Abstract
Ten alkaloids (1−10), with sophoridine (1) as the most abundant component, were obtained from the whole plants of Oxytropis ochrocephala Bunge. Furthermore, eight new sophoridine derivatives (11−16, 20, 21), with modification on the C-14 position of 1 were synthesized. All compounds (1−16, 20, 21) were evaluated for antiproliferative activity against five human tumor cell lines. Among them, the newly synthesized derivative 20 exhibited the best inhibitory activity against the tested cell lines. Its activity was increased by more than fourfold as compared with parent compound 1.
Keywords: Oxytropis ochrocephala, Poisonous plant, Antiproliferative, Sophoridine derivatives
Graphical Abstract
Locoweed is a common name for specific plants poisonous to livestock and belonging to the genera Oxytropis and Astragalus, which are widely distributed in Eastern and Central Asia, Western North and South America, and Australia1–2. Livestock ingesting locoweed can develop a chronic neurological disease, characterized by a staggering gait, and muscular incoordination, giving rise to the vivid name ‘locoweed’ for these poisonous plants.2–3. Today, locoweed is a main threat to rangelands worldwide1. In China, locoweeds cover over 11 m ha, amounting to 3.3% of the total western grassland2.
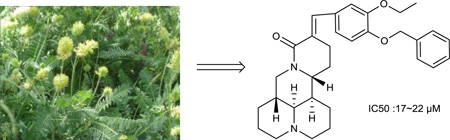
The plant Oxytropis ochrocephala Bunge is one of the common locoweeds found on the western grassland in China2, 4. It spreads 3 m ha across Qinghai, Ningxia, Gansu and Xizang provinces of China. In recent years, due to overgrazing, salinization and damage from drought and rodents, this poisonous plant has grown very so rapidly and even become the dominant species in some places2. On the other hand, if the chemical constituents in this plant had a therapeutic use, harm to the grassland could be mitigated.
To date, some efforts have been made to study locoism and poisonous alkaloid constituents of O. ochrocephala. As a result, five alkaloids, including one indolizidine and four quinolizidine alkaloids, were isolated previously from this plant5. Quinolizidine alkaloids exhibit broad pharmacological effects, such as antibacterial, antipyrotic, antipyretic, antiarrhythmic, antiasthmatic, antiulcerative, antivirus and antineoplastic properties.6–7 However, the antiproliferative activities of the quinolizidine alkaloids isolated from O. ochrocephala remain unclear.
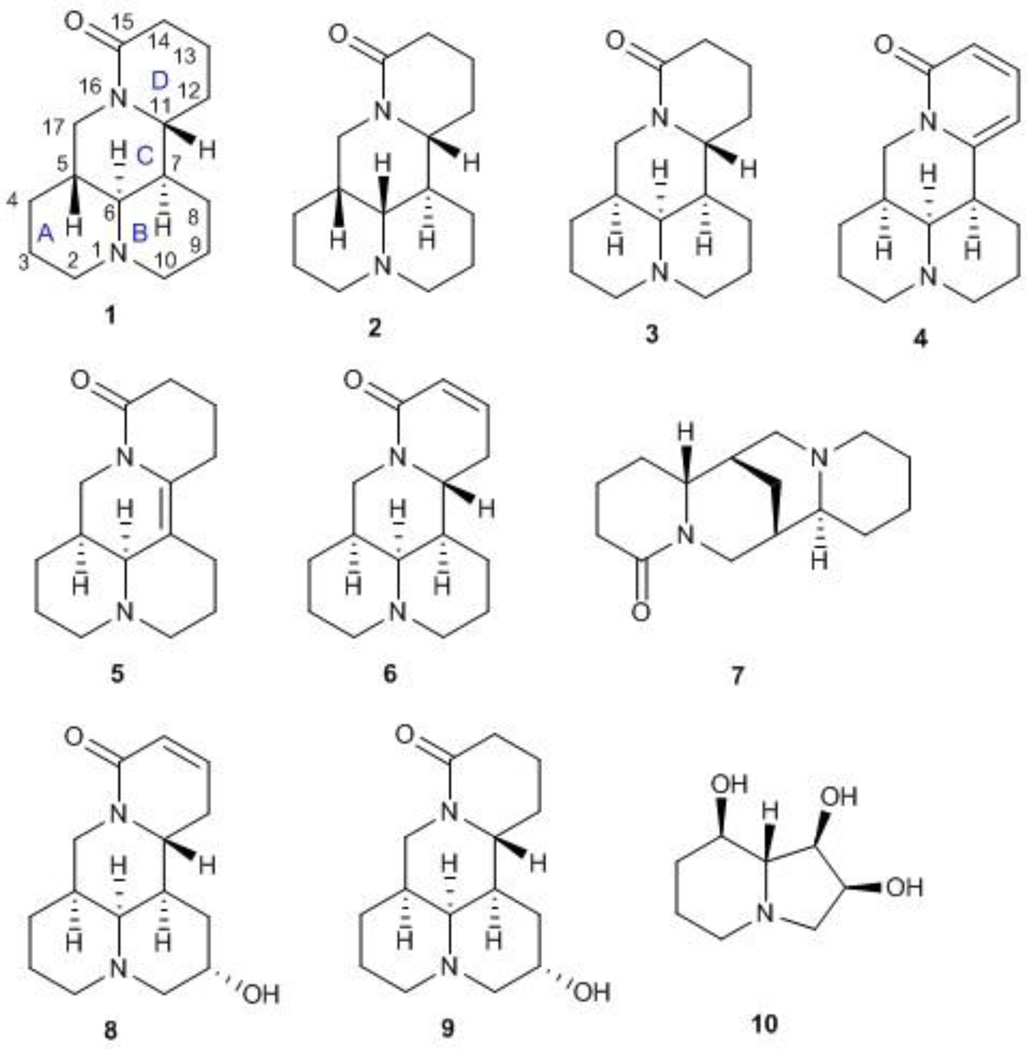
As part of our ongoing research program on the identification of alkaloids from Oxytropis ochrocephala, ten known alkaloids (Figure 1), sophoridine (1), isosophoridine (2), matrine (3), sophoramine (4), 7,11-dehydromatrine (5), sophocarpine (6), lupanine (7), (+)-9α-hydroxymatrine (8), (−)-9α-hydroxysophocarpine (9) and swainonine (10)8–15 were obtained from the whole plants of Oxytropis ochrocephala. Among them, compounds 1–9 are quinolizidine alkaloids, while compound 10 is an indolizidine alkaloid. Moreover, in our study, sophoridine (1, Figure 1), obtained in 5 gram quantity, provided an ideal starting material for further modifications. Compound 1 is one of three main chemical ingredients of Fufang Kushen injection, which was approved by the Chinese FDA (CFDA) in 1995 as an anticancer drug for treating non-small cell lung carcinoma, liver cancer, and gastric cancer in combination with other anticancer drugs.16–19 While some synthetic derivatives of 1 with an open D ring have exhibited good antiproliferative activity20–21, no research on the activity as well as structure-activity relationship (SAR) correlations of derivatives of 1 with four intact rings has been reported. In the present study, eight new derivatives of 1 with various substituents on the C-14 position were synthesized.
Figure 1.
The structures of alkaloids from Oxytropis ochrocephala
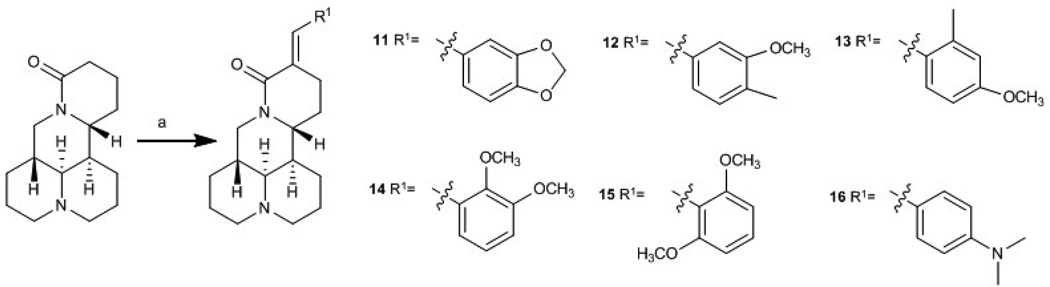
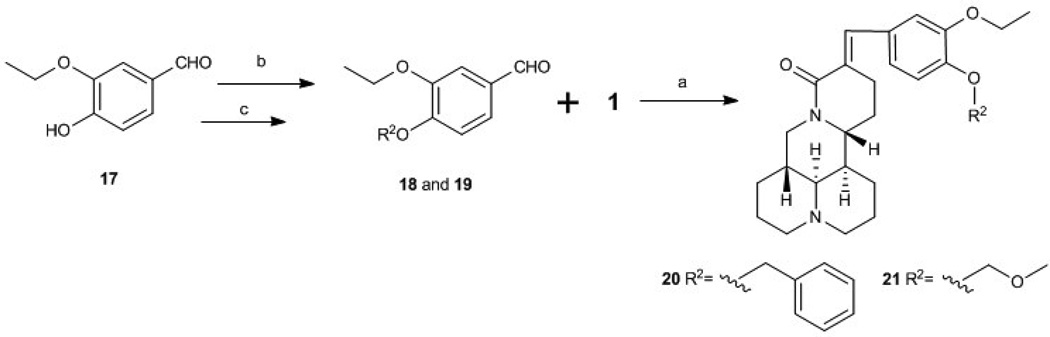
With 1 as starting material, eight new sophoridine derivatives were synthesized. As shown in Scheme 1, aldol reaction of various aromatic aldehydes with 1 produced compounds 11−16 (Scheme 1). Addition of a benzyl or methoxymethyl group on OH-4 of 17 produced 18 and 19, respectively (Scheme 2). Compounds 18 and 19 were further reacted with 1 to yield 20 and 21, respectively.
Scheme 1.
Synthesis of sophoridine derivations 11−16. (a) Aromatic aldehyde, NaH, THF, reflux, 6 h
Scheme 2.
Synthesis of sophoridine derivations 20 and 21. (b) BnBr, 10% K2CO3, rt; (c) MomCl, DIPEA, 0 °C to rt
The anti-proliferative activities of the natural alkaloids (1–10) and newly synthesized derivatives (11−16, 20, 21) against five tumor cell lines (A549, KB, KB-VIN, MDA-MB-231, MCF7) were evaluated by the sulforhodamine B (SRB) colorimetric assay. Paclitaxel was used as the positive control. The results are summarized in Table 1.
Table 1.
Antiproliferative Activity of Natural (1–10) and Synthetic (11–16, 20, 21) Alkaloids
| Compd | IC50 (µM) | |||||
|---|---|---|---|---|---|---|
| R1 | A549 | KB | KB-VIN | MDA-MB-231 | MCF7 | |
| 1 | >80 | >80 | >80 | >80 | >80 | |
| 2–10 | >40 | >40 | >40 | >40 | N/Da) | |
| 11 | 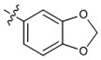 |
>40 | >40 | >40 | >40 | N/D |
| 12 | 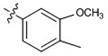 |
42 ± 0.94 | 58 ± 2.1 | 72 ± 1.6 | 45 ± 7.8 | 54 ± 21 |
| 13 | 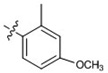 |
74 ± 1.9 | 74 ± 4.0 | >80 | 67 ± 1.7 | >80 |
| 14 | 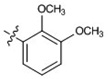 |
>80 | 71 ± 0.61 | >80 | 68 ± 4.7 | >80 |
| 15 | 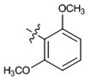 |
>40 | >40 | >40 | >40 | N/D |
| 16 | 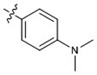 |
>40 | >40 | >40 | >40 | N/D |
| 20 | 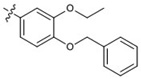 |
17.6 ± 0.31 | 20.7 ± 0.56 | 21.6 ± 2.011 | 21.9 ± 0.54 | 20.5 ± 1.49 |
| 21 | 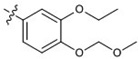 |
>40 | >40 | >40 | >40 | N/D |
| PXL (nM)b | 0.03 ± 0.03 | 0.04 ± 0.03 | 2600 ± 160 | 18 ± 3.2 | 3.9 ± 1.3 | |
N/D: not determined.
: IC50 of paclitaxel (PXL) is represented in nM.
At 40 µM, none of the naturally occurring indolizidine and quinolizidine alkaloids (1−10) from O. ochrocephala exhibited inhibitory activity against the five tested human tumor cell lines. Compound 1 was inactive even at 80 µM. As compared with 1, neither a conformation change at C-6 (2) or C-5 (3) nor the presence of unsaturation in the D or C ring (4−6) resulted in significant antiproliferative activity. However, among the eight newly synthesized derivatives with a substituted phenylmethylene group on C-14, some beneficial effects on activity were seen. Regarding the substitution pattern on the phenyl ring, 2-methyl-4-methoxy substituted 13 was less active than 3-methoxy-4-methyl substituted 12. Notably, when the 3,4-substituted pattern was retained, with an ethoxy group at position-3 and a benzyloxy group at position-4, the resulting compound, 20, showed significantly increased antiproliferative activity, with IC50 values around 20 µM against all five cancer cell lines, fourfold higher than those of 1. Interestingly, a change from a benzyloxy (20) to methoxymethoxy (21) group on the same position of the phenyl ring did not lead to increased antiproliferative activity. Thus, in this limited data set, the benzyloxy moiety is crucial for activity. Notably, compound 20 was equipotent against KB and MDR-subline KB-VIN, suggesting that this compounds is not a P-gp substrate. Finally, compound 16 with a dimethylamino group at position-4 of the phenyl ring was not active indicating that this particular basic group is not good for antiproliferative activity.
Ten natural alkaloids from O. ochrocephala and eight synthetic sophoridine analogues were evaluated for antiproliferative activity. Among them, synthetic derivative 20 exhibited the best inhibitory activity against five human tumor cell lines. The activity results and SAR correlations of this compound series were reported. Our research produced fundamental information for further study and exploitation of the poisonous plant O. ochrocephala.
Supplementary Material
Acknowledgments
The work was financially supported by the Special Scientific Research Fund of Agriculture Public Welfare Industry (Grant No. 201203062), the Chinese National Natural Science Foundation (31360084), the Natural Science Foundation of Guizhou Province (20102273), China Scholarship Council (CSC) to Cheng-jian Tan, and partial support from NIH Grant CA177584 from the National Cancer Institute awarded to Kuo-Hsiung Lee. K.Y. Hsieh was supported by the Teaching and Learning Excellence Program from Kaohsiung Medical University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data (experimental details and compound characterization for all synthesized compounds) associated with this article can be found, in the online version, at
References and notes
- 1.Panter KE, Gardner DR, Lee ST, Pfister JA, Rolphs MH, Stegelmeier BL, James LF. In: Veterinary Toxicology: Basic and Clinical Principles. Gupta RC, editor. New York: Academic Press; 2007. p. 825. [Google Scholar]
- 2.Lu H, Wang SS, Zhou QW, Zhao YN, Zhao BY. Rangeland J. 2012;34:609. [Google Scholar]
- 3.Molyneux RJ, James LF. Science. 1982;216:190. doi: 10.1126/science.6801763. [DOI] [PubMed] [Google Scholar]
- 4.Academia Sinica. Flora of China. 2. Vol. 42. Beijing: Science Press; 1998. p. 21. [Google Scholar]
- 5.Cao GR, Li SJ, Duan DX, Zhao XW. J. Northwest Sci-Tech Univ. Agric. For. 1989;17:1. [Google Scholar]
- 6.Meng XZ, Hu XQ, Zhang RM, You YT. Chin. Tradit. Herb. Drug. 1994;25:61. [Google Scholar]
- 7.Wang LS, You YJ, Wang SQ, Liu X, Liu BM, Wang JN, Lin X, Chen MS, Liang G, Yang H. Bioorg. Med. Chem. Lett. 2012;22:4100. doi: 10.1016/j.bmcl.2012.04.069. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Wu XD, He J, Li GT, Peng LY, Li Y, Song LD, Zhao QS. Chem. Nat. Compd. 2014;50:876. [Google Scholar]
- 9.Galasso V, Asaro F, Berti F, Pergolese B, Kovac B, Pichierri F. Chem. Phys. 2006;330:457. [Google Scholar]
- 10.Xiao P, Li JS, Saito K, Murakoshi I, Ohmiya S. Chem. Pharm. Bull. 1996;44:1951. [Google Scholar]
- 11.Ling JY, Zhang GY, Cui ZJ, Zhang CK. J. Chromatogr. A. 2007;1145:123. doi: 10.1016/j.chroma.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 12.Przybyl AK, Kubicki M. Tetrahedron. 2011;67:7787. [Google Scholar]
- 13.Zhao BY, Liu ZY, Wang JJ, Sun LS, Wang ZX, Wang YC. Agric. Sci. China. 2009;8:115. [Google Scholar]
- 14.Negrete R, Cassels BK, Eckhardt G. Phytochemistry. 1983;22:2069. [Google Scholar]
- 15.Xiao P, Kubo H, Komiya H, Higashiyama K, Yan YN, Li JS, Ohmiya S. Phytochemistry. 1999;50:189. [Google Scholar]
- 16.Gao LM, Han YX, Wang YP, Li YH, Shan YX, Li X, Peng ZG, Bi CW, Zhang T, Du NN, Jiang JD, Song DQ. J. Med. Chem. 2011;54:869. doi: 10.1021/jm101325h. [DOI] [PubMed] [Google Scholar]
- 17.Ye G, Zhu HY, Li ZX, Ma CH, Fan MS, Sun ZL, Huang CG. Biomed. Chromatogr. 2007;21:655. doi: 10.1002/bmc.805. [DOI] [PubMed] [Google Scholar]
- 18.Li YM, Min G, Xue Q, Chen L, Liu W, Chen H. Biomed. Chromatogr. 2004;18:619. doi: 10.1002/bmc.348. [DOI] [PubMed] [Google Scholar]
- 19.Hu PY, Zheng Q, Chen H, Wu ZF, Yue PF, Yang M. Zhongguo Xinyao Zazhi. 2012;21:2662. [Google Scholar]
- 20.Li X, Zhao WL, Jiang JD, Ren KH, Du NN, Li YB, Wang YX, Bi CW, Shao RG, Song DQ. Bioorg. Med. Chem. Lett. 2011;21:5251. doi: 10.1016/j.bmcl.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 21.Bi CW, Zhang CX, Li YH, Tang S, Wang SG, Shao RG, Fu HG, Su F, Song DQ. Med. Chem. Lett. 2014;5:1225. doi: 10.1021/ml500289h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.