Summary
Overexpression of miR-374a inhibited lung adenocarcinoma cell proliferation, migration and invasion. miR-374a suppressed lung adenocarcinoma cell proliferation and invasion via targeting TGFA gene expression. High TGFA gene expression strongly correlated with poor survival in patients with lung adenocarcinoma.
Abstract
Aberrant expression of miR-374a has been reported in several types of human cancers, including lung cancer. However, the functional significance and molecular mechanisms underlying the role of miR-374a in lung cancer remain largely unknown. We found that the expression of miR-374a was significantly downregulated in lung adenocarcinoma tissues compared to adjacent normal lung tissues in samples included in The Cancer Genome Atlas. Functional studies revealed that overexpression of miR-374a led to inhibition of lung adenocarcinoma cell proliferation, migration and invasion and that miR-374a negatively regulated transforming growth factor-alpha (TGFA) gene expression by directly targeting the 3′-UTR of TGFA mRNA. Treating lung adenocarcinoma cells with TGF-α neutralizing antibody resulted in suppression of cell proliferation and invasion, which mimicked the action of miR-374a. Additionally, TGFA gene expression was significantly higher in tumor tissues compared to adjacent normal tissue and high TGFA gene expression strongly correlated with poor survival in patients with lung adenocarcinoma. Taken together, our studies suggest that miR-374a suppresses lung adenocarcinoma cell proliferation and invasion via targeting TGFA gene expression. Our findings may provide novel treatment strategies for lung adenocarcinoma patients.
Introduction
Lung cancer is the leading cause of cancer deaths in men and the second leading cause of cancer deaths in women worldwide, accounting for about 13% (1.8 million) of total cancer diagnoses in 2012 (1). Of the histologic types of lung cancer, adenocarcinoma has surpassed squamous cell carcinoma as the most common type of primary lung cancer (2). Despite recent advances in the diagnosis and management of lung cancer, the prognosis for patients with lung cancer remains poor worldwide, with 5-year relative survival currently at 18% (3). A better understanding of the molecular mechanisms underlying the development and progression of lung cancer is essential for identifying novel and effective therapeutic targets.
MicroRNAs (miRNAs) are small, endogenous, non- coding RNAs that negatively regulate gene expression at the posttranscriptional level by binding to the 3′ untranslated region (3′-UTR) of their target mRNAs, leading to mRNA degradation or suppression of protein translation (4). Accumulating evidence suggests that miRNAs can modulate various cellular processes and may act as either oncogenes or tumor suppressor genes in different types of human cancer, including lung cancer (5). Indeed, miR-17~92, miR-21, miR-221 and miR-222 have been reported to promote lung tumorigenesis, while tumor suppressive miRNAs, such as let-7, miR-15/16, miR-34/449, miR-200, miR-205 and miR-145 have been shown to suppress lung tumorigenesis (6,7). These deregulated miRNAs may be involved in multiple malignancy-related processes, such as tumor initiation, proliferation, angiogenesis, invasion or metastasis. Growing evidence also demonstrates that miRNAs may serve as biomarkers for diagnosis or prognosis in different types of human cancer. For instance, low expression of let-7, miR-34a or miR-218 predicted poor survival in patients with lung cancer (8–11).
Recently, aberrant expression of miR-374a has been reported in several different types of human cancer, including lung cancer (12,13), prostate cancer (14), breast cancer (15–17) and osteosarcoma (18). Notably, miRNA arrays analyzing the expression profile of 858 miRNAs in non-small cell lung cancer showed that high expression of miR-374a increased overall survival, whereas low expression was associated with a significantly poorer prognosis (12). Very recently, miR-374a was identified as one of the 13 miRNAs in a serum microRNA signature (the miR-Test) for lung cancer early detection (19). Furthermore, miRNA array profiles of paclitaxel-resistant and paclitaxel-sensitive lung cancer A549 cells showed that miR-374a was down-regulated in the paclitaxel-resistant cells (20). While previous data suggest that miR-374a plays a role in lung cancer, the mechanism by which miR-374a functions via target genes in lung cancer remains largely unknown. In this study, we queried publicly available data from The Cancer Genome Atlas (TCGA) (21–23) to compare miR-374a expression levels between lung adenocarcinoma tissues and adjacent normal lung tissues. Then, we investigated the function and downstream target of miR-374a in lung adenocarcinoma cells.
Materials and methods
Expression of miR-374a and the TGFA gene in human lung adenocarcinoma tissue samples and survival analysis
Expression levels of miR-374a and the TGFA gene in lung adenocarcinoma tissues and adjacent normal tissues were obtained from TCGA dataset and an algorithm developed by Li et al. (21–23) (available at http://starbase.sysu.edu.cn). TGFA gene expression levels (Affymetrix probe IDs: 205015_s_at, 211258_s_at and 205016_at) were extracted from publicly-available microarray data of lung adenocarcinoma patients and related to survival using the online analysis tool Kaplan–Meier Plotter (http://kmplot.com) (24). Hazard ratios with 95% confidence intervals and log-rank P values were calculated.
Cell culture and transfection
Human lung adenocarcinoma A549 and PC-9 cells from the American Type Culture Collection (ATCC, Manassas, VA) were cultured in RPMI 1640 medium (Gibco/Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin at 37°C in a humidified atmosphere with 5% CO2. A549 and PC-9 cells were transiently transfected with hsa-miR-374a mimic or miRNA mimic-negative control (NC) (Ambion/Life Technologies, Grand Island, NY) using Lipofectamine RNAiMAX Reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol. For migration, invasion, colony formation, quantitative real-time PCR (qRT-PCR) and Western blotting, cells were collected 48h after transfection. Co-transfection of the miRNA mimic and plasmid DNA was conducted using Lipofectamine 2000 Reagent (Life Technologies, Grand Island, NY).
Cell proliferation and colony formation assays
Lung adenocarcinoma A549 and PC-9 cells were seeded into 96-well plates at a density of 2×103 cells/well in 100 μl of RPMI 1640 and tested by 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays at different time points. Briefly, 20 μl of 5mg/ml solution of MTT (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline was added to each well. The plates were then incubated for 4h at 37ºC. The supernatant was removed, and 150 μl of dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) was added to each well. The MTT formazan products were dissolved by shaking the plates at room temperature for 10min, and the absorbance was subsequently measured on a BioTek Plate Reader (BioTek Instruments, Winooski, VT) using a test wavelength of 490nm. For the plate colony formation assay, cells were plated in six-well plates at a density of 500 cells per well and were cultured with RPMI 1640 supplemented with 10% fetal bovine serum for 10 days. At the end of the incubation period, the cells were washed twice with phosphate-buffered saline, fixed in methanol and dyed with crystal violet. Three independent experiments were performed.
Trans-well migration and invasion assays
Cell migration and invasion assays were performed in a 24-well plate with 8 µm pore size chamber inserts (Corning, New York, NY). Cells in serum-free medium were seeded into upper chambers coated with or without Matrigel (Corning, New York, NY) for invasion and migration assays, respectively. For the migration assay, 5×104 cells were re-suspended in 100 μl of serum-free medium and placed in the upper compartment of a Trans-well chamber. For the invasion assay, 1×105 cells were plated in the top chamber with a Matrigel-coated membrane. The lower chambers were filled with 600 ul of RPMI 1640 containing 10% fetal bovine serum as nutritional attractants. After 12h of incubation at 37°C, cells on the top surface of the insert were manually removed by wiping with a cotton swab. Cells that invaded or migrated to the bottom surface of the insert were fixed in 100% pre-cooling methanol for 30min, stained with crystal violet, then subjected to microscopic inspection. Five visual fields of each insert were randomly chosen and counted under a light microscope.
Bioinformatics analysis of miR374a target genes
The biological targets of miRNA were predicted using the algorithms TargetScan, miRDB, PicTar and PITA (25–28).
RNA isolation and quantitative real-time PCR
Total RNA was isolated from A549 and PC-9 cells using the miRNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. RNA concentrations were determined using the NanoDrop. 500ng of total RNA from each sample was subjected to reverse transcription using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Expression of the TGFA gene was detected using RT2 qPCR Primer Assays and RT2 SYBR Green Mastermixes (Qiagen, Valencia, CA) in an ABI 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Relative gene expression was normalized to the expression of GAPDH and was calculated using the 2(-ΔΔCT) method (29).
Western blotting
Cells were lysed using the RIPA Lysis and Extraction Buffer (Life Technologies, Grand Island, NY) supplemented with protease inhibitors (Roche, Indianapolis, IN). The total protein was quantified with a Pierce BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s protocol. Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking in phosphate-buffered saline/Tween-20 containing 5% non-fat milk at room temperature for 1h, the membrane was incubated with primary antibody at 4°C overnight using human TGF-alpha antibody (1 µg/ml; R&D Systems, Minneapolis, MN). Then, the membrane was incubated with anti-goat IgG secondary antibody conjugated with horseradish peroxidase (1:5000; Santa Cruz Biotechnology, Dallas, TX). GAPDH was used as a loading control. Proteins were visualized with LumiGLO chemiluminescent substrate (Cell Signaling Technology, Danvers, MA) and X-ray film was used to capture images.
Plasmid construction and luciferase reporter assay
The 3′-UTR sequence of the TGFA gene predicted to interact with miR-374a or a mutated sequence within the predicted target site was synthesized and inserted into the PmeI and XbaI sites of the pmirGLO Vector (Promega, Madison, WI). The sequences of the wild-type and mutated TGFA gene 3′-UTR were 5′-AAACTAGCGGCCGCTAGTTATCTTTATTATAATAAACCT-3′, and 5′-AAACTAGCGGCCGCTAGTTATCTTGCTGCTCCTAAACCT-3′, respectively (mutated bases were underlined). The wild-type or mutant luciferase reporter constructs, together with the pRL-TK Vector (Promega, Madison, WI), were co-transfected into A549 cells with miR-374a mimic or mimic-NC by lipofectamine 2000 (Life Technologies, Grand Island, NY). Forty-eight hours after transfection, firefly and renilla luciferase activity was measured by the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer’s instruction. Firefly luciferase activity was normalized to renilla luciferase activity. Three independent experiments were performed and the data were presented as mean ± SD.
TGF-α neutralizing antibody treatment
Cells were treated with human TGF-α neutralizing antibody (5 µg/ml) or IgG control antibody (R&D Systems, Minneapolis, MN) for 48 or 72h. The Neutralization Dose (ND50) is typically 0.4–0.8 µg/ml in the presence of 3ng/ml recombinant human TGF-α. MTT, colony formation and invasion assays were performed after treatment.
Statistical analysis
Data were shown as mean ± SD. Differences between experimental groups and controls were assessed by Student’s t-test. Two-tailed tests were used, and a P value of 0.05 or less was considered statistically significant.
Results
MiR-374a was significantly downregulated in lung adenocarcinoma
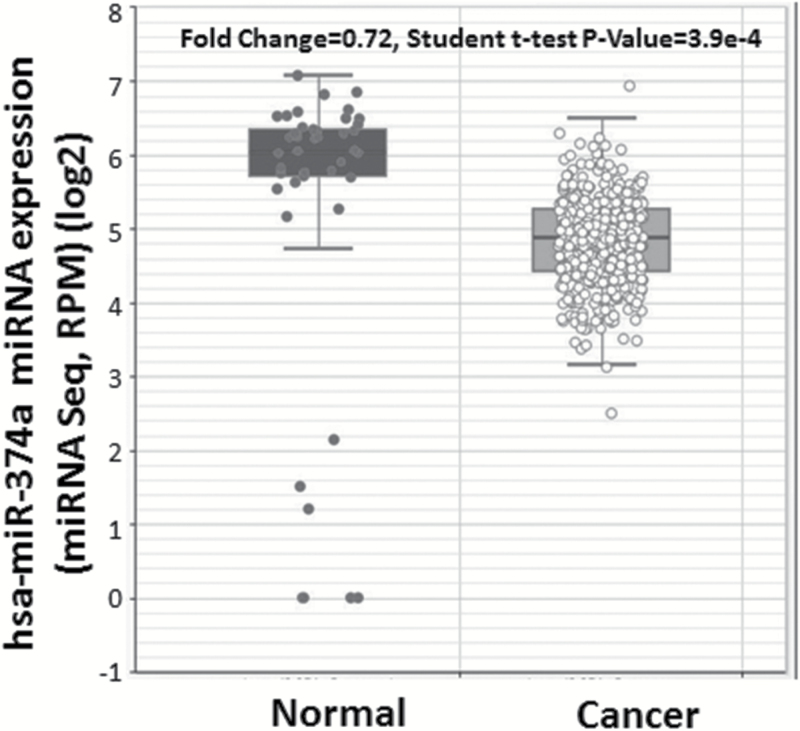
We compared miR-374a expression levels in 430 lung adenocarcinoma tissue samples with 46 adjacent normal lung tissues using existing data from TCGA and following algorithms posted on http://starbase.sysu.edu.cn. The expression of miR-374a was significantly lower in lung adenocarcinoma tissues compared to adjacent normal tissues (fold change = 0.72, P = 3.9×10–4) (Figure 1).
Figure 1.
MiR-374a expression in lung adenocarcinoma and adjacent normal tissues. MiR-374a expression levels of 430 lung adenocarcinoma tumor tissues and 46 adjacent normal tissues were obtained from TCGA and algorithms available at http://starbase.sysu.edu.cn.
Overexpression of miR-374a inhibited lung adenocarcinoma cell proliferation, migration and invasion in vitro
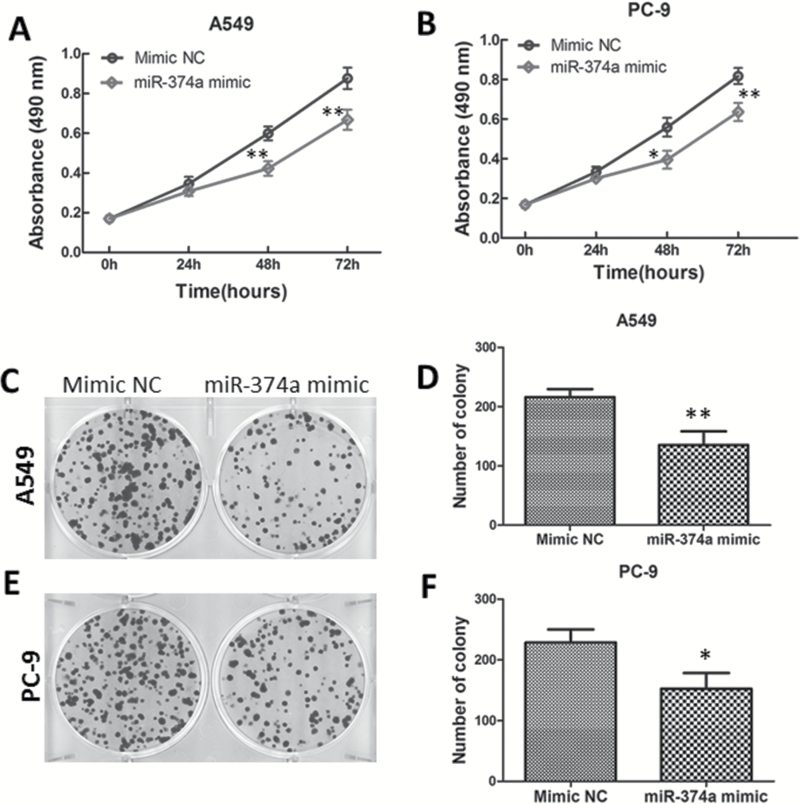
To investigate the biological functions of miR-374a in lung adenocarcinoma, a cell proliferation (MTT) assay was performed after transient transfection of miR-374a mimic or mimic-NC in A549 and PC-9 cells. As shown in Figure 2A and B, cells transfected with miR-374a mimic showed reduced proliferation compared with those transfected with miRNA mimic-NC in both A549 and PC-9 cells. To evaluate the long-term effects of miR-374a on cell proliferation, a colony formation assay was employed. A549 cells transfected with miR-374a mimic displayed fewer colonies than cells transfected with mimic-NC (Figure 2C and D). Consistently, miR-374a had a similar effect on the colony formation ability in PC-9 cells (Figure 2E and F), suggesting that miR-374a negatively regulated cell proliferation of lung adenocarcinoma cells.
Figure 2.
Overexpression of miR-374a inhibits lung adenocarcinoma cell proliferation. (A, B) A549 and PC-9 cells transfected with miR-374a mimic or mimic-NC were subjected to MTT assay to analyze the cell viability at indicated time points. (C–F) A549 and PC-9 cells transfected with miR-374a mimic or mimic-NC were subjected to colony formation assay for 10 days. Data are presented as means of three independent experiments. *P < 0.05; **P < 0.01. P value indicates statistical significance analyzed by Student’s t-test.
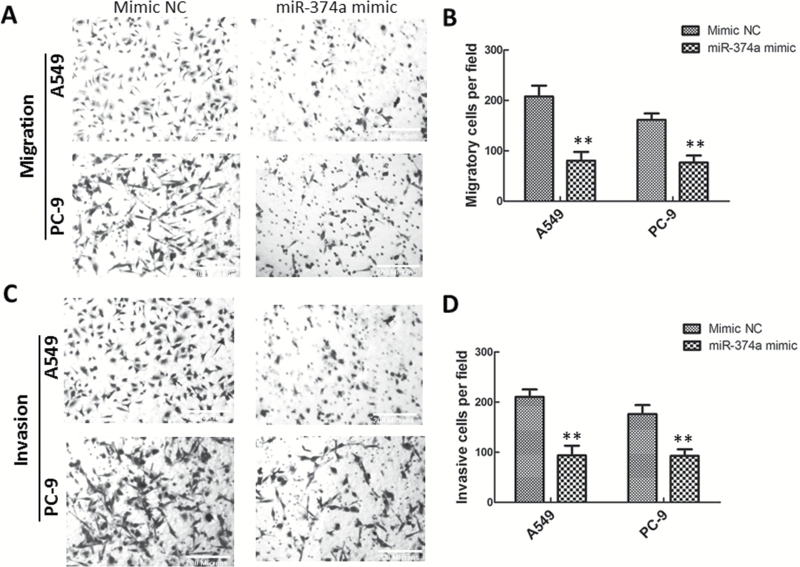
To explore whether miR-374a would affect migration and invasion of lung adenocarcinoma cells, we transiently transfected A549 and PC-9 cells with miR-374a mimic or miRNA-NC. Cell migration and invasion were assessed using Transwell assays. Overexpression of miR-374a resulted in decreased A549 cell migration (Figure 3A and B) and penetration (Figure 3C and D) compared with negative control cells. Similarly, migration and invasion of PC-9 cells were also impeded by miR-374a overexpression (Figure 3A-D).
Figure 3.
MiR-374a impedes lung adenocarcinoma cell migration and invasion in vitro. (A, B) Representative images (A) and quantification (B) of the Transwell migration assay with A549 and PC-9 cells transfected with miR-374a mimic or mimic-NC. (C, D) Representative images (C) and quantification (D) of the Transwell invasion assay with A549 and PC-9 cells transfected with miR-374a mimic or mimic-NC. Data are represented as mean ± SD. **P < 0.01.
MiR-374a directly targeted TGFA mRNA and negatively regulated TGFA gene expression
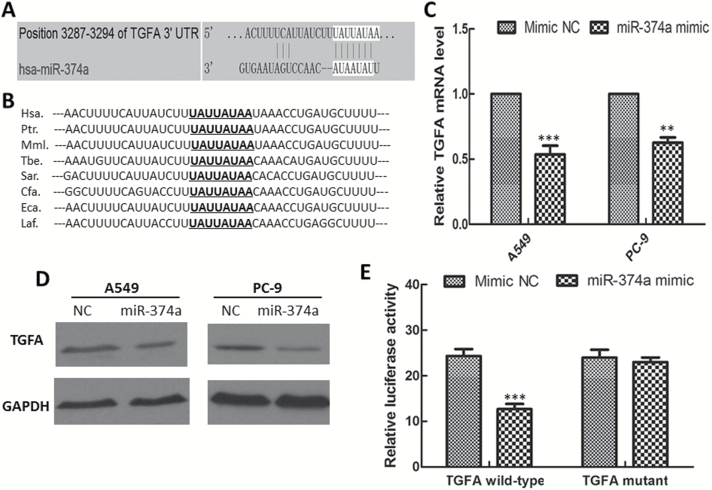
To elucidate the molecular mechanism by which miR-374a exerts its inhibitory effect on lung adenocarcinoma cells, miRNA target-predicted algorithms (TargetScan, miRDB, PicTar and PITA) were used to find potential target genes that mediate cell growth, migration and invasion. One putative target gene, transforming growth factor-alpha (TGF-α, TGFA), which has been widely reported to be involved in tumor growth and progression (30–32), showed high Target and PicTar scores (81 and 1.62, respectively), and a low PITA score (−4.14) (Figure 4A). Moreover, the potential miR-374a targeting site at the 3′-UTR of TGFA mRNA was highly conserved across species (Figure 4B). We further determined the mRNA expression of TGFA gene by qRT-PCR in lung adenocarcinoma cells transfected with miR-374a mimic or negative control. Overexpression of miR-374a significantly reduced the expression of TGFA gene in both A549 and PC-9 cells (Figure 4C). Furthermore, Western blotting revealed that overexpression of miR-374a significantly decreased the expression of TGFA protein in both A549 and PC-9 cells (Figure 4D).
Figure 4.
MiR-374a negatively regulates TGFA gene expression and directly targets the 3′-UTR of TGFA gene. (A) TGFA gene was predicted as a direct target of miR-374a (TargetScan). (B) The potential miR-374a targeting site at the 3′-UTR of TGFA gene was highly conserved across species. (C) TGFA gene expression levels in A549 and PC-9 cells treated by miR-374a mimic and mimic-NC for 48h, as detected by quantitative real-time PCR assay. (D) TGFA protein level in A549 and PC-9 cells treated by miR-374a mimic and mimic-NC for 48h, as detected by Western blotting. (E) Relative luciferase activity was analyzed after luciferase reporter plasmids with TGFA 3′-UTR constructs (either wild-type or mutant) or control reporter plasmid were co-transfected in A549 cells with miR-374a mimic or mimic-NC, respectively. The data shown are the means ± SD of three individual experiments. **P < 0.01; ***P < 0.001.
To determine whether the TGFA gene is regulated by miR-374a via direct binding to its 3′-UTR, we employed a dual-luciferase reporter system with luciferase reporter vectors containing either the wild-type or the mutant 3′-UTR of the TGFA gene. The relative luciferase activity was reduced in A549 cells co-transfected with luciferase reporter pmirGLO-3′UTR-Wild and miR-374a mimic as compared with negative control cells. However, this suppressive effect was abolished by the mutation in the miR-374a target sequence (Figure 4E). Taken together, these data suggest that miR-374a negatively regulates TGFA gene expression by directly binding to its putative binding site in the 3′-UTR sequence of the TGFA gene.
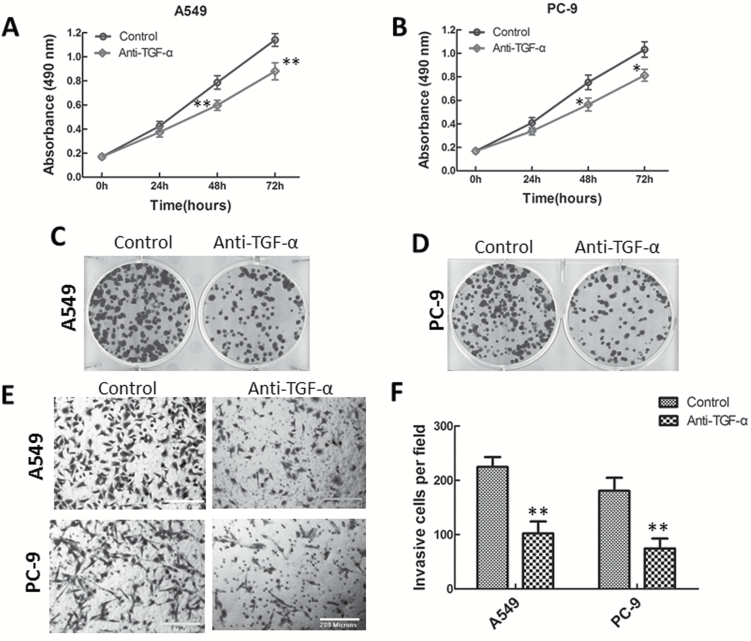
Treatment with TGF-α neutralizing antibody suppressed lung adenocarcinoma cell proliferation and invasion
To demonstrate whether the suppressive effect of miR-374a was mediated by repression of TGFA gene expression, we treated A549 and PC-9 cells with human TGF-α neutralizing antibody (5 µg/ml) or IgG control antibody, and performed MTT, colony formation and invasion assays. Treatment of lung adenocarcinoma cells with TGF-α neutralizing antibody for 48 and 72h significantly inhibited cell proliferation of these two cell lines (Figure 5A and B). We also analyzed the potential role of TGF-α in colony formation by blocking its biological activity with a neutralizing antibody. The colony formation of A549 and PC-9 cells was markedly suppressed by addition of TGF-α neutralizing antibody (Figure 5C and D). Moreover, TGF-α neutralizing antibody caused a significant decrease in lung adenocarcinoma cell invasion (Figure 5E and F), similar to the effect observed on miR-374a overexpression. Collectively, neutralization of TGF-α mimicked the inhibitory effects of miR-374a on lung adenocarcinoma cells.
Figure 5.
TGF-α neutralizing antibody suppresses lung adenocarcinoma cell proliferation and invasion. A549 and PC-9 cells were treated with the human TGF-α neutralizing antibody or the IgG control antibody, and cell proliferation (MTT) assay (A, B), colony formation (C, D) and Transwell invasion (E, F) assays were performed. Data are represented as mean ± SD. Student’s t-test was used to analyze the statistical significance of differences between groups.*P < 0.05; **P < 0.01.
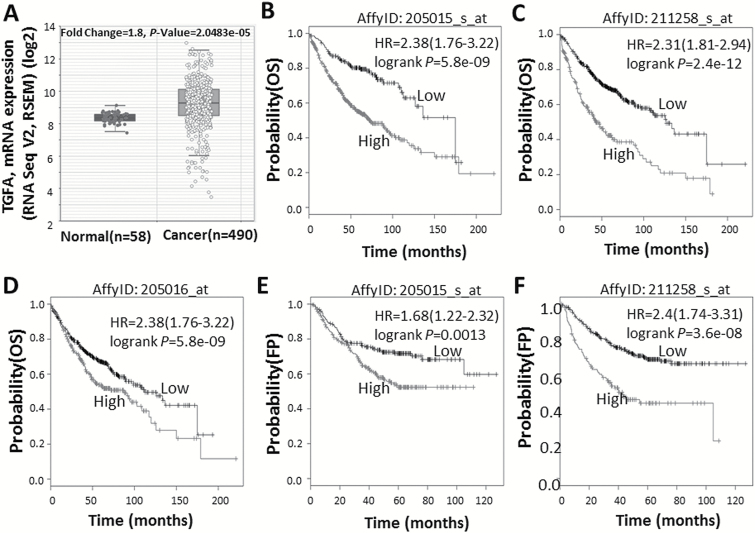
High TGFA mRNA expression predicted poor survival in patients with lung adenocarcinoma
TGFA gene expression level was significantly higher in lung adenocarcinoma tissues compared to adjacent normal tissues (fold change =1.80, P = 2.0×10–5) (Figure 6A), based on data from TCGA and algorithms available at http://starbase.sysu.edu.cn. To further clarify the role of the TGFA gene in lung adenocarcinoma, we used the Kaplan–Meier Plotter online survival analysis tool (www.kmplot.com/lung) to evaluate the relationship between TGFA mRNA expression and the clinical outcome of lung adenocarcinoma patients (n = 866). As shown in Figure 6, high TGFA mRNA expression strongly correlated with poor overall survival (Affymetrix probe IDs: 205015_s_at, 211258_s_at and 205016_at) (Figure 6B–D) and first progression (Affymetrix probe IDs: 205015_s_at and 211258_s_at) survival time in lung adenocarcinoma patients (Figure 6E and F).
Figure 6.
High TGFA mRNA expression predicts poor survival in lung adenocarcinoma patients. (A) TGFA mRNA expression levels of 490 lung adenocarcinoma tissues and 58 adjacent normal tissues were obtained from TCGA and algorithms available at http://starbase.sysu.edu.cn. (B–F) Kaplan–Meier curves for overall survival (OS) (B–D) and first progression survival time (E, F) were created using the Kaplan–Meier Plotter (www.kmplot.com) with lung adenocarcinoma patients classified according to high and low TGFA gene expression (Affymetrix probe IDs: 205015_s_at, 211258_s_at and 205016_at). Hazard ratio (with 95% confidence interval) and log-rank P values were calculated.
Discussion
In this study, we found that the expression of miR-374a was significantly down-regulated in lung adenocarcinoma tissues compared to adjacent normal lung tissues in samples included in TCGA. Functional studies revealed that overexpression of miR-374a led to inhibition of cell proliferation, migration and invasion in lung adenocarcinoma A549 and PC-9 cells. Our study further revealed that miR-374a negatively regulated TGFA gene expression by directly targeting the 3′-UTR of TGFA mRNA. Furthermore, treating lung adenocarcinoma cells with TGF-α neutralizing antibody resulted in suppression of cell proliferation and invasion, which mimicked the action of miR-374a.
We found that miR-374a-mediated TGFA gene silencing led to significant inhibitory effects on proliferation, migration and invasion of lung adenocarcinoma cells. As a member of the epidermal growth factor family that binds to and activates the epidermal growth factor receptor (EGFR), TGF-α has been well known as a crucial mediator of oncogenesis and malignant progression (30–32). TGF-α/EGFR signaling promotes the growth and spread of cancer cells and creates a tumor microenvironment that is conducive to metastasis (33). Autocrine regulation through EGFR by the TGF-α ligand has also been implicated in epithelial tumor development and tumor progression (34,35). Mounting evidence indicates that an abnormally high expression of TGFA occurs in a variety of cancers, including lung cancer. When TGF-α expression was compared in tumor and corresponding normal lung tissue, overexpression of TGF-α was found in approximately 60% of the tumor samples (36). In the non-small cell lung cancer, overexpression of TGF-α correlates significantly with the metastasis of lymph nodes (37). Our data from the Kaplan–Meier Plotter shows that high TGFA gene expression predicts poor survival in lung adenocarcinoma patients. In line with this, previous immunohistochemical studies also found that TGF-α is commonly overexpressed in lung adenocarcinomas, and high expression of TGF-α is associated with poor prognosis (38,39). Moreover, the status of TGF-α in serum may be an important predictor of resistance to gefitinib among patients with advanced non-small cell lung cancer (40). In addition, the human lung adenocarcinoma cell lines A-549 and PC-9 produce TGF-α, while the addition of monoclonal antibody against TGF-α inhibits cell growth (41). Similarly, in the present study, we found that lung adenocarcinoma cell proliferation was significantly inhibited by addition of a TGF-α neutralizing antibody. TGF-α regulates cell proliferation and cancer progression through activation of multiple pathways. For example, TGF-α can bind to EGFR and lead to the activation of major downstream signaling cascades, including the Ras/Raf/MAPK and PI3K/AKT pathways, which in turn promote cell proliferation, invasion and metastasis (30,31). Collectively, these studies provide strong evidence of a vital role for TGF-α in lung cancer prognosis.
Our results further showed that overexpression of miR-374a significantly reduced the expression of TGFA with respect to both mRNA and protein levels in lung adenocarcinoma cells. It should be noted that incomplete inhibition of TGFA expression was observed after miR-374a transfection. In addition, we did not find a significant reverse correlation between expression levels of miR-374a and the TGFA gene in TCGA samples. Bioinformatics predictions, in combination with functional experiments, have demonstrated that the networks of microRNA biology and function in human cancers are sophisticated (42). A single miRNA may target multiple putative target mRNAs, while a single gene may be modulated by multiple miRNAs. The TGFA gene is one of the targets of miR-374a, as predicted by bioinformatics analysis. We showed that miR-374a negatively regulates TGFA gene expression by directly binding to its putative binding site in the 3′-UTR sequence of the TGFA gene. Besides miR-374a, the TGFA gene is the target of many miRNAs as predicted by bioinformatics analysis. Recent studies have identified several other miRNAs that may negatively regulate TGFA gene expression at the posttranscriptional level. For example, miR-376c, which is significantly decreased in multiple cancer types, suppresses cell proliferation and invasion in osteosarcoma by directly targeting TGFA, whereas forced expression of TGFA restores the effects of miR-376c and increases osteosarcoma cell growth, migration and invasion (43). Similarly, another study observed that TGF-α and miR-152 are inversely expressed in resected prostate cancer specimens, and miR-152 may act as a tumor suppressor by targeting TGF-α at the posttranscriptional level in prostate cancer cell lines (44). Additionally, Morante et al. (45) found that miR-8 ensures both a correct glial architecture and spatiotemporal control of Spitz protein synthesis via direct binding to Spitz 3′-UTR. Spitz is a (TGF-α)-like ligand that triggers EGFR activation to promote neuroepithelial proliferation and neuroblast formation.
In accord with previous observations regarding miR-374a (12), our functional studies demonstrated that re-expression of miR-374a had an inhibitory effect on lung adenocarcinoma cells. Slattery et al. (46) recently utilized large population-based data from 1141 colorectal cancer (CRC) cases (745 colon cancers and 396 rectal cancers) to replicate previously reported associations between 121 miRNAs and disease stage and survival. Interestingly, they found that miR-374a significantly reduced the hazard of dying for all colorectal cancer cases, regardless of tumor site. Moreover, miR-374a was recognized as a ‘protective’ miRNA in triple negative breast cancer; upregulation of miR-374a correlated with better prognosis (15). However, previous studies also reported that miR-374a promotes cell invasion by targeting SRCIN1 in gastric cancer (47) and activating Wnt/β-catenin signaling in breast cancer (17). miRNA may exert opposite functions in different tissues or under different cancer contexts by targeting different genes. For example, miR-125b, a ‘double-edged’ miRNA, targets multiple mRNAs which are tissue- and tumor-specifically expressed, resulting in either oncogenic or tumor suppressive modes of action in different tumor types (48). Other miRNAs, such as miR-7, miR-182 and miR-183, exhibit both pro- and anticancer activities in a context-dependent manner (6). In addition to its role in lung adenocarcinoma, miR-374a is significantly downregulated in lung squamous cell carcinoma tissues in samples included in TCGA. Therefore, it would be of interest to evaluate the role of miR-374a in lung squamous cell carcinoma and explore its potential targets, including TGFA, in future studies.
In summary, we found that miR-374a inhibited lung adenocarcinoma cell proliferation and invasion, at least partially, through repression of TGFA gene expression via targeting its 3′-UTR. The newly-identified miR-374a-mediated TGFA gene silencing may facilitate a better understanding of the molecular mechanisms of lung cancer progression and present a new strategy for treatment of patients with lung adenocarcinoma.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
This study was supported in part by the National Institutes of Health (U01CA161045), Department of Defense (W81XWH-13-1-0168) and the Vanderbilt Molecular and Genetic Epidemiology of Cancer training program (R25CA160056).
Supplementary Material
Acknowledgements
We thank Regina Courtney and Jie Wu for their laboratory support and Nancy Kennedy for assistance with editing and manuscript preparation. Laboratory assays were partially performed at the Survey and Biospecimen Shared Resource, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30CA068485).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- EGFR
epidermal growth factor receptor
- miRNAs
microRNAs
- MTT
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide.
References
- 1. Torre L.A., et al. (2015) Global cancer statistics, 2012. CA. Cancer J. Clin., 65, 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Nakamura H., et al. (2014) Worldwide trend of increasing primary adenocarcinoma of the lung. Surg. Today, 44, 1004–1012. [DOI] [PubMed] [Google Scholar]
- 3. Siegel R.L., et al. (2015) Cancer statistics, 2015. CA. Cancer J. Clin., 65, 5–29. [DOI] [PubMed] [Google Scholar]
- 4. Huntzinger E., et al. (2011) Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat. Rev. Genet., 12, 99–110. [DOI] [PubMed] [Google Scholar]
- 5. Hata A., et al. (2015) Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci. Signal., 8, re3. [DOI] [PubMed] [Google Scholar]
- 6. Qi J., et al. (2012) MicroRNAs and lung cancers: from pathogenesis to clinical implications. Front. Med., 6, 134–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joshi P., et al. (2014) MicroRNAs in lung cancer. World J. Methodol., 4, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yendamuri S., et al. (2011) MicroRNA biomarkers in lung cancer: MiRacle or quagMiRe? Transl. Res., 157, 209–215. [DOI] [PubMed] [Google Scholar]
- 9. Takamizawa J., et al. (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res., 64, 3753–3756. [DOI] [PubMed] [Google Scholar]
- 10. Gallardo E., et al. (2009) miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis, 30, 1903–1909. [DOI] [PubMed] [Google Scholar]
- 11. Wu D.W., et al. (2010) Paxillin predicts survival and relapse in non-small cell lung cancer by microRNA-218 targeting. Cancer Res., 70, 10392–10401. [DOI] [PubMed] [Google Scholar]
- 12. Võsa U., et al. (2011) Identification of miR-374a as a prognostic marker for survival in patients with early-stage nonsmall cell lung cancer. Genes. Chromosomes Cancer, 50, 812–822. [DOI] [PubMed] [Google Scholar]
- 13. Wang Q.Z., et al. (2009) Potential uses of microRNA in lung cancer diagnosis, prognosis, and therapy. Curr. Cancer Drug Targets, 9, 572–594. [DOI] [PubMed] [Google Scholar]
- 14. Erdmann K., et al. (2014) Elevated expression of prostate cancer-associated genes is linked to down-regulation of microRNAs. BMC Cancer, 14, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cascione L., et al. (2013) Integrated microRNA and mRNA signatures associated with survival in triple negative breast cancer. PLoS One, 8, e55910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J.Y., et al. (2013) Effects of differential distribution of microvessel density, possibly regulated by miR-374a, on breast cancer prognosis. Asian Pac. J. Cancer Prev., 14, 1715–1720. [DOI] [PubMed] [Google Scholar]
- 17. Cai J., et al. (2013) MicroRNA-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis. J. Clin. Invest., 123, 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Namløs H.M., et al. (2012) Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS One, 7, e48086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montani F., et al. (2015) miR-Test: a blood test for lung cancer early detection. J. Natl. Cancer Inst., 107, djv063. [DOI] [PubMed] [Google Scholar]
- 20. Chatterjee A., et al. (2014) miR-17-5p downregulation contributes to paclitaxel resistance of lung cancer cells through altering beclin1 expression. PLoS One, 9, e95716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J.H., et al. (2014) starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res., 42, D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang J.H., et al. (2011) starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res., 39, D202–D209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cancer Genome Atlas Research Network et al. (2013) The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet., 45, 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Győrffy B., et al. (2013) Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One, 8, e82241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis B.P., et al. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell, 120, 15–20. [DOI] [PubMed] [Google Scholar]
- 26. Wong N., et al. (2015) miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res., 43, D146–D152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krek A., et al. (2005) Combinatorial microRNA target predictions. Nat. Genet., 37, 495–500. [DOI] [PubMed] [Google Scholar]
- 28. Kertesz M., et al. (2007) The role of site accessibility in microRNA target recognition. Nat. Genet., 39, 1278–1284. [DOI] [PubMed] [Google Scholar]
- 29. Livak K.J., et al. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 30. Asami K., et al. (2014) Epidermal growth factor receptor tyrosine kinase inhibitors for non-small cell lung cancer. World J. Clin. Oncol., 5, 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holbro T., et al. (2003) The ErbB receptors and their role in cancer progression. Exp. Cell Res., 284, 99–110. [DOI] [PubMed] [Google Scholar]
- 32. Hynes N.E., et al. (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer, 5, 341–354. [DOI] [PubMed] [Google Scholar]
- 33. Sasaki T., et al. (2013) The role of epidermal growth factor receptor in cancer metastasis and microenvironment. Biomed. Res. Int., 2013, 546318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sporn M.B., et al. (1985) Autocrine growth factors and cancer. Nature, 313, 745–747. [DOI] [PubMed] [Google Scholar]
- 35. Sporn M.B., et al. (1980) Autocrine secretion and malignant transformation of cells. N. Engl. J. Med., 303, 878–880. [DOI] [PubMed] [Google Scholar]
- 36. Salomon D.S., et al. (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol., 19, 183–232. [DOI] [PubMed] [Google Scholar]
- 37. Yun F., et al. (2013) Clinicopathological significance of PTEN and PI3K/AKT signal transduction pathway in non-small cell lung cancer. Int. J. Clin. Exp. Pathol., 6, 2112–2120. [PMC free article] [PubMed] [Google Scholar]
- 38. Tateishi M., et al. (1991) Prognostic implication of transforming growth factor alpha in adenocarcinoma of the lung–an immunohistochemical study. Br. J. Cancer, 63, 130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tateishi M., et al. (1990) Immunohistochemical evidence of autocrine growth factors in adenocarcinoma of the human lung. Cancer Res., 50, 7077–7080. [PubMed] [Google Scholar]
- 40. Ishikawa N., et al. (2005) Increases of amphiregulin and transforming growth factor-alpha in serum as predictors of poor response to gefitinib among patients with advanced non-small cell lung cancers. Cancer Res., 65, 9176–9184. [DOI] [PubMed] [Google Scholar]
- 41. Imanishi K., et al. (1989) Inhibition of growth of human lung adenocarcinoma cell lines by anti-transforming growth factor-alpha monoclonal antibody. J. Natl. Cancer Inst., 81, 220–223. [DOI] [PubMed] [Google Scholar]
- 42. Iorio M.V., et al. (2012) microRNA involvement in human cancer. Carcinogenesis, 33, 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Jin Y., et al. (2013) MicroRNA-376c inhibits cell proliferation and invasion in osteosarcoma by targeting to transforming growth factor-alpha. DNA Cell Biol., 32, 302–309. [DOI] [PubMed] [Google Scholar]
- 44. Zhu C., et al. (2013) miR-152 controls migration and invasive potential by targeting TGFα in prostate cancer cell lines. Prostate, 73, 1082–1089. [DOI] [PubMed] [Google Scholar]
- 45. Morante J., et al. (2013) Conserved miR-8/miR-200 defines a glial niche that controls neuroepithelial expansion and neuroblast transition. Dev. Cell, 27, 174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Slattery M.L., et al. (2015) An evaluation and replication of miRNAs with disease stage and colorectal cancer-specific mortality. Int. J. Cancer, 137, 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu X., et al. (2015) miR-374a promotes cell proliferation, migration and invasion by targeting SRCIN1 in gastric cancer. FEBS Lett., 589, 407–413. [DOI] [PubMed] [Google Scholar]
- 48. Banzhaf-Strathmann J., et al. (2014) Good guy or bad guy: the opposing roles of microRNA 125b in cancer. Cell Commun. Signal., 12, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.