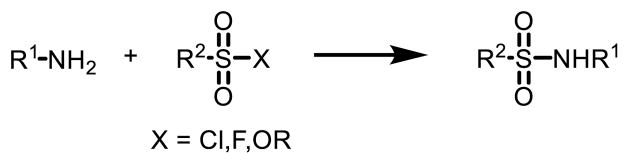Abstract
The impact of the development of sulfur therapeutics is instrumental to the evolution of the pharmaceutical industry. Sulfur-derived functional groups can be found in a broad range of pharmaceuticals and natural products. For centuries, sulfur continues to maintain its status as the dominating heteroatom integrated into a set of 362 sulfur-containing FDA approved drugs (besides oxygen or nitrogen) through the present. Sulfonamides, thioethers, sulfones and Penicillin are the most common scaffolds in sulfur containing drugs, which are well studied both on synthesis and application during the past decades. In this review, these four moieties in pharmaceuticals and recent advances in the synthesis of the corresponding core scaffolds are presented.
Keywords: sulfur containing drugs, sulfonamide, thioether, sulfones, sulfur dioxide fixation, organic synthesis
INTRODUCTION
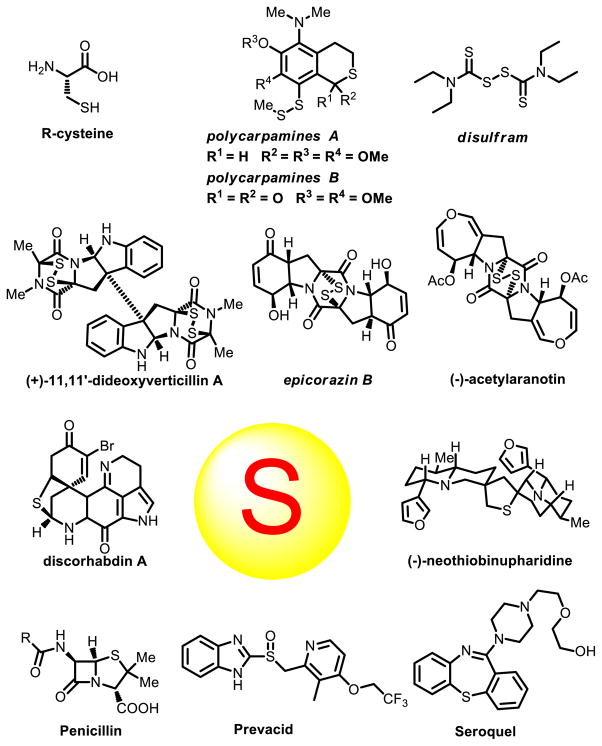
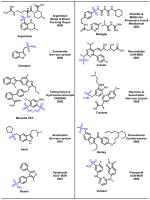
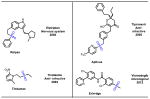
Sulfur containing compounds often show different biological activities and serve important functions in applications in the pharmaceutical industry [1]. Variety of sulfur containing scaffolds widely exists in natural products and drugs (Fig. 1). For instance, epidithiodiketopiperazine (ETP), characterized by sulfur atoms and a diketopiperazine structure, comprises a large number of metabolites, which display a range of biological activities including antiviral, antibacterial, antiallergic, antimalarial and cytotoxic properties [2]. Prevacid is a proton-pump inhibitor (PPI) which inhibits the stomach’s production of gastric acids [3]. Seroquel is an atypical antipsychotic approved for the treatment of schizophrenia, bipolar disorder, and in the XR version along with a selective serotonin reuptake inhibitor (SSRI) to treat major depressive disorder [4]. In this review, several key scaffolds of sulfur containing pharmaceuticals, namely sulfonamides, thioethers, sulfones and Penicillin and its derivatives, are illustrated and some recent synthetic approaches are discussed.
Fig. 1.
Representative sulfur containing scaffolds in natural products and pharmaceuticals
SULFONAMIDE CONTAINING DRUGS
From a historical perspective, sulfonamides have been a leading constituent in new drugs since the first appearance in the 1930s [5], occupying six decades over the past 100 years.
Sulfonamide drugs were the first antibiotics to be used systemically, and paved the way for the new antibiotic revolution in medicine. Nevertheless, antibiotics are not the only function of sulfonamides. Table 1 showed a list of sulfonamide containing scaffolds in pharmaceutical molecules with different indications. For example, cyclothiazide is a diuretic and antihypertensive that was originally introduced in the United States in 1963 by Eli Lilly [6].
Table 1.
ALM = alimentary tract & metabolism; AIN = anti-infective; BBO = blood & blood forming organs; CAR = cardiovascular system; DER = dermatological; END = endocrine system; GUS = genito-urinary & sex hormones; MSK = musculo-skeletal system; ONC = oncological; RES = respiratory system; SEN = sensory organs.
SYNTHESIS OF SULFONAMIDES
One of the most conventional routes to sulfonamide involves the direct N-S bond formation via an addition-elimination process (Fig. 2). Sulfonyl chloride is a common substrate in this type of reactions, which reacts with aryl or alkyl amines to afford the corresponding sulfonamide in large scale [9].
Fig. 2.
Conventional routes to sulfonamide
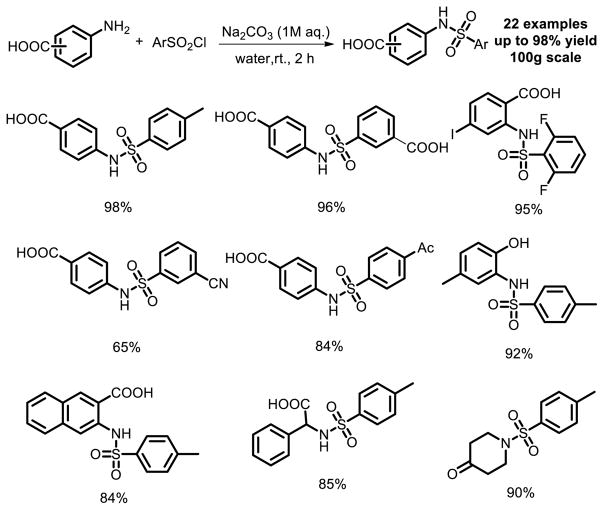
To date, such an approach is a general method for the preparation of sulfonamide in pharmaceutical industry which still needs further improvement. In 2006, a facile sulfonamide synthesis in water under pH control was reported by Deng and co-workers [10]. The desired sulfonamide was afforded in up to 98% yield and with greater than 95% purity by simply acidifying the solution with concentrated HCl to pH=2.0 and collecting precipitated product after the reaction. Furthermore, the reaction was easily scalable to 100 grams. This method is also suitable for various amino compounds and arylsulfonyl chlorides (Fig. 3).
Fig. 3.
Environmentally benign sulfonamide synthesis in water.
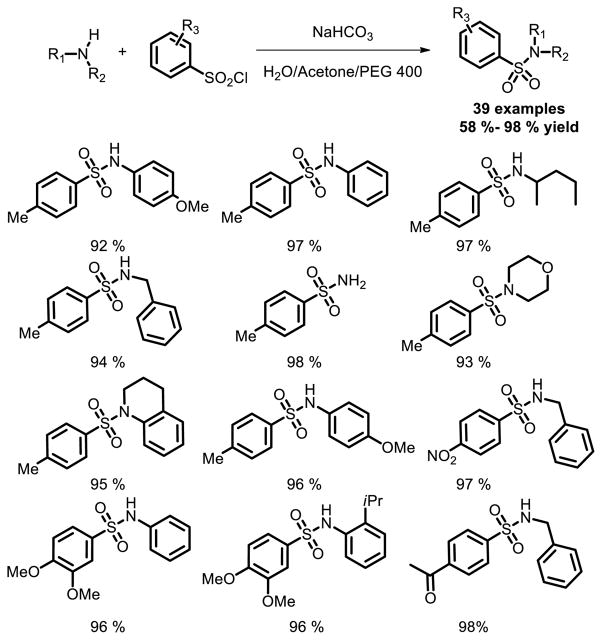
Flow chemistry has rapidly turned into one of the techniques that may revolutionize the synthetic sector of drug discovery in recent decades. In 2013, Gioiello and coworkers reported their work on the synthesis of sulfonamides by employing flow-based chemistry [11]. This work could therefore support medicinal chemistry program at various stages as the hit-to-lead, lead-optimization and lead candidate scale-up (Fig. 4).
Fig. 4.
Sulfonamide synthesis involving flow chemistry.
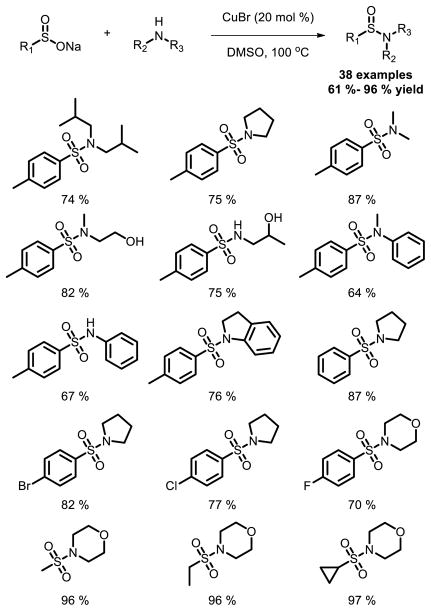
Although sulfonyl chloride is often utilized in the preparation, the difficulties associated with sulfonamide synthesis stem not from the amination reaction, but rather from the preparation of sulfonyl chlorides. As an alternative, a new and convenient method for the construction of sulfonamides via a copper-catalyzed oxidative coupling between sodium sulfinates and amines with O2 (1 atm) or DMSO as the oxidant was described by Jiang et al [12] (Fig. 5).
Fig. 5.
Cu catalyzed sulfonamide synthesis between sodium sulfinates and amines
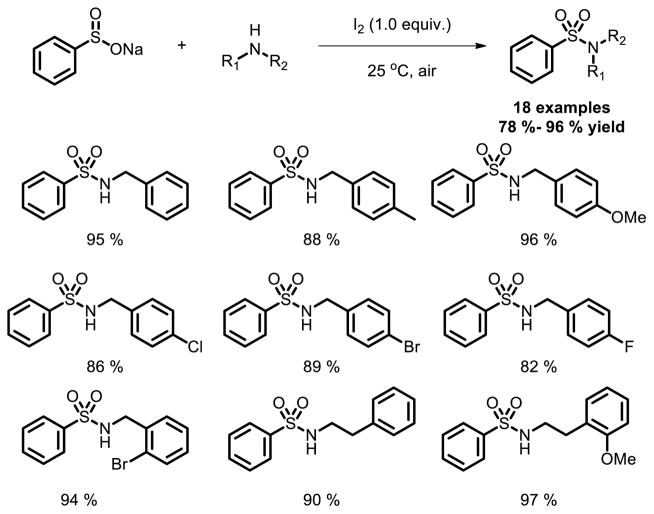
Very recently, another simple protocol has been developed for the synthesis of sulfonamides from sodium sulfinates and various amines through an iodine-mediated S-N bond formation reaction at room temperature [13]. This metal-free methodology showed broad functional group tolerance, which provides a practical, cost-effective, efficient and green approach to various sulfonamides. More than 40 sulfonamides were synthesized by this method. Mild conditions and wide substrate scope make it possible to be utilized in the pharmaceutical industry (Fig. 6).
Fig. 6.
Sulfonamide synthesis via a metal free oxidative coupling.
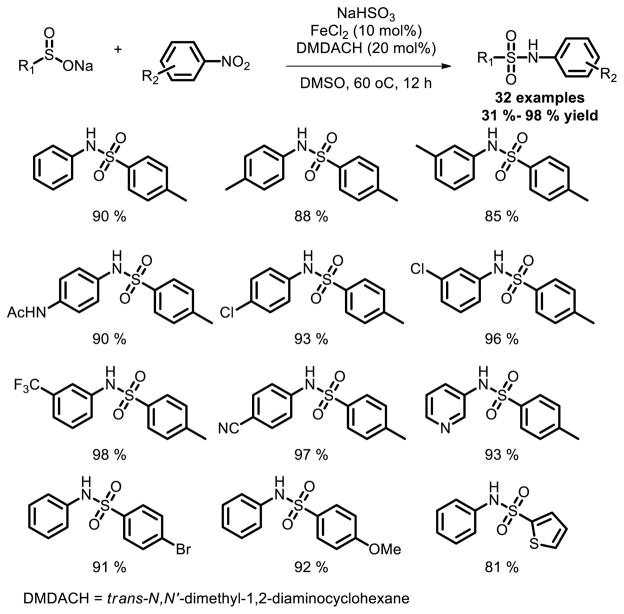
Luo et al. reported their FeCl2-catalyzed system for the construction of sulfonamide directly from nitroarenes and sodium arylsulfinates under mild conditions [14]. It’s noteworthy that they use nitroarenes instead of conventional aromatic amines as nitrogen source. In terms of substrate scope, nitroarenes either bearing electron-donating groups, such as methyl, methoxy, amido, or electron-withdrawing groups, such as chloro, cyano, trifluoromethyl, carbonyl, ester and carboxylic acid generated the desired products in good to excellent yields (Fig. 7).
Fig. 7.
Fe catalyzed sulfonamide synthesis using nitroarenes as the nitrogen sources.
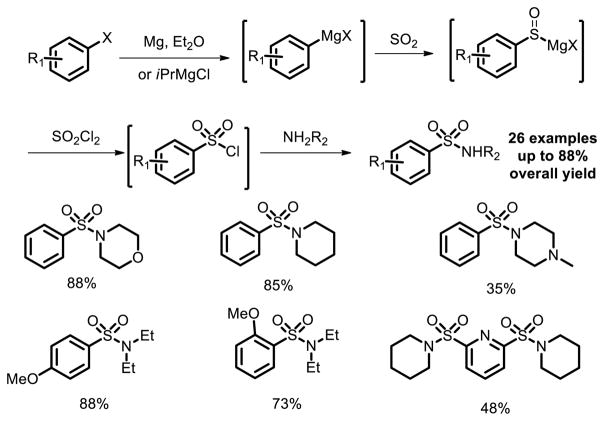
Among the methods for introducing sulfonyl building blocks, fixation of sulfur dioxide into small molecules is promising and attractive. The methods have been extensively applied in the construction of some drugs [15]. In 2003, Barrett reported an one-pot procedure for the preparation of aryl- and heteroaryl substituted sulfonamides from commercially available and inexpensive aryl and heteroaryl bromides and iodides [16]. Sulfur dioxide was utilized in the reaction as an electrophilic reagent to react with Grignard reagents to introduce sulfur atom. After quenching with SO2Cl2, the sulfonyl chloride was formed in situ, which was then attacked by the amine to give the corresponding sulfonamides in moderate to good yields (Fig. 8).
Fig. 8.
Sulfonamide synthesis using SO2 fixation protocol.
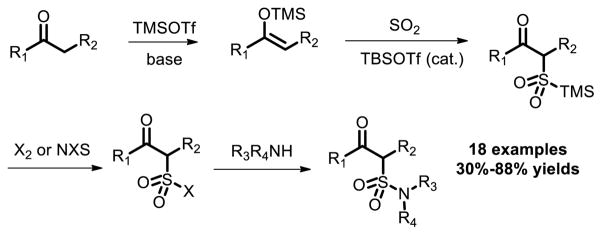
In 2004, Vogel and co-workers found that silyl enol ethers derived from acylic and cyclic ketones underwent ene reaction with SO2 in the presence of Lewis acid with the generation of the corresponding silyl β-oxoalkanesulfinates, which were brominated (Br2 or NBS) or chlorinated (NCS or Cl2) to produce the corresponding sulfonyl halides [17]. The sulfonyl halides subsequently reacted with primary and secondary amines to give the corresponding sulfonamides (Fig. 9).
Fig. 9.
Sulfur dioxide mediated one-pot, three- and four-component syntheses of polyfunctional sulfonamides.
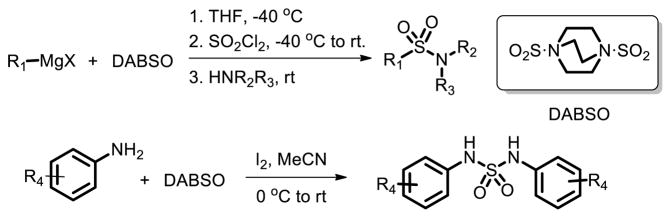
The difficulties associated with the handling and use of a toxic gaseous reagent limit the application of SO2 in organic synthesis. To deal with this problem, DABCO-bis(sulfur dioxide), DABSO, which is a bench-stable colorless solid, was utilized to instead of SO2 gas [18]. Willis et al. demonstrated that DABSO, can be used as an effective replacement for gaseous sulfur dioxide in a number of established processes, leading the synthesis of sulfonamides (Fig. 10).
Fig. 10.
DABSO used instead of SO2 in sulfonamide synthesis.
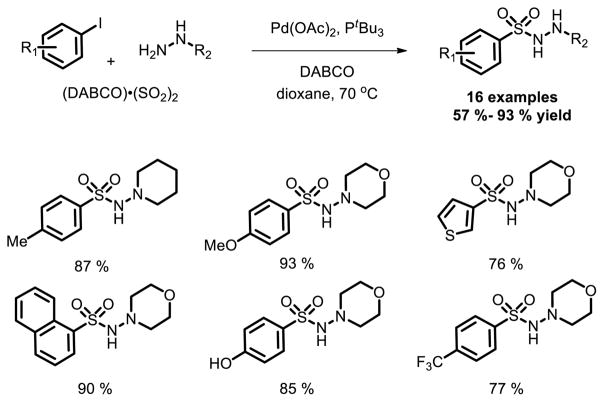
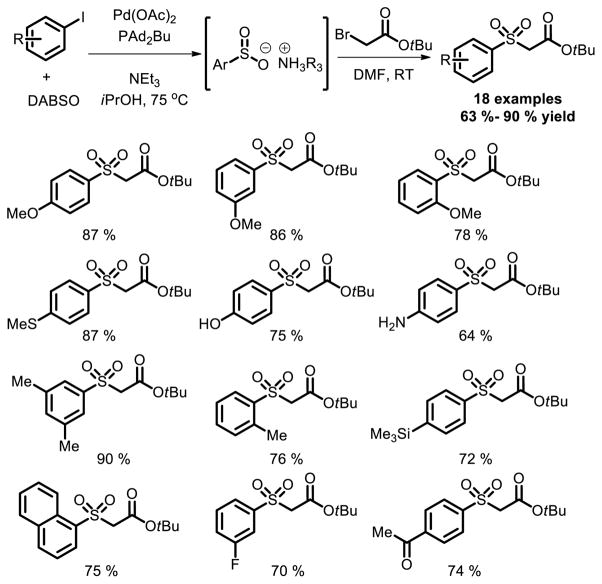
Furthermore, Willis and co-workers demonstrated the feasibility to prepare C-SO2-N linkage using a palladium-catalyzed aminosulfonylation process for the first time [19]. The utilization of DABSO was the key to the success of the reaction. Efficient aminosulfonylation reactions between a range of aryl iodides and N,N-dialkylhydrazines, providing aryl N-aminosulfonamides in good to excellent yields was achieved (Fig. 11).
Fig. 11.
Pd catalyzed aminosulfonylation of aryl halides using DABSO as sulfur source.
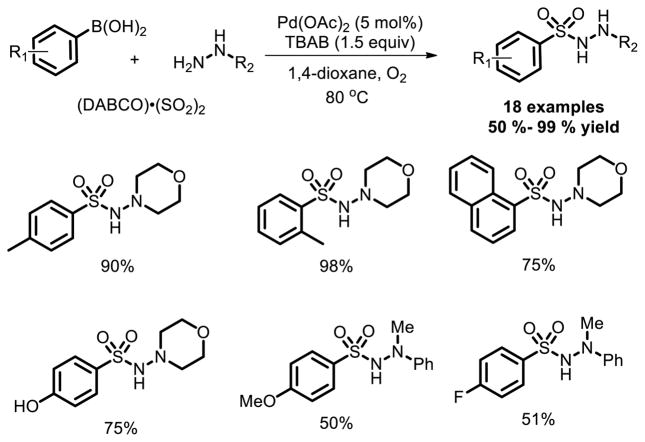
In the last five years, fixation of sulfur dioxide into small molecules was well investigated as an effective approach to prepare N-aminosulfonamides and sulfones. An efficient route to aryl N-aminosulfonamides via a palladium-catalyzed three-component coupling of arylboronic acids, DABSO and hydrazines in the presence of oxygen (1 atm) was reported by Wu and co-workers in 2012 [20]. Various sensitive functional groups were compatible under the reaction conditions (Fig. 12).
Fig. 12.
Pd catalyzed three-component coupling of arylboronic acids, DABSO and hydrazines.
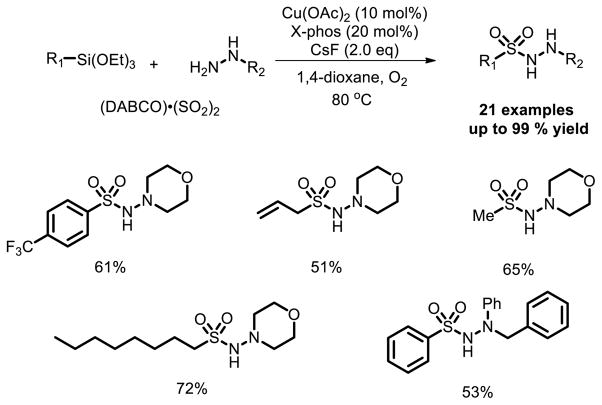
A three-component reaction of triethoxysilanes, DABSO, and hydrazines catalyzed by copper(II) acetate was reported by Wang et al. [21], leading to N-aminosulfonamides in good yields. This is the first example of using a copper catalyzed aminosulfonylation process through the insertion of sulfur dioxide. Aryl-, alkenyl- and alkyl groups can be incorporated into the final products (Fig. 13).
Fig. 13.
Cu-catalyzed three-component reaction of triethoxysilanes, sulfur dioxide, and hydrazines.
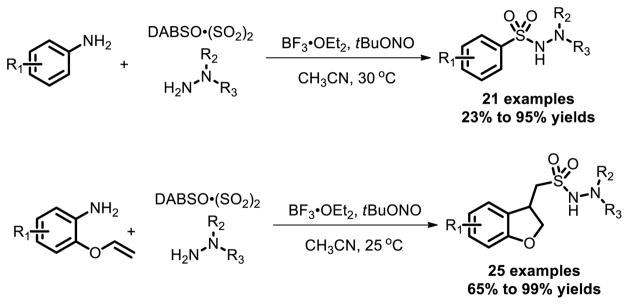
A metal free coupling of aromatic and heteroaromatic amines with DABSO and hydrazines, leading to aryl N-aminosulfonamides in good to excellent yields, was reported by Wu et al. in 2014 [22]. Different functional groups including ester, hydroxyl, chloro and trifluoromethyl groups are compatible under these conditions. Subsequently, they disclosed that when 2-(allyloxy)anilines were used instead of amines, a cascade reaction was triggered to give the 1-(2,3-dihydrobenzofuran-3-yl)-methanesulfonohydrazides in good yields [23]. This cascade intramolecular 5-exo-cyclization and insertion of SO2 reaction was a radical process (Fig. 14).
Fig. 14.
Functionalized N-aminosulfonamides synthesis via free radical reactions.
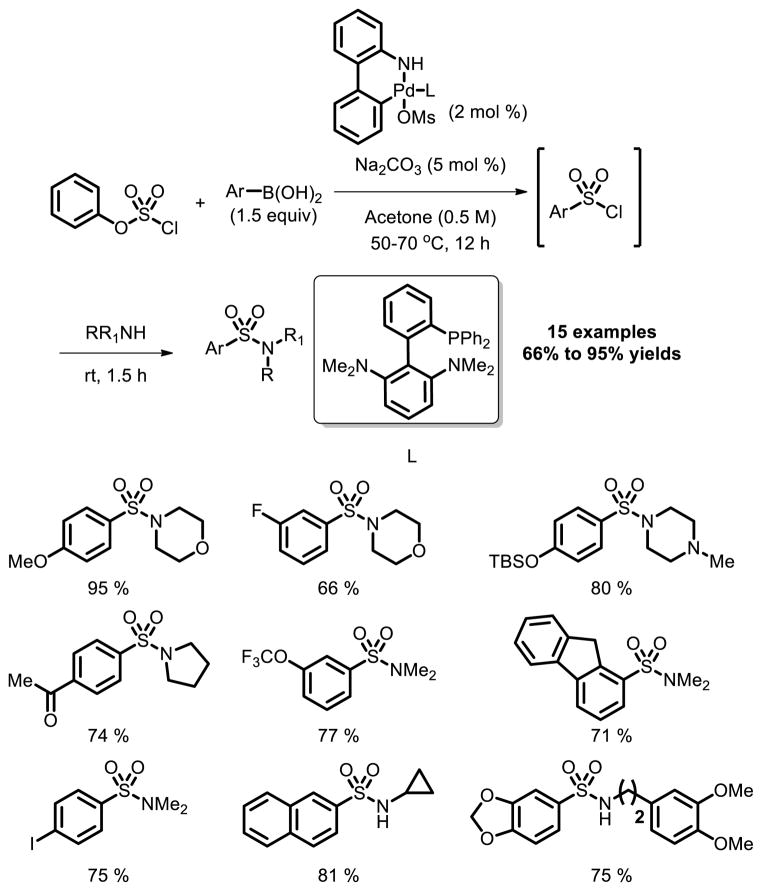
While these examples represent an important achievement in sulfonylation chemistry, amine nucleophiles remain incompatible with these types of couplings. To address this limitation, a new protocol was developed by Buchwald and co-workers [24]. They demonstrated that phenyl chlorosulfate represents an excellent [SO2Cl]+ synthon in the context of Pd catalyzed Suzuki Miyaura cross-coupling, which provided different kinds of sulfonamides in good to excellent yields.
THIOETHER CONTAINING DRUGS
Thioethers represents (8.8%) the third most exemplified constituent of the sulfur containing drugs [8]. The representative pharmaceuticals including cimetidine, thiethylperazine, pergolide etc are summarized in Table 2.
Table 2.
ALM = alimentary tract & metabolism; AIN = anti-infective; BBO = blood & blood forming organs; CAR = cardiovascular system; DER = dermatological; END = endocrine system; GUS = genito-urinary & sex hormones; MSK = musculo-skeletal system; ONC = oncological; RES = respiratory system; SEN = sensory organs.
SYNTHESIS OF THIOETHERS
Traditional methods for the formation of C-S bond often take place in polar solvents, such as HMPA, and at elevated temperatures [25]. In 1978, Migita and co-workers first reported a Pd(0) catalyzed cross-coupling reactions of aryl iodide/bromide with thiols to give the corresponding thioethers [26]. In this pioneering work, thiophenol and thiols were both transferred to the thioether smoothly (Fig. 16).
Fig. 16.
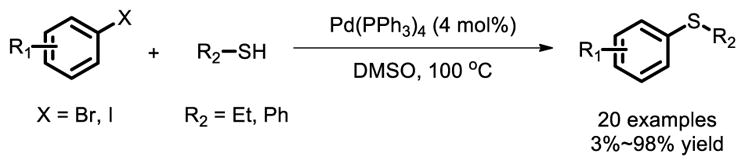
Synthesis of aryl thioethers via Pd catalyzed Ullman coupling.
In the next three decades, this type of transformation was well studied by organic chemists. A remarkable work was reported by Hartwig and co-workers in 2006 [27]. Using a dative ligand. they developed a general, highly efficient and functional-group-tolerant catalyst system for the coupling of aryl halides and triflates with thiols that typically occur with turnover numbers (TONs) that are 2 or 3 orders of magnitude higher than those of related couplings by previous catalysts (Fig. 17).
Fig. 17.
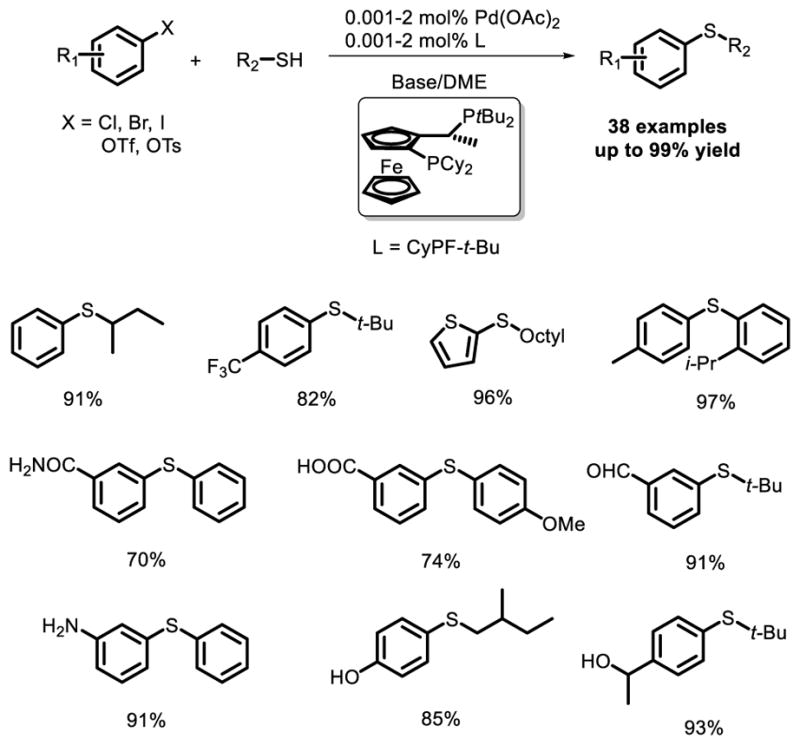
Long-lived catalyst for the Pd-catalyzed coupling of aryl halides with thiols.
A Cu-catalyzed efficient procedure for the synthesis of diaryl sulfides has been described by Zhou and co-workers [28]. The aryl thiocyanate was formed in the first step, followed by hydrolysis to a thiolate ion, which reacted with aryl halide to afford the aryl thioether (Fig. 18).
Fig. 18.
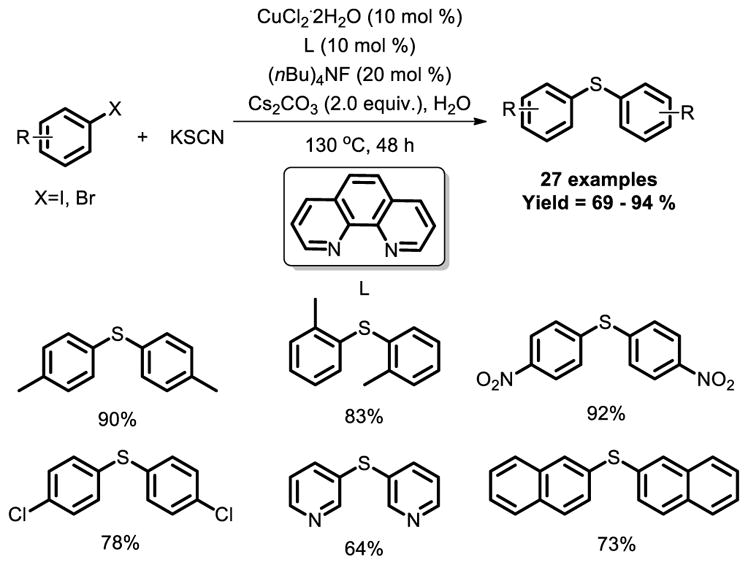
Copper-catalyzed carbon-sulfur bond formation protocol in water.
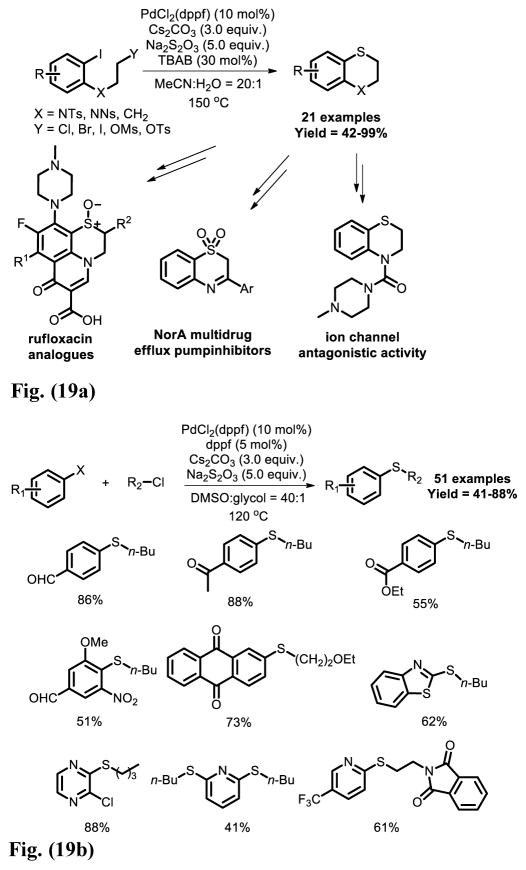
In 2013, Jiang and co-workers developed a Pd-catalyzed double carbon-sulfur bond formation using sodium thiosulfate as sulfurating reagent [29] (Fig. 19a). This method provides an efficient way for the synthesis of substituted 1,4-benzothiazine derivates which are structural elements to a variety of pharmaceutically active compounds and natural products. In their subsequent work, the intermolecular sulfur atom transfer between aryl halide and alkyl thiosulfate was achieved to give a variety of thiosulfate [30] (Fig. 19b).
Fig. 19.
Fig. 19a. Intramolecular direct cross-coupling using Na2S2O3 as a sulfurating reagent.
Fig. 19b. Intermolecular direct cross-coupling access to diverse aromatic sulfides using Na2S2O3 as a sulfurating reagent.
A highly efficient Cu-catalyzed dual C S bonds formation reaction, proceeding in alcohol and water under air, was reported by Jiang et al [31], in which inodorous stable Na2S2O3 is used as a sulfurating reagent (Fig. 20a). As a novel sulfur source, Na2S2O3 shows its irreplaceability in this system. In contrast with organic thiols and thiophenols, its intrinsic properties helped us to accomplish this S atom transfer reaction in a highly efficient manner. Further study indicated that this transformation could take place between 1-aryltriazenes, Na2S2O3 and alkyl halides at room temperature, in which water functioned as a green solvent [32] (Fig. 20b).
Fig. 20.
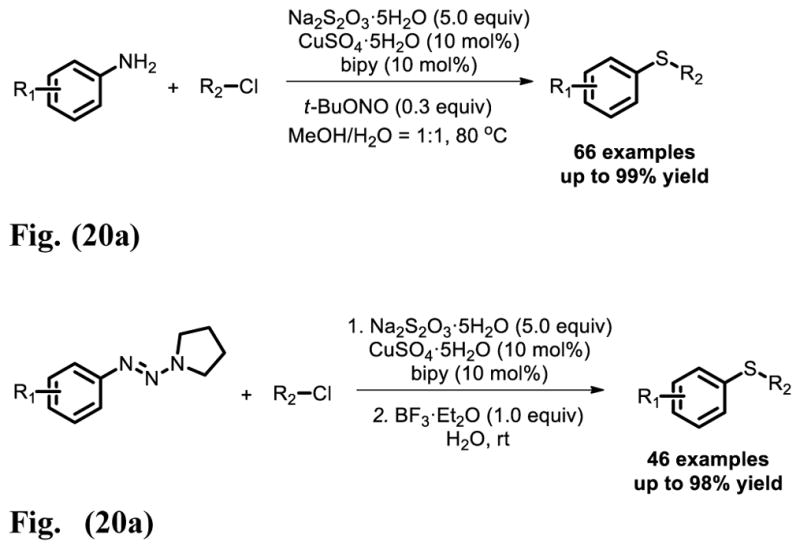
Fig. 20a. Cu-Catalyzed S-transfer reaction: from amines to sulfides.
Fig. 20b. Cu-Catalyzed S-transfer reaction: from 1-aryltriazenes to sulfides.
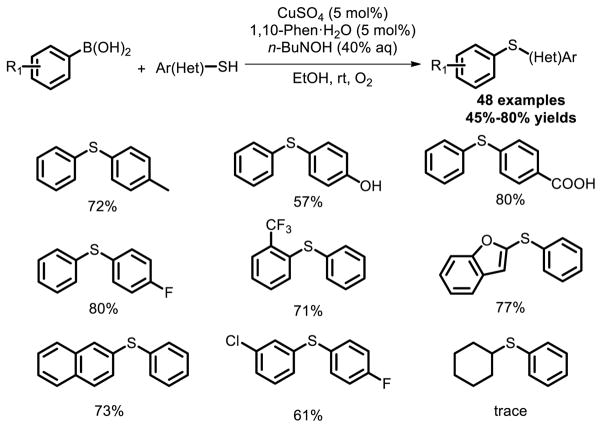
Another novel and highly efficient Cu catalyzed protocol for S-arylation reaction of thiols is through oxidative coupling with aryl and heteroaryl boronic acids at room temperature [33]. A wide variety of thiols and arylboronic acids had been screened, and most of the substrates afforded the desired S-arylation products in good to excellent yields under mild conditions (Fig. 21).
Fig. 21.
Chan Lam-type S-arylation of thiols with boronic acids.
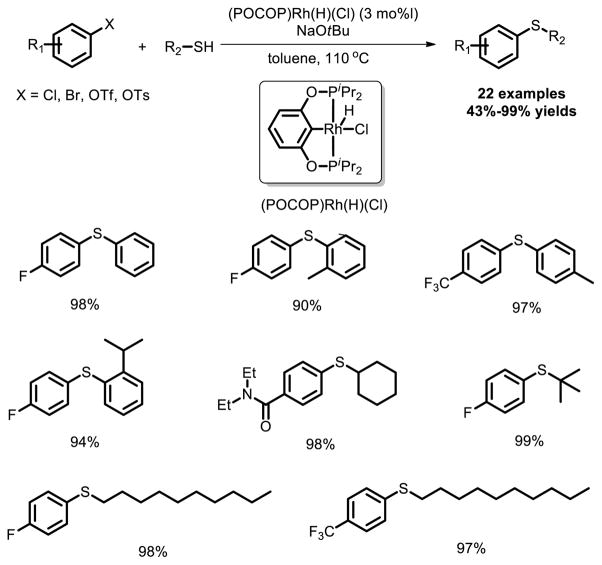
Recently, a well-defined (POCOP)Rh system for the catalytic coupling of aryl bromides and chlorides with aryl and alkyl thiols was reported by Ozerov et al [34]. (POCOP)Rh(H)(Cl) was demonstrated to be an active precatalyst for the coupling of aryl chlorides and bromides with aryl and alkyl thiols under reasonable conditions (Fig. 22).
Fig. 22.
(POCOP)Rh catalyst for the coupling of aryl halides with thiols.
SULFONE CONTAINING DRUGS
Sulfones are found in numerous medicines and drug candidates under development for the treatment of a host of diseases impacting human health worldwide (Table 3). For example, Diazoxide is a potassium channel activator, which causes local relaxation in smooth muscle by increasing membrane permeability to potassium ions. This switches off voltage-gated calcium ion channels, preventing calcium flux across the sarcolemma and activation of the contractile apparatus.
Table 3.
ALM = alimentary tract & metabolism; AIN = anti-infective; BBO = blood & blood forming organs; CAR = cardiovascular system; DER = dermatological; END = endocrine system; GUS = genito-urinary & sex hormones; MSK = musculo-skeletal system; ONC = oncological; RES = respiratory system; SEN = sensory organs.
Diazoxide is often used as a vasodilator in the treatment of acute hypertension or malignant hypertension [35].
SYNTHESIS OF SULFONES
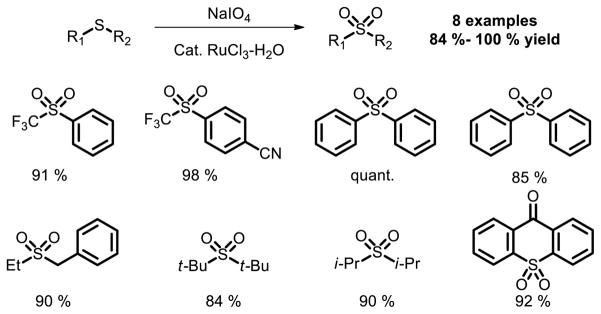
Sulfone is most commonly prepared by the oxidation of a precursor sulfide [36], which is usually obtained from a conventional nucleophile electrophile combination. However, the use of an oxidative route precludes the presence of oxidation-sensitive functional groups, and the corresponding sulfide precursors often require the use of foul-smelling thiols for their preparation (Fig. 23).
Fig. 23.
Ru catalyzed oxidation of thioethers to sulfones.
Since a breakthrough was made by Willis for the generation of aryl N-aminosulfonamides using bench-stable reliable DABSO as the S source, the rapid development for the insertion of sulfur dioxide into small molecules has been reported [37]. Willis and co-workers developed a simple and efficient process for the palladium-catalyzed conversion of aryl and heteroaryl halides into the corresponding ammonium sulfinates, which can be converted into sulfones smoothly (Fig. 24).
Fig. 24.
Pd catalyzed synthesis of ammonium sulfinates from aryl halides and DABSO.
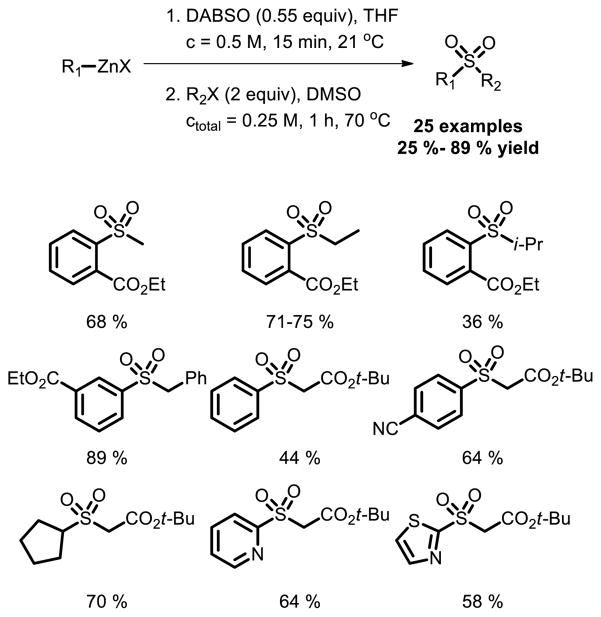
Rocke and co-workers reported that organozinc reagents reacted with DABSO and the resulting zinc sulfinate salts were alkylated in situ to afford sulfones [38]. This transformation has a broad scope and is compatible with a wide range of functional groups including nitrile, secondary carbamates, and nitrogen-containing heterocycles (Fig. 25).
Fig. 25.
Synthesis of sulfones from organozinc reagents, DABSO, and alkyl halides.
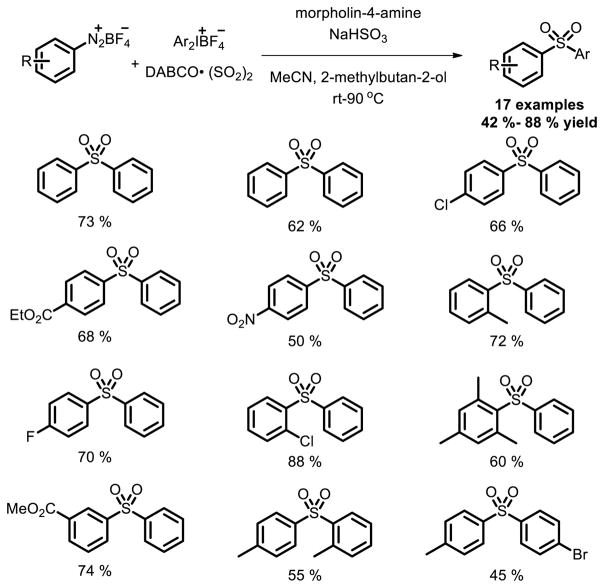
Wu et al. described a useful method for synthesis sulfones via a reaction of aryldiazonium tetrafluoroborates, DABSO and aryliodonium tetrafluoroborates under metal-free conditions [39]. N-aminosulfonamide was the intermediate in this transformation. It’s noteworthy that the reaction was carried out under mild conditions which enabled the protocol to be attractive for further construction of sulfones containing drugs (Fig. 26).
Fig. 26.
Synthesis of sulfones from aryldiazonium tetrafluoroborates, DABSO, and aryliodonium tetrafluoro-borates.
PENICILLINS AND CORRESPONDING DRUGS
Penicillins, the well-known antibiotics containing sulfur atom, play a significant role in fighting against syphilis, or infections caused by staphylococci and streptococci. Though certain kinds of bacteria are resistant to Penicillins, they are still the popular antibiotics in the treatment of bacterial infections caused by susceptible, usually Gram-positive, organisms [40].
SYNTHESIS OF PENICILLINS
Penicillin is a secondary metabolite of certain species of Penicillium and is produced when growth of the fungus is inhibited by stress. It is not produced during active growth. The mass production of penicillins is mainly based on biosynthesis and semisynthesis. The first chemical synthesis of penicillin was accomplished by John C. Sheehan in 1957 [41]. Although the initial synthesis developed by Sheehan was not appropriate for mass production of penicillins, one of the intermediate compounds in their synthesis 6-aminopenicillanic acid (6-APA), was the nucleus of penicillin. It can be obtained from the fermentation brew of the Penicillium mold and used as the main building block for the preparation of numerous semisynthetic penicillins.
In addition, chemists have still taken efforts to develop new chemical methods to achieve the core structure of penicillins. In the 1970s, Maki et al. reported intramolecular radical cyclization to give penicillin derivatives [42]. Upon irradiation in acetonitrile, β-lactam led to 3-methylenecepham methyl ester and 3-methyl-2-cephem methyl ester. The dilution proved to have a dramatic effect on the course of this cyclization process, as β- and α-(benzothiazoylthiomethyl)penam derivatives were formed preferentially at higher concentration (Fig. 27).
Fig. 27.
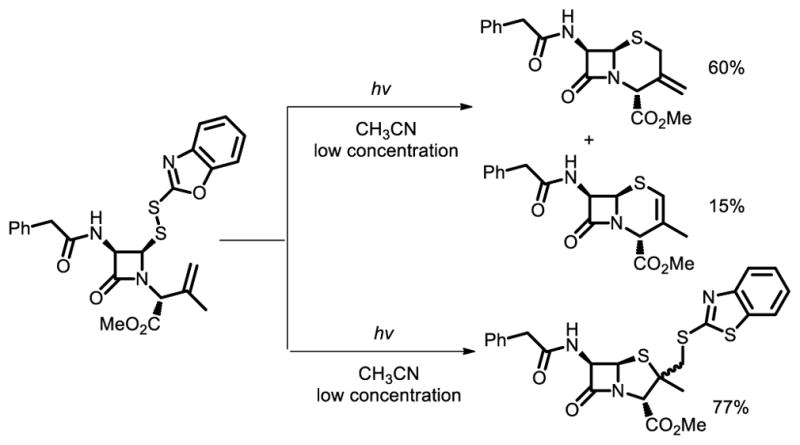
Synthesis of penicillin derivatives via light promoted radical cyclization.
Cabri and co-workers have extensively studied the transition metal-mediated version of this kind of radical process [43]. Initially, they found that both Fe(III) and Mn(III) could promote this reaction and later developed catalytic versions, using the same metals (Fig. 28a). They have also developed catalytic Fe(III) Cu(II) and Mn(III) Cu(II) variants, which furnished α-methyl-substituted penicillin in a highly stereoselective manner (Fig. 28b).
Fig. 28.
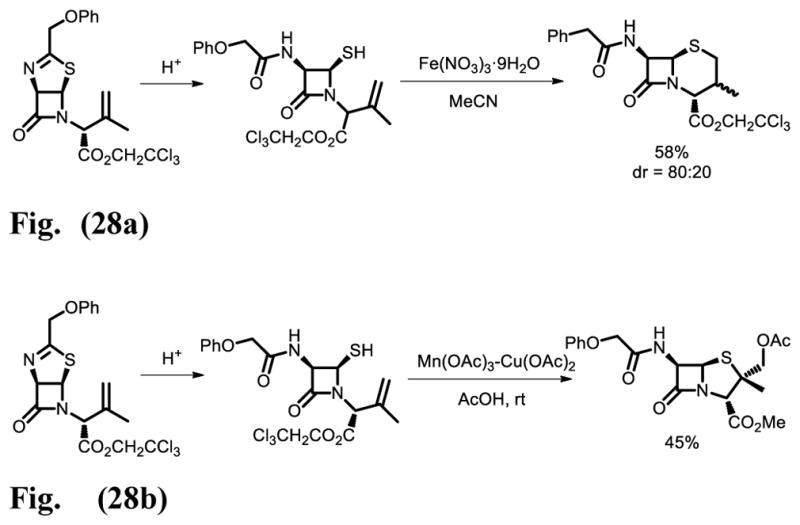
Fig. 28a. Synthesis of penicillin derivatives via Fe(III) promoted radical cyclization.
Fig. 28b. Synthesis of penicillin derivatives via Mn(III) Cu(II) promoted radical cyclization.
A concise and flexible route to bicyclic sulfur-based β-lactam was reported by Hales and co-workers in 1997 [44]. azomethine ylide strategy for assembling bicyclic β-lactams constitutes a simple, versatile, and above all, direct synthesis of penams and penems. This chemistry permits access to a range of penicillins, some of which are difficult to prepare by conventional methods (Fig. 29).
Fig. 29.
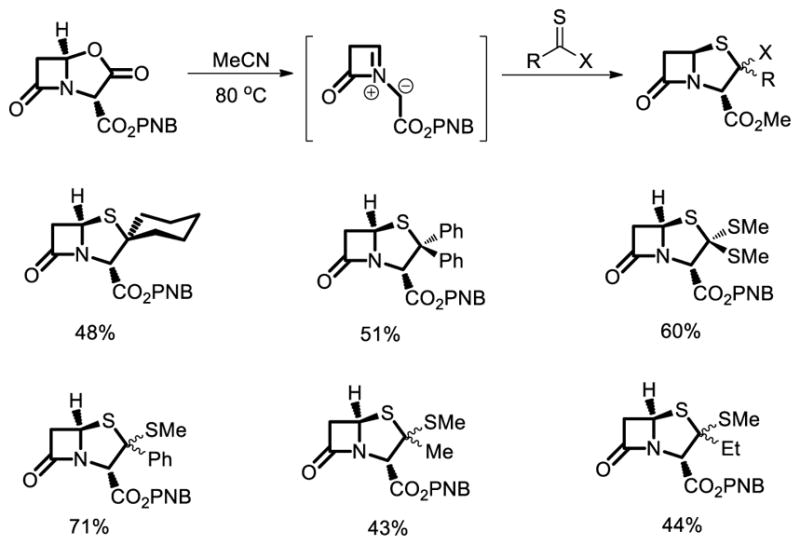
A direct approach to penams via azomethine ylide.
A rigid framework with stereochemistry different than that of penicillin, is designed to be a suitable scaffold for the development of compounds inhibiting pilus formation in uropathogenic Escherichia coli was synthesized by Almqvist and co-workers [45]. The obtained compounds will be evaluated as chaperone inhibitors in the near future. The excellent and reproducible yields in the synthesis of the β-lactam and the convenience with which Meldrum’s acid derivatives are prepared constitute a platform for future development of statistically diverse libraries of optically active β-lactams.
CONCLUSION
In summary, the most common sulfur containing drugs and recent advances in the synthesis of the core scaffolds, including sulfonamides, thioethers, sulfones and penicillins are presented. Other sulfur containing moieties such as thiophenes and thiazoles can be found in pharmaceutical molecules as well. Although many novel protocols have been developed, some challenges still remain. More general methods and process to synthesize sulfur containing pharmaceutical molecules are still urgent to be developed.
Fig. 15.
Synthesis of aryl sulfonamides via Pd catalyzed chlorosulfonylation of arylboronic acids.
Fig. 30.
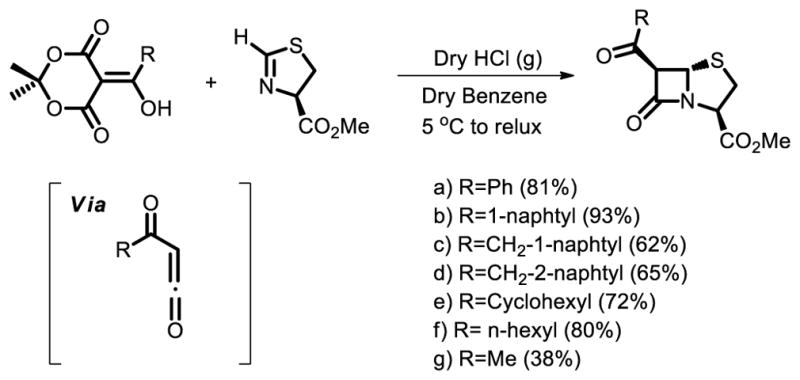
A stereoselective synthesis of optically active β-lactams from Meldrum’s acid derivatives.
Table 4.
ALM = alimentary tract & metabolism; AIN = anti-infective; BBO = blood & blood forming organs; CAR = cardiovascular system; DER = dermatological; END = endocrine system; GUS = genito-urinary & sex hormones; MSK = musculo-skeletal system; ONC = oncological; RES = respiratory system; SEN = sensory organs.
Acknowledgments
Financial support was provided by NBRPC (973 Program, 2015CB856600), NSFC (21472050, 21272075), NCET (120178), DFMEC (20130076110023), Fok Ying Tung Education Foundation (141011), the program for Shanghai Rising Star (15QA1401800), Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, and the Changjiang Scholar and Innovative Research Team in University.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.a) Bernardi F, Csizmadia IG, Mangini A. In: Organic Sulfur Chemistry. Theoretical and Experimental Advances. Bernardi F, Csizmadia IG, Mangini A, editors. Elsevier; Amsterdam, The Netherlands: 1985. [Google Scholar]; b) Block E. Reactions of Organosulfur Compounds. Academic Press; New York: 1978. [Google Scholar]; c) Patai S, Rappoport Z. The Chemistry of Organic Selenium and Tellurium Compounds. John Wiley & Sons; New York: 1986–1987. [Google Scholar]; d) McReynolds MD, Dougherty JM, Hanson PR. Synthesis of phosphorus and sulfur heterocycles via ring-closing olefin metathesis. Chem Rev. 2004;104:2239–2258. doi: 10.1021/cr020109k. [DOI] [PubMed] [Google Scholar]; e) Ager DJ. Silicon-containing carbonyl equivalents. Chem Soc Rev. 1982;11:493. [Google Scholar]
- 2.a) Guo H, Sun B, Gao H, Chen X, Liu S, Yao X, Liu X, Che Y. Diketopiperazines from the cordyceps-colonizing fungus Epicoccum nigrum. J Nat Prod. 2009;72:2115–2119. doi: 10.1021/np900654a. [DOI] [PubMed] [Google Scholar]; b) Wang J-M, Ding G-Z, Fang L, Dai J-G, Yu S-S, Wang Y-H, Chen X-G, Ma S-G, Qu J, Xu S, Du D. Thiodiketopiperazines produced by the endophytic fungus Epicoccum nigrum. J Nat Prod. 2010;73:1240–1249. doi: 10.1021/np1000895. [DOI] [PubMed] [Google Scholar]
- 3.Piscitelli SC, Goss TF, Wilton JH, D’Andrea DT, Goldstein H, Schentag JJ. Effects of ranitidine and sucralfate on ketoconazole bioavailability. Antimicrob Agents Chemother. 1991;35:1765–1771. doi: 10.1128/aac.35.9.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thase ME, MacFadden W, Weisler RH, Chang W, Paulsson BR, Khan A, Calabrese JR, Bolder G. Efficacy of quetiapine monotherapy in bipolar I and II depression: A double-blind, placebo-controlled study (The BOLDER II Study) Journal of Clinical Psychopharmacology. 2006;26:600–609. doi: 10.1097/01.jcp.0000248603.76231.b7. [DOI] [PubMed] [Google Scholar]
- 5.Lesch JE. The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine. Oxford University Press; 2007. [Google Scholar]
- 6.Sittig M. Pharmaceutical Manufacturing Encyclopedia. Park Ridge, Noyes Publications; N.J., U.S.A: 1988. p. 1756. [Google Scholar]
- 7.For a list of top drugs by year, see: [Accessed on April 24, 2015]; http://cbc.arizona.edu/njardarson/group/toppharmaceuticalsposter.
- 8.Ilardi EA, Vitaku E, Njardarson JT. Data-mining for sulfur and fluorine: an evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J Med Chem. 2014;57:2832–2842. doi: 10.1021/jm401375q. [DOI] [PubMed] [Google Scholar]
- 9.Anderson KK. In: Sulfonic Acids and Their Derivatives in Comprehensive Organic Chemistry. Barton DHR, Ollis WD, Jones DN, editors. Vol. 3. Pergamon Press; Oxford: 1979. pp. 331–350. [Google Scholar]
- 10.Deng X, Mani NS. A facile, environmentally benign sulfonamide synthesis in water. Green Chem. 2006;8:835–838. [Google Scholar]
- 11.Gioiello A, Rosatelli E, Teofrasti M, Filipponi P, Pellicciari R. Building a sulfonamide library by eco-friendly flow synthesis. ACS Comb Sci. 2013;15:235–239. doi: 10.1021/co400012m. [DOI] [PubMed] [Google Scholar]
- 12.Tang X, Huang L, Qi C, Wu X, Wu W, Jiang H. Copper-catalyzed sulfonamides formation from sodium sulfinates and amines. Chem Commun. 2013;49:6102–6104. doi: 10.1039/c3cc41249k. [DOI] [PubMed] [Google Scholar]
- 13.Wei W, Liu C, Yang D, Wen J, You j, Wang H. Metal-free direct construction of sulfonamides via iodine-mediated coupling reaction of sodium sulfinates and amines at room temperature. Adv Synth Catal. 2015;357:987–992. [Google Scholar]
- 14.Zhang W, Xie J, Rao B, Luo M. Iron-catalyzed N arylsulfonamide formation through directly using nitroarenes as nitrogen sources. J Org Chem. 2015;80:3504–3511. doi: 10.1021/acs.joc.5b00130. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, Fan C, Wu J. Fixation of sulfur dioxide into small molecules. Org Biomol Chem. 2015;13:1592–1599. doi: 10.1039/c4ob02139h. [DOI] [PubMed] [Google Scholar]
- 16.Pandya R, Murashima T, Tedeschi L, Barrett AGM. Facile one-pot synthesis of aromatic and heteroaromatic sulfonamides. J Org Chem. 2003;68:8274–8276. doi: 10.1021/jo034643j. [DOI] [PubMed] [Google Scholar]
- 17.Bouchez LC, Dubbaka SR, Turks M, Vogel P. Sulfur dioxide mediated one-pot, three- and four-component syntheses of polyfunctional sulfonamides and sulfonic esters: study of the stereoselectivity of the ene reaction of sulfur dioxide. J Org Chem. 2004;69:6413–6418. doi: 10.1021/jo049047j. [DOI] [PubMed] [Google Scholar]
- 18.Woolven H, Gonzalez-Rodriguez C, Marco I, Thompson AL, Willis MC. DABCO-bis(sulfur dioxide), DABSO, as a convenient source of sulfur dioxide for organic synthesis: utility in sulfonamide and sulfamide preparation. Org Lett. 2011;13:4876–4878. doi: 10.1021/ol201957n. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen B, Emmett EJ, Willis MC. Palladium-catalyzed ami-nosulfonylation of aryl halides. J Am Chem Soc. 2010;132:16372–16373. doi: 10.1021/ja1081124. [DOI] [PubMed] [Google Scholar]
- 20.Ye S, Wu J. A palladium-catalyzed three-component coupling of arylboronic acids, sulfur dioxide and hydrazines. Chem Commun. 2012;48:7753–7755. doi: 10.1039/c2cc33725h. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Xue L, Wang Z. A copper-catalyzed three-component reaction of triethoxysilanes, sulfur dioxide, and hydrazines. Org Lett. 2014;16:4056–4058. doi: 10.1021/ol5018849. [DOI] [PubMed] [Google Scholar]
- 22.Zheng D, Li Y, An Y, Wu J. Aminosulfonylation of aromatic amines, sulfur dioxide and hydrazines. Chem Commun. 2014;50:8886–8888. doi: 10.1039/c4cc03032j. [DOI] [PubMed] [Google Scholar]
- 23.An Y, Zheng D, Wu J. Synthesis of 1-(2,3-dihydrobenzofuran-3-yl)-methanesulfonohydrazides through insertion of sulfur dioxide. Chem Commun. 2014;50:11746–11748. doi: 10.1039/c4cc05401f. [DOI] [PubMed] [Google Scholar]
- 24.Debergh JR, Niljianskul N, Buchwald SL. Synthesis of aryl su-lfonamides via palladium-catalyzed chlorosulfonylation of arylboronic acids. J Am Chem Soc. 2013;135:10638–10641. doi: 10.1021/ja405949a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindley J. Copper assisted nucleophilic substitution of arylhalog-en. Tetrahedron. 1984;40:1433–1456. [Google Scholar]
- 26.Kosugi M, Shimizu T, Migita T. Reactions of aryl halides with thiolate anions in the presence of catalytic amounts of tetrakis(triphenylphosphine)palladium preparation of aryl sulfides. Chem Lett. 1978:13–14. [Google Scholar]
- 27.Fernandez-Rodriguez MA, Shen Q, Hartwig JF. A General and long-lived catalyst for the palladium-catalyzed Coupling of aryl halides with thiols. J Am Chem Soc. 2006;128:2180–2181. doi: 10.1021/ja0580340. [DOI] [PubMed] [Google Scholar]
- 28.Ke F, Qu F, Jiang Z, Li Z, Wu D, Zhou X. An efficient copper-catalyzed carbon-sulfur bond formation protocol in water. Org Lett. 2011;13:454–457. doi: 10.1021/ol102784c. [DOI] [PubMed] [Google Scholar]
- 29.Qiao Z, Liu H, Xiao X, Fu Y, Li Y. Efficient access to 1, 4-benzothiazine: palladium-catalyzed double C-S bond formation using Na2S2O3 as sulfurating reagent. Org Lett. 2013;15:2594–2597. doi: 10.1021/ol400618k. [DOI] [PubMed] [Google Scholar]
- 30.Qiao Z, Wei J, Jiang X. Direct cross-coupling access to diverse aromatic sulfide: palladium catalyzed double C S bond construction using Na2S2O3 as a sulfurating reagent. Org Lett. 2014;16:1212–1215. doi: 10.1021/ol500112y. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Pu J, Jiang X. A highly efficient Cu-Catalyzed S-transfer reaction: from amine to sulfide. Org Lett. 2014;16:2692–2695. doi: 10.1021/ol5009747. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Li Y, Zhang X, Jiang X. Sulfide synthesis through copper-catalyzed C S bond formation under biomolecule-compatible conditions. Chem Commun. 2015;51:941–944. doi: 10.1039/c4cc08367a. [DOI] [PubMed] [Google Scholar]
- 33.Xu HJ, Zhao YQ, Feng T, Feng YS. Chan Lam-type S-arylation of thiols with boronic acids at room temperature. J Org Chem. 2012;77:2878–2884. doi: 10.1021/jo300100x. [DOI] [PubMed] [Google Scholar]
- 34.Timpa SD, Pell CJ, Ozerov OV. A well-defined (POCOP)-Rh catalyst for the coupling of aryl halides with thiols. J Am Chem Soc. 2014;136:14772–14779. doi: 10.1021/ja505576g. [DOI] [PubMed] [Google Scholar]
- 35.Hamersvelt HWV, Kloke HJ, Jong DJD, Koene RA, Huysmans FT. Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention? J Hypertens. 1996;14:1041–1045. [PubMed] [Google Scholar]
- 36.Su W. An efficient method for the oxidation of sulfides to sulfones. Tetrahedron Lett. 1994;35:4955–4958. [Google Scholar]
- 37.Emmett EJ, Hayter BR, Willis MC. Palladium-catalyzed synthesis of ammonium sulfinates from aryl halides and a sulfur dioxide surrogate: a gas- and reductant-free process. Angew Chem Int Ed. 2014;53:10204–10208. doi: 10.1002/anie.201404527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocke BN, Bahnck KB, Herr M, Lavergne S, Mascitti V, Perreault C, Polivkova J, Shavnya A. Synthesis of sulfones from organozinc reagents, DABSO, and alkyl halides. Org Lett. 2014;16:154–157. doi: 10.1021/ol4031233. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Li W, Zheng D, Fan X, Wu J. Tetrahedron. 2015 In press, http://dx.doi.org/10.1016/j.tet.2015.03.101.
- 40.Garrod LP. Relative antibacterial activity of three Penicillins. Br Med J. 1960;1:527–529. doi: 10.1136/bmj.1.5172.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheehan JC, Henery L, Kenneth R. The Total Synthesis of Penicillin V. J Am Chem Soc. 1957;79:1262–1263. [Google Scholar]
- 42.a) Maki Y, Sako M. Tetrahedron Lett. Photochemical formation of 3-methylenecepham. 1976;17:4291. [Google Scholar]; b) Maki Y, Sako M. Concentration-dependent photoreaction of benzothiazolyldithioazetidinones: novel photochemical formation of penam derivatives. J Chem Soc, Chem Commun. 1978;19:836. [Google Scholar]
- 43.a) Cabri W, Candiani I, Bedeschi A, Santi R. Metal-promoted thiyl radical cyclizations in β-lactam antibiotics. Tetrahedron Lett. 1992;33:4783. [Google Scholar]; b) Cabri W, Borghi D, Arlandini E, Sbraletta P, Bedeschi A. Metal-catalyzed and -promoted cyclizations for the synthesis of cephalosporins: a possible DAOC/DAC synthetase biomimetic process. Tetrahedron. 1993;49:6837. [Google Scholar]; c) Cabri W, Candiani L, Bedeschi A. Iron(III) copper(II) and manganese(III) copper(II) promoted cyclizations: a new stereoselective approach towards-methyl substituted penicillin derivatives. J Chem Soc, Chem Commun. 1994;5:597. [Google Scholar]
- 44.Planchenault D, Wisedale R, Gallagher T, Hales NJ. A Direct and Convergent Approach to Penams and Penems. J Org Chem. 1997;62:3438–3439. [Google Scholar]
- 45.Emtenals H, Soto D, Hultgren SJ, Marshall GR, Almqvist F. Stereoselective Synthesis of Optically Active â-Lactams, Potential Inhibitors of Pilus Assembly in Pathogenic Bacteria. Org Lett. 2000;2:2065–2067. doi: 10.1021/ol0059899. [DOI] [PubMed] [Google Scholar]