Abstract
Due to its unique capability of visualizing optical absorption in deep tissues, photoacoustic tomography is increasingly used in biomedical imaging. Among various types of transducer arrays, the linear array is perhaps the most widely used in photoacoustic tomography, because it is commercially available and readily allows ultrasound imaging. However, the three-dimensional imaging capability of a linear array is limited, due to its poor elevational resolution. While various scanning schemes have been proposed to address this problem, they all suffer from long scanning time. To address this issue, we introduce slit-enabled three-dimensional photoacoustic tomography. The metal slit, placed at the array focus, causes the incoming photoacoustic waves to diffract along the elevation direction and hence significantly improves the elevation detection aperture and resolution. We tested the new system in both phantoms and animals. The slit improves the elevation resolution by ten times without compromising scanning time.
Photoacoustic (PA) tomography (PAT) is playing an increasingly important role in biomedical imaging. The hybrid nature of PAT allows acquisition of high-resolution images beyond the optical diffusion limit [1–4]. Among various transducer arrays used in PAT, linear transducer arrays are commonly seen due to their low cost, hand-held convenience and easy adaption to clinical applications [5–8]. However, the three-dimensional (3D) imaging capability of a linear array is limited, because its elevation resolution is much worse than the axial and lateral spatial resolutions. For instance, a Philips L7-4 array has 0.144 mm axial and 0.298 mm lateral resolutions, but only a 1.5 mm elevation resolution (at the acoustic focus). Over the past few years, multiple methods have been proposed to address this issue. Gateau et al. combined linear and rotational scanning to achieve nearly isotropic 3D spatial resolution [9]. Schwarz et al. proposed a bi-directional scan method with two array positions perpendicular to each other [10]. In principle, all these methods improve elevation resolution by converting the elevation direction into axial or lateral directions. However, such complicated scanning geometry often requires prolonged scanning times.
In this letter, we propose a fundamentally different approach to improve elevation resolution. Our method is based on acoustic diffraction through a thin slit placed along the acoustic focus of the array (Fig. 1). The thin slit diffracts the incoming photoacoustic waves and hence improves the receiving aperture along elevation direction. As shown in Fig. 1, in a conventional linear-array PAT system (Fig. 1a), due to elevation focus from the acoustic lens, photoacoustic waves coming out of the focal zone cannot be received by the transducer. The loss in elevation receiving aperture limits the corresponding spatial resolution. The metal slit (with a width close to the 300 µm central acoustic wavelength) eliminates the acoustic focus, allowing waves coming out of the focal zone to still reach the transducer (Fig. 1b). The increased receiving aperture can greatly improve the elevation resolution. Compared to other approaches, our method does not require any modification on scanning geometry — only one elevation scan is needed as in conventional 3D PAT. This unique feature possesses significant advantage over existing approaches [9, 10].
Figure 1.
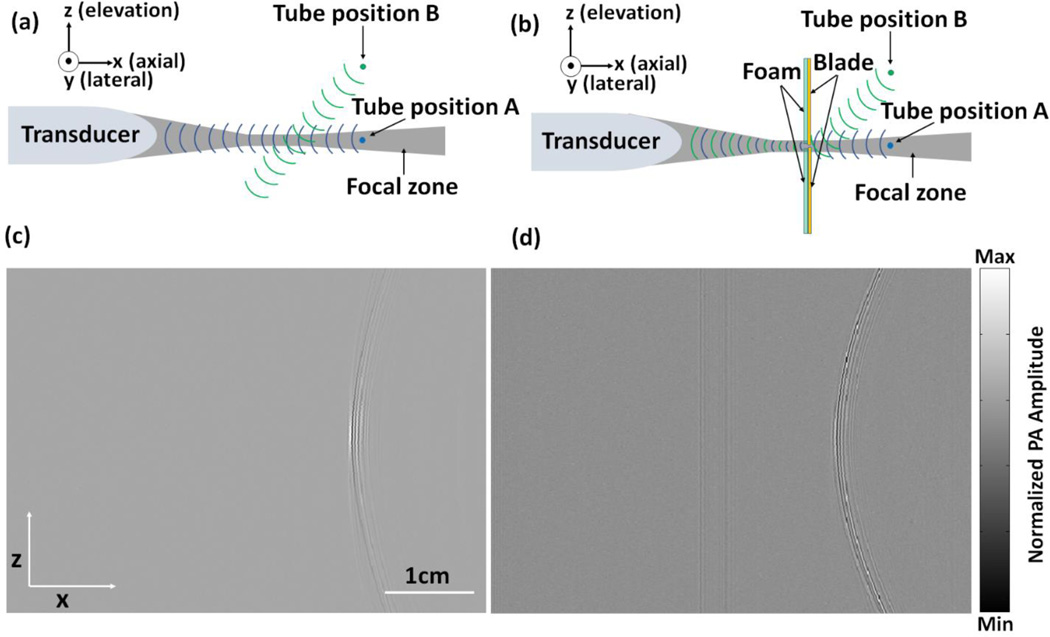
Principle of slit-enabled PAT. (a) A schematic drawing of conventional PAT and its receiving aperture along elevation direction. (b) A schematic drawing of slit-enabled PAT and its receiving aperture along elevation direction. The metal slit is placed in between the transducer array and the object. (c) Raw channel data (x–z view) of a tube filled with black ink imaged in conventional PAT. (d) Raw channel data (x–z view) of the same tube imaged in slit-enabled PAT.
To validate our assumption, we first imaged a tube filled with black ink. The tube is placed along the lateral direction (y axis) of the array and can be scanned along the elevation direction (z axis) through a translation stage. In the z-x plane, the tube looks like a point source (Figs. 1a and 1b). Acoustic signals are received by a 128-element linear transducer array (ATL/Philips L7-4) with 5 MHz central frequency and elevation focus at 25 mm. Light illumination was achieved by an Nd:YAG laser (Surelite SL III-10, Continuum) with <10 ns pulses width and 10 Hz pulse repetition frequency (PRF). The output wavelength is 532 nm and the energy of each pulse is 18 mJ. PA signals received by the L7-4 array were multiplexed and digitalized by a 64-channel ultrasound data acquisition system (Vantage, Verasonics Inc., Redmond, Washington). The thin slit was formed by two metal blades (0.5 mm thickness). The bottom blade was fixed in position while the top blade was mounted on a translation stage, which allowed easy and precise control of the slit opening. While our blade thickness is not multiples of half acoustic wavelength in stainless steel (0.58 mm), a small portion of sound energy may still transmit directly through the plate [11]. To prevent this, we glued a 5 mm foam on the back surface of the blade (facing the transducer) as an acoustic absorber. As a demonstration of the principle, we scanned over 40 mm with 0.1 mm step size. The entire scan took 80 seconds (40 mm/0.1mm/5 Hz).
Figure 1c shows a segment of raw-channel data (in z-x plane) acquired without the use of the metal slit. Because of the limited receiving aperture along elevation direction (the gray-colored region represents the acoustic receiving zone), the transducer gradually misses tube signal while it is moved from position A to position B. Thus in Fig. 1c, only the central region shows strong tube signal. Once the metal slit has been added (Fig. 1b), incoming acoustic waves will diffract through the slit, which changes the wave propagation direction. The tube can now be detected within the entire 40-mm scanning range (Fig. 1d). In principle, the slit can be placed anywhere along the lateral direction. We chose the acoustic focal position because it offers the highest detection sensitivity and minimum signal loss.
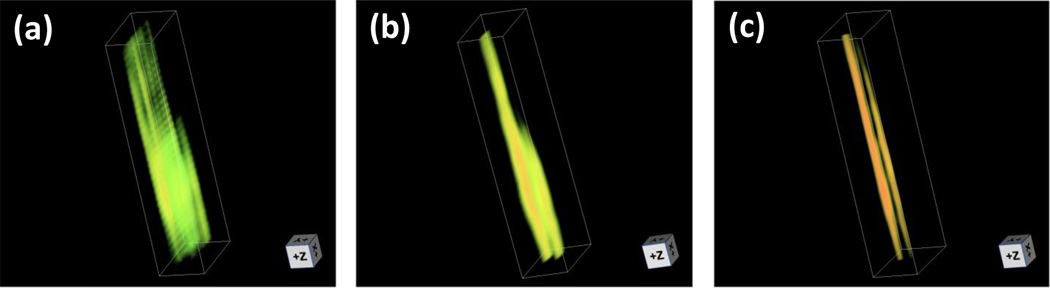
Reconstructed images of the single tube are shown in Fig. 2. For the conventional PAT, we used two reconstruction methods. The first method reconstructs each 2D image individually and then all images are stacked to form a 3D image. This is the most commonly used approach to form a 3D image from linear array [8]. The second method reconstructs the same dataset in 3D using the focal-line concept [12], which improves the elevation resolution up to the size of the elevation focal height (~1.5 mm). Image reconstruction with slit is similar to the focal-line reconstruction approach [12]. The delay time of each point source in 3D space is calculated based on the following two principles: (a) wave propagation in x-y plane is unaffected by the slit; and (b) wave propagation in z-x plane has two segments, first from point source to slit and then from slit to transducer element. For simplicity, we name the three different reconstruction/imaging methods as 2D-stack PAT, 3D-focal-line PAT, and slit-PAT. As shown in Fig. 2a and Visualization 1, 2D-stack PAT has the poorest elevation resolution (along z-axis). The tube can barely be identified. While 3D-focal-line PAT provides better images of tube structure, the tube image is still blurred along elevation direction (Fig. 2b and Visualization 2). As expected, slit-PAT offers the highest elevation resolution and the tube can be clearly identified (Fig. 2c and Visualization 3). It should be noted that, in all these images, we saw two tubes instead of one due to the boundary built up effect along axial direction [13]. The distance between the two “tubes” is quantified to be 0.5 mm, which is the inner diameter of the tube.
Figure 2.
Reconstructed images of a single tube. (a) 2D-stack PAT image (Visualization 1). (b) 3D-focal-line PAT image (Visualization 2). (c) Slit-PAT image (Visualization 3).
Table 1 summarizes the elevation resolution and signal-to-noise ratio (SNR) of three imaging/reconstruction methods. The elevation resolution is defined as the full-width-at-half-maximum (FWHM) of the first boundary of the tube along the elevation direction. It can be seen that 2D-stack PAT provides the worst elevation resolution. With focal-line reconstruction, the resolution was improved by two time and the value is close to the height of the elevation focus (1.5 mm). Slit-PAT further improves resolution by almost five times to 0.33 mm, which is close to the 0.3 mm slit opening. In total, slit-PAT offers ten times better elevation resolution than 2D-stack PAT. Because the slit also blocks some incoming photoacoustic signals, we analyzed the SNR. The signal intensity was calculated by averaging signals within a small region in the tube. Noise was estimated by calculating the standard deviation of signals in a background region. Main source of noise was the electronic noise in the PAT data acquisition system. As shown in table 1, the slit-PAT SNR is actually four times better than that of 2D-stack PAT. This is due to the fact that, in slit-PAT, the transducer receives signal from all 400 scanning positions, whose data are coherently summed during reconstruction. This procedure reduces the noise by 20 times. In terms of signal intensity, the slit reduces the signal by 5 times (0.3 mm slit opening/1.5 mm elevation focal height). Thus the overall improvement in SNR is 4 times . As expected, 3D-focal-line PAT provides the highest SNR. However, due to the limited receiving aperture, its SNR is only two times better than that of slit-PAT, but with five times worse elevation resolution. The resolution and SNR results demonstrate that slit-PAT remarkably improves the elevation resolution without compromising the SNR.
Table 1.
Elevation resolution and SNR analysis of different imaging/reconstruction methods
| 2D-stack PAT | 3D-focal-line PAT | Slit-PAT | |
|---|---|---|---|
| Resolution | 3.52 mm | 1.67 mm | 0.33 mm |
| SNR | 16.2 | 136.7 | 62.7 |
To further demonstrate the imaging capability, we imaged a mouse abdomen in situ (Fig. 3). In addition to the hemoglobin contrast, we also used ZnBNc nanonaps as an intestine-confined contrast agent, as previously reported [14]. To match the absorption peak of ZnBNc nanonaps (710 nm), we used light coming out of an optical parametric oscillator (OPO) laser pumped by the Nd:YAG laser. The experiment was performed on a 23 g female nude mouse been fasted overnight with access to only water. Before the experiment, the mouse was administered via gavage 0.2 mL of ZnBNc nanonaps with an absorbance of 500 at 710 nm. Thirty minutes after gavage, the mouse was sacrificed and mounted vertically on the system with the abdomen facing the transducer array (Fig. 3). In both the conventional and slit-enabled PAT, we scanned vertically over 25 mm, which covers the entire small intestine region. For better illustration, the reconstructed 3D image was maximum amplitude projected and depth-encoded along the axial direction of the array (posterior direction of the animal body).
Figure 3.
A 3D drawing of the in situ experiment setup.
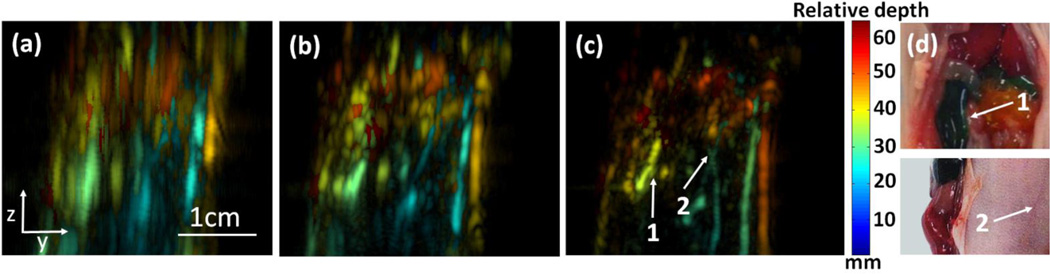
Figure 4a shows the depth-encoded image of 2D-stack PAT. Anatomical features or structures were not recognizable, due to the poor elevation resolution. Figure 4b shows depth-encoded image of 3D-focal-line PAT, where some skin vessels could be recognized. However, body structures were still difficult to recognize. Figure 4c shows the depth-encoded image of slit-PAT. Here we can clearly identify the intestine and several additional skin vessels. In particular, a pair of crossed skin vessels (arrow 2) is only made visible with the high elevation resolution. These features agree well with photos of exposed animal acquired after the experiment (Fig. 4d). Corresponding 3D images of Figs. 4a–4c are shown in Visualizations 4–6, respectively.
Figure 4.
In situ experiment of mouse abdomen. (a) Depth-encoded image of 2D-stack PAT (Visualization 4). (b) Depth-encoded image of 3D-focal-line PAT (Visualization 5). (c) Depth-encoded image of slit-PAT (Visualization 6). Arrows 1 and 2 point to intestine and crossed skin vessels, respectively. (d) Photos of exposed animal, acquired after the PAT experiment. Arrows 1 and 2 points to the same features respectively as in (c). Abdomen and skin were fully intact for (a)–(c).
In summary, we developed slit-enabled PAT, which significantly improves the elevation resolution of the linear transducer array. For the L7-4 array used in our experiment, the elevation resolution was improved ten times to 0.33 mm, which is close to the lateral resolution (0.298 mm) and is not far from the axial resolution (0.144 mm). Besides improvement in elevation resolution, the slit also improves the SNR due to a larger receiving aperture. We demonstrated the performance of the system in both phantom and in situ and the improvements are obvious. Compared to existing approaches to improve elevation resolution [8, 9], our method has the highest imaging speed and requires no change to the scanning geometry. Thus it can be easily adapted to any existing linear-array PAT systems. Due to limitations in laser pulse repetition rate (10 Hz), we imaged the animal only in situ (as animal motion prevents 3D reconstruction). However, kHz high power pulsed lasers are commercially available and can be used for high speed in vivo imaging. Also, the current setup cannot achieve true isotropic resolution in 3D due to the large element pitch, which defines the lateral resolution. In future studies, we can use a linear phased array or a custom-designed array with smaller pitch to address this issue. Nevertheless, with broad availability of linear arrays, we expect our method to further advance the image quality and application of PAT.
Acknowledgments
This work was sponsored in part by University at Buffalo startup funding, the SUNY Brain Network of Excellence Big Idea Award, and the National Institutes of Health [R21EY026411 and DP5OD017898 (J.F.L.)].
Full References
- 1.Xia J, Yao J, Wang LV. Photoacoustic tomography: principles and advances. Electromagnetic waves (Cambridge, Mass.) 2014;147:1. doi: 10.2528/pier14032303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335:1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia J, Wang LV. Small-animal whole-body photoacoustic tomography: a review. Biomedical Engineering, IEEE Transactions on. 2014;61:1380–1389. doi: 10.1109/TBME.2013.2283507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia J, Kim C, F Lovell J. Opportunities for Photoacoustic-Guided Drug Delivery. Current drug targets. 2015;16:571–581. doi: 10.2174/1389450116666150707100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gateau J, Caballero MÁA, Dima A, Ntziachristos V. Three-dimensional optoacoustic tomography using a conventional ultrasound linear detector array: whole-body tomographic system for small animals. Medical physics. 2013;40:013302. doi: 10.1118/1.4770292. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Li L, Zhu L, Xia J, Wang LV. Multiview Hilbert transformation for full-view photoacoustic computed tomography using a linear array. Journal of biomedical optics. 2015;20:066010–066010. doi: 10.1117/1.JBO.20.6.066010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, Xia J, Li L, Wang L, Wang LV. In Isotropic-resolution linear-array-based photoacoustic computed tomography through inverse Radon transform. SPIE BiOS2015 International Society for Optics and Photonics. pp 93230I-93230I-6. [Google Scholar]
- 8.Needles A, Heinmiller A, Sun J, Theodoropoulos C, Bates D, Hirson D, Yin M, Foster FS. Development and initial application of a fully integrated photoacoustic micro-ultrasound system. Ultrasonics, Ferroelectrics, and Frequency Control, IEEE Transactions on. 2013;60:888–897. doi: 10.1109/TUFFC.2013.2646. [DOI] [PubMed] [Google Scholar]
- 9.Gateau J, Gesnik M, Chassot J-M, Bossy E. Single-side access, isotropic resolution, and multispectral three-dimensional photoacoustic imaging with rotate-translate scanning of ultrasonic detector array. Journal of biomedical optics. 2015;20:056004–056004. doi: 10.1117/1.JBO.20.5.056004. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz M, Buehler A, Ntziachristos V. Isotropic high resolution optoacoustic imaging with linear detector arrays in bi-directional scanning. Journal of biophotonics. 2015;8:60–70. doi: 10.1002/jbio.201400021. [DOI] [PubMed] [Google Scholar]
- 11.Michaud M, Leong T, Swiergon P, Juliano P, Knoerzer K. Design parameters of stainless steel plates for maximizing high frequency ultrasound wave transmission. Ultrasonics sonochemistry. 2015;26:56–63. doi: 10.1016/j.ultsonch.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Xia J, Guo Z, Maslov K, Aguirre A, Zhu Q, Percival C, Wang LV. Three-dimensional photoacoustic tomography based on the focal-line concept. Journal of biomedical optics. 2011;16 doi: 10.1117/1.3625576. 090505-090505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Z, Li L, Wang LV. On the speckle-free nature of photoacoustic tomography. Medical physics. 2009;36:4084–4088. doi: 10.1118/1.3187231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Jeon M, Rich LJ, Hong H, Geng J, Zhang Y, Shi S, Barnhart TE, Alexandridis P, Huizinga JD. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nature nanotechnology. 2014;9:631–638. doi: 10.1038/nnano.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]