Abstract
Introduction
Little is known about the impact of childhood cancer on the personal income of survivors. We compared income between survivors and siblings, and determined factors associated with income.
Methods
As part of the Swiss Childhood Cancer Survivor Study (SCCSS), a questionnaire was sent to survivors, aged ≥18 years, registered in the Swiss Childhood Cancer Registry (SCCR), diagnosed at age <21 years, who had survived ≥5 years after diagnosis of the primary tumor. Siblings were used as a comparison group. We asked questions about education, profession and income and retrieved clinical data from the SCCR. We used multivariable logistic regression to identify characteristics associated with income.
Results
We analyzed data from 1’506 survivors and 598 siblings. Survivors were less likely than siblings to have a high monthly income (>4’500 CHF), even after we adjusted for socio-demographic and educational factors (OR = 0.46, p<0.001). Older age, male sex, personal and parental education, and number of working hours were associated with high income. Survivors of leukemia (OR = 0.40, p<0.001), lymphoma (OR = 0.63, p = 0.040), CNS tumors (OR = 0.22, p<0.001), bone tumors (OR = 0.24, p = 0.003) had a lower income than siblings. Survivors who had cranial irradiation, had a lower income than survivors who had no cranial irradiation (OR = 0.48, p = 0.006).
Discussion
Even after adjusting for socio-demographic characteristics, education and working hours, survivors of various diagnostic groups have lower incomes than siblings. Further research needs to identify the underlying causes.
Introduction
Today, most childhood cancer patients (>80%) survive after treatment in developed countries[1, 2]. Late effects such as poor physical or mental health, functional impairments or activity limitations are well studied[3, 4]. Other factors are understudied, including the impact of childhood cancer on later earning capacity and income level. Income and educational attainment are linked, and studies have shown that cancer treatment during school years, combined with late effects, can lower the chance that survivors will excel at school[5–10]. But little is known about the later income situation of survivors. Income is a relevant factor for subjective well-being[11]. Some studies have assessed the personal income of survivors, but these included only small populations of survivors (N = 48–219)[12–16], or focused on specific diagnostic groups (osteosarcoma[17], retinoblastoma[18] or central nervous system [CNS] tumors and hematological malignancies[19]). Their results are inconsistent. Some studies found that income in survivors does not differ from controls[12, 15–17], and others found that their income is lower[14, 18, 19]. Researchers from the US Childhood Cancer Survivor Study investigated income and occupational outcomes in 4845 survivors of all diagnostic groups and 1727 siblings[20]. They found that survivors had earned less than siblings in all occupational fields. However, it remains unknown how underlying socio-demographic and clinical factors affect income in survivors.
Our goal was 1) to compare income between survivors and their siblings; 2) to assess the effects of socio-demographic characteristics on income in survivors and siblings (gender; age at survey; migration background; language region of Switzerland; parental education level; number of own children; work situation; personal education); 3) to compare income between survivors from different diagnostic groups, and to assess clinical characteristics on income in survivors (diagnosis, treatment modalities, age at diagnosis, relapse status).
Methods
The Swiss Childhood Cancer Survivor Study (SCCSS)
The Swiss Childhood Cancer Survivor Study (SCCSS) is a population-based, long-term follow-up study of all patients registered in the Swiss Childhood Cancer Registry (SCCR), who were diagnosed 1976–2005 at ≤21 years, and who survived ≥5 years after diagnosis.[21] The SCCR includes all children and adolescents in Switzerland diagnosed with leukemia, lymphoma, CNS tumors, malignant solid tumors, or Langerhans cell histiocytosis (LCH).[22] For this analysis, we included all survivors and siblings, who were aged ≥18 years at survey.
During 2007–2013, we traced addresses and sent a questionnaire to all survivors.[21, 23] Non-responders were mailed a second copy of the questionnaire. If they again failed to respond, we contacted them by phone. We asked survivors who were contacted in 2012 and earlier for their consent to contact their siblings for our comparison group. If survivors agreed, we sent the same questionnaire to siblings, without including cancer-related questions. Those who did not respond were sent another copy 4–6 weeks later, but were not contacted by phone. We used questionnaires similar to those used in US and UK childhood cancer survivor studies.[24, 25] We added questions about health behaviors and socio-demographic measures similar to those used in the Swiss Health Survey 2007[26] and the Swiss Census 2000.[27] Ethics approval was granted through the ethics committee of the canton of Bern to the SCCR.
Assessment of income
We asked survivors and siblings to select one of the following categories to report their personal monthly net income: ≤ 3'000 Swiss Francs (CHF); 3'001–4'500 CHF; 4'501–6'000 CHF; 6'001–9'000 CHF; and, >9'000 CHF. Net income was gross income from which social insurance and retirement insurance were subtracted. Net income includes income allocated for taxes and health insurance.
Assessment of socio-demographic characteristics
Socio-demographic characteristics were divided into baseline socio-demographic characteristics (characteristics present already at birth), and secondary socio-demographic characteristics (characteristics that occurred after the cancer diagnosis) that may be influenced by cancer or its treatment.
The questionnaire assessed the following baseline socio-demographic characteristics for survivors and siblings: gender; age at survey; migration background; language region of Switzerland; and, parental education level. We considered participants who fulfilled one of the following criteria to have a migration background: not born in Switzerland; no Swiss citizenship at birth; or, at least one parent is not a Swiss citizen. Switzerland has different language regions, and health behaviors and mortality differ between them[28]. We coded the language region of participants as German, French, or Italian speaking. Personal and parental education level fell into three categories: compulsory schooling (≤9 years of schooling); secondary education (vocational training or upper secondary education); and, tertiary education (university or technical college education). We assessed the following secondary socio-demographic characteristics for survivors and siblings: number of own children; work situation; and, personal education. Participants were asked if they were employed and how many hours they worked per week. Unemployed participants were divided into these categories: educational training; receiving disability insurance (not on educational training); currently looking for a job (no educational training, no disability insurance); and, not looking for a job (no educational training, no disability insurance).
Assessment of clinical data
The SCCR routinely collects clinical data. We extracted diagnosis, treatment modalities (surgery, chemotherapy, radiotherapy including the area of radiation, and bone marrow transplantation), age at diagnosis, and relapse status (yes/no) of survivors from the SCCR. We coded diagnoses according to the International Classification of Childhood Cancer, 3rd edition (ICCC-3),[29] and put treatment modalities into hierarchical order for analysis: chemotherapy (may include surgery); surgery only (includes no other treatments), bone marrow transplantation (may include surgery and/or chemotherapy); and, radiotherapy, not cranial and radiotherapy, including cranial (may include surgery and/or chemotherapy and/or bone marrow transplantation).
The questionnaire asked survivors if their sight was severely impaired or if they were blind in one or both eyes, were deaf in one or both ears and if they had had a limb amputated. It also asked survivors if they currently experienced any kind of late effects of childhood cancer or its treatment (yes/no). We defined late effects as a physical or mental problem that resulted from the cancer or its treatment.
Statistical analysis
First, we used Chi2 tests to compare baseline and secondary socio-demographic characteristics and income in survivors and siblings.
Second, we analyzed the association between socio-demographic characteristics and having a higher income (>4’500 CHF per month) with logistic regressions including survivors and siblings. We ran the analysis separately for baseline socio-demographic characteristics unaffected by cancer or its treatment (age, gender, language region, migration background and parental education), and for secondary socio-demographic characteristics that may be affected by the cancer or its treatment (having own children, weekly working hours and own education). We chose the income of >4’500 CHF as cut-off because it surpasses the recently discussed minimum wage of 4’000 CHF per month. We determined the effect of baseline socio-demographic characteristics on income by using univariable regressions. We included factors associated (p<0.05) with income in a multivariable logistic model. We also used univariable regressions to explore the association between secondary socio-demographic characteristics and income. We then included relevant (p<0.005) baseline and secondary socio-demographic characteristics associated with income in a multivariable logistic regression. Interaction of study group and gender was tested using likelihood ratio tests. We chose to test interaction for study group to find out if and how socio-demographic characteristics have a different effect on survivors than on siblings. We tested interaction for gender since it is known that income differences partially can be explained by gender.
Third, to find out if income differs between survivors of different types of tumors, we analyzed the association of diagnostic group with income in a multivariable logistic regression that included survivors and siblings. Siblings served as a reference group.
Fourth, we analyzed the association of clinical characteristics, including treatment, age at diagnosis, relapse status, blindness, deafness, amputation and perceived late effects, with income in a multivariable logistic regression that included only survivors.
We used the propensity score method[30] to standardize siblings to the survivor population for gender, age at survey, migration background and language region in all analyses that included siblings. We included robust variance estimation for clustered data to account for dependence of observations between survivors and their siblings. The criterion for statistical significance was a 2-sided p-value < 0.05. We used Stata, version 12.1 (StataCorp LP, College Station, TX) for all analyses.
Results
Socio-demographic characteristics
Of 4’111 survivors eligible for the SCCSS, we excluded 1’625 survivors for this study because they were aged <18 years at time of survey. Of the remaining 2’486 survivors address was not available for 129. Thus 2’357 survivors received our questionnaire. We used data from 1’506 survivors (response = 64%; S1 Table) and 598 siblings (response = 62%). Of the survivors, 48% were female, mean age at survey was 29.3 years (range: 18–55; SD = 7.5), and 26% had a migration background. Most survivors came from German speaking regions of Switzerland (70%, Table 1). The parents of most survivors had a secondary education (73%). We standardized the sibling population on age, gender, migration background, language region and parental education in accordance with the survivor population. More siblings (23%) than survivors (15%) had children (p<0.001). Fewer survivors (13%) reached tertiary education than siblings (21%; p<0.001). More siblings (84%) than survivors (78%) were employed (p = 0.016), with 56% of siblings and 57% of survivors in full time employment. Fewer siblings (10%) than survivors (13%) were in educational training.
Table 1. Socio-demographic characteristics of survivors and siblings.
| Survivors | Siblings | Siblings weighteda | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 1’506 | N = 598 | N = 598 | ||||||||
| n | (%b) | n | (%b) | pc | (%b) | pd | ||||
| Baseline socio-demographic characteristics | ||||||||||
| Female gender | 719 | (48) | 359 | (60) | <0.001 | (49) | 0.693 | |||
| Age at survey (years) | <0.001 | |||||||||
| 18-<25 | 490 | (33) | 116 | (19) | (30) | 0.727 | ||||
| 25-<30 | 394 | (26) | 146 | (24) | (28) | |||||
| 30-<35 | 275 | (18) | 108 | (18) | (18) | |||||
| 35-<40 | 181 | (12) | 74 | (12) | (12) | |||||
| ≥40 | 166 | (11) | 154 | (26) | (13) | |||||
| Migration background | <0.001 | 0.313 | ||||||||
| No | 1117 | (74) | 506 | (85) | (77) | |||||
| Yes | 389 | (26) | 92 | (15) | (23) | |||||
| Language region | <0.001 | 0.516 | ||||||||
| German | 1054 | (70) | 483 | (81) | (73) | |||||
| French | 403 | (27) | 104 | (17) | (25) | |||||
| Italian | 49 | (3) | 11 | (2) | (2) | |||||
| Highest parental education | 0.541 | 0.882 | ||||||||
| Compulsory schooling | 153 | (11) | 61 | (11) | (10) | |||||
| Secondary education | 1028 | (73) | 421 | (75) | (74) | |||||
| Tertiary education | 234 | (17) | 82 | (15) | (16) | |||||
| Secondary socio-demographic factors | ||||||||||
| Do you have children? | <0.001 | <0.001 | ||||||||
| No | 1’271 | (84) | 413 | (69) | (77) | |||||
| Yes, 1–2 children | 202 | (13) | 134 | (22) | (18) | |||||
| Yes, 3 or more children | 33 | (2) | 51 | (9) | (5) | |||||
| Personal education | <0.001 | <0.001 | ||||||||
| Compulsory schooling | 127 | (9) | 0 | (0) | (0) | |||||
| Secondary education | 1150 | (78) | 460 | (81) | (79) | |||||
| Tertiary education | 194 | (13) | 107 | (19) | (21) | |||||
| Employment situation | <0.001e | 0.016 | ||||||||
| Employed for | 1’174 | (78) | 516 | (86) | (84) | |||||
| ≥40 hours per week (full time) | 834 | (57) | 314 | (53) | (56) | |||||
| 30–39 hours per week | 113 | (8) | 65 | (11) | (8) | |||||
| 20–29 hours per week | 87 | (6) | 46 | (8) | (7) | |||||
| 10–19 hours per week | 52 | (4) | 48 | (8) | (8) | |||||
| 0–9 hours per week | 53 | (4) | 39 | (7) | (6) | |||||
| Not employed | 332 | (22) | 82 | (14) | (16) | |||||
| Educational training | 187 | (13) | 46 | (8) | (10) | |||||
| Looking for a jobf | 52 | (4) | 20 | (3) | (4) | |||||
| Not looking for a jobf | 35 | (2) | 12 | (2) | (1) | |||||
| Disability insuranceg | 37 | (2) | 2 | (<1) | (<1) | |||||
| Monthly income | ||||||||||
| Income in CHF | <0.001 | 0.002 | ||||||||
| ≤ 3'000 | 580 | (41) | 205 | (35) | (36) | |||||
| 3'001–4’500 | 396 | (28) | 128 | (22) | (24) | |||||
| 4'501–6’000 | 271 | (19) | 149 | (26) | (25) | |||||
| 6'001–9’000 | 131 | (9) | 87 | (15) | (13) | |||||
| >9’000 | 41 | (3) | 12 | (2) | (2) | |||||
NOTE: Percentages are based upon available data for each variable. Abbreviations: n, number; n.a., not applicable; CHF, Swiss Francs.
aSibling population is standardized on age, gender, migration background, language region, parental education according to the survivor population. All numbers in siblings are based upon weighted percentages, therefore p-values do not apply to variables for which siblings were weighted
bColumn percentages are given
cp-value calculated from Chi2 statistics that compare survivors and siblings
dp-value calculated from Chi2 statistics that compare survivors and weighted siblings
ep-value refers to comparison of survivors and siblings working vs. not working
fdoes not include those currently on educational training
gdoes not include those on educational training or working
The most frequent cancer diagnoses were leukemia (31%), lymphoma (22%) and CNS tumors (13%; Table 2).
Table 2. Clinical characteristics of survivors.
| Survivors N = 1’506 | ||
|---|---|---|
| n | (%)a | |
| Diagnosis (ICCC-3) | ||
| I Leukemia | 466 | (31) |
| II Lymphoma | 338 | (22) |
| III CNS | 197 | (13) |
| IV Neuroblastoma | 49 | (3) |
| V Retinoblastoma | 27 | (2) |
| VI Renal tumor | 67 | (4) |
| VII Hepatic tumor | 8 | (1) |
| VIII Bone tumor | 78 | (5) |
| IX Soft tissue sarcoma | 94 | (6) |
| X Germ cell tumor | 86 | (6) |
| XI & XII Other tumorsb | 50 | (3) |
| Langerhans cell histiocytosis | 46 | (3) |
| Treatmentc | ||
| Chemotherapy | 1’149 | (76) |
| Surgery | 960 | (64) |
| Radiotherapy | ||
| No | 924 | (61) |
| Yes, excluding cranial | 351 | (23) |
| Yes, including cranial | 231 | (15) |
| Bone marrow transplantation | 67 | (4) |
| Age at diagnosis (years) | ||
| 0–5 | 471 | (31) |
| >5–10 | 333 | (22) |
| >10–15 | 488 | (32) |
| >15–20 | 214 | (14) |
| Had relapse | 152 | (11) |
| Severe impairment or blindness on one or both eyes | 104 | (7) |
| Deaf on one or both ears | 10 | (1) |
| Amputation | 85 | (6) |
| Reported severe late effects from cancer | 548 | (38) |
NOTE: Percentages are based upon available data for each variable. Abbreviations: CNS, Central Nervous System; ICCC-3, International Classification of Childhood Cancer—Third Edition; n, number; n.a.
aColumn percentages are given
bOther malignant epithelial neoplasms, malignant melanomas, and other or unspecified malignant neoplasms
c“chemotherapy” may include surgery, “surgery only” includes no other treatments, “bone marrow transplantation” may include surgery and/or chemotherapy, “Radiotherapy, not cranial” and “Radiotherapy, including cranial” may include surgery and/or chemotherapy and/or bone marrow transplantation.
Income in survivors and siblings
Survivors had lower income than siblings (p = 0.002): More survivors than siblings reported a low monthly income of ≤ 3'000 CHF (41% of survivors vs. 36% of siblings) and 3'001–4'500 CHF (28% of survivors vs. 24% of siblings; Table 1). Fewer survivors than siblings reported a medium monthly income of 4'501–6'000 CHF (19% of survivors vs. 25% of siblings) and 6'001–9'000 CHF (9% of survivors vs. 13% of siblings). The proportion of survivors and siblings who reported a high income of >9'000 CHF was similar (3% of survivors vs. 2% of siblings).
Socio-demographic characteristics associated with income
Results from logistic regressions showed that survivors were less likely to have a monthly income of >4’500 CHF than siblings, whether the analysis was unadjusted (OR = 0.54, p<0.001; Table 3, column A), adjusted for baseline socio-demographic characteristics (OR = 0.57, p = 0.001; Table 3, column B), or adjusted for baseline and secondary socio-demographic characteristics (OR = 0.46, p<0.001, Table 3, column C). Older participants (OR ranging from 4.23–11.90, p<0.001) and those with a tertiary education (OR = 2.14, p = 0.002) were more likely to have a monthly income of >4’500 CHF. Females (OR = 0.46, p<0.001), and those who worked less than 40 hours per week (OR ranging from 0.01–0.33, p<0.001) were less likely to have a monthly income of >4’500 CHF.
Table 3. Socio-demographic predictors of monthly income >4’500 CHF in survivors and siblingsa.
| A) Univariable analysis | B) Multivariable analysis including baseline socio-demographic characteristicsb | C)Multivariable analysis including baseline and secondary socio-demographic characteristicsc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ORd | 95% CI | p-value | ORd | 95% CI | p-value | ORd | 95% CI | p-value | |
| Study group | |||||||||
| Sibling | 1 | 1 | 1 | ||||||
| Survivor | 0.54 | 0.43–0.69 | <0.001 | 0.57 | 0.43–0.75 | 0.001 | 0.46 | 0.33–0.64 | <0.001 |
| Basline socio-demographic characteristics (before cancer) | |||||||||
| Age at survey | |||||||||
| 18-<25 years | 1 | 1 | <0.001e | 1 | <0.001e | ||||
| 25-<30 years | 5.65 | 3.49–9.15 | <0.001 | 5.83 | 3.44–9.88 | <0.001 | 4.31 | 2.52–7.39 | <0.001 |
| 30-<35 years | 9.22 | 5.62–15.15 | <0.001 | 9.70 | 5.66–16.63 | <0.001 | 7.05 | 4.08–12.19 | <0.001 |
| 35-<40 years | 10.87 | 6.38–18.53 | <0.001 | 11.49 | 6.57–20.07 | <0.001 | 10.23 | 5.68–18.41 | <0.001 |
| ≥40 years | 12.88 | 7.74–21.44 | <0.001 | 11.86 | 6.78–20.73 | <0.001 | 11.90 | 5.96–23.76 | <0.001 |
| Gender | |||||||||
| Male | 1 | 1 | 1 | ||||||
| Female | 0.31 | 0.24–0.40 | <0.001 | 0.29 | 0.21–0.39 | <0.001 | 0.46 | 0.32–0.65 | <0.001 |
| Language region | |||||||||
| German | 1 | n.a. | n.a. | ||||||
| French/Italian | 1.13 | 0.79–162 | 0.497 | ||||||
| Migration | |||||||||
| No | 1 | n.a. | n.a. | ||||||
| Yes | 0.85 | 0.59–1.21 | 0.361 | ||||||
| Parental education | |||||||||
| Compulsory schooling | 0.73 | 0.43–1.25 | 0.251 | 0.51 | 0.27–0.96 | 0.038 | 0.57 | 0.32–1.02 | 0.057 |
| Secondary education | 1 | 1 | 0.043 e | 1 | 0.153e | ||||
| Tertiary or university education | 0.65 | 0.45–0.93 | 0.019 | 0.73 | 0.50–1.08 | 0.119 | 0.88 | 0.55–1.40 | 0.579 |
| Secondary socio-demographic characteristics (after cancer) | |||||||||
| Having children | |||||||||
| No children | 1 | n.a. | 1 | 0.364e | |||||
| 1 to 2 children | 1.71 | 1.18–2.46 | 0.004 | 1.25 | 0.74–2.11 | 0.411 | |||
| >2 children | 1.22 | 0.63–2.35 | 0.562 | 0.71 | 0.36–1.40 | 0.323 | |||
| Working hours | |||||||||
| ≥40 hours | 1 | n.a. | 1 | <0.001e | |||||
| 30–39 hours | 0.38 | 0.24–0.60 | <0.001 | 0.33 | 0.19–0.59 | <0.001 | |||
| 20–29 hours | 0.07 | 0.04–0.15 | <0.001 | 0.05 | 0.02–0.11 | <0.001 | |||
| 10–19 hours | 0.01 | <0.01–0.07 | <0.001 | 0.01 | <0.01–0.06 | <0.001 | |||
| 0–9 hours | 0.04 | 0.02–0.07 | <0.001 | 0.04 | 0.02–0.09 | <0.001 | |||
| Personal education | |||||||||
| Compulsory schooling | 0.25 | 0.13–0.49 | <0.001 | n.a. | 0.55 | 0.22–1.36 | 0.196 | ||
| Secondary education | 1 | 1 | <0.002e | ||||||
| Tertiary or university education | 3.01 | 2.07–4.39 | <0.001 | 2.14 | 1.34–3.43 | 0.002 | |||
aSibling population is standardized on age, gender, migration background and language region according to the survivor population
bMultivariable analysis, including baseline socio-demographic variables that were significant in the univariable analysis
cMultivariable analysis, including baseline socio-demographic variables and secondary socio-demographic characteristics that occurred after cancer was diagnosed and were significant in the univariable analysis
dOR for having a monthly income of >4500 CHF
eglobal p-value calculated with Wald test
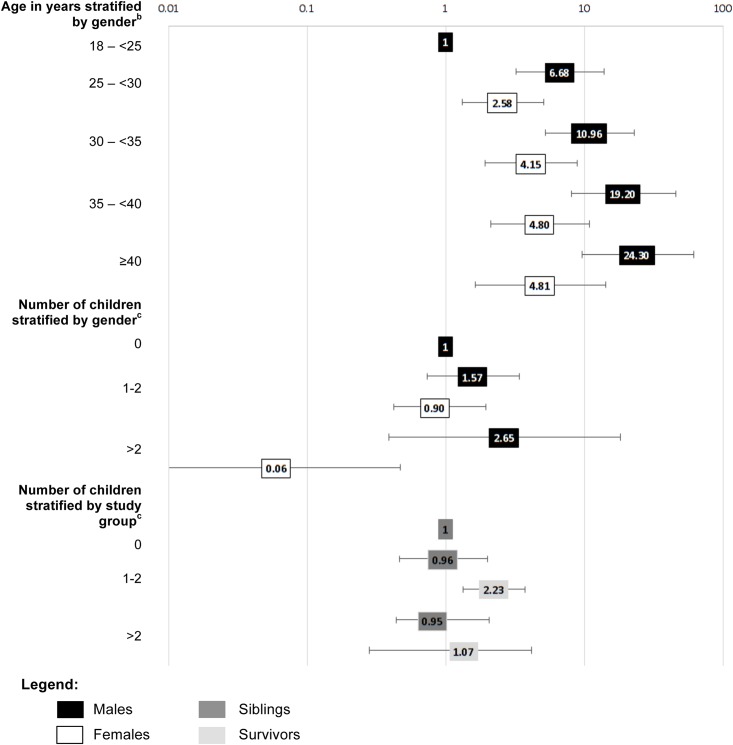
Some predictors differed between females and males (S2 Table) and between survivors and siblings (S3 Table). Significant differences are displayed in Fig 1: Men’s income increased more steeply with age than women’s (p = 0.005). Women with >2 children were less likely to have an income of >4’500 CHF (OR = 0.06, p = 0.007) than men with no children. We also found an interaction between study groups and having own children (p = 0.035). Though survivors with 1–2 children were more likely than siblings with no children to have an income of >4’500 CHF (OR = 2.23, p = 0.002), income in siblings was not influenced by the number of children.
Fig 1. Interaction effects on socio-demographic predictors of monthly income >4’500 CHF.
Fig 1 shows interaction effects of gender and study group on socio-demographic predictors of monthly income >4’500 CHF. aResults were retrieved from multivariable logistic regression adjusted for baseline and secondary socio-demographic variables that were significant in the univariable model (Table 3). An OR<1 means that the respective group is less likely to have a monthly income of >4’500 CHF; bReference group are those aged 18 –<25 years; cReference group are those with 0 children.
Clinical characteristics associated with income
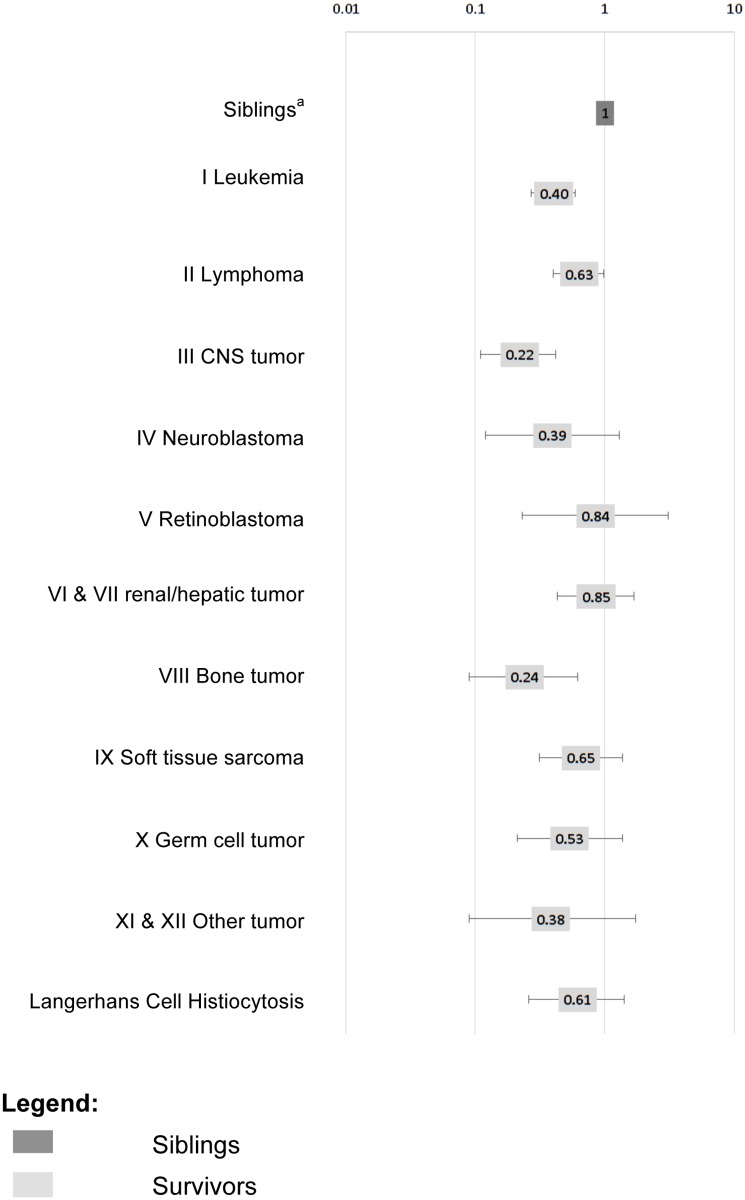
After we adjusted for baseline and secondary socio-demographic characteristics, we found survivors of leukemia (OR = 0.40, p<0.001), lymphoma (OR = 0.63, p = 0.040), CNS tumors (OR = 0.22, p<0.001), and bone tumors (OR = 0.24, p = 0.003) were less likely than siblings to have a monthly income of >4’500 CHF (Fig 2).
Fig 2. Association of diagnostic group with having a monthly income of >4’500 CHF.
Fig 2 shows the association of diagnostic group with having a monthly income of >4’500 CHF compared to siblings. aMultivariable analysis showing ORs adjusted for baseline and secondary socio-demographic variables that were significant in the univariable model (Table 3); bSibling population is standardized on age, gender, migration background and language region according to the survivor population.
Survivors treated by cranial irradiation (OR = 0.48, p = 0.006) were less likely than survivors treated by chemotherapy alone to have a monthly income of >4’500 CHF (Table 4). Those diagnosed aged >15–20 years were more likely than those diagnosed aged 0–5 years to have a monthly income of >4’500 CHF. Only in the univariable model were survivors who relapsed or had late effects less likely to have a higher income. All survivors who were deaf on one or both ears were in the lower income category.
Table 4. Association of clinical characteristics with having a monthly income of >4’500 CHF in survivors–results from univariable and multivariable logistic regression.
| Univariable analysis | Multivariable analysisa | |||||
|---|---|---|---|---|---|---|
| OR | 95%-CI | p-value | OR | 95%-CI | p-value | |
| Treatment b | ||||||
| Chemotherapy | 1 | 1 | 0.021e | |||
| Surgery only | 1.13 | 0.79–1.61 | 0.514 | 1.42 | 0.72–2.77 | 0.306 |
| Bone marrow transplant | 0.72 | 0.26–1.98 | 0.525 | 1.56 | 0.34–7.06 | 0.567 |
| Radiotherapy, not cranial | 1.60 | 1.20–2.11 | 0.001 | 1.13 | 0.70–1.83 | 0.611 |
| Radiotherapy, including cranial | 0.74 | 0.51–1.07 | 0.107 | 0.48 | 0.29–0.81 | 0.006 |
| Age at diagnosis | ||||||
| 0–5 years | 1 | 1 | 0.073e | |||
| >5–10 years | 1.41 | 1.02–1.96 | 0.038 | 1.61 | 0.98–2.63 | 0.060 |
| >10–15 years | 1.42 | 1.05–1.91 | 0.021 | 1.19 | 0.73–1.92 | 0.491 |
| >15–20 years | 3.67 | 2.58–5.23 | <0.001 | 2.09 | 1.05–4.16 | 0.035 |
| Relapse | ||||||
| No | 1 | 1 | ||||
| Yes | 0.65 | 0.43–0.98 | 0.038 | 0.69 | 0.38–1.25 | 0.222 |
| Blind on one or both eyes | ||||||
| No | 1 | n.ac | ||||
| Yes | 0.74 | 0.48–1.14 | 0.173 | |||
| Deaf on one or both ears | ||||||
| No | 1 | n.ad | ||||
| Yes | All have lower income. | |||||
| Amputation | ||||||
| No | 1 | n.ac | ||||
| Yes | 1.07 | 0.66–1.74 | 0.781 | |||
| Perceived late effects | ||||||
| No | 1 | 1 | ||||
| Yes | 0.77 | 0.61–0.98 | 0.034 | 0.92 | 0.63–1.34 | 0.665 |
aThe analysis is adjusted for baseline and secondary socio-demographic variables that were significant in the univariable model (Table 3) and for diagnostic group
b“chemotherapy” may include surgery, “surgery only” includes no other treatments, “bone marrow transplant” may include surgery and/or chemotherapy, “Radiotherapy, not cranial” and “Radiotherapy, including cranial” may include surgery and/or chemotherapy and/or bone marrow transplantation
cBeing blind in one or both eyes and amputation were not significantly associated (p-value was ≥0.05) with a monthly income of >4’500 CHF in the univariable model and were therefore not included in the multivariable model
dBeing deaf on one or both ears perfectly predicts having an income of <4500 CHF, thus this variable could not be included into the model
eglobal p-value calculated with Wald test
Discussion
This is the first study analyzing the association of socio-demographic and clinical characteristics with personal income in survivors. We found that income in survivors was lower than in siblings. Socio-demographic characteristics such as age, gender, working hours and education of the parents affect income. Survivors of leukemia, lymphoma, CNS tumors and bone tumors were likely to have an income lower than their siblings. Of survivors, those treated with cranial radiotherapy were most likely to have lower income.
Income in survivors and comparison groups
Our results are in line with other studies. The US Childhood Cancer Survivor Study (CCSS) includes survivors diagnosed between 1970 and 1986. One study including 4’845 survivors and 1’727 siblings aged >25, all currently employed, found that survivors had a lower yearly income than siblings for all occupations[20]. The Norwegian Cancer Registry analyzed the yearly income of childhood cancer survivors of CNS tumors (n = 222) and hematological malignancies (n = 202), aged 25–44 years, and diagnosed between 1970–1997. More survivors of CNS tumors (14%) and hematological malignancies (15%) had a yearly income of <10’000 Euro than the general population (6%)[19]. Even after controlling for several socio-demographic factors affecting income (age, gender, parental education, personal education, working hours, number of own children) our study showed income to be lower in survivors than in siblings. Possible factors for low income in survivors compared to siblings might be that survivors make different career choices or receive different job offers than siblings.
Socio-demographic characteristics associated with income
Few studies investigated how socio-demographic characteristics were associated with personal income. The CCSS[20] found that women were less likely in full-time managerial or professional occupations than males. Typically, full-time managerial or professional occupations are associated with high income compared to blue collar or service jobs. We also found that women and those with lower education have a lower income, which lines up with data from the general population of Switzerland, where men earn 24% more than women[31], and where higher education usually leads to higher income[32]. Thus the same factors associated with income observed in women from the general population also holds true for the population of survivors. Since our analysis controlled for various socio-demographic factors, lower incomes in survivors might be due to other factors such as personal career choice or discriminatory salary offers.
Clinical characteristics associated with income
Few studies compared personal income between diagnostic groups. We found that survivors of CNS tumors had a lower income than siblings, in line with the results of the Norwegian study that compared survivors to the general population[19]. A US study found that survivors of CNS tumors earned less than other survivors and controls[13]. Cognitive late effects, caused by cranial irradiation or surgery might affect income in CNS survivors. We also found that survivors of leukemia, lymphoma and bone tumors also earned less than other survivors. For leukemia, this was also seen in Norway[19]. Further research needs to identify reasons for a lower income in leukemia survivors. We found no studies that explicitly examined income of lymphoma and bone tumor survivors, but it has been reported that lymphoma survivors can experience cognitive deficits from treatment[33]; and that amputee survivors of bone tumors can have deficits in education and employment[9].
Few studies assessed the effect of cranial irradiation on income. A Japanese study found that survivors treated with radiotherapy had lower annual incomes[12]. They believed the disparity was explained by a higher proportion of survivors who were studying at time of survey in the group of irradiated survivors. In the CCSS survivors treated with high dose cranial irradiation were less likely to be work in managerial or professional occupations than survivors treated by other means [20]. Survivors treated with cranial irradiation have more cognitive problems[34, 35] and thus lower educational achievements than healthy peers[36], all of which might contribute to lowering income.
We found that survivors diagnosed at age >15–20 years had higher incomes than survivors diagnosed at age 0–5 years. However, we did not find other studies that analyzed the effect of age at diagnosis on income. But others found lower occupational positions and education in survivors: The CCSS found that survivors diagnosed when they were younger were less often found in managerial or professional occupations than survivors diagnosed when they were older[20]. In a Swedish study, survivors of lymphoblastic leukemia with young age at diagnosis attained a lower level of education and were less often employed than controls[37]. Young age at diagnosis is a risk factor for cancer-related cognitive dysfunction[38]. Those cognitive deficits and being less often employed in managerial or professional occupations in survivors diagnosed at young age might lead to lower income.
Clinical implication
Long treatment periods may explain why survivors earn less, since that pushes back their educational training and may cause them to start working later than their peers in the general population. Survivor income may also start to climb later than sibling income. In an earlier study, we found that the gap in educational achievements between survivors and the general population became smaller when we included those aged ≥27 years old[6]. Continuous research for a shorter and less toxic treatment of childhood cancer as well as educational support during and after treatment might help to improve and accelerate education, which might increase income. From our study we do not know if survivors choose jobs that pay less, if they are offered lower salaries than siblings when they apply for the same job or if specific late effects from treatment such as fatigue affects income. Further studies should include longitudinal assessment of income and career, and the employers’ attitude towards childhood cancer survivors.
Limitations and Strengths
Because income was only assessed at one time point we could not measure changes of income levels over time. We could not estimate income in non-responders, so we could not determine if their income differed systematically from responders. However, almost all of our respondents answered our question about personal income (4% missing values in survivors and 2% in siblings). Our population-based approach was a strength. The distribution of diagnostic groups in our study was equivalent to the distribution in the Swiss population of childhood cancer survivors. However, non-responders differed from responders. Non-responders included more males, more survivors of lymphoma and CNS tumors, were less often treated with chemotherapy and older at diagnosis. We weighted the sibling population to maximize comparability to the survivor cohort. Stratifying analysis by diagnostic groups, and analyzing the effect of treatment and other cancer-related characteristics, allowed us to show specific survivor groups at risk for lower income.
Conclusion
Survivors in a variety of diagnostic groups earn less than siblings, even after we adjusted for socio-demographic characteristics, education and working hours. Follow-up studies should investigate how income changes in survivors over time. Further they should assess underlying reasons for lower income beyond socio-demographic characteristics, including survivor’s preferences for certain job fields and how survivors are acting and being treated when their income is determined by employers.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank all childhood cancer survivors and their parents who participated in our survey. We thank the study team of the SCCSS (Rahel Kuonen, Erika Brantschen-Berclaz, Julia Koch, Fabienne Liechti), the data managers of the SPOG (Claudia Anderegg, Nadine Beusch, Rosa-Emma Garcia, Franziska Hochreutener, Friedgard Julmy, Nadine Lanz, Heike Markiewicz, Genevieve Perrenoud, Annette Reinberger, Renate Siegenthaler, Verena Stahel) and the team of the SCCR (Vera Mitter, Elisabeth Kiraly, Marlen Spring, Christina Krenger, Priska Wölfli). We also thank Kali Tal for her editorial assistance.
This study was supported by Cancer League Aargau (www.krebsliga-aargau.ch), the Bernese Cancer League (www.bernischekrebsliga.ch), Swiss Cancer Research (grant 02631-08-2010), and the Swiss Cancer League (grant KLS-3412-02-2014). GM was supported by the Swiss National Science Foundation (GM: Ambizione-Fellowship-grant PZ00P3_121682, PZ00P3-141722). BDS was supported by a Swiss National Science Foundation fellowship (PZ00P3_147987). MS was supported by the SNF (ProDoc: PDFMP3_141775). The work of the Swiss Childhood Cancer Registry is supported by the Swiss Paediatric Oncology Group (www.spog.ch), Schweizerische Konferenz der kantonalen Gesundheitsdirektorinnen und–direktoren (www.gdk-cds.ch), Swiss Cancer Research (www.krebsforschung.ch), Kinderkrebshilfe Schweiz (www.kinderkrebshilfe.ch), Ernst-Göhner Stiftung, Stiftung Domarena, CSL Behring (www.cslbehring.ch) and National Institute of Cancer Epidemiology and Registration (www.nicer.ch).
The members of the Swiss Paediatric Oncology Group (SPOG) Scientific Committee are: Dr. med. R. Angst, Aarau; PD Dr. med. M. Ansari, Geneva; PD Dr. med. M. Beck Popovic, Lausanne; Dr. med. P. Brazzola, Bellinzona; Dr. med. J. Greiner, St. Gallen; Prof. Dr. med. M. Grotzer, Zurich; Prof. Dr. med. K. Leibundgut, Bern; Prof. Dr. med. F. Niggli, Zurich; PD Dr. med. J. Rischewski, Lucerne; Prof. Dr. med. N. von der Weid, Basel.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Cancer League Aargau (www.krebsliga-aargau.ch), the Bernese Cancer League (www.bernischekrebsliga.ch), and Swiss Cancer Research (grant 02631-08-2010) and the Swiss Cancer League (grant KLS-3412-02-2014). GM was supported by the Swiss National Science Foundation (GM: Ambizione-Fellowship-grant PZ00P3_121682, PZ00P3-141722). BDS was supported by a Swiss National Science Foundation fellowship (PZ00P3_147987). MS was supported by the SNF (ProDoc: PDFMP3_141775). The work of the Swiss Childhood Cancer Registry is supported by the Swiss Paediatric Oncology Group (www.spog.ch), Schweizerische Konferenz der kantonalen Gesundheitsdirektorinnen und –direktoren (www.gdk-cds.ch), Swiss Cancer Research (www.krebsforschung.ch), Kinderkrebshilfe Schweiz (www.kinderkrebshilfe.ch), Ernst-Göhner Stiftung, Stiftung Domarena, CSL Behring (www.cslbehring.ch) and National Institute of Cancer Epidemiology and Registration (www.nicer.ch). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, et al. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer. 2009;45(6):992–1005. 10.1016/j.ejca.2008.11.042 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63(1):11–30. 10.3322/caac.21166 . [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. [DOI] [PubMed] [Google Scholar]
- 4.Hudson MM, Oeffinger KC, Jones K, Brinkman TM, Krull KR, Mulrooney DA, et al. Age-Dependent Changes in Health Status in the Childhood Cancer Survivor Cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014. 10.1200/JCO.2014.57.4863 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieluweit U, Debatin KM, Grabow D, Kaatsch P, Peter R, Seitz DC, et al. Educational and vocational achievement among long-term survivors of adolescent cancer in Germany. Pediatric blood & cancer. 2011;56(3):432–8. 10.1002/pbc.22806 . [DOI] [PubMed] [Google Scholar]
- 6.Kuehni CE, Strippoli MP, Rueegg CS, Rebholz CE, Bergstraesser E, Grotzer M, et al. Educational achievement in Swiss childhood cancer survivors compared with the general population. Cancer. 2012;118(5):1439–49. Epub 2011/08/09. 10.1002/cncr.26418 [DOI] [PubMed] [Google Scholar]
- 7.Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B, et al. Social Outcomes in the Childhood Cancer Survivor Study Cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(14):2390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robison LL, Green DM, Hudson M, Meadows AT, Mertens AC, Packer RJ, et al. Long-term outcomes of adult survivors of childhood cancer. Cancer. 2005;104(11 Suppl):2557–64. [DOI] [PubMed] [Google Scholar]
- 9.Nagarajan R, Neglia JP, Clohisy DR, Yasui Y, Greenberg M, Hudson M, et al. Education, employment, insurance, and marital status among 694 survivors of pediatric lower extremity bone tumors: a report from the childhood cancer survivor study. Cancer. 2003;97(10):2554–64. [DOI] [PubMed] [Google Scholar]
- 10.Pang JW, Friedman DL, Whitton JA, Stovall M, Mertens AC, Robison LL, et al. Employment status among adult survivors in the Childhood Cancer Survivor Study. Pediatric blood & cancer. 2008;50(1):104–10. [DOI] [PubMed] [Google Scholar]
- 11.Kahneman D, Deaton A. High income improves evaluation of life but not emotional well-being. Proc Natl Acad Sci U S A. 2010;107(38):16489–93. 10.1073/pnas.1011492107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida Y, Honda M, Kamibeppu K, Ozono S, Okamura J, Asami K, et al. Social outcomes and quality of life of childhood cancer survivors in Japan: a cross-sectional study on marriage, education, employment and health-related QOL (SF-36). International journal of hematology. 2011;93(5):633–44. Epub 2011/04/27. 10.1007/s12185-011-0843-6 . [DOI] [PubMed] [Google Scholar]
- 13.Hays DM, Landsverk J, Sallan SE, Hewett KD, Patenaude AF, Schoonover D, et al. Educational, occupational, and insurance status of childhood cancer survivors in their fourth and fifth decades of life. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1992;10(9):1397–406. Epub 1992/09/01. [DOI] [PubMed] [Google Scholar]
- 14.Dolgin MJ, Somer E, Buchvald E, Zaizov R. Quality of life in adult survivors of childhood cancer. Soc Work Health Care. 1999;28(4):31–43. . [DOI] [PubMed] [Google Scholar]
- 15.Felder-Puig R, Formann AK, Mildner A, Bretschneider W, Bucher B, Windhager R, et al. Quality of life and psychosocial adjustment of young patients after treatment of bone cancer. Cancer. 1998;83(1):69–75. . [DOI] [PubMed] [Google Scholar]
- 16.Evans SE, Radford M. Current lifestyle of young adults treated for cancer in childhood. Arch Dis Child. 1995;72(5):423–6. Epub 1995/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson HS, Mulvihill JJ, Byrne J. Late effects of therapy in adult survivors of osteosarcoma and Ewing's sarcoma. Medical and pediatric oncology. 1992;20(1):6–12. . [DOI] [PubMed] [Google Scholar]
- 18.Byrne J, Fears TR, Whitney C, Parry DM. Survival after retinoblastoma: long-term consequences and family history of cancer. Medical and pediatric oncology. 1995;24(3):160–5. . [DOI] [PubMed] [Google Scholar]
- 19.Johannesen TB, Langmark F, Wesenberg F, Lote K. Prevalence of Norwegian patients diagnosed with childhood cancer, their working ability and need of health insurance benefits. Acta oncologica. 2007;46(1):60–6. . [DOI] [PubMed] [Google Scholar]
- 20.Kirchhoff AC, Krull KR, Ness KK, Park ER, Oeffinger KC, Hudson MM, et al. Occupational outcomes of adult childhood cancer survivors: A report from the childhood cancer survivor study. Cancer. 2011. Epub 2011/01/20. 10.1002/cncr.25867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuehni CE, Rueegg CS, Michel G, Rebholz CE, Strippoli M-PF, Niggli FK, et al. Cohort profile: The Swiss Childhood Cancer Survivor Study. Int J Epidemiol. 2012;41(6):1553–64. 10.1093/ije/dyr142 PubMed Central PMCID: PMC2464. [DOI] [PubMed] [Google Scholar]
- 22.Michel G, von der Weid NX, Zwahlen M, Adam M, Rebholz CE, Kuehni CE. The Swiss Childhood Cancer Registry: rationale, organisation and results for the years 2001–2005. Swiss medical weekly. 2007;137(35–36):502–9. Epub 2007/11/09. 2007/35/smw-11875. . [DOI] [PubMed] [Google Scholar]
- 23.Wengenroth L, Schindler M, Kuonen R, Kuehni CE. Krebs als Kind oder Teenager: das Leben danach—Survivorship-Forschung im Schweizer Kinderkrebsregister. Schweizer Krebsbulletin. 2014;34(4):292–5. [Google Scholar]
- 24.Hawkins MM, Lancashire ER, Winter DL, Frobisher C, Reulen RC, Taylor AJ, et al. The British Childhood Cancer Survivor Study: Objectives, methods, population structure, response rates and initial descriptive information. Pediatric blood & cancer. 2008;50(5):1018–25. [DOI] [PubMed] [Google Scholar]
- 25.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(14):2308–18. Epub 2009/04/15. 10.1200/JCO.2009.22.3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebherr R, Marquis J, Storni M, Wiedenmayer G. Gesundheit und Gesundheitsverhalten in der Schweiz 2007—Schweizerische Gesundheitsbefragung. Neuchâtel: Bundesamt für Statistik; 2010. [Google Scholar]
- 27.Germann U. Abschlussbericht zur Volkszählung 2000. Neuchâtel: Bundesamt für Statistik; 2005. [Google Scholar]
- 28.Faeh D, Bopp M, Swiss National Cohort Study G. Educational inequalities in mortality and associated risk factors: German—versus French-speaking Switzerland. BMC public health. 2010;10:567 10.1186/1471-2458-10-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103(7):1457–67. [DOI] [PubMed] [Google Scholar]
- 30.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate behavioral research. 2011;46(3):399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linder E. Auf dem Weg zur Lohngleichheit! Tatsachen und Trends. Bern: Eidgenössisches Büro für die Gleichstellung von Frau und Mann (EBG) Bundesamt für Statistik (BFS); 2013. [Google Scholar]
- 32.Cangemi V, Capezzali E, Chételat M, Farine A, Häfliger J, Rouvinez Mauron A. Schweizerische Lohnstrukturerhebung 2012. In: (BFS) BfS, editor. Neuchâtel: 2015. [Google Scholar]
- 33.von der Weid NX. Adult life after surviving lymphoma in childhood. Support Care Cancer. 2008. [DOI] [PubMed] [Google Scholar]
- 34.Clanton NR, Klosky JL, Li C, Jain N, Srivastava DK, Mulrooney D, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(11):2559–68. 10.1002/cncr.25797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadan-Lottick NS, Zeltzer LK, Liu Q, Yasui Y, Ellenberg L, Gioia G, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. Journal of the National Cancer Institute. 2010;102(12):881–93. Epub 2010/05/12. 10.1093/jnci/djq156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langeveld NE, Ubbink MC, Last BF, Grootenhuis MA, Voute PA, De Haan RJ. Educational achievement, employment and living situation in long-term young adult survivors of childhood cancer in the Netherlands. Psychooncology. 2003;12(3):213–25. [DOI] [PubMed] [Google Scholar]
- 37.Holmqvist AS, Wiebe T, Hjorth L, Lindgren A, Ora I, Moell C. Young age at diagnosis is a risk factor for negative late socio-economic effects after acute lymphoblastic leukemia in childhood. Pediatric blood & cancer. 2010;55(4):698–707. Epub 2010/07/01. 10.1002/pbc.22670 [DOI] [PubMed] [Google Scholar]
- 38.Castellino SM, Ullrich NJ, Whelen MJ, Lange BJ. Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors. Journal of the National Cancer Institute. 2014;106(8). 10.1093/jnci/dju186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.