics. Am Cancer Soc. 2000;50:6–11. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Etzioni R, Berry KM, Legler JM, Shaw P. Prostate-specific antigen testing in black and white men: an analysis of medicare claims from 1991–1998. Urology. 2002;59(2):251–255. doi: 10.1016/s0090-4295(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Can Facts and Figures. Atlanta: American Cancer Society; 2014. pp. 1–72. [Google Scholar]
- 5.Kelsey JL, Gannon MD. Epidemiology of BC. Epidemiol Rev. 1990;12:228–240. doi: 10.1093/oxfordjournals.epirev.a036056. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health. What is CAM (CAM)? Publication No D156. Bethesda, MS: National Center for Complementary and Alternative Medicine; [Accessed March 11, 2015]. (updated 2005 April 5; cited 2005 May 3). Available from: http://nccam.nih.gov/health/whatiscam/ [Google Scholar]
- 7.Sarma AV, Schottenfeld D. Prostate cancer incidence, mortality and survival trends in the US: 1981–2001. Semin Urol Oncol. 2002;20(1):3–9. doi: 10.1053/suro.2002.30390. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Prostate Cancer Treatments. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 9.Eisenburg D, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 10.Joshi BS, Kaul PN. Alternative medicine: herbal drugs and their critical appraisal – part 1. Prog Drug Res. 2001;56:1–76. [PubMed] [Google Scholar]
- 11.Jasaka M, Ohigashi H, Takegaki T, et al. Bitter steroid glucosides, Vernoniosides A1, A2, A3 and related B1 from possible medicinal plant-Vernonia amygdalina used by wild chimpanzee. Tetrahedron. 1992;48(4):625–632. [Google Scholar]
- 12.Abdullaev FL. Plant-derived agent against cancer. In: Gupta SK, editor. Pharmacology and Therapeutics in the New Millen. New Delhi: Narosa Publishing House; 2001. pp. 345–354. [Google Scholar]
- 13.Bhat KP, Lantvit D, Christov K, et al. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Can Res. 2001;61(20):7456–7463. [PubMed] [Google Scholar]
- 14.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94(19):10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farombi EO. African indigenous plants with chemotherapeutic potentials and biotechnological approach to the production of bioactive prophylactic agents. Afr J Biotechnol. 2003;12:662–671. [Google Scholar]
- 16.Heber D, Bowerman S. Applying science to changing dietary patterns. J Nutr. 2001;131(11 Suppl):3078S–3081S. doi: 10.1093/jn/131.11.3078S. [DOI] [PubMed] [Google Scholar]
- 17.Burkill HM. Families A–D. 2nd. Vol. 1. Vol. 960 Kew, Richmond: Royal Botanic Gardens; 1985. The useful plants of West tropical Africa. [Google Scholar]
- 18.Jang M, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Drugs Exp Clin Res. 1999;25(2–3):65–77. [PubMed] [Google Scholar]
- 19.Kobayashi T, Nakata T, Kuzumaki T. Effect of flavonoids on cell cycle progression in prostate cancer cells. Cancer Lett. 2000;176(1):17–23. doi: 10.1016/s0304-3835(01)00738-8. [DOI] [PubMed] [Google Scholar]
- 20.Lagergren TPJ, Hansen H, Wolk A, Nyren O. Fruits and vegetables consumption in the prevention of esophageal and cardiac cancers. Eur J Cancer Prev. 2001;10(4):365–369. doi: 10.1097/00008469-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Lippman SM, Lotan R. Advances in the development of retinoids as chemopreventive agents. J Nutr. 2000;130:479S–482S. doi: 10.1093/jn/130.2.479S. [DOI] [PubMed] [Google Scholar]
- 22.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70(3):461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 23.Pezzuto JM. Plant-derived anticancer agents. Biochem Pharmacol. 1997;53(2):121–133. doi: 10.1016/s0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- 24.Pirani JF. The effects of phytotherapeutic agents on prostate cancer: An overview of recent clinical trials of PC SPEC. Urology. 2001;58(2 Suppl 1):36–38. doi: 10.1016/s0090-4295(01)01240-7. [DOI] [PubMed] [Google Scholar]
- 25.Richardson MA. Biopharmacologic and herbal therapies for cancer: research update from NCCAM. J Nutr. 2001;131(11S):3037S–3040S. doi: 10.1093/jn/131.11.3037S. [DOI] [PubMed] [Google Scholar]
- 26.Rock CL, Moskowitz A, Huizar B, et al. High vegetable and fruit diet intervention in premenopausal women with cervical intraepithelial neoplasia. J Am Diet Assoc. 2001;101(10):1167–1174. doi: 10.1016/S0002-8223(01)00286-3. [DOI] [PubMed] [Google Scholar]
- 27.Simopoulos AP. The Mediterranean diets: What is so special about the diet of Greece? the scientific evidence. J Nutr. 2001;131(11 Suppl):3065S–3073S. doi: 10.1093/jn/131.11.3065S. [DOI] [PubMed] [Google Scholar]
- 28.Singletary K. Natural products and cancer chemoprevention. J Nutr. 2000;130:465–466. doi: 10.1093/jn/130.2.465S. [DOI] [PubMed] [Google Scholar]
- 29.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131(11 Suppl):3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 30.Tan GT, Lee S, Lee IS, et al. Natural-product inhibitors of human DNA ligase 1. Biochem J. 1996;313(pt 3):993–1000. doi: 10.1042/bj3140993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatman D, Mo H. Volatile isoprenoid constituents of fruits, vegetables and herbs cumulatively suppress the proliferation of murine B16 melanoma and human HL-60 Leukemia cells. Cancer Lett. 2002;175(2):129–139. doi: 10.1016/s0304-3835(01)00723-6. [DOI] [PubMed] [Google Scholar]
- 32.Udeani GO, Gerhauser C, Thomas CF, et al. Cancer chemopreventive activity mediated by deguelin, a naturally occurring rotenoid. Cancer Res. 1997;57(16):3424–3428. [PubMed] [Google Scholar]
- 33.Uhegbu FO. Dietary secondary Amines and liver hepatoma in Port Harcourt, Nigeria. Plant Foods Hum Nutr. 1997;51(3):257–263. doi: 10.1023/a:1007947815017. [DOI] [PubMed] [Google Scholar]
- 34.Crowell P. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129:775S–778S. doi: 10.1093/jn/129.3.775S. [DOI] [PubMed] [Google Scholar]
- 35.Wargovich MJ, Wood C, Hollis DM, Zander ME. Herbals, cancer prevention and health. J Nutr. 2001;131(11):3034S–3036S. doi: 10.1093/jn/131.11.3034S. [DOI] [PubMed] [Google Scholar]
- 36.Zeegers MP, Goldbohm RA, Van den Brandt PA. Consumption of vegetables and fruits and urothelial cancer incidence: a prospective study. Cancer Epidemiol Biomarkers Prev. 2001;10(11):1121–1128. [PubMed] [Google Scholar]
- 37.Tinley LT, Deborah AR, Rachel ML, et al. Taccalonolides E and A: plant-derived steroids with microtubule-stabilizing activity. Cancer Res. 2003;63:3211–3220. [PubMed] [Google Scholar]
- 38.Izevbigie EB. Discovery of water soluble anticancer agents (Edotides) from vegetables found in Benin City, Nigeria. Exp Biol Med. 2003;228:293–298. doi: 10.1177/153537020322800308. [DOI] [PubMed] [Google Scholar]
- 39.Klafke N, Mahler C, von Hagens C, et al. A complex nursing intervention of complementary and alternative medicine (CAM) to increase quality of life in patients with breast and gynecologic cancer undergoing chemotherapy: study protocol for a partially randomized patient preference trial. Trials. 2015;16(51) doi: 10.1186/s13063-014-0538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians' attitudes toward CAM and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495–501. doi: 10.1093/ecam/nel036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Njan AA, Adza B, Agaba AG, Byarugaba D, Díaz-Llera S, Bangsberg DR. The analgesic and antiplasmodial activities and toxicology of Vernonia amygdalina. J Med Food. 2008;11:574–581. doi: 10.1089/jmf.2007.0511. [DOI] [PubMed] [Google Scholar]
- 42.Iwalokun BA, Efedede BU, Alabi-Sofunde JA, Oduala T, Magbagbeola OA, Akinwande AI. Hepatoprotective and antioxidant activities of Vernonia amygdalina on acetaminophen-induced hepatic damage in mice. J Med Food. 2006;9(4):524–530. doi: 10.1089/jmf.2006.9.524. [DOI] [PubMed] [Google Scholar]
- 43.Alabi DA, Oyero LA, Jimoh, Amusa NA. Fungitoxic and phytotoxic effect of Vernonia amygdalina Del., Bryophyllum pinnantus Kurz, Ocimum gratissimum (Closium) L and Eucalyptna globules (Caliptos) Labill water extracts on cowpea and cowpea seedling pathogens in Ago-Iwoye, South Western Nigeria. World J Agric Sci. 2005;1:70–75. [Google Scholar]
- 44.Osinubi AAA. Effects of Vernonia amygdalina and chlorpropamide on blood glucose levels. Med J Islam World Acad Sci. 1996;16:115–119. [Google Scholar]
- 45.Nwanjo HU, Nwokoro EA. Antidiabetic and biochemical effects of aqueous extract of Vernonia amygdalina leaf in normoglycaemic and diabetic rats. J Innov Life Sci. 2004;7:6–10. [Google Scholar]
- 46.Akah PA, Okafor CI. Hypoglycemic effect of Vernonia amygdalina in experimental rabbits. Plant Med Res. 1992;1:6–10. [Google Scholar]
- 47.Ijeh II, Ejike CECC. Current perspectives on the medicinal potentials of Vernonia amygdalina Del. J Med Plant Res. 2011;5(7):1051–1061. [Google Scholar]
- 48.Arhoghro EM, Ekpo KE, Anosike EO, Ibeh GO. Effect of aqueous extract of bitter leaf (Vernonia amygdalina Del.) on carbon tetrachloride induced liver damage in albino Wistar rats. Eur J Sci Res. 2009;26:122–130. [Google Scholar]
- 49.Nwanjo HU. Efficacy of aqueous leaf extract of Vernonia amygdalina on plasma lipoprotein and oxidative status in diabetic rat models. Niger J Physiol Sci. 2005;20:39–42. [PubMed] [Google Scholar]
- 50.Owu DU, Ben EE, Antai AB, et al. Stimulation of gastric acid secretion and intestinal motility by Vernonia amygdalina extract. Fitoterapia. 2007;79:2. doi: 10.1016/j.fitote.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Kupchan SM, Hemingway RJ, Karim A, Werner D. Tumor inhibitors 47: Vernodalin and vernomygdin, two new cytotoxic sesquiterpene lactones from Vernonia amygdalina Del. J Org Chem. 1969;34(12):3908–3911. doi: 10.1021/jo01264a035. [DOI] [PubMed] [Google Scholar]
- 52.Akachuku CO. Growth of bitter leaf (Vernonia amygdalina, Del., Compositae) and nutritive values of its processed and unprocessed leaves. Discov Innov. 2001;13:227–233. [Google Scholar]
- 53.Egedigwe CA. thesis. Umudike: Department of Biochemistry, Michael Okpara University of Agriculture; 2010. Effect of Dietary Incorporation of Vernonia amygdalina and Vernonia colorata on Blood Lipid Profile and Relative Organ Weights in Albino Rats. [Google Scholar]
- 54.Huffman MA, Page JE, Sukhedo MVK, et al. Leaf swallowing by chimpanzees: a behavioral adaptation for the control of strong nematode infections. Int J Primatol. 1996;72:475–503. [Google Scholar]
- 55.Moundipa FP, Kamini G, Melanie F, et al. In vitro amoebicidal activity of some medicinal plants of the Bamun region (Cameroon) Afr J Tradit Complement Altern Med. 2005;2(2):113–121. [Google Scholar]
- 56.Momoh MA, Muhamed U, Agboke AA, et al. Immunological effect of aqueous extract of VA and a known immune booster called immunace and their admixtures on HIV/AIDS clients: a comparative study. Asian Pac J Trop Biomed. 2012;2(3):181–184. doi: 10.1016/S2221-1691(12)60038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Butler GW, Bailey RW. Chemistry and Biochemistry of Herbage. Vol. 1. London: Academic Press; 1973. [Google Scholar]
- 58.Ologunde MO, Ayorinde FO, Shepard RK, Afolabi OA, Oke OL. Sterols of seed oils of Vernonia galanesis, Amaranthus cruentus, Amaranthus caudatus, Amaranthus hybrids and Amaranthus hypochondriacus growth in the humid tropics. J Food Agric. 1992;58:221–225. [Google Scholar]
- 59.Bonsi MLK, Osuji PO, Tuah AK. Effect of supplementing teff straw with different levels of leucaena or sesbania leaves on the degradabilities of teff straw, sesbania, leucaena, tagasaste and vernonia and on certain rumen and blood metabolites in Ethiopian Menz sheep. Anim Feed Sci Technol. 1995;52:101–129. [Google Scholar]
- 60.Bonsi MLK, Osuji PO, Tuah AK, et al. Vernonia amygdalina as a supplement to teff straw (Eragrostis tef.) fed to Ethiopian Menz sheep. Agrofor Syst. 1995;31:229–241. [Google Scholar]
- 61.Harborne JB. Phytochemical Methods. Vol. 278 London: Chapman and Hall; 1973. [Google Scholar]
- 62.Majolagbe ON, Adebayo EA, Aina DO, et al. Phytochemical screening and inhibition studies of the ethanolic and aqueous extracts of Vernonia amygdalina using microbes isolated from refuse dung hill as test organisms. Asian J Sci Technol. 2014;5(7):395–399. [Google Scholar]
- 63.Cimanga RK, Tona L, Mesia K, et al. In vitro antiplasmodial activity of extracts and fractions of seven medicinal plants used in the Democratic Republic of Congo. J Ethnopharmacol. 2004;93:27–32. doi: 10.1016/j.jep.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Sayed MD. Traditional medicine in health care. J Ethnophamacol. 1980;2(1):19–22. doi: 10.1016/0378-8741(80)90023-9. [DOI] [PubMed] [Google Scholar]
- 65.Jenett-Siems K, Froelich S, Onegi B, et al. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected East African plants used for the treatment of malaria. Planta Med. 2006;72 [Google Scholar]
- 66.Oyugi DA. thesis. Jackson (MS): Department of Biology, Jackson State University; 2007. Quality Assurance: Chemical Fingerprinting of Vernonia amygdalina Extracts with Anti-Proliferative Activities on MCF-7 BCous Cells. [Google Scholar]
- 67.Izevbigie EB, Howard CB, Lee KS. V. amygdalina: folk medicine, analysis, and potential applications for cancer treatment. Curr Pharm Anal. 2008;4:20–24. doi: 10.2174/157341208783497579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oyugi D, Xuan L, Lee K, Hill B, Izevbigie EB. Activity markers of the anti-breast carcinoma cell growth fractions of Vernonia amygdalina extracts. Exp Biol Med. 2009;234:410–417. doi: 10.3181/0811-RM-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venton DL, Kim SO, Breton GC. In: Economic and Medicinal Plants Research. Wagner H, Farnsworth WR, editors. London: Academic press; pp. 323–351. [Google Scholar]
- 70. [Accessed March 11, 2015];Success stories: Taxol. Available from: http://dtp.nci.nih.gov/timeline/flash/success_stories/S2_taxol.htm.
- 71.Jakoby WB, Ziegler DM. The enzymes of detoxification. J Biol Chem. 1990;265(34):20715–20718. [PubMed] [Google Scholar]
- 72.Howard CB, Stevens J, Izevbigie EB, et al. Time and dose-dependent modulation of phase 1 and phase 2 gene product expression in response to treatment of MCF-7 cells with a natural anticancer agent. J Cell Mol Biol. 2003;49(7):1057–1065. [PubMed] [Google Scholar]
- 73.Izevbigie EB, Bryant JL, Walker A. A novel natural inhibitor of extracellular signal-related kinases on human breast cancer cell growth. Exp Biol Med. 2004;229:163–169. doi: 10.1177/153537020422900205. [DOI] [PubMed] [Google Scholar]
- 74.Opata MM. thesis. Jackson (MS): Department of Biology, Jackson State University; 2006. Biochemical and Biophysical Evidence for Vernonia amygdalina Extracts Anticancer Actions. [Google Scholar]
- 75.Opata M, Izevbigie E. Aqueous Vernonia amygdalina extracts alter MCF-7 cell membrane permeability and efflux. Int J Environ Res Public Health. 2006;3(2):174–179. doi: 10.3390/ijerph2006030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Florack DE, Stickema WJ. Thionins: properties, possible biological roles and mechanisms of action. Plant Mol Biol. 1994;26:25–37. doi: 10.1007/BF00039517. [DOI] [PubMed] [Google Scholar]
- 77.Carrasco L, Vazquez D, Hernandez-Lucas C, Carbonero P, García-Olmedo F. Thionins: plant peptides that modify membrane permeability in cultured mammalian cells. Eur J Biochem. 1981;116:185–189. doi: 10.1111/j.1432-1033.1981.tb05317.x. [DOI] [PubMed] [Google Scholar]
- 78.Fregene A, Newman L. Breast cancer in Sub-Saharan Africa: how does it relate to breast cancer in African-American women? Cancer. 2005;103:1540–1550. doi: 10.1002/cncr.20978. [DOI] [PubMed] [Google Scholar]
- 79.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: Factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6) doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 80.Gresham LJ. thesis. Jackson (MS): Department of Biology, Jackson State University; 2008. Anti-Mitogenic Actions of V. amygdalina Extracts on Estrogen Receptor Negative Human Breast Tumoral Cells: Implication in Health Disparity. [Google Scholar]
- 81.Gresham LJ, Ross J, Izevbigie EB. Vernonia amygdalina: anticancer activity, authentication, and adulteration detection. Intl J Environ Res Public Health. 2008;5(5):342–348. doi: 10.3390/ijerph5050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ross JC. thesis. Jackson (MS): Department of Biology, Jackson State University; 2010. Additive Cytotoxic effects of Vernonia amygdalina Extracts and Tamoxifen in Human Carcinoma Cells. [Google Scholar]
- 83.Demeritte TL. thesis. Jackson (MS): Department of Chemistry, Jackson State University; 2010. Evaluation of the Protective Activity of Vernonia amygdalina on Acrylonitrile-Induced Genotoxicity in Rat Astrocytes. [Google Scholar]
- 84.Yedjou C, Izevbigie E, Tchounwou P. Preclinical assessment of Vernonia amygdalina leaf extracts as DNA damaging anticancer agent in the management of breast cancer. Int J Environ Res Pub Health. 2008;5(5):337–341. doi: 10.3390/ijerph5050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hill BJ. thesis. Jackson (MS): Department of Biology, Jackson State University; 2010. Ethanolic Extracts of Vernonia amygdalina Inhibit Spindle Formation of Microtubules in HELA Cells. [Google Scholar]
- 86.Yedjou CD, Izevbigie E, Tchounwou PB. Vernonia amygdalina-induced growth arrest and apoptosis of BC (MCF-7) cells. Pharmacol Pharm. 2013;4(1) doi: 10.4236/pp.2013.41013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cameron KS. dissertation. Jackson (MS): Department of Biology, Environmental Science, Jackson State University; 2010. Sensitivity of Taxol-Resistant Human Prostate Cancerous Cells to Aqueous Vernonia amygdalina Extracts. [Google Scholar]
- 88.Howard CB, Cameron K, Gresham L, et al. Environmental carcinogens and sensitization of Paclitaxel and Vincristine by Vernonia amygdalina extracts. Afr Environ Perspect. 2011;1:103–114. [Google Scholar]
- 89.Cameron KS, Howard CB, Izevbigie EB, Hilla BJ, Tchounwou PB. Sensitivity and mechanisms of taxol-resistant prostate adenocarcinoma cells to Vernonia amygdalina extract. Exp Toxicol Pathol. 2013;65:759–765. doi: 10.1016/j.etp.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McDowell R. thesis. Jackson (MS): Department of Biology, Jackson State University; 2013. Enhanced Genotypic and Apoptotic Responsiveness in Estrogen Receptor Negative Breast Cancerous Cells Exposed to Aqueous Vernonia amygdalina Extracts versus Paclitaxel. [Google Scholar]
- 91.Johnson WK. dissertation. Jackson (MS): Department of Biology, Environmental Science, Jackson State University; 2015. The Molecular Mechanisms of Vernonia amygdalina-Induced Toxicity in Prostatic Adenocarcinoma Cells. [Google Scholar]
- 92.Howard CB, Pervin S. Aqueous VA extract inhibits breast cancerous cell growth; Poster presented at: Transdisciplinary Collaborations: Evolving Dimensions of US and Global Health Equity, National Harbor, Maryland, The Minority Health and Health Disparities Grantees' Conference, formerly known as the International Symposium on Minority Health and Health Disparities, NIMHD, NIH, DHHS; December 1–3. [Google Scholar]
- 93.Howard CB, Pervin S. Vernonia amygdalina extract surpasses paclitaxel toward inhibition of triple negative breast cancerous cell-induced tumor growth. The Minority Health and Health Disparities Grantees' Conference, NIMHD, NIH, DHHS; December 1–3. [Google Scholar]
- 94.Syad AN, Devi KP. Botanics: a potential source of new therapies for Alzheimer's disease? Bot Targets Ther. 2014;4:11–26. [Google Scholar]
- 95.Cragg GM, Beutler JA, Jones WP. The American Society of Pharmacognosy: 50 years of progress in natural products research. American Society Pharmacognosy; 1959–2009. [Accessed March 11, 2015]. Available from: http://www.pharmacognosy.us/wordpress/wp-content/uploads/Front_Matter.pdf. [Google Scholar]
- 96.Brunken U, Schmidt M, Dressler S, Janssen T, Thiombiano A, Zizka G. West African plants - A Photo Guide. Forschungsinstitut Senckenberg; Frankfurt/Main, Germany: 2008. [Accessed October 30, 2015]. Available from: www.westafrican-plants.senckenberg.de. [Google Scholar]