Abstract
The majority of disseminated cryptococcal infections occur in patients with acquired immunodeficiency syndrome (AIDS), with only 11-14% of cases occurring in patients without AIDS. Most non-AIDS related cases (75%) occur in patients with another immune deficiency. Here, we present the first case of mucocutaneous cryptococcal disease in an immunocompetent host, review the epidemiology of risk factors associated with disseminated cryptococcal disease, and describe a rational workup for a possible acquired immunodeficiency. While rare, 25% of non-AIDS related cryptococcal disease will occur in individuals without an identifiable immunodeficiency and should prompt a work up for cell-mediated immunodeficiency and monitored for closely for progression of other opportunistic infections.
Case Report
A 57 year old female with a history of hypertension, seizure disorder, possible sarcoidosis, remote meningioma resection, and a 30 pack-year smoking history presented with one month of progressive swelling and pain over her manubrium and right chest wall and associated constitutional symptoms including fevers, chills, night sweats, and unintentional weight loss. Initially, she was placed on cephalexin for a lower left dental abscess. The pain and swelling did not improve with 7 days of treatment. She had two teeth extracted and cephalexin was switched to amoxicillin. A week later, two crowns were placed and another tooth was extracted. The swelling and pain of her left jaw progressed and she subsequently developed left sided neck and left shoulder pain for which cyclobenzaprine and clindamycin was prescribed. One week later (eight weeks from the onset of symptoms) she returned to her PCP with discrete chest wall masses and was sent to the Emergency Center (EC) for evaluation.
Thirteen years ago, she developed three tender, painful nodules on her left shin, which were biopsied revealing granulomas; she was told they might represent sarcoidosis. She had no pulmonary complaints at that time, or subsequently. The nodules resolved after 6 months of oral prednisone; she has not been on prednisone or other immunosuppressive medications in the ensuing twelve years leading to this presentation. Hypertension was diagnosed more than ten years prior and was well controlled on amlodipine. She underwent a meningioma resection as a teenager with resultant seizure disorder controlled by anti-epileptics. Her medications included amlodipine, carbamazepine, acetaminophen, ibuprofen, tramadol, and oxycodone.
She was born and raised in Texas, has no history of recent travel, and works as a home health aide. She is married and monogamous with no history of sexually transmitted diseases. She smokes tobacco (30 pack-years) and denies drug or alcohol use. She has no known exposures to individuals with tuberculosis, has never been in or worked in shelters or jails, and has had annual negative PPDs for her employment. She denies unpasteurized food intake, exposure to chemicals, or exposure to birds or other animals.
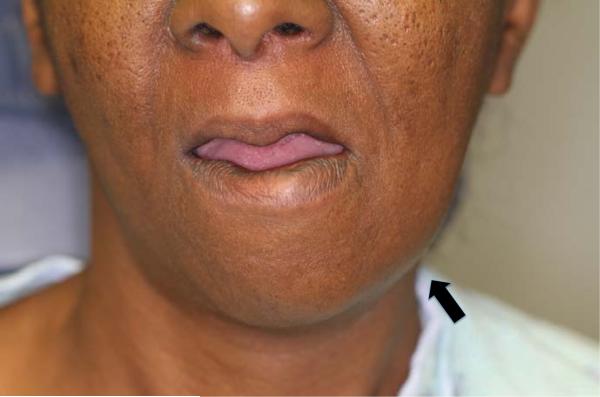
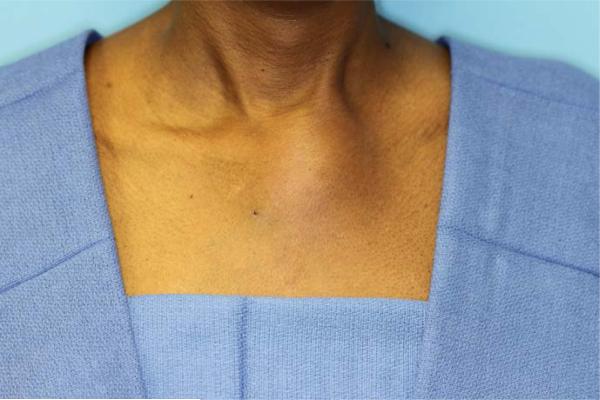
Physical exam revealed a maximum temperature of 99.4 Fahrenheit and otherwise normal vital signs. Her oral exam was significant for a 1 cm raised fluctuant lesion noted on the left alveolar ridge and mild blunting of her mandibular angle on the left (Figure 1). Her neck exam was benign, without thyromegaly or lymphadenopathy. She had an approximately 6 cm mildly erythematous, firm, and tender mass overlying the left side of the manubrium without crepitus (Figure 2). A prominence of the right chest overlying her 3rd rib was palpable and mildly tender. The remainder of her exam was normal.
Figure 1.
One centimeter, raised fluctuant mass blunting the left mandibular angle.
Figure 2.
Six-centimeter erythematous, firm, tender mass overlying the left manubrium.
Laboratory studies revealed a WBC count of 9.4 K/uL, Hgb 11.8 g/dL, HgbA1c 6.8%, (previously within normal limits), CRP 6.34 mg/dL, erythrocyte sedimentation rate 77 mm/Hr, alkaline phosphatase of 510 U/L, serum albumin 2.8 g/dL, HIV 1/2 serology negative, CD4 count 687 cells/uL (37%). Her electrolytes and renal function were normal.
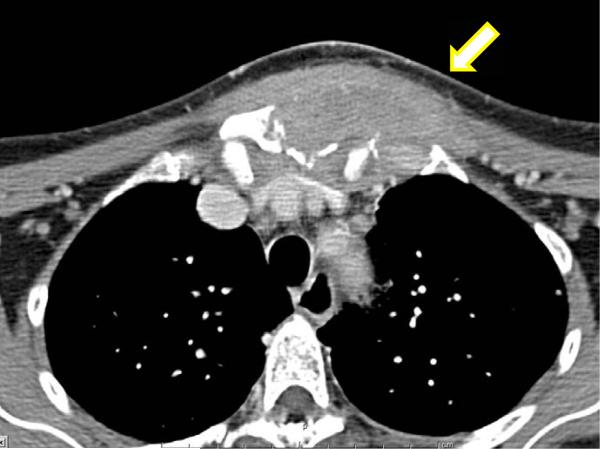
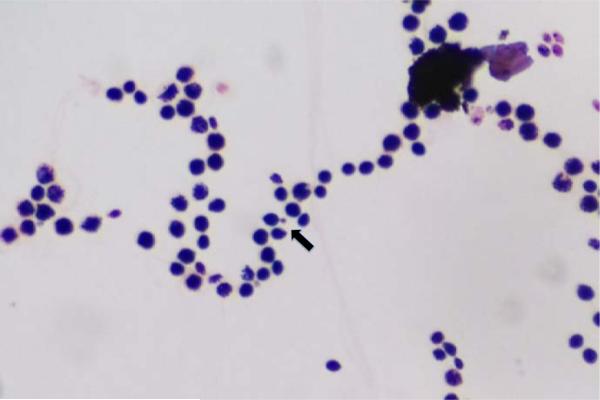
Computed tomography (CT) of the neck and chest with and without contrast showed complex soft tissue masses of the right pectoral region and the parasternal region with osseous erosion of the underlying bone (right anterior 3rd rib and manubrium), mediastinal and hilar lymphadenopathy, and multiple mandibular lucencies corresponding with recent dental extractions, as well as a 1.5×1.5×1.0 cm radiolucency posterior to the body of the left mandible (Figure 3). A CT guided biopsy of the sternal mass and right pectoral mass was performed. Gram stain revealed narrow budding fungal bodies (Figure 4). Culture grew Cryptococcus neoformans (typing confirmed neoformans not gattii). Pathological exam of core biopsy revealed acute and chronic inflammation, granulation tissue with fibrotic changes, and focal ossification with no evidence of malignancy. Serum Cryptococcal antigen was positive at a titer of 1,024. A lumbar puncture was performed, which revealed normal opening pressure and 2 WBC's; glucose and protein were within normal limits and Cryptococcal antigen was negative.
Figure 3.
Computed tomography of the chest shows a complex soft tissue mass of the right pectoral and presternal region including osseous erosion of the 3rd rib and manubrium.
Figure 4.
Gram stain of narrow budding fungal bodies (arrow) which grew Cryptococcus neoformans.
She was initially placed on clindamycin for antimicrobial coverage against oral flora including Actinomyces species. When the sternal cultures revealed Cryptococcus, she was placed on fluconazole 400 mg PO per day. Oral examination was significant for a fluctuant lesion on the left posterior mandibular alveolar ridge (Figure 5). Purulence was expressed from the lesion and sent for culture. A biopsy and debridement of the left mandibular abscess was performed; pathology revealed granulomatous inflammation and culture a grew Cryptococcus neoformans. After eight days on oral fluconazole, she underwent surgical debridement of the sternal and chest wall sites with partial resection of the left sternoclavicular joint and placement of amphotericin B beads. She underwent a second debridement with removal of beads and placement of a pectoral flap. Cultures from all sternal and chest wall surgical debridements were sterile. She was given six months of Fluconazole 400 mg PO daily and followed in Infectious Diseases Clinic without further infections.
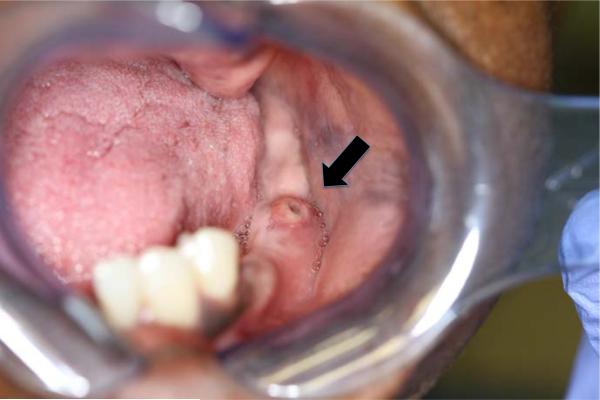
Figure 5.
Investigation of the oral cavity revealed a fluctuant lesion of the left posterior mandible, which drained pus growing Cryptococcus neoformans.
Discussion
Cryptococcus was first described clinically by the surgeon Abraham Buschke [1] and pathologist Otto Bussee in 1894 when they isolated the organisms from a gumma-like lesion of a tibia of a 31-year-old female. Although Cryptococcus is a ubiquitous yeast that can cause disease in healthy hosts, the majority of patients have predisposing immunodeficiencies, most commonly advanced AIDS (Table 1).
Table 1.
Review of underlying associated medical conditions associated with HIV-negative cryptococcal disease.
| Study | Number of Cases of HIV-Negative Cryptococcus | No known comorbidities or drugs | Mortality | On immune-suppressive drugs | Malignancy | Rheumatologic disease | Chronic pulm Disease | Diabetes | Solid Organ Transplant | Other | Cirrhosis | ESRD | CD4 lymphocytopenia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lewis (1972) | 32 | 6 (18%) | 14 (44%) | 11 | 10 | 2 | 4 | 3 | NR | NR | NR | NR | NR |
| Dromer (1996) | 83 | 20 (24%) | 22 (26%) | 6 | 23 | 20 | NR | 3 | NR | NR | 3 | NR | 3 |
| Hajjeh (1998) | 135 | 44 (32%) | 23 (27%) | 25 | 31 | 17 | 16 | 30 | 6 | 8 | 5 | 5 | NR |
| Pappas (2000) | 251 | 66 (26%) | 91 (30%) | 85 | 68 | 41 | 38 | NR | 54 | 51 | NR | NR | NR |
| Mirza (2003) | 124 | 22 (17%) | 26 (21%) | NR | 25 | 16 | 17 | 28 | NR | NR | NR | NR | NR |
| Kietiburanakul (2006) |
37 | 11 (29%) | 10 (27%) | 15 | 6 | 6 | NR | 5 | NR | NR | 2 | NR | NR |
| Total | 662 | 169 (25%) | 186 (28%) | 142 | 163 | 95 | 75 | 69 | 60 | 59 | 10 | 5 | 3 |
NR = Not reported.
Oral fungal infections are most commonly due to Candida, although rarely other fungal pathogens have been described [2]. Aspergillosis, Blastomycosis, Coccidioidomycosis, Cryptococcosis, Fusariosis, Geotrichosis, Histoplasmosis, Mucormycosis, Paracoccidiomycosis, Penicilliosis, and Sporotrichosis have all been described intra-orally [3]. Oral and mucocutaneous cryptococcal infections have rarely been documented in the literature, but none, as yet, have been reported in an immunocompetent patient. A review of the literature reveals six cases of cryptococcal infection with oral manifestations. Five occurred in AIDS patients with lesions of the palate [4,5], maxilla [6] and tongue [7]; the most recently reported case occurred in an immunosuppressed stem cell transplant recipient [8] with a lesion of the tongue. Another case was reported of an AIDS patient with disseminated Cryptococcal disease found to have Cryptococcus neoformans in the salivary glands on autopsy without any oral symptoms pre-mortem [9].
Cryptococcus skeletal infections occur in 5-10% of disseminated disease, often involving bony prominences and infrequently joints [10,11]. Isolated cases of osteomyelitis are less common. A review of the literature from 1956 to 1998 identified 40 cases of isolated cryptococcal osteomyelitis [12]. The most common presentation was involvement of vertebrae, followed by femur, tibia, and rib. Single sites of involvement occurred in 75% of patients with the rest having multiple sites of infection. Sarcoidosis was the most common underlying disease, followed by tuberculosis and systemic steroid treatment. Initial symptoms of cryptococcal osteomyelitis included swelling and soft-tissue tenderness. In cases with joint involvement, typical synovitis manifestations were observed on exam [13]. Patients were generally a febrile and had normal white blood cell counts at presentation [13,14]. Serum cryptococcal antigen was rarely detected. Most cases of osteomyelitis are successfully treated medically using amphotericin B or fluconazole in combination with surgical debridement [11,13,14]. Since the disease is rare, the role of surgical management is unclear with some advising debridement [15] and others advocating medical management alone [14].
Since the AIDS epidemic has begun, two large surveillance studies [15,16] have evaluated Cryptococcal disease occurring in both HIV-negative and HIV-positive patients. These studies verify that Cryptococcal disease occurs predominantly in AIDS patients, with only 11-14% of all Cryptococcal disease occurring in HIV-negative individuals. HIV-negative patients who developed Cryptococcal disease, 70% in onestudy and 82% in the other, had at least one underlying medical condition (Table 1). Compared to patients with HIV-related Cryptococcal disease, HIV-negative patients had higher mortality.
In the pre-AIDS era, a case series from 1953 to 1970 at the University of Iowa and the Iowa VA Hospital described 32 cases of Cryptococcal disease. Eighty-two percent of these cases occurred in patients with underlying medical illnesses, most commonly lymphoma (31%), chronic pulmonary disease (18%), or cardiac (15%) disease. Two-thirds of patients had CNS or pulmonary disease; there were no cases of osteomyelitis and one case of skin and soft tissue infection. Mortality in this series of patients was 44% [17]. In more recent case series from France [18] and Thailand [19], 83 and 37 cases, respectively, of Cryptococcal disease in HIV-negative patients were described. The majority of cases (76% and 71%, respectively) occurred in patients on immunosuppressive drugs or in patients with associated comorbidities.
In a surveillance study of 15 medical centers from 1990-1996, Pappas et al. [20] described 251 definitive and 40 probable cases of Cryptococcal disease in HIV-negative patients. In this large series of 251 HIV-negative patients with Cryptococcal disease, 90% of cases involved pulmonary or CNS disease with only 10% presenting without pulmonary or CNS involvement. Almost one-third of cases occurred in patients on chronic corticosteroids. Overall, 74% of patients were on immunosuppressive medications or had associated diseases such as cancer, organ transplant, or chronic pulmonary disease (Table 1). Worse outcomes occurred in patients with disease not involving the CNS or lung perhaps due to delay in diagnosis, atypical presentations such as cellulitis, or severity of underlying illness.
Since the majority ofdisseminated Cryptococcal disease occurs in AIDS patients or patients with underlying immunodeficiencies, this patient's presentation triggered a workup for an undiagnosed immunodeficiency. Case reports have described Cryptococcal disease in patients with deficiencies of cell-mediated immunity, such as patients with IL-2 deficiency [21], Hyper-IgE syndrome [22] and associated CD40/CD40Ligand signaling deficiencies [23] and Jak3 missense mutations [24].
In 2012, investigators from the NIH, Thailand and Taiwan described patients with Cryptococcal disease in association with neutralizing auto-antibodies to IFN-γ [25] and GM-CSF [26]. PBMCs and serum were evaluated from 52 patients with disseminated non-tuberculous mycobacterial disease and 45 cases of other opportunistic infections, 10 of which included Cryptococcal disease. These patients were HIV-negative and had no other known immunodeficiency. Serum from each of these patients had IgG that was shown to inhibit IFN-γ-mediated STAT1 phosphorylation. Rosen et al. retrospectively evaluated 67 samples of patients with Cryptococcal meningitis and no known immunodeficiency [27]. Seven of the 67 patients were found to have biologically active auto-antibodies against GM-CSF. Anti-GM-CSF antibodies were not found in 180 healthy controls. Studies of our patient's serum performed at the NIH were negative for auto-antibodies to IFN-γ, GM-CSF, TNF-α, IL-12, IL-17A and IL-6. She was followed in clinic without recurrence or presentation of other opportunistic infections [28].
In conclusion, we present a case of a 57-year old immunocompetent woman who had disseminated cryptococcal disease involving the mandible and left sternoclavicular joint that progressed to manubrial and rib osteomyelitis and adjacent soft tissue abscesses. Both the mandibular and manubrial abscesses grew Cryptococcus neoformans. She had a normal CD4 count, CD4 percentage and was HIV-negative. In light of recent reports of circulating cytokine auto-antibodies in immunocompetent patients with disseminated cryptococcal and cryptococcal meningitis, serum was sent to the NIH for anti-cytokine antibodies studies, which were negative. Our patient carried the diagnosis of sarcoidosis, which has been associated with cryptococcal disease, but at the time of her presentation to us, she had no evidence of active disease and had not taken immunosuppressive medications for thirteen years. The patient's only identifiable immunodeficiency was a new diagnosis of diabetes with a hemoglobin A1c of 6.8%. However, it is not clear if the infection or diabetes came first. Thus, the basis for our patient's predisposition to disseminated cryptococcal disease, if any, remains uncertain.
References
- 1.Buschke A. Über eine durch coccidien hervergerufene krankheit des menschen. Dtsch Med. Wochenschr. 1895;21:14. [Google Scholar]
- 2.Neville BW, Damm DD, Allen CM, Bouquot JE. Neville, Dammn, Allen, Bouquot Oral and axillofacial pathology. 3rd Ed. WB Saunder; Philadelphia: 2009. Fungal and protozoal diseases. pp. 224–237. [Google Scholar]
- 3.Samaranayake LP, Leung WK, Jin L. Oral mucosal fungal infections. Periodontology 2000. 2009;49:39–59. doi: 10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 4.Glick M, Cohen SG, Cheney RT, Crooks GW, Greenberg MS. Oral manifestations of disseminated Cryptococcus neoformans in a patient with acquired immunodeficiency syndrome. Oral Surg Oral Med Oral Pathol. 1987;64(4):454–459. doi: 10.1016/0030-4220(87)90152-6. [DOI] [PubMed] [Google Scholar]
- 5.Tzerbos F, Kabani S, Booth D. Cryptococcosis as an Exclusive Oral Presentation. J Oral Maxillofac Surg. 1992;50(7):759–760. doi: 10.1016/0278-2391(92)90115-g. [DOI] [PubMed] [Google Scholar]
- 6.Dodson TB, Perrott DH, Leonard MS. Nonhealing Ulceration of Oral Mucosa. J Oral Maxillofac Surg. 1989;47(8):849–852. doi: 10.1016/s0278-2391(89)80045-x. [DOI] [PubMed] [Google Scholar]
- 7.Lynch DP, Naftolin LZ. Oral Cryptococcus neoformans infection in AIDS. Oral Surg Oral Med Oral Pathol. 1987;64(4):449–453. doi: 10.1016/0030-4220(87)90151-4. [DOI] [PubMed] [Google Scholar]
- 8.Reinstadler DR, Dadwal S, Maghami E. Cryptococcal Tongue Lesion in a Stem Cell Transplant Patient: First Reported Case. Case Reports in Otolaryngology. 2012;4:517415. doi: 10.1155/2012/517415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monteii RA, Hofman P, Michiels IF, Loubiere R. Oral eryptococcosis: case report of salivary gland involvement in an AIDS patient. J Oral Pathol Med. 1997;26(1):53–56. doi: 10.1111/j.1600-0714.1997.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 10.Flannery MT, Rivera L, Casanas B. Cryptococcal Neoformans Osteomyelitis-A Case Report and Review of Literature. Medical Case Reports. 2012;3(1):23–25. [Google Scholar]
- 11.Collins VP. Bone involvement in cryptococcosis. Am J Roentgenol. 1950;63:102. [PubMed] [Google Scholar]
- 12.Liu FY. Cryptococcal Osteomyelitis: Case Report and Review. Diagn Microbiol Infect Dis. 1998;30(1):33–35. doi: 10.1016/s0732-8893(97)00190-9. [DOI] [PubMed] [Google Scholar]
- 13.Wildstein MS, Martin SM, Glaser JA. Cryptococcal osteomyelitis in a 20-year-old male with sarcoidosis. Spine J. 2005;5(4):467–470. doi: 10.1016/j.spinee.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Behrman RE, Masci JR, Nicholas P. Cryptococcal skeletal infections: case report and review. Rev Infect Dis. 1990;12(2):181–190. doi: 10.1093/clinids/12.2.181. [DOI] [PubMed] [Google Scholar]
- 15.Mirza SA, Phelan M, Rimland D, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992-2000. Clin Infect Dis. 2003;36(6):789–794. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 16.Hajjeh RA, Conn LA, Stephens DS, et al. Cryptococcosis: Population-Based Multistate Active Surveillance and Risk Factors in Human Immunodeficiency Virus–Infected Persons. The Journal of Infectious Diseases. 1999;179(2):449–454. doi: 10.1086/314606. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JL, Rabinovich S. The wide spectrum of cryptococcal infections. Am J Med. 1972;53(3):315–322. doi: 10.1016/0002-9343(72)90174-x. [DOI] [PubMed] [Google Scholar]
- 18.Dromer F, Mathoulin S, Dupont B, Brugiere O, Letenneur L. Comparison of the Efficacy of Amphotericin B and Fluconazole in the Treatment of Cryptococcosis in Human Immunodeficiency Virus-Negative Patients: Retrospective Analysis of 83 Cases. Clin Infect Dis. 1996;22(Suppl 2):SI54–160. doi: 10.1093/clinids/22.supplement_2.s154. [DOI] [PubMed] [Google Scholar]
- 19.Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, Sungkanuparph S. Cryptococcosis in human immunodeficiency virus-negative patients. Int J Infect Dis. 2006;10(1):72–78. doi: 10.1016/j.ijid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Pappas PG, Perfect JR, Cloud GA, et al. Cryptococcosis in Human Immunodeficiency Virus–Negative Patients in the Era of Effective Azole Therapy. Clin Infect Dis. 2001;33(5):690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 21.Sorenson RU, Boehm KD, Kaplan D, Berger M. Cryptococcal osteomyelitis and cellular immunodeficiency associated with interleukin-2 deficiency. J Pediatr. 1992;121(6):873–879. doi: 10.1016/s0022-3476(05)80331-2. [DOI] [PubMed] [Google Scholar]
- 22.Hutto JO, Bryan CS, Greene FL, White CJ, Gallin JI. Cryptococcosis of the colon resembling Crohn's disease in a patient with the hyperimmunoglobulinemia E-recurrent infection (Job's) syndrome. Gastroenterology. 1988;94(3):808–812. doi: 10.1016/0016-5085(88)90257-0. [DOI] [PubMed] [Google Scholar]
- 23.Pietrella D, Lupo P, Perito S, Mosci P, Bistoni F, Vecchiarelli A. Disruption of CD40/CD40L interaction influences the course of Cryptococcus neoformans infection. FEMS Immunol Med Microbiol. 2004;40(1):63–70. doi: 10.1016/S0928-8244(03)00297-9. [DOI] [PubMed] [Google Scholar]
- 24.Aslum Z, Al-Saud B, Al-Ghonaium A, et al. The Pediatric Infectious Disease Journal. 2012;31(2):p204–206. doi: 10.1097/INF.0b013e318239c3b3. [DOI] [PubMed] [Google Scholar]
- 25.Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367(8):725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen LB, Freeman AF, Yang LM, et al. Anti–GM-CSF Autoantibodies in Patients with Cryptococcal Meningitis. J Immunol. 2013;190(8):3959–3966. doi: 10.4049/jimmunol.1202526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One. 2013;8(3):e60431. doi: 10.1371/journal.pone.0060431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc. 2013;124:61–79. [PMC free article] [PubMed] [Google Scholar]