Abstract
Background
Brain metastasis is a common endpoint in patients suffering from malignant melanoma. However, little is known about factors that predispose to brain metastases.
Objective
We performed a retrospective clinical and pathological investigation of melanoma patients with brain metastases in order to better characterise this patient population.
Methods
193 melanoma patients with brain metastasis histologically diagnosed between 1990 and 2015 at the University Hospital Zurich were retrospectively identified and further specified for sex, age at diagnosis and detection of brain metastasis, and localisation. In addition, data were extracted regarding the subtype of primary melanoma, Breslow tumour thickness, Clark Level, mutation status, extent of metastatic spread and history of a second melanoma.
Results
We found a significant male predominance (n = 126/193; 65%; p < 0.001). Breslow tumour thickness showed a wide range from 0.2 to 12.0 mm (n = 99; median 2.3 mm). 14 of 101 melanomas (14%) were classified as T1, thereof 11 (79%) were found in men. In 32 of 193 patients (17%), the primary melanoma was unknown.
Conclusions
Of special interest in our series is the high incidence of male predominance (79%) in cases of thin metastasing melanoma (14%), implicating genetic or epigenetic (hormonal) gender differences underlying tumour progression. Additionally, the high percentage of unknown primary melanoma (17%), at least partly representing completely regressed melanomas, indicates the importance of immune surveillance in melanoma progression.
Introduction
The development of brain metastases, reported to occur in up to 25% of patients with cancer, is a common and devastating complication of many systemic malignancies [1]. Cutaneous melanoma is a highly aggressive cancer with a propensity for brain metastases. The reported incidence of cerebral metastases is variable and dependent on whether the source is clinical or autopsy data, the patient population, and other factors. A study analysing 700 melanoma patients treated at Roswell Park Memorial Institute from 1972 to 1978 found that 125/700 (18%) developed brain metastases [2]. In contrast, an autopsy study reported melanoma as the most commonly encountered source of brain metastasis. The incidence of melanoma brain metastasis was recorded as 91%, followed by breast (37%) and lung (34%) cancer [3]. An important event during brain metastasis, at least for some cancers, is the extravasation of cancer cells from the vascular lumina across the blood-brain barrier, which consists of the vascular endothelium and surrounding cells, into the brain parenchyma [4]. Not all cancers seem to share the same propensity to cross the blood-brain barrier. Of note, microRNA-containing vesicles capable of destroying the blood-brain barrier have recently been identified as a mechanism in breast cancer [5].
With particular reference to melanoma, the biology and mechanisms of brain metastasis have received little attention in the literature. Recently, in vitro and in vivo studies by Lugassy and Barnhill demonstrated the capacity of melanoma cells to spread to local and regional (and perhaps even distant) sites via migration along the external (abluminal) surfaces of vascular channels without intravasation [6]. This revolutionary new paradigm of tumour spread has been termed extravascular migratory metastasis and may partially explain why brain metastases are so frequently encountered in melanoma.
In order to better understand the characteristics of this patient population, we retrospectively analysed 193 patients with melanoma brain metastases, diagnosed between 1990 and 2015.
Patients and Methods
Study population
Patients were admitted at the University Hospital Zurich and some provided written informed consent in accordance with the Declaration of Helsinki. As not all patients were able to do so, Ethics Commission Zurich, Switzerland, also approved the here reported melanoma study (approval number KEK-ZH-Nr. 2014–0193 and Stv. 16–2007, amendment 2014).
The electronic database of the Department of Pathology at the University Hospital Zurich, existing since 1990, was searched for melanoma patients with histologically confirmed brain metastasis between 1990 and 2015. In the clinical electronic database, which dates to 1995, patients with histologically confirmed melanoma brain metastasis were further investigated for sex, age at diagnosis of primary melanoma, the sequence and extent of metastatic spread.
In addition, we were interested in identifying patients without lymphatic spread, indicating primary hematogenous or extravascular migratory dissemination. The primary melanoma was characterised according to anatomic location, subtype, Breslow tumour thickness and Clark Level. Brain metastases were further classified as either solitary or multiple. Since 2009 every initial melanoma metastasis was tested for the BRAF mutation status, followed by the NRAS mutation analysis in BRAF wild-type tumours
Follow-up data were extracted, including the time interval between primary diagnosis and manifestation of brain metastases and survival time. Survival information was collected in collaboration with the Cancer Registry Zurich and Zug, Switzerland.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics, version 21.0 (Armonk, NY: IBM Corp.). Results of the descriptive statistics were expressed as numbers, percentages and median with range. Data were analysed using the binominal test, chi-square test, Mann-Whitney U test and the Kruskal-Wallis test. Overall survival was analysed using Kaplan-Meier curves. Median survival is reported with 95% confidence intervals (CI). Groups were compared using the log-rank test. All statistical tests were two-sided and an exact p-value < 0.05 was considered to be statistically significant.
Results
Patients
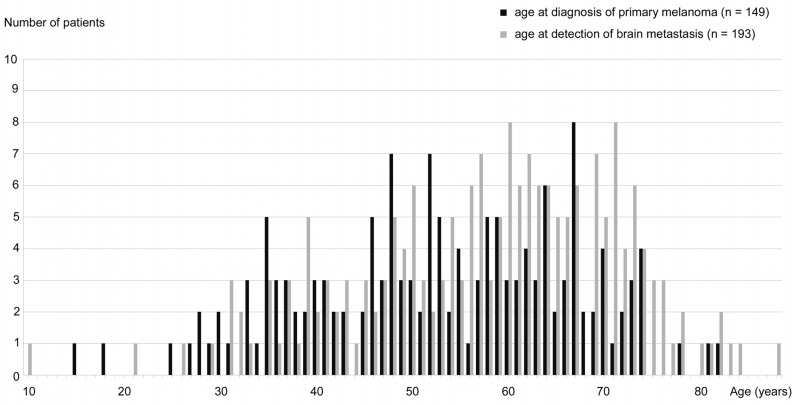
193 patients with melanoma brain metastasis were identified. In this population, there was a significant male predominance (126/193 men (65%) versus 67/193 women (35%); p < 0.001). The median age at diagnosis of primary melanoma was 53.0 years (n = 149; range 15–82 years) (Fig 1A). There was no significant age difference between men and women. The median age at detection of brain metastasis was 60.0 years (n = 193; range 10–88 years) (Fig 1B). There was no significant age difference between men and women.
Fig 1. Age distribution.
(a) Black: age at diagnosis of primary melanoma (n = 149). (b) Grey: age at detection of brain metastasis (n = 193).
155 patients could be analysed with respect to the clinical time course. In the majority of patients (n = 108/155; 70%), brain metastasis was detected later than metastasis to other organs. In 34 patients (22%) brain metastases were simultaneous (within 2 months) with metastases to other organs. Eight patients (5%) presented with brain metastasis before metastasis to other organs. 5 patients (3%) harboured only brain metastasis. At the initial diagnosis of brain metastases, 98 of 193 patients (51%) presented with a solitary and 95 of 193 patients (49%) with multiple brain tumours.
15 of 193 patients (8%) had a history of a second primary melanoma.
Primary melanoma
The back was the most frequent location in males (26 of 90 melanomas; 29%) and the lower extremities in females (14 of 49 melanomas; 29%). Males tended to have melanomas located on the chest and abdomen (p = 0.06), whereas location on the arms was observed more frequently in females (p = 0.07). Mucosal melanomas were significantly more common in women (p = 0.02), taking into account that vaginal primary melanoma accounted for 2 of 5 mucosal locations (40%) in women (Table 1).
Table 1. Location of the primary melanoma according to gender.
| Total cases | Male | Female | p-value | |
|---|---|---|---|---|
| n = 139 | n = 90 | n = 49 | (male vs female) | |
| (100%) | (100%) | (100%) | ||
| Back | 35 (25%) | 26 (29%) | 9 (18%) | n.s. |
| Legs | 28 (20%) | 14 (16%) | 14 (29%) | n.s. |
| Head and neck | 26 (19%) | 20 (22%) | 6 (12%) | n.s. |
| Chest and abdomen | 23 (17%) | 19 (21%) | 4 (8%) | 0.06 |
| Arms | 20 (14%) | 9 (10%) | 11 (22%) | 0.07 |
| Mucosa | 6 (4%) | 1 (1%) | 5 (10%) | 0.02 |
| Iris | 1 (1%) | 1 (1%) | 0 (0%) | n.s. |
n.s., not significant.
Nodular melanoma (NM) was the most common subtype (44 of 105; 42%), followed by superficial spreading melanoma (SSM) in 30 of 105 (29%). There was no significant gender difference in these two subtypes (Table 2).
Table 2. Subtypes of primary melanoma according to gender.
| Total cases | Male | Female | p-value | |
|---|---|---|---|---|
| n = 105 | n = 72 | n = 33 | (male vs female) | |
| (100%) | (100%) | (100%) | ||
| NM | 44 (42%) | 33 (46%) | 11 (33%) | n.s. |
| SSM | 30 (29%) | 22 (31%) | 8 (24%) | n.s. |
| ALM | 7 (7%) | 3 (4%) | 4 (12%) | n.s. |
| LMM | 7 (7%) | 4 (6%) | 3 (9%) | n.s. |
| Mucosal | 6 (6%) | 1 (1%) | 5 (15%) | 0.01 |
| Other variants | 11 (11%) | 9 (13%) | 2 (6%) | n.s. |
NM, nodular melanoma; SSM, superficial spreading melanoma; ALM, acral lentiginous melanoma; LMM, lentigo maligna melanoma; n.s., not significant.
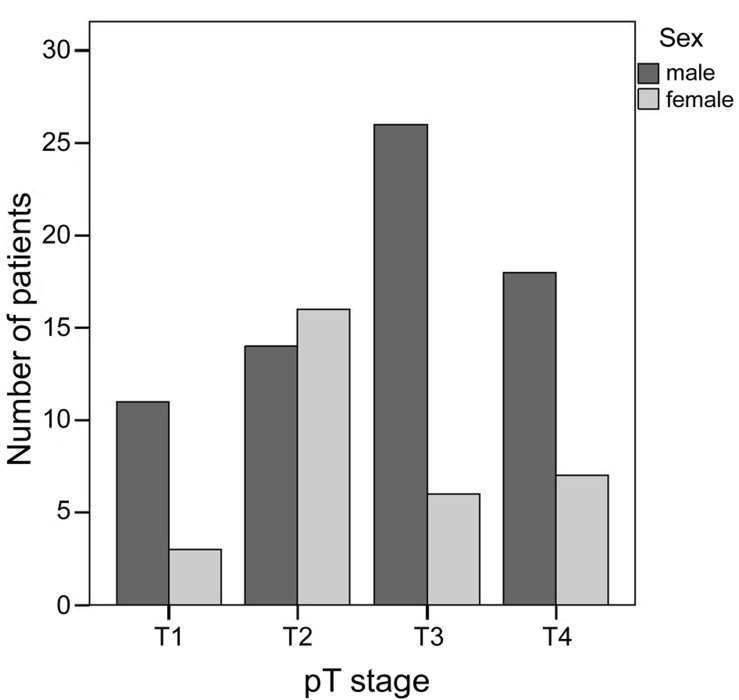
In 101 melanomas, the T classification of the AJCC/UICC staging system [7] was reported. 14 melanomas (14%) were classified as T1 (≤ 1.00 mm thickness), 30 (30%) as T2 (1.01–2.00 mm thickness), 32 (32%) as T3 (2.01–4.00 mm thickness) and 25 (25%) as T4 (> 4 mm thickness). There is a significant gender difference with regard to the T stages (p = 0.02). Males account for 11 out of 14 (79%) T1 melanomas, 14 out of 30 (47%) T2 melanomas, 26 out of 32 (81%) T3 melanomas and 18 out of 25 (72%) T4 melanomas (Fig 2).
Fig 2. Comparison of T stages between males and females.
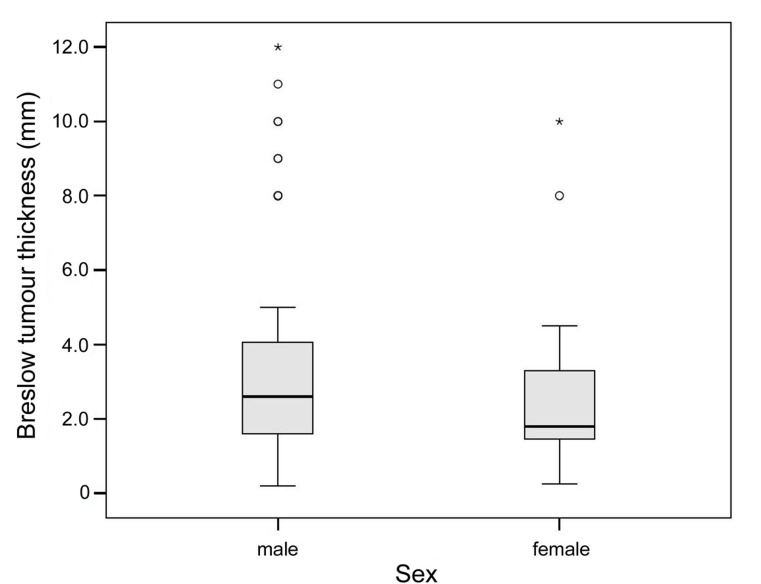
Breslow tumour thickness was reported in 99 primary melanomas and had median of 2.3 mm and a range of 0.2 to 12.0 mm. There was no significant difference in Breslow tumour thickness between men and women (Fig 3). Median tumour thickness was 2.6 mm in men (n = 67; range 0.2–12.0 mm) and 1.8 mm in women (n = 32; range 0.3–10.0 mm). Clark level was reported in 94 melanomas: 3 melanomas (3%) were classified as Clark Level II, 23 (25%) as Clark Level III, 60 (64%) as Clark Level IV and 8 (9%) as Clark Level V.
Fig 3. Comparison of Breslow tumour thickness between males and females.
Thirty-two of 193 (17%) patients had an unknown primary melanoma, 20 (63%) thereof were males.
Mutation status
The mutation status was known in 55 of 193 (28%) patients. In 22 (40%) patients melanomas were BRAF wild-type, 23 (42%) showed a BRAF mutation, and 10 (18%) a NRAS mutation. There was a significant difference in the age at detection of brain metastasis according to the mutational status (p = 0.01). The median age at detection of brain metastasis was 56.0 years in BRAF mutated tumours (range 35–71 years), 59.5 years in NRAS mutated tumours (range 35–76 years) and 69.5 years in BRAF wild-type tumours (range 40–75 years).
Comparing patients with and without lymph node metastasis
Information about lymph node involvement was available in 126 patients. In this cohort, 108 (86%) patients showed lymph node metastasis at the time when brain metastasis was diagnosed, whereas 18 (14%) did not. Among 58 patients with positive nodes, 24 patients (41%) were classified as N1, while the N2 and N3 stages contained 17 patients each (29%). Metastases, apart from lymph nodes and brain, involved the skin, lung, liver, kidney, bone, and other sites in 124 of 193 patients (64%) in the course of their disease.
We found a significant difference in melanoma subtype between patients with and without lymph node metastasis (p = 0.006). All 26 patients with SSM manifested lymphatic spread concurrent with the diagnosis of brain metastasis. In contrast, the absence of lymph node metastasis was found in 3 out of 4 lentigo maligna melanomas (LMM) and in 10 out of 42 NM (24%). NM accounted for the largest group of lymph node negative patients (10/16; 63%) (Table 3). There was no significant difference between patients with and without lymph node metastasis with respect to Breslow tumour thickness. However, there was a significant difference in Breslow tumour thickness between malignant melanoma of NM and SSM types (p < 0.001). Breslow tumour thickness ranged from 1.4 to 9.0 mm in NM (n = 39; median 3.6 mm) and from 0.2 to 4.9 mm in SSM (n = 27; median 1.5 mm).
Table 3. Subtypes of primary melanoma according to the presence or absence of lymph node metastasis.
| Total cases | N+ | N- | p-value | |
|---|---|---|---|---|
| n = 90 | n = 74 | n = 16 | (N+ vs N-) | |
| NM | 42 (100%) | 32 (76%) | 10 (24%) | n.s. |
| SSM | 26 (100%) | 26 (100%) | 0 (0%) | 0.004 |
| ALM | 6 (100%) | 6 (100%) | 0 (0%) | n.s. |
| LMM | 4 (100%) | 1 (25%) | 3 (75%) | 0.02 |
| Mucosal | 3 (100%) | 2 (67%) | 1 (33%) | n.s. |
| Other variants | 9 (100%) | 7 (78%) | 2 (22%) | n.s. |
NM, nodular melanoma; SSM, superficial spreading melanoma; ALM, acral lentiginous melanoma; LMM, lentigo maligna melanoma; N+, lymph node positive melanoma; N-, lymph node negative melanoma; n.s., not significant.
History of a second melanoma was significantly associated with the absence of lymph node metastasis (p = 0.003).
We found no significant difference between patients with and without lymph node metastasis with respect to sex, age at detection of primary melanoma or brain metastasis, occurrence of metastases apart from the lymph nodes and the brain, number of brain metastases, Clark Level and mutation status.
We found no significant difference in the location of primary melanoma with respect to age at detection of brain metastasis.
Comparing cases with known and unknown primary melanoma
There was no significant difference between patients with known and unknown primary melanoma regarding sex, age at detection of brain metastasis, presence or absence of lymph node metastasis, occurrence of metastases apart from the lymph nodes and the brain, number of brain metastases and mutation status.
Compared to other primary melanomas, lymph node and visceral (other than brain) metastases associated with unknown primary melanomas showed a significantly different clinical time course (p = 0.04). In unknown primary melanomas, they were more commonly synchronous (within 2 months) with brain metastases (9/19 cases; 47%). In contrast, most known primary melanomas (99/136; 73%) manifested the sequential development of lymph node or visceral metastases, later followed by brain metastases as the final step in tumour progression (for details consult S1 Dataset).
Follow-up data
Survival and follow-up data were considered as reliable only if patients were dead or reported alive within one year before the end of data collection. 166/193 (86%) patients fulfilled these criteria and were included in the statistical survival analysis. 17 of these 166 well documented patients (10%) were still alive in 2015 or 2016. Of those, 2 patients had a melanoma of unknown primary. In the group of patients with unreliable follow-up data, 21/27 (78%) carried the diagnosis of brain metastases 10 years or longer ago. Considering the short median survival after diagnosis of brain metastasis (0.5 years) in patients with reliable follow-up, it is probable that most patients with poor follow-up data are also dead (for details consult S2 Dataset).
The median age at death was 61.2 years (n = 149; range 24–89 years). There was no significant age difference between men and women. The median time between diagnosis of primary melanoma and of brain metastasis was 3.4 years (n = 149; range 0–33.2 years) without a significant difference between men and women. Breslow tumour thickness neither significantly influenced this time interval. The median survival after detection of brain metastasis was 0.47 years (95% CI 0.39 to 0.66 years) with a borderline significantly better prognosis for women (n = 56; median survival 0.71 years; 95% CI 0.43 to 1.0 years) compared to men (n = 110; median survival 0.43 years; 95% CI 0.32 to 0.56 years; p = 0.05). Breslow tumour thickness did not influence survival after brain metastasis. For the 17 patients who are still alive, the median follow-up after diagnosis of brain metastasis was 1.9 years, range 0.2–13.5 years.
Discussion
In this study of 193 melanoma patients with brain metastases, men (65%) were more frequently affected than women (35%). Gender is widely accepted as an independent prognostic factor in predicting outcome for patients with cutaneous melanoma [8–10]. The predominance of men with brain metastases is congruent with a previous study showing increased risk in men for visceral metastases and a correspondingly higher mortality rate [10]. As men have additional unfavourable primary melanoma characteristics such as increased tumour thickness, ulceration, axial location and older age [11], there is uncertainty as to whether this gender difference is due to behavioural factors or an inherent biological difference. Negligence with respect to health care is often suggested to explain this gender difference for thick melanomas, as we observed in this study. Aside from nodal status, Breslow tumour thickness remains among the most important prognostic indicators for melanoma. Additionally, tumour thickness has been reported as the only prognostic confounder affecting gender survival differences in one study [10]. Therefore, our finding of a significant gender difference with regard to T stages (p = 0.02) is of considerable interest. Of special note is the high percentage (14 of 101; 14%) of T1 stage melanomas with clear-cut male predominance (79% men). This observation is supported by previous studies indicating that female survival advantage relates at least partially to biological differences, either tumour or host related [10, 12–14]. Interestingly a recent study demonstrated that sex differences in melanoma survival is not related to mitotic rate of the primary tumour [15]. In our study men displayed a borderline significantly shorter median survival after detection of brain metastasis compared to women (p = 0.05). This observation underscores a survival benefit in women. However, interpretation of survival data is limited by the different treatment regimens in this study cohort. Vice versa, the prognostic value of the current dataset is enhanced by the fact that all patients underwent surgery at least once for histologic confirmation of brain metastasis.
Thin T1 melanomas have an excellent prognosis; the range of melanoma associated deaths reported in the literature however is substantial (1–8% at 10 years [7, 16–18]). In addition, many studies regarding melanoma recurrence and survival are reported with end points of 5 to 10 years. Especially for thin melanomas, this period of surveillance is too short since approximately 10% of lesions show recurrence beyond 10 years [19]. Many large multicentre survival studies have failed to make adjustments to differentiate between death from all causes and death due to disease. In such cases, longer observation periods may result in falsely high results. Accordingly, our results with the endpoint of brain metastases provide more robust survival information. Importantly, our data illustrate that there is a significant male predominance for thin melanomas with subsequent brain metastasis.
Interestingly, we found 17% of patients with unknown primary melanomas. This incidence is much higher than previously reported (3.2% [20] and 0.9% [21], respectively). In routine clinical practice, metastatic melanoma with unknown primary is considered to originate from completely regressed primary melanomas. From our experience, especially in immune compromised patients after organ transplantation [22], we fully recognise the importance of immune surveillance in preventing the development and progression of cancer [23]. Regression is common in melanoma, particularly in thin lesions with radial growth phase. Its incidence across all melanoma tumour thicknesses amounts to 10–35% [24], and up to 58% in thin melanomas (< 0.75 mm) [25]. Regression has the following histologically defined phases: An early phase characterised by an area of tumour accompanied by tumour infiltrating lymphocytes (TILS) and associated with necrosis of tumour cells. In the intermediate phase, tumour gradually replaced by granulation tissue and partly pigmented macrophages. In the late phase, the histological picture is dominated by scar with partly pigmented macrophages. In the final phase, melanoma cells are completely replaced by scar tissue, thus precluding the histological diagnosis of melanoma [26]. Clark et al. have shown in 1989 that TILS are associated with good prognosis and many authors have confirmed this observation since then [9, 24, 25]. On the contrary, prognostic significance of regression, particularly in patients with thin melanomas, has been lately controversially debated. Some studies have found that the presence of regression is a poor prognostic indicator [26–31], while others found that it has no effect or even a benefit on the risk of recurrence or survival [32–35]. Possible limitation of some multi-institutional and multinational studies might be methodical inconsistencies, for example not performing slide reviews. Additionally, assessment of regression has high interobserver variability [25, 28, 36]. Interestingly several studies have shown that thin melanomas with extensive late regression have higher risk for metastatic disease [26, 29–31]. Our results coincide with these findings.
In our patients with unknown primaries, which at least partially represent completely regressed melanomas, there was a significant difference in the pattern of metastatic spread (p = 0.04). Compared to other primary melanomas, lymph node and visceral (other than brain) metastases associated with unknown primary melanomas were more commonly synchronous (within 2 months) with brain metastases (in 9/19 cases; 47%), indicating an aggressive clinical course. In contrast, most known primary melanomas (99/136; 73%) manifested the sequential development of lymph node or visceral metastases, later followed by brain metastases as the final step in tumour progression. However, this difference might result from a diagnostic bias because all patients included in the study had brain metastasis. Unfortunately, a prospective approach to confirm our results is not feasible.
Absence of lymph node metastases was found in 16 out of 90 (18%) melanoma patients at the time of initial diagnosis of brain metastasis. Interestingly, most of those patients had primary melanomas of nodular type (10/16; 63%) with Breslow tumour thickness between 1.7 and 5.0 mm. In contrast, all 26 patients with SSM type melanoma showed lymph node metastases, and their tumours were significantly thinner when compared to NM type melanomas (p < 0.001) (Table 3). These observations suggest that tumour thickness plays an important role in primary hematogenous metastasis.
Furthermore, patients without lymphatic spread had significantly greater numbers of multiple primary melanomas (p = 0.003), indicating a special genetic and/ or immunological status in this subgroup.
Interestingly, a recent study identified more frequent BRAF or NRAS mutations in brain metastases compared to primaries and metastasis at other sites [37]. The NRAS mutation was reported as an independent prognostic factor in metastatic melanoma [38]. Furthermore, melanomas harbouring NRAS or BRAF mutations were associated with a trend towards worse survival and a greater likelihood of brain metastases at the time of initial diagnosis [37]. In our cohort the BRAF and NRAS mutation status was documented in 55 of 193 patients (29%): 22 patients (40%) manifested BRAF wild-type, 23 (42%) BRAF mutations, and 10 (18%) NRAS mutations. The latter results are congruent with those found in the literature [39]. Depending upon the mutational status of the patient, there was a significant difference in patient age at the time of detection of brain metastases (p = 0.01). The median age at detection of brain metastasis was 56.0 years in BRAF mutated tumours, 59.5 years in NRAS mutated tumours and 69.5 years in BRAF wild-type tumours. This age difference between patients with BRAF and NRAS mutations is consistent with that reported in the literature [38, 40–41].
Conclusions
What emerges from this study is a clearer picture of factors associated with brain metastasis in patients suffering from malignant melanoma. Accordingly, our results provide a framework for the reliable stratification of metastatic potential, which in turn should facilitate rational clinical planning and treatment.
Of special interest are the higher incidence of thin melanomas (14%) with male predominance (79%) and the higher incidence of unknown primaries (17%) (representing at least partly completely regressed melanomas) with metastatic disease if compared with the literature. These findings illustrate the importance of gender (genetic or epigenetic) and immune surveillance in melanoma progression.
Supporting Information
(XLSX)
(XLSX)
Acknowledgments
We thank Dr. Miriam Wanner from the Cancer Registry Zurich and Zug, Switzerland, for contributing follow-up data of our patient population.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Kaal EC, Niel CG, Vecht CJ. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4(5):289–98. 10.1016/S1474-4422(05)70072-7 . [DOI] [PubMed] [Google Scholar]
- 2.Madajewicz S, Karakousis C, West CR, Caracandas J, Avellanosa AM. Malignant melanoma brain metastases. Review of Roswell Park Memorial Institute experience. Cancer. 1984;53(11):2550–2. . [DOI] [PubMed] [Google Scholar]
- 3.Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer. 1963;16:781–7. . [DOI] [PubMed] [Google Scholar]
- 4.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. 10.1016/j.nbd.2003.12.016 . [DOI] [PubMed] [Google Scholar]
- 5.Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716 10.1038/ncomms7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnhill RL, Lugassy C. Angiotropic malignant melanoma and extravascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology. 2004;36(5):485–90. 10.1080/00313020412331282708 . [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Gershenwald JE, Soong S-j, Thompson JF, Atkins MB, Byrd DR, et al. Final Version of 2009 AJCC Melanoma Staging and Classification. Journal of Clinical Oncology. 2009;27(36):6199–206. 10.1200/jco.2009.23.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw HM, McGovern VJ, Milton GW, Farago GA, McCarthy WH. Histologic features of tumors and the female superiority in survival from malignant melanoma. Cancer. 1980;45(7):1604–8. . [DOI] [PubMed] [Google Scholar]
- 9.Clark WH Jr, Elder DE, Guerry DT, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81(24):1893–904. . [DOI] [PubMed] [Google Scholar]
- 10.Joosse A, de Vries E, Eckel R, Nijsten T, Eggermont AM, Holzel D, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol. 2011;131(3):719–26. 10.1038/jid.2010.354 . [DOI] [PubMed] [Google Scholar]
- 11.Scoggins CR, Ross MI, Reintgen DS, Noyes RD, Goydos JS, Beitsch PD, et al. Gender-Related Differences in Outcome for Melanoma Patients. Annals of Surgery. 2006;243(5):693–700. 10.1097/01.sla.0000216771.81362.6b . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joosse A, Collette S, Suciu S, Nijsten T, Lejeune F, Kleeberg UR, et al. Superior outcome of women with stage I/II cutaneous melanoma: pooled analysis of four European Organisation for Research and Treatment of Cancer phase III trials. J Clin Oncol. 2012;30(18):2240–7. 10.1200/JCO.2011.38.0584 . [DOI] [PubMed] [Google Scholar]
- 13.Joosse A, Collette S, Suciu S, Nijsten T, Patel PM, Keilholz U, et al. Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage III to IV melanoma: a pooled analysis of five European organisation for research and treatment of cancer randomized controlled trials. J Clin Oncol. 2013;31(18):2337–46. 10.1200/JCO.2012.44.5031 . [DOI] [PubMed] [Google Scholar]
- 14.Lasithiotakis K, Leiter U, Meier F, Eigentler T, Metzler G, Moehrle M, et al. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer. 2008;112(8):1795–804. 10.1002/cncr.23359 [DOI] [PubMed] [Google Scholar]
- 15.Joosse A, van der Ploeg AP, Haydu LE, Nijsten TE, de Vries E, Scolyer RA, et al. Sex differences in melanoma survival are not related to mitotic rate of the primary tumor. Annals of surgical oncology. 2015;22(5):1598–603. 10.1245/s10434-014-4166-8 . [DOI] [PubMed] [Google Scholar]
- 16.Sahin S, Rao B, Kopf AW, Lee E, Rigel DS, Nossa R, et al. Predicting ten-year survival of patients with primary cutaneous melanoma: corroboration of a prognostic model. Cancer. 1997;80(8):1426–31. . [PubMed] [Google Scholar]
- 17.Kalady MF, White RR, Johnson JL, Tyler DS, Seigler HF. Thin melanomas: predictive lethal characteristics from a 30-year clinical experience. Annals of surgery. 2003;238(4):528–35; discussion 35–7. 10.1097/01.sla.0000090446.63327.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinnon JG, Yu XQ, McCarthy WH, Thompson JF. Prognosis for patients with thin cutaneous melanoma: long-term survival data from New South Wales Central Cancer Registry and the Sydney Melanoma Unit. Cancer. 2003;98(6):1223–31. 10.1002/cncr.11624 . [DOI] [PubMed] [Google Scholar]
- 19.Crowley NJ, Seigler HF. Relationship between disease-free interval and survival in patients with recurrent melanoma. Arch Surg. 1992;127(11):1303–8. . [DOI] [PubMed] [Google Scholar]
- 20.Kamposioras K, Pentheroudakis G, Pectasides D, Pavlidis N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit Rev Oncol Hematol. 2011;78(2):112–26. 10.1016/j.critrevonc.2010.04.007 . [DOI] [PubMed] [Google Scholar]
- 21.Geller AC, Clapp RW, Sober AJ, Gonsalves L, Mueller L, Christiansen CL, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31(33):4172–8. 10.1200/JCO.2012.47.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Euvrard S, Kanitakis J, Claudy A. Skin Cancers after Organ Transplantation. New England Journal of Medicine. 2003;348(17):1681–91. 10.1056/NEJMra022137 . [DOI] [PubMed] [Google Scholar]
- 23.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. 10.1111/j.1365-2567.2007.02587.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–10. . [DOI] [PubMed] [Google Scholar]
- 25.Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25(7):869–75. 10.1200/JCO.2006.08.9755 . [DOI] [PubMed] [Google Scholar]
- 26.McGovern VJ, Shaw HM, Milton GW. Prognosis in patients with thin malignant melanoma: influence of regression. Histopathology. 1983;7(5):673–80. 10.1111/j.1365-2559.1983.tb02279.x [DOI] [PubMed] [Google Scholar]
- 27.Shaw HM, McCarthy SW, McCarthy WH, Thompson JF, Milton GW. Thin regressing malignant melanoma: significance of concurrent regional lymph node metastases. Histopathology. 1989;15(3):257–65. . [DOI] [PubMed] [Google Scholar]
- 28.Cook MG, Spatz A, Brocker EB, Ruiter DJ. Identification of histological features associated with metastatic potential in thin (<1.0 mm) cutaneous melanoma with metastases. A study on behalf of the EORTC Melanoma Group. J Pathol. 2002;197(2):188–93. 10.1002/path.1093 . [DOI] [PubMed] [Google Scholar]
- 29.Gromet MA, Epstein WL, Blois MS. The regressing thin malignant melanoma: a distinctive lesion with metastatic potential. Cancer. 1978;42(5):2282–92. . [DOI] [PubMed] [Google Scholar]
- 30.Guitart J, Lowe L, Piepkorn M, Prieto VG, Rabkin MS, Ronan SG, et al. Histological characteristics of metastasizing thin melanomas: a case-control study of 43 cases. Arch Dermatol. 2002;138(5):603–8. . [DOI] [PubMed] [Google Scholar]
- 31.Slingluff CL Jr, Vollmer RT, Reintgen DS, Seigler HF. Lethal "thin" malignant melanoma. Identifying patients at risk. Annals of surgery. 1988;208(2):150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris KT, Busam KJ, Bero S, Patel A, Brady MS. Primary cutaneous melanoma with regression does not require a lower threshold for sentinel lymph node biopsy. Annals of surgical oncology. 2008;15(1):316–22. 10.1245/s10434-007-9675-2 . [DOI] [PubMed] [Google Scholar]
- 33.Ribero S, Gualano MR, Osella-Abate S, Scaioli G, Bert F, Sanlorenzo M, et al. Association of Histologic Regression in Primary Melanoma With Sentinel Lymph Node Status: A Systematic Review and Meta-analysis. JAMA Dermatol. 2015;151(12):1301–7. 10.1001/jamadermatol.2015.2235 . [DOI] [PubMed] [Google Scholar]
- 34.Ribero S, Osella-Abate S, Sanlorenzo M, Savoia P, Astrua C, Cavaliere G, et al. Favourable prognostic role of regression of primary melanoma in AJCC stage I-II patients. The British journal of dermatology. 2013;169(6):1240–5. 10.1111/bjd.12586 . [DOI] [PubMed] [Google Scholar]
- 35.White RL Jr, Ayers GD, Stell VH, Ding S, Gershenwald JE, Salo JC, et al. Factors predictive of the status of sentinel lymph nodes in melanoma patients from a large multicenter database. Annals of surgical oncology. 2011;18(13):3593–600. 10.1245/s10434-011-1826-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang S, Barnhill RL, Mihm MC Jr, Sober AJ. Histologic regression in malignant melanoma: an interobserver concordance study. J Cutan Pathol. 1993;20(2):126–9. . [DOI] [PubMed] [Google Scholar]
- 37.Thumar J, Shahbazian D, Aziz SA, Jilaveanu LB, Kluger HM. MEK targeting in N-RAS mutated metastatic melanoma. Mol Cancer. 2014;13:45 10.1186/1476-4598-13-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakob JA, Bassett RL Jr, Ng CS, Curry JL, Joseph RW, Alvarado GC, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118(16):4014–23. 10.1002/cncr.26724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas NE, Edmiston SN, Alexander A, Groben PA, Parrish E, Kricker A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncology. 2015;1(3):359–68. 10.1001/jamaoncol.2015.0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekedahl H, Cirenajwis H, Harbst K, Carneiro A, Nielsen K, Olsson H, et al. The clinical significance of BRAF and NRAS mutations in a clinic-based metastatic melanoma cohort. British Journal of Dermatology. 2013;169(5):1049–55. 10.1111/bjd.12504 [DOI] [PubMed] [Google Scholar]
- 41.Edlundh-Rose E, Egyhazi S, Omholt K, Mansson-Brahme E, Platz A, Hansson J, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res. 2006;16(6):471–8. 10.1097/01.cmr.0000232300.22032.86 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.