Abstract
Cross-sectional relationships were examined between regional brain volumes and mitochondrial DNA (mtDNA) 8-hydroxy-2-deoxyguanosine (8-oxo-dG) in peripheral blood mononuclear cells (PBMCs) of 47 HIV patients [mean age 51 years; 81% with HIV RNA ≤50 copies/mL] on combination antiretroviral therapy. The gene-specific DNA damage and repair assay measured mtDNA 8-oxo-dG break frequency. Magnetic resonance imaging was performed at 3 T. Higher mtDNA 8-oxo-dG was associated with lateral ventricular enlargement and with decreased volumes of hippocampus, pallidum, and total subcortical gray matter, suggesting the involvement of systemic mitochondrial-specific oxidative stress in chronic HIV-related structural brain changes and cognitive difficulties. Clarification of the mechanism may provide potential therapeutic targets.
Keywords: Atrophy, Brain, Neuroinflammation, Oxidative stress, Pallidum, ROS
1. Introduction
Entry of the human immunodeficiency virus (HIV) into the brain is believed to occur during acute infection via infected monocytes that migrate across the blood–brain barrier (Gartner, 2000; Persidsky et al., 2000). HIV antiretroviral drugs suppress plasma viral load and increase CD4 + T-cell count (Collier et al., 1996) but have variable central nervous system (CNS) penetration and effectiveness against infected monocytes (Enting et al., 1998; Letendre et al., 2008). Protected HIV reservoirs are established in brain perivascular macrophages and parenchymal microglia (Kaul et al., 2005) and other CNS cell types (Bagasra et al., 1996). While viral proteins can injure neurons directly (Meucci et al., 1998; Liu et al., 2000), the predominant pathway to neuronal damage and death in HIV/AIDS is probably indirect and mediated by proinflammatory cytokines secreted by HIV-infected or activated macrophages and microglia (Adle-Biassette et al., 1999; Kaul et al., 2001). Immune activation and neuroinflammation often persist even after combination antiretroviral therapy (cART) has reduced plasma HIV RNA to undetectable levels (Anthony et al., 2005; Eden et al., 2007). Many cART-treated patients exhibit clinical features of HIV-associated neurocognitive disorders (HAND) such as psychomotor slowing and impaired verbal and visual memory (Sacktor et al., 2002). Systemic inflammation may also contribute to persistent HAND in this population (Eden et al., 2007).
Studies of Alzheimer's disease (AD) (Griffin et al., 1998; Nunomura et al., 2001), Parkinson's disease (PD) (Sawada et al., 2006; Jenner & Olanow, 1996) and amyotrophic lateral sclerosis (Almer et al., 2002; Ferrante et al., 1997) point to a connection between inflammation and elevated oxidative stress. Oxidative stress is linked to a range of neurodegenerative disorders (reviewed by Andersen (2004)). A mediator of apoptosis and necrosis (Higuchi, 2003), oxidative stress arises from excessive amounts of reactive oxygen species (ROS) which overwhelm cellular antioxidant defenses (Andersen, 2004). In normal and pathological metabolism, most ROS are formed as toxic by-products of oxidative phosphorylation within mitochondria (Turrens, 2003; Lesnefsky et al., 2001; Wallace, 2000) during synthesis of adenosine triphosphate. Mitochondrial DNA (mtDNA) has a high mutation rate compared to that of nuclear DNA (Nachman et al., 1996), possibly due to the proximity of mtDNA to mitochondrial ROS production (Wallace et al., 2010). Emerging data suggest that mitochondrial dysfunction plays a critical role in inflammation. Through ROS production, alterations in mitochondria may amplify cellular responses to cytokine-induced inflammation in osteoarthritis (Vaamonde-Garcia et al., 2012). Mitochondrial dysfunction also increases allergic airway inflammation (Aguilera-Aguirre et al., 2009), which is modulated by mitochondrial ROS production that activates the inflammasome and leads to production of the cytokine IL-1β (Kim et al., 2014). The generation of inflammatory mediators may induce additional mitochondrial dysfunction and oxidative damage, perpetuating a vicious cycle of inflammation.
The majority of metabolic enzymes are downregulated in HIV-infected cells (van't Wout et al., 2003). Neurotoxic HIV proteins that increase ROS production have been implicated in HAND; for example, HIV envelope glycoprotein (gp120) impairs glucose metabolism and neuronal function (Kimes et al., 1991; Vignoli et al., 2000). ROS production is induced by mitochondrial dysfunction in HIV disease (Macho et al., 1995; Gil et al., 2003). Mitochondrial toxicity (Brinkman et al., 1998) and oxidative stress (Lagathu et al., 2007) can be also caused by antiretroviral drugs, especially nucleoside reverse transcriptase inhibitors (NRTIs) which are components of current cART regimens (Hulgan et al., 2003). In particular, use of zidovudine (ZDV) causes mtDNA depletion (Semino-Mora et al., 1994), shown to impair mitochondrial protein synthesis in a rat model (Masini et al., 1999), and didanosine (ddI) and stavudine (d4T) have been related to mitochondrial damage (Blanco et al., 2003). Oxidative stress can lead to declines in mitochondrial function, which in turn hamper energy production and further elevate ROS levels.
Although neuroimaging of patients on cART reveals HIV-related changes in subcortical and cortical brain structure (Aylward et al., 1993; Thompson et al., 2005; Becker et al., 2011; Kallianpur et al., 2012, 2013), little in vivo research has addressed HIV-related brain structural change in the context of oxidative damage. Neuronal injury detected in the brains of HIV patients (Harezlak et al., 2011; Gray et al., 2000; Ellis et al., 2007) is believed to involve apoptosis and ROS-mediated injury to cellular lipids and proteins (Gray et al., 2000). A widely used marker of mitochondrial oxidative DNA damage is the ROS-induced lesion 8-hydroxy-2-deoxyguanosine (8-oxo-dG) (Valavanidis et al., 2009). Our study investigated the associations of peripheral oxidative stress with regional brain volumes and cognition in chronic HIV infection. Specifically, we examined relationships of mtDNA 8-oxo-dG in peripheral blood mononuclear cells (PBMCs) to regional brain volumes and to neuropsychological (NP) measures.
2. Materials and methods
2.1. Participants
Neuropsychological and regional brain volume data, obtained in a subset of cART-treated HIV patients from the Hawaii Aging with HIV Cohort-Cardiovascular Disease (HAHC-CVD) study (Shikuma et al., 2012), were examined for cross-sectional associations with mitochondrial data from the parent investigation. The substudy population comprised 47 HIV-seropositive (HIV+) individuals aged 40 years and older. Participants were recruited through referrals from our clinic at the Hawaii Center for AIDS, community physicians, community advisory board members, and AIDS service organizations. Individuals were eligible for the study if they were ≥ 40 years old, had a documented history of HIV infection, had been on stable cART ≥ 3 months, and if their primary language was English and they could understand and provide informed consent. Exclusion criteria were: (i) uncontrolled major affective disorder; (ii) active psychosis; (iii) loss of consciousness > 5 min; (iv) pregnancy or breast-feeding; (v) factors precluding MRI (e.g., claustrophobia); and (vi) any past or present condition (e.g., central nervous system infection, traumatic brain injury, stroke, substance abuse) deemed by the evaluating physician to introduce confounding variables. All study participants underwent a general and focused HIV/neurological history and physical exam, neuroimaging, and neuropsychological testing. Nadir CD4 count, years since HIV diagnosis, and date of cART initiation were determined by subject self-report. Proximity to HIV diagnosis (i.e., duration of HIV seropositivity) served as a proxy for duration of infection. Past or current use of ZDV, d4T or ddI was noted. Blood specimens were obtained. Plasma HIV RNA and CD4 cell counts were performed by a local commercial Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory. Levels of mtDNA-specific 8-oxo-dG were measured in PBMCs. Each participant provided written informed consent. The University of Hawaii Committee on Human Studies approved the study.
2.2. Neuroimaging
MRI was performed on a 3.0-Tesla Philips Medical Systems Achieva scanner equipped with an 8-channel head coil (InVision Imaging, Honolulu). For each subject, a high-resolution anatomical volume was acquired with a sagittal T1-weighted 3D turbo field echo (T1W 3D TFE) sequence (echo time TE/repetition time TR = 3.2 ms/6.9 ms; flip angle 8°; slice thickness 1.2 mm with no gap; in-plane resolution 1.0 mm2; field of view 256 × 256 mm2). To obtain regional brain volumes, structural MRI data were processed with FreeSurfer (version 5.0, https://surfer.nmr.mgh.harvard.edu/) (Dale et al., 1999; Fischl et al., 2002; Fischl et al., 1999, 2004) in a procedure that includes skull-stripping (Segonne et al., 2004), intensity normalization (Sled et al., 1998), Talairach transformation, subcortical white matter and deep gray matter segmentation (Fischl et al., 2002, 2004), and cortical gray/white matter boundary and pial surface reconstruction (Dale et al., 1999). Quality assurance of processed data was performed visually, and cortical surfaces and subcortical segmentations were checked prior to statistical analysis. FreeSurfer's estimate of intracranial volume (ICV) is a reliable measure for regional brain volume normalization (Buckner et al., 2004).
2.3. Neuropsychological testing
Subjects completed a comprehensive neuropsychological test battery that assessed cognitive domains affected by HIV. It included the following tests: Choice and Sequential Reaction Time from the California Computerized Assessment Package (CalCAP); the Rey Auditory Verbal Learning Test (RAVLT); Rey-Osterrieth Complex Figure (RCF) Copy and Recall; Trail Making Test, Parts A and B; Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol; WAIS-III Letter-Number Sequencing; Grooved Pegboard (dominant and nondominant hands); Verbal Fluency Test (FAS); Animal Naming; Boston Naming Test (BNT), the WAIS-R Digit Span (Forward and Backward); Delis-Kaplan Executive Function System (D-KEFS) Color Word Interference Test, condition 3; and Timed Gait. Raw NP test scores were z-transformed by use of standard demographically adjusted normative data. Composite, domain-specific z-scores were derived by averaging z-scores of the appropriate individual sub-tests (NPZpm [psychomotor speed]: WAIS-R Digit Symbol Subtest; Trail-Making Test, Part B; Grooved Pegboard, non-dominant hand; NPZlrn_mem [learning and memory]: RAVLT Total; RAVLT Delayed Recall; Rey-Osterreith Complex Figure Test– Delayed Recall; NPZef [executive functioning]: Verbal Fluency Test (FAS); Stroop Color Word Interference Test; Trail-Making Test, Part B; and NPZwm [working memory]: RAVLT_B; WAIS-III Letter Number Sequencing). NPZglobal, a global z-score reflecting overall neurocognitive performance, was the arithmetic mean of z-scores on 14 NP tests (WAIS-R Digit Symbol Subtest; Trail-Making Test, Parts A and B; Grooved Pegboard, dominant hand and non-dominant hand; Timed Gait; CalCAP Choice; CalCAP Sequence; RAVLT Total; RAVLT Delayed Recall; Rey-Osterrieth Complex Figure Test– Delayed Recall; Verbal Fluency Test (FAS); Animal Naming; D-KEFS Color Word Interference Test).
2.4. Mitochondrial analyses
DNA was isolated using a Qiagen DNA isolation kit (Qiagen, Inc., Valencia, CA). Eight μg of total DNA was digested with Pvu II for one hour at 37 °C. MtDNA 8-oxo-dG was measured as follows: 4 μg of total DNA was treated with 10 units of human 8-oxo-guanine DNA glycosylase (hOGG1) in 1X REC™ Buffer 6 (20 mM Tris-Cl (pH 8.0), 1 mM DTT, 1 mM EDTA, 0.1 mg/mL BSA) for 1 h at 37 °C in a reaction volume of 20 μL (Trevigen, Inc., Gaithersburg, MD). For analysis, 5 μL of REC 5X Loading Buffer (10 mM HEPES-KOH (pH 7.4)), 10 mM EDTA, 6 M urea, 50% glycerol, and 0.2% bromophenol blue were added. The tubes were heated at 95 °C for 5 min and then fast-cooled to 4 °C. Cleaved and non-cleaved products were resolved on 0.75% alkaline agarose gels. DNA was transferred to nylon membranes using standard Southern Blot methodology. Human mitochondrial probes to cytochrome b were labeled with digoxigenin-deoxyuridine triphosphate (Roche Applied Science, Indianapolis, IN) during a linear PCR amplification. Blots were probed and processed for chemiluminescent detection using Roche protocols. Membranes were developed on chemilumiimager (Roche). Quantitation of the break frequency of mtDNA 8-oxo-dG was based on comparing the undigested and digested mtDNA break frequencies calculated using the Poisson distribution, with units reported in break frequency (BF) (Gerschenson et al., 2009).
2.5. Statistical analysis
Volumes of 13 brain regions (thalamus, caudate, putamen, globus pallidus, hippocampus, amygdala, nucleus accumbens, cerebellar white matter, cerebellar gray matter, cortical gray matter, cerebral white matter, total subcortical gray matter, and lateral ventricles) were obtained by summing over left and right brain hemispheres. Descriptive statistics were provided for patient demographics, clinical variables, mtDNA 8-oxo-dG, NP z-scores, and regional brain volumes. For ZDV, d4T and ddI (separately), a “history of use” variable was defined to include either prior or current use. Another variable called “history of ZDV, d4T and/or ddI use” reflected past or present use of any of these three NRTIs. MtDNA 8-oxo-dg was treated as a dichotomous categorical variable (zero vs. positive) only for comparisons between subject groups with and without PBMC mtDNA breaks (mtDNA 8-oxo-dG > 0 and mtDNA 8-oxo-dG = 0, respectively). In analyses involving correlation or multivariable linear regression, mtDNA 8-oxo-dG was viewed as a continuous variable. Mann–Whitney or chi-square tests compared differences in demographic and clinical parameters, NP z-scores, and regional brain volumes between groups characterized by the presence or absence of PBMC mtDNA breaks. Relationships of mtDNA 8-oxo-dG levels to continuous demographic and clinical parameters, regional volumes and NP z-scores were also assessed by Pearson correlation (and, when restricted to subjects with mtDNA 8-oxo-dG > 0, by Spearman correlation).
Multiple linear regression examined associations between regional brain volumes (dependent variables) and mtDNA 8-oxo-dG, controlling for age, nadir CD4 cell count and ICV (head size). Low nadir CD4 count has been related to brain atrophy (Cohen et al., 2010) and increased risk of cognitive impairment (Ellis et al., 2011) in HIV-infected individuals on cART. Gender, years of education and years since HIV diagnosis were tested as covariates in the regression but had no significant effects and were not retained. Squares of the semipartial correlations were computed to determine the proportion of variance of each regional volume explained by mtDNA 8-oxo-dG after taking into account the other independent variables. MtDNA 8-oxo-dG data were inspected for outliers and non-normal distribution, the effects of which were examined in post-hoc analyses. For all regression models, histograms of the standardized residuals were visually checked for normality, and plots of standardized residuals examined to ensure that the residuals were normally distributed around the regression line. Homogeneity of variances was assessed using plots of the standardized residuals against predicted (fitted) values.
SPSS and StatView 5.0 (SAS Institute Inc., Cary, NC) were utilized for statistical analyses. P-values <0.05 were considered statistically significant and 0.05 ≤ p ≤ 0.1 indicative of trends. For multivariable regression analyses, all p-values are uncorrected for multiple comparisons, but associations meeting the Bonferroni criterion (p < 0.05/13 = 0.004) are noted.
3. Results
3.1. Subject characteristics
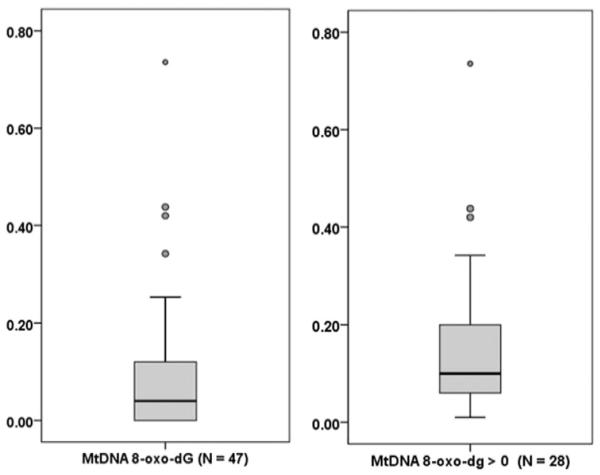
We studied 47 HIV-infected individuals [mean age: 50.9 ± 7.3 years; predominantly male (87%); mean CD4 cell count = 462.6 ± 201.4 cells/mm3; median (interquartile range [IQR]) nadir CD4 count = 140.0 (34.0–251.5) cells/mm3; mean duration of HIV infection=13.5 ± 6.8 years; mean duration of cART = 11.5 ± 6.0 years]. Plasma HIV RNA was undetectable (< 50 copies/mL) in 38 (81%) of the study participants, and the remaining 9 subjects had a median plasma HIV RNA count of 180 copies/mL (range: 53–15,700). Twenty-eight individuals had nonzero (positive) mtDNA 8-oxo-dG levels in PBMCs. Median (IQR) mtDNA 8-oxo-dG values for the entire study sample and for subjects with positive mtDNA 8-oxo-dG were 0.04 (0–0.13) and 0.10 (0.06–0.21) BF, respectively. Distributions of the mtDNA 8-oxo-dG data are shown in Fig. 1. Most demographic and clinical parameters were well balanced between groups defined by the presence or absence of PBMC mtDNA breaks (Table 1), although subjects with mtDNA 8-oxo-dG > showed trends toward higher current and nadir CD4 counts.
Fig. 1.
Data distributions of peripheral blood mononuclear cell mitochondrial DNA (mtDNA) 8-hydroxy-2-deoxyguanosine (8-oxo-dG) (break frequency). Left: full distribution; right: distribution of positive values. For each boxplot, the middle dark line shows the median; the shaded box indicates the 1st (Q1) and 3rd (Q3) quartiles. Whiskers extend the box range to the extreme data points or, if there are outliers, by 1.5×(Q3-Q1) in either direction; circles are outliers (< Q1 − 1.5×[Q3 − Q1] or > Q3 + 1.5×[Q3 − Q1]).
Table 1.
Study population: demographics, clinical variables, and mitochondrial DNA 8-oxo-dG inperipheral blood mononuclear cells. Data are given as mean ± standard deviation, n (%) or median (interquartile range [IQR]). P-values for group differences were obtained by chi-squared or Mann–Whitney test.
| Variable | All subjects | MtDNA 8-oxo-dG = 0 | MtDNA 8-oxo-dG > 0 | p |
|---|---|---|---|---|
| N | 47 | 19 | 2 | 8– |
| Age (years) | 50.9 ± 7.3 | 49.4 ± 6.7 | 51.9 ± 7.6 | 0.24 |
| Male gender | 41 (87.2%) | 17 (90%) | 24 (86%) | 0.99 |
| Education (years)a | 14.4 ± 2.1 | 14.2 ± 2.1 | 14.6 ± 2.1 | 0.58 |
| Race/ethnicity (Caucasian) | 24 (51%) | 10 (53%) | 14 (50%) | 0.99 |
| Duration of HIV infection (years) | 13.5 ± 6.8 | 11.7 ± 6.9 | 14.8 ± 6.5 | 0.12 |
| Duration of cART (years) | 11.5 ± 6.0 | 10.0 ± 6.1 | 12.4 ± 5.9 | 0.18 |
| CD4 count (cells/mm3) | 462.6 ± 201.4 | 393.3 ± 205.6 | 509.6 ± 187.7 | 0.05 |
| Nadir CD4 count (cells/mm3)b | 140.0 (34.0–251.5) | 67.0 (10.0–230.0) | 183.5 (76.3–269.8) | 0.07 |
| Undetectable plasma HIV RNA (<50 copies/mL) | 38 (81%) | 14 (74%) | 24 (86%) | 0.45 |
| MtDNA 8-oxo-dG (BF) | 0.04 (0–0.13) | 0 (0–0) | 0.10 (0.06–0.21) | – |
| History of ZDV use | 25 (53%) | 8 (42%) | 17 (61%) | 0.21 |
| History of d4T use | 18 (38%) | 6 (32%) | 12 (43%) | 0.44 |
| History of ddI use | 10 (21%) | 3 (16%) | 7 (25%) | 0.45 |
| History of ZDV, d4T and/or ddI use | 31 (66%) | 10 (53%) | 21 (75%) | 0.11 |
N = 44.
N = 45.
Complete neuropsychological data were obtained for 38 participants, with NP test scores incomplete for 5 subjects and lacking for 4. NP test performance was slightly worse in working memory and learning and memory than in other neurocognitive domains: NPZlrn_mem = −0.20 ± 1.04 (N = 41); NPZwm = −0.18 ± 0.78 (N = 43); NPZpm = 0.28 ± 0.63 (N = 42); NPZ ef = 0.16 ± 0.99 (N = 43); and NPZglobal = −0.05 ± 0.50 (N = 38). Structural MRI yielded volumes of the following brain regions: thalamus (13,023.6 ± 1186.2 mm3), caudate (7292.3 ± 768.3 mm3), putamen (11,438.0 ± 1406.7 mm3), pallidum (3315.7 ± 404.8 mm3), hippocampus (7934.8 ± 804.8 mm3), amygdala (3803.2 ± 495.9 mm3), nucleus accumbens (1325.8 ± 209.4 mm3), cerebellar white matter (30,936.5 ± 3805.4 mm3), cerebellar gray matter (87,417.6 ± 9232.1 mm3), cortical gray matter (430,782.3 ± 35,072.00 mm3), cerebral white matter (490,764.5 ± 55,875.4 mm3), subcortical gray matter (164,534.5 ± 14,469.7 mm3), and lateral ventricles (22,798.9 ± 12,097.5 mm3). Intracranial volume, a covariate in the statistical analyses, was 1451.2 ± 204.8 cm3.
3.2. Comparison of subject groups with mtDNA 8-oxo-dG = 0 and mtDNA 8-oxo-dG > 0
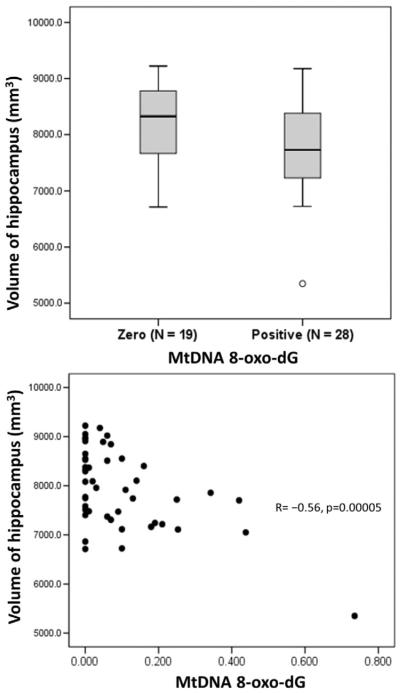
The groups with and without mtDNA breaks did not differ significantly in demographic or clinical characteristics (Table 1). Compared to subjects with mtDNA 8-oxo-dG = 0, those with mtDNA 8-oxo-dG > 0 had higher current and nadir CD4 count but the differences did not reach significance. NP z-scores did not differ between groups. There were no group differences in history of ZDV use; history of d4T use; history of ddI use; or history of ZDV, d4T and/or ddI use. Current use and past use were also examined separately for each drug, and the group differences (not shown in Table 1) were non-significant. Similarly, Mann–Whitney tests found no significant difference in any regional brain volumes between subjects with mtDNA 8 oxo-dG = 0 and mtDNA 8-oxo-dG > 0. The group with mtDNA breaks showed a trend toward smaller volumes of hippocampus (positive mtDNA 8-oxo-dG: 7765.5 ± 816.2 mm3 vs. zero mtDNA 8-oxo-dG: 8184.3 ± 738.6 mm3; p = 0.07) and pallidum (positive mtDNA 8 oxo-dG: 3224.9 ± 378.2 mm3 vs. zero mtDNA 8-oxo-dG: 3449.5 ± 415.3 mm3; p = 0.09). (Both p-values were 0.03 when group differences were assessed by analysis of covariance adjusting for ICV.) Fig. 2 (top) shows the distributions of hippocampal volume for both the zero and positive mtDNA 8-oxo-dG groups.
Fig. 2.
Top: boxplots showing total volumes of hippocampus for subject groups with zero and positive values of mitochondrial DNA (mtDNA) 8-hydroxy-2-deoxyguanosine (8-oxo-dG) in peripheral blood mononuclear cells; p = 0.07 by Mann–Whitney test. Bottom: total volumes of hippocampus plotted against mtDNA 8-oxo-dG for entire study population.
3.3. Correlation of mtDNA 8-oxo-dG with other parameters
Pearson correlation showed no relationship between mtDNA 8-oxo-dG and years of education, years since HIV diagnosis, or years on cART. MtDNA 8-oxo-dG correlated with age (R = 0.31, p = 0.04), and at trend-level with NPZ lrn_mem (R = 0.30, p = 0.06) nadir CD4 (R = 0.29, p = 0.05) and current CD4 count (R = 0.27, p = 0.06). Higher mtDNA 8-oxo-dG levels correlated with reduced volumes of hippocampus (R = −0.56, p = 0.000046), amygdala (R = −0.48, p = 0.001), pallidum (R = −0.32, p = 0.027) and total subcortical gray matter (R = −0.36, p = 0.013). Hippocampal volumes are plotted against mtDNA 8-oxo-dG in Fig. 2 (bottom). When analyses were restricted to subjects with mtDNA 8-oxo-dG > 0, mtDNA 8-oxo-dG still correlated with hippocampal (Spearman ρ = −0.55, p = 0.003) and amygdalar (ρ = −0.52, p = 0.004) volumes, and there was a trend correlation between mtDNA 8-oxo-dG and volume of the thalamus (ρ = −0.33, p = 0.09).
3.4. Adjusted associations between mtDNA 8-oxo-dG and regional brain volumes
Higher mtDNA 8-oxo-dG levels correlated with decreased volumes of several brain regions after controlling for age, CD4 nadir count and ICV (Table 2). P-values in the table are uncorrected for multiple comparisons, but volumetric associations of mtDNA 8-oxo-dG with hippocampus, pallidum, total subcortical gray matter and lateral ventricles survive application of a stringent Bonferroni correction (p < 0.0039 = .05/13). (The same relationships satisfy the sequentially rejective Holm–Bonferroni criterion (Holm, 1979), often considered more powerful than the classical Bonferroni test.) MtDNA 8-oxo-dG was directly associated with lateral ventricular volume (p = 0.0023) and inversely associated with volumes of hippocampus (p = 0.0003), pallidum (p = 0.0032) and subcortical gray matter (p = 0.0013). Relationships between higher mtDNA 8-oxo-dG and decreased volumes of amygdala (p = 0.013), putamen (p = 0.025) and cerebellar gray matter (p = 0.015) did not satisfy multiple comparison correction criteria.
Table 2.
Associations between regional brain volumes and mitochondrial 8-oxo-dG by multiple regression.
| Brain region (volume) |
Predictor variables |
β | p | Fraction of variance due to mtDNA 8-oxo-dG |
|---|---|---|---|---|
| Hippocampus | Age | −0.14 | 0.273 | |
| CD4 nadir | 0.10 | 0.412 | ||
| ICV | 0.36 | 0.0040 | ||
| MtDNA 8-oxo-dG | −0.52 | 0.0003* | ||
| 0.21 | ||||
| Amygdala | Age | −0.28 | 0.045 | |
| CD4 nadir | 0.07 | 0.591 | ||
| ICV | 0.34 | 0.0086 | ||
| MtDNA 8-oxo-dG | −0.36 | 0.013 | ||
| 0.10 | ||||
| Pallidum | Age | −0.16 | 0.256 | |
| CD4 nadir | 0.34 | 0.013 | ||
| ICV | 0.35 | 0.0077 | ||
| MtDNA 8-oxo-dG | −0.44 | 0.0032* | ||
| 0.15 | ||||
| Thalamus | Age | −0.23 | 0.092 | |
| CD4 nadir | −0.02 | 0.853 | ||
| ICV | 0.50 | 0.0002 | ||
| MtDNA 8-oxo-dG | −0.23 | 0.096 | ||
| 0.04 | ||||
| Caudate | Age | −0.26 | 0.082 | |
| CD4 nadir | 0.11 | 0.415 | ||
| ICV | 0.46 | 0.0014 | ||
| MtDNA 8-oxo-dG | −0.17 | 0.248 | ||
| 0.02 | ||||
| Putamen | Age | −0.28 | 0.040 | |
| CD4 nadir | 0.29 | 0.027 | ||
| ICV | 0.42 | 0.0014 | ||
| MtDNA 8-oxo-dG | −0.32 | 0.025 | ||
| 0.08 | ||||
| Nucleus accumbens | Age | −0.25 | 0.105 | |
| CD4 nadir | 0.12 | 0.397 | ||
| ICV | 0.31 | 0.032 | ||
| MtDNA 8-oxo-dG | −0.25 | 0.112 | ||
| 0.05 | ||||
| Cerebellar WM | Age | −0.18 | 0.230 | |
| CD4 nadir | −0.08 | 0.585 | ||
| ICV | 0.32 | 0.024 | ||
| MtDNA 8-oxo-dG | −0.27 | 0.089 | ||
| 0.06 | ||||
| Cerebellar GM | Age | −0.06 | 0.688 | |
| CD4 nadir | 0.25 | 0.079 | ||
| ICV | 0.43 | 0.0023 | ||
| MtDNA 8-oxo-dG | −0.38 | 0.015 | ||
| 0.11 | ||||
| Cortical GM | Age | −0.14 | 0.199 | |
| CD4 nadir | −0.01 | 0.934 | ||
| ICV | 0.72 | <0.0001 | ||
| MtDNA 8-oxo-dG | −0.16 | 0.183 | ||
| 0.02 | ||||
| Cerebral WM | Age | −0.14 | 0.189 | |
| CD4 nadir | 0.01 | 0.950 | ||
| ICV | 0.72 | <0.0001 | ||
| MtDNA 8-oxo-dG | −0.21 | 0.064 | ||
| 0.03 | ||||
| Subcortical GM | Age | −0.13 | 0.296 | |
| CD4 nadir | 0.25 | 0.039 | ||
| ICV | 0.55 | <0.0001 | ||
| MtDNA 8-oxo-dG | −0.43 | 0.0013* | ||
| 0.14 | ||||
| Lateral ventricles | Age | 0.33 | 0.006 | |
| CD4 nadir | −0.11 | 0.307 | ||
| ICV | 0.49 | <0.0001 | ||
| MtDNA 8-oxo-dG | 0.38 | 0.0023* | ||
| 0.11 |
Significant when Bonferroni-corrected for multiple comparisons (p < 0.004).
Associations between regional volumes and mtDNA 8-oxo-dG, adjusted for age, ICV and nadir CD4, were examined for dependence on the extreme mtDNA 8-oxo-dG outlier (0.74). The same data point corresponds to the outlier (5349 mm3) in the hippocampal volume distribution (Fig. 2, top). With the outlier removed from analyses, brain regions whose volumetric associations with mtDNA 8-oxo-dG had been significant after correction for multiple comparisons remained significantly related to mtDNA 8-oxo-dG: hippocampus (β = −0.40, p = 0.0071), pallidum (β = −0.35, p = 0.015), subcortical gray matter (β = −0.31, p = 0.019), and lateral ventricles (β = 0.26, p = 0.030). Discarding large mtDNA 8-oxo-dG values is somewhat undesirable since the impact of high break frequency is of interest, but the regression coefficients and p-values obtained when the outlier is excluded indicate the robustness of the main results.
Given the skewed distribution of mtDNA 8-oxo-dG (Fig. 1), we tested the effects of transforming these data although predictor variables in regression models need not be normally distributed. (The dependent variables, regional volumes, were approximately Gaussian in distribution.) For the full study population, skewness due to zero values of mtDNA 8-oxo-dG could not be eliminated but was reduced by square root and cube root transformations. The associations between mtDNA 8-oxo-dG and volumes of hippocampus, pallidum, subcortical gray matter, and lateral ventricles (all p < 0.01) showed little change in β coefficients. For the subject group with nonzero (positive) values of mtDNA 8-oxo-dG, log-transformation of the data achieved a normal mtDNA 8-oxo-dG distribution. MtDNA 8-oxo-dG was negatively associated with hippocampal volume (β = −0.38, p = 0.021), controlling for age and ICV (nadir CD4 was dropped as it had a non-significant effect and because of low sample size). Square root and cube root transformations of mtDNA 8-oxo-dG produced results roughly similar to those found for the log-transformed data. (Regression coefficients cannot be directly compared when using different transformations of the data.)
4. Discussion
ROS-mediated mtDNA injury, mitochondrial dysfunction and oxidative stress play important roles in neurodegenerative processes. MtDNA is 10 to 100 times more sensitive than nuclear DNA to oxidative stress (Yakes & Van Houten, 1997) and thus highly susceptible to oxidative damage. In a cycle central to the free radical theory of aging, elevated mitochondrial ROS levels lead to mtDNA damage which then causes further mitochondrial and respiratory chain deficiency and accumulation of ROS (Harman, 1956, 1972). HIV-associated mitochondrial dysfunction results in increases in ROS production and indices of overall oxidative stress (Macho et al., 1995; Gil et al., 2003). Peripheral macrophages are related to activated microglia (Davis et al., 1994) which are largely responsible for generation of free radicals in the CNS (Klegeris & McGeer, 2000). Neurotoxins released by the activated microglia trigger inflammatory processes linked to neurodegeneration. Oxidative stress-mediated neuroinflammation is a probable contributor to neuronal loss in HIV (reviewed by Gray et al. (2000)); pronounced neuroinflammation occurs even in cART-treated, cognitively normal HIV patients (Anthony et al., 2005). Our study identified a link between brain structural change and elevated peripheral oxidative stress in well-controlled HIV disease. Volumes of multiple brain regions correlated negatively with mitochondrial DNA 8-oxo-dG in PBMCs of HIV-infected individuals after adjustment for age, intracranial volume and nadir CD4 count. These findings were substantiated by an association between increased mtDNA 8-oxo-dG and lateral ventricular dilatation indicating central brain atrophy. After correction for multiple comparisons, higher mtDNA 8-oxo-dG levels were associated with enlargement of the lateral ventricles and with smaller volumes of hippocampus, pallidum, and subcortical gray matter in regression models with moderate R (Persidsky et al., 2000). The amygdala, putamen, and cerebellar gray matter were also volumetrically associated with PBMC mtDNA 8-oxo-dG though the p-values did not pass the strict Bonferroni criterion.
Our most statistically significant result was the relationship between increased PBMC mtDNA 8-oxo-dG break frequency and reduced hippocampal volume. HIV proteins damage the hippocampus via oxidative stress: for example, hippocampal neurons are susceptible to transregulatory protein (Tat)-induced injury and apoptosis (Maragos et al., 2003) which occurs through mitochondrial dysfunction (Kruman et al., 1998). The hippocampus is a site of ongoing neuroinflammation even in HIV patients on suppressive cART. A postmortem study by Anthony et al. (Anthony et al., 2005) examined microglial and macrophage activation in the basal ganglia and hippocampus of HIV-infected, cognitively normal individuals whose plasma viral load had been suppressed by cART to below 50 copies/mL. Hippocampal neuroinflammation in these subjects exceeded that seen in HIV-encephalitis patients during the pre-cART era. It was concluded that the site of microglial/macrophage activation in patients with chronic suppressed HIV infection may be shifting to the hippocampus, perhaps because of therapy itself (Anthony et al., 2005). The basal ganglia, too, showed a pre- and post-cART difference in neuroinflammation (Anthony et al., 2005) that is consistent with the associations we observed between mtDNA break frequency and volumes of pallidum, putamen, and total subcortical gray matter.
The hippocampus is vulnerable to oxidative stress in general. In a rat model of neuroinflammation in AD, increased microglial activation and working memory deficits (Hauss-Wegrzyniak et al., 1998, 1999a, 1999b) as well as striking hippocampal atrophy (Hauss-Wegrzyniak et al., 2000) were produced by infusion of lipopolysaccharide into the ventricular system. Severity of neuronal damage in rat hippocampal slices correlates with ROS levels (Schurr & Gozal, 2011). Prominent in the human hippocampus are fast neuronal network oscillations in the gamma frequency range (~ 30–90 Hz), which involve high oxygen consumption requiring maximal mitochondrial oxidative capacity (Kann et al., 2011). These gamma oscillations provide a structure for information processing and contribute to cognitive memory formation and sensory processing (reviewed by Bartos et al. (2007)). The role played by hippocampal gamma oscillations in encoding and retrieving working memories (Montgomery & Buzsaki, 2007; van Vugt et al., 2010), and the well-established involvement of the hippocampus in learning and memory (O'Reilly & Rudy, 2001), support our study findings since the participants had lower test scores both in working memory and in learning and memory. Because the amygdala was also volumetrically related to mtDNA 8-oxo-dG, the limbic system may be particularly susceptible to ROS-induced oxidative stress. This conjecture is strengthened by the fact that the relationships between mtDNA oxidative damage and volumes of hippocampus and amygdala appeared to be driven by the subject group with positive mtDNA 8-oxo-dG values. Moreover, the associations we found between mtDNA 8-oxo-dG and volumes of hippocampus and cerebellar gray matter are interesting in light of published work on the effect of oxidative stress on brain neurons. Hippocampal CA1 neurons are selectively vulnerable to oxidative stress (Wang et al., 2005; Wilde et al., 1997). Neurons in cerebellar gray matter (but not cerebral cortex) show a differential response to conditions involving oxidative stress: in particular, the cerebellar granule layer is sensitive to oxidative stress and can undergo extensive neuronal death (Wang et al., 2009).
The trend associations of mtDNA 8-oxo-dG with higher current and nadir CD4 counts may be attributable to earlier initiation of therapy in subjects with positive mtDNA 8-oxo-dG, although duration of cART did not correlate with mtDNA 8-oxo-dG or differ significantly between groups with and without PBMC mtDNA oxidative damage. A greater proportion of subjects with mtDNA 8-oxo-dG > 0 had a history of ZDV, d4T and/or ddI use. Antiretroviral drugs may cause accumulation of ROS and induce neuronal injury and death in vitro (Akay et al., 2014), and were proven centrally neurotoxic in vivo in two animal models (Akay et al., 2014). HIV patients treated with NRTIs have reduced mtDNA content in PBMCs (Chiappini et al., 2004). However, PBMCs of HIV-infected individuals naïve to antiretroviral therapy also exhibit mitochondrial dysfunction involving decreased oxidative phosphorylation activity (Miro et al., 2004) and mtDNA levels (Chiappini et al., 2004; Miro et al., 2004; Casula et al., 2005; Miura et al., 2003), suggesting that HIV infection itself contributes to mitochondrial toxicity (Maagaard & Kvale, 2009).
Our study limitations included the cross-sectional design, modest sample size and lack of HIV-negative subjects. Additionally, the participants were aged 40 years and older. A larger, longitudinal study that includes younger individuals is warranted to confirm and clarify the impact of HIV-induced oxidative stress on the brain.
5. Conclusion
Peripheral mitochondrial DNA oxidative damage was associated with reduced hippocampal and subcortical gray matter volumes in chronic stable HIV disease. To our knowledge this is the first reported connection between oxidative stress in the peripheral compartment and brain structure in HIV infection. Our data corroborate the hypothesized involvement of oxidative stress in HIV-related brain neuronal loss in the era of effective cART, and suggest that PBMC mtDNA 8-oxo-dG should be investigated as a predictor of brain atrophy in individuals with mild symptoms of HAND. Regional brain volumetric loss may be moderated in part by mitochondrial dysfunction or by a common HIV-related process in the midst of viral suppression that affects both mtDNA and the brain. Clarification of the mechanism may eventually lead to treatments that inhibit neurodegeneration by decreasing oxidative stress and restoring mitochondrial function in this vulnerable population.
Acknowledgments
The authors thank the clinical and laboratory staff of the Hawaii Center for AIDS, the staff at InVision Imaging, and especially the patients in the Hawaii Aging with HIV-CVD cohort. This study was supported by R21 N5080656 (KJK), R01 HL095135 (MG, CMS), R21 NS087951-01 A1 (BKN), U54 RR026136 (KJK, CMS, DCC, BKN), and U54 MD007584 (KJK, CMS, DCC, BKN).
References
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol. Appl. Neurobiol. 1999;25(2):123–133. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J. Immunol. 2009;183(8):5379–5387. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, Gannon PJ, Mankowski J, Dorsey JL, Buch AM, Cross SA, Cook DR, Pena MM, Andersen ES, Christofidou-Solomidou M, Lindl KA, Zink MC, Clements J, Pierce RC, Kolson DL, Jordan-Sciutto KL. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J. Neurovirol. 2014;20(1):39–53. doi: 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almer G, Teismann P, Stevic Z, Halaschek-Wiener J, Deecke L, Kostic V, Przedborski S. Increased levels of the pro-inflammatory prostaglandin PGE2 in CSF from ALS patients. Neurology. 2002;58(8):1277–1279. doi: 10.1212/wnl.58.8.1277. [DOI] [PubMed] [Google Scholar]
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat. Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J. Neuropathol. Exp. Neurol. 2005;64(6):529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43(10):2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10(6):573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011 doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco F, Garcia-Benayas T, Jose de la Cruz J, Gonzalez-Lahoz J, Soriano V. First-line therapy and mitochondrial damage: different nucleosides, different findings. HIV Clin. Trials. 2003;4(1):11–19. doi: 10.1310/HF1J-3P6K-1K9H-AGPY. [DOI] [PubMed] [Google Scholar]
- Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12(14):1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Casula M, Bosboom-Dobbelaer I, Smolders K, Otto S, Bakker M, de Baar MP, Reiss P, de Ronde A. Infection with HIV-1 induces a decrease in mtDNA. J. Infect. Dis. 2005;191(9):1468–1471. doi: 10.1086/429412. [DOI] [PubMed] [Google Scholar]
- Chiappini F, Teicher E, Saffroy R, Pham P, Falissard B, Barrier A, Chevalier S, Debuire B, Vittecoq D, Lemoine A. Prospective evaluation of blood concentration of mitochondrial DNA as a marker of toxicity in 157 consecutively recruited untreated or HAART-treated HIV-positive patients. Lab. Investig. 2004;84(7):908–914. doi: 10.1038/labinvest.3700113. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J. Neurovirol. 2010;16(1):25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC, Coombs RW, Schoenfeld DA, Bassett RL, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe VJ, Friedman HM, Merigan TC, Reichman RC, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS clinical trials group. N. Engl. J. Med. 1996;334(16):1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davis EJ, Foster TD, Thomas WE. Cellular forms and functions of brain microglia. Brain Res. Bull. 1994;34(1):73–78. doi: 10.1016/0361-9230(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after N 4 years of effective highly active antiretroviral therapy. J. Infect. Dis. 2007;196(12):1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 2007;8(1):33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Enting RH, Hoetelmans RM, Lange JM, Burger DM, Beijnen JH, Portegies P. Antiretroviral drugs and the central nervous system. AIDS. 1998;12(15):1941–1955. doi: 10.1097/00002030-199815000-00005. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, Kowall NW, Brown RH, Jr., Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 1997;69(5):2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gartner S. HIV infection and dementia. Science. 2000;287(5453):602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- Gerschenson M, Kim C, Berzins B, Taiwo B, Libutti DE, Choi J, Chen D, Weinstein J, Shore J, da Silva B, Belsey E, McComsey GA, Murphy RL. Mitochondrial function, morphology and metabolic parameters improve after switching from stavudine to a tenofovir-containing regimen. J. Antimicrob. Chemother. 2009;63(6):1244–1250. doi: 10.1093/jac/dkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil L, Martinez G, Gonzalez I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tapanes R, Perez J, Leon OS. Contribution to characterization of oxidative stress in HIV/ AIDS patients. Pharmacol. Res. 2003;47(3):217–224. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- Gray F, Adle-Biassette H, Brion F, Ereau T, le Maner I, Levy V, Corcket G. Neuronal apoptosis in human immunodeficiency virus infection. J. Neurovirol. 2000;6(Suppl. 1):S38–S43. [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer's disease: the potential role of a 'cytokine cycle' in disease progression. Brain Pathol. 1998;8(1):65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J. Am. Geriatr. Soc. 1972;20(4):145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vraniak P, Wenk GL. The effects of a novel NSAID on chronic neuroinflammation are age dependent. Neurobiol. Aging. 1999a;20(3):305–313. doi: 10.1016/s0197-4580(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Willard LB, Del Soldato P, Pepeu G, Wenk GL. Peripheral administration of novel anti-inflammatories can attenuate the effects of chronic inflammation within the CNS. Brain Res. 1999b;815(1):36–43. doi: 10.1016/s0006-8993(98)01081-6. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer's disease. Brain Res. 1998;780(2):294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Galons JP, Wenk GL. Quantitative volumetric analyses of brain magnetic resonance imaging from rat with chronic neuroinflammation. Exp. Neurol. 2000;165(2):347–354. doi: 10.1006/exnr.2000.7469. [DOI] [PubMed] [Google Scholar]
- Higuchi Y. Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem. Pharmacol. 2003;66(8):1527–1535. doi: 10.1016/s0006-2952(03)00508-2. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6(2):65–70. [Google Scholar]
- Hulgan T, Morrow J, D'Aquila RT, Raffanti S, Morgan M, Rebeiro P, Haas DW. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin. Infect. Dis. 2003;37(12):1711–1717. doi: 10.1086/379776. [DOI] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996;47(6 Suppl. 3):S161–S170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK, Shikuma C. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb. Cortex. 2012;22(9):2065–2075. doi: 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur KJ, Shikuma C, Kirk GR, Shiramizu B, Valcour V, Chow D, Souza S, Nakamoto B, Sailasuta N. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013 doi: 10.1212/WNL.0b013e318291903f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O, Huchzermeyer C, Kovacs R, Wirtz S, Schuelke M. Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain. 2011;134:345–358. doi: 10.1093/brain/awq333. Pt 2. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl. 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kim SR, Kim DI, Kim SH, Lee H, Lee KS, Cho SH, Lee YC. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014;5:e1498. doi: 10.1038/cddis.2014.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimes AS, London ED, Szabo G, Raymon L, Tabakoff B. Reduction of cerebral glucose utilization by the HIV envelope glycoprotein Gp-120. Exp. Neurol. 1991;112(2):224–228. doi: 10.1016/0014-4886(91)90073-l. [DOI] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. Interaction of various intracellular signaling mechanisms involved in mononuclear phagocyte toxicity toward neuronal cells. J. Leukoc. Biol. 2000;67(1):127–133. doi: 10.1002/jlb.67.1.127. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp. Neurol. 1998;154(2):276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lagathu C, Eustace B, Prot M, Frantz D, Gu Y, Bastard JP, Maachi M, Azoulay S, Briggs M, Caron M, Capeau J. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir. Ther. 2007;12(4):489–500. [PubMed] [Google Scholar]
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia–reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001;33(6):1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat. Med. 2000;6(12):1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Maagaard A, Kvale D. Mitochondrial toxicity in HIV-infected patients both off and on antiretroviral treatment: a continuum or distinct underlying mechanisms? J. Antimicrob. Chemother. 2009;64(5):901–909. doi: 10.1093/jac/dkp316. [DOI] [PubMed] [Google Scholar]
- Macho A, Castedo M, Marchetti P, Aguilar JJ, Decaudin D, Zamzami N, Girard PM, Uriel J, Kroemer G. Mitochondrial dysfunctions in circulating T lymphocytes from human immunodeficiency virus-1 carriers. Blood. 1995;86(7):2481–2487. [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117(1):43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- Masini A, Scotti C, Calligaro A, Cazzalini O, Stivala LA, Bianchi L, Giovannini F, Ceccarelli D, Muscatello U, Tomasi A, Vannini V. Zidovudine-induced experimental myopathy: dual mechanism of mitochondrial damage. J. Neurol. Sci. 1999;166(2):131–140. doi: 10.1016/s0022-510x(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc. Natl. Acad. Sci. U. S. A. 1998;95(24):14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro O, Lopez S, Martinez E, Pedrol E, Milinkovic A, Deig E, Garrabou G, Casademont J, Gatell JM, Cardellach F. Mitochondrial effects of HIV infection on the peripheral blood mononuclear cells of HIV-infected patients who were never treated with antiretrovirals. Clin. Infect. Dis. 2004;39(5):710–716. doi: 10.1086/423176. [DOI] [PubMed] [Google Scholar]
- Miura T, Goto M, Hosoya N, Odawara T, Kitamura Y, Nakamura T, Iwamoto A. Depletion of mitochondrial DNA in HIV-1-infected patients and its amelioration by antiretroviral therapy. J. Med. Virol. 2003;70(4):497–505. doi: 10.1002/jmv.10423. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc. Natl. Acad. Sci. U. S. A. 2007;104(36):14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman MW, Brown WM, Stoneking M, Aquadro CF. Nonneutral mitochondrial DNA variation in humans and chimpanzees. Genetics. 1996;142(3):953–963. doi: 10.1093/genetics/142.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol. Rev. 2001;108(2):311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Zheng J, Miller D, Gendelman HE. Mononuclear phagocytes mediate blood–brain barrier compromise and neuronal injury during HIV-1-associated dementia. J. Leukoc. Biol. 2000;68(3):413–422. [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J. Neurovirol. 2002;8(2):136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson's disease. J. Neural Transm. Suppl. 2006;70:373–381. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- Schurr A, Gozal E. Aerobic production and utilization of lactate satisfy increased energy demands upon neuronal activation in hippocampal slices and provide neuroprotection against oxidative stress. Front. Pharmacol. 2011;2:96. doi: 10.3389/fphar.2011.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Semino-Mora MC, Leon-Monzon ME, Dalakas MC. Effect of L-carnitine on the zidovudine-induced destruction of human myotubes. Part I: L-carnitine prevents the myotoxicity of AZT in vitro. Lab. Investig. 1994;71(1):102–112. [PubMed] [Google Scholar]
- Shikuma CM, Seto T, Liang CY, Bennett K, DeGruttola V, Gerschenson M, Stein JH, Budoff M, Hodis HN, Delaney JA, Ogata-Arakaki D, Pramyothin P, Chow D. Vitamin D levels and markers of arterial dysfunction in HIV. AIDS Res. Hum. Retrovir. 2012;28(8):793–797. doi: 10.1089/aid.2011.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc. Natl. Acad. Sci. U. S. A. 2005;102(43):15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaamonde-Garcia C, Riveiro-Naveira RR, Valcarcel-Ares MN, Hermida-Carballo L, Blanco FJ, Lopez-Armada MJ. Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 2012;64(9):2927–2936. doi: 10.1002/art.34508. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009;27(2):120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- Vignoli AL, Martini I, Haglid KG, Silvestroni L, Augusti-Tocco G, Biagioni S. Neuronal glycolytic pathway impairment induced by HIV envelope glycoprotein gp120. Mol. Cell. Biochem. 2000;215(1–2):73–80. doi: 10.1023/a:1026590916661. [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Schulze-Bonhage A, Litt B, Brandt A, Kahana MJ. Hippocampal gamma oscillations increase with memory load. J. Neurosci. 2010;30(7):2694–2699. doi: 10.1523/JNEUROSCI.0567-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial defects in cardiomyopathy and neuromuscular disease. Am. Heart J. 2000;139(2):S70–S85. doi: 10.1067/mhj.2000.103934. Pt 3. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pal R, Chen XW, Limpeanchob N, Kumar KN, Michaelis EK. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res. Mol. Brain Res. 2005;140(1–2):120–126. doi: 10.1016/j.molbrainres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Wang X, Zaidi A, Pal R, Garrett AS, Braceras R, Chen XW, Michaelis ML, Michaelis EK. Genomic and biochemical approaches in the discovery of mechanisms for selective neuronal vulnerability to oxidative stress. BMC Neurosci. 2009;10:12. doi: 10.1186/1471-2202-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde GJ, Pringle AK, Wright P, Iannotti F. Differential vulnerability of the CA1 and CA3 subfields of the hippocampus to superoxide and hydroxyl radicals in vitro. J. Neurochem. 1997;69(2):883–886. doi: 10.1046/j.1471-4159.1997.69020883.x. [DOI] [PubMed] [Google Scholar]
- van 't Wout AB, Lehrman GK, Mikheeva SA, O'Keeffe GC, Katze MG, Bumgarner RE, Geiss GK, Mullins JI. Cellular gene expression upon human immunodeficiency virus type 1 infection of CD4(+)-T-cell lines. J. Virol. 2003;77(2):1392–1402. doi: 10.1128/JVI.77.2.1392-1402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 1997;94(2):514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]