Abstract
Aspergillus flavus colonizes numerous oil seed crops such as corn, peanuts, treenuts and cotton worldwide, contaminating them with aflatoxin and other harmful potent toxins. In the phylogenetically related model fungus Aspergillus nidulans, the methyltransferase, RmtA, has been described to be involved in epigenetics regulation through histone modification. Epigenetics regulation affects a variety of cellular processes, including morphogenesis and secondary metabolism. Our study shows that deletion of rmtA in A. flavus results in hyperconidiating colonies, indicating that rmtA is a repressor of asexual development in this fungus. The increase in conidiation in the absence of rmtA coincides with greater expression of brlA, abaA, and wetA compared to that in the wild type. Additionally, the rmtA deletion mutant presents a drastic reduction or loss of sclerotial production, while forced expression of this gene increased the ability of this fungus to generate these resistant structures, revealing rmtA as a positive regulator of sclerotial formation. Importantly, rmtA is also required for the production of aflatoxin B1 in A. flavus, affecting the expression of aflJ. Furthermore, biosynthesis of additional metabolites is also controlled by rmtA, indicating a broad regulatory output in the control of secondary metabolism. This study also revealed that rmtA positively regulates the expression of the global regulatory gene veA, which could contribute to mediate the effects of rmtA on development and secondary metabolism in this relevant opportunistic plant pathogen.
Introduction
The genus Aspergillus includes some of the most harmful fungal species known. Aspergillus flavus is one of these organisms, known mostly for its impact in agriculture. This fungus is an opportunistic pathogen of important oil seed crops, such as corn, peanuts, sorghum, cotton and treenuts. It efficiently disseminates in fields by producing asexual spores called conidia, infecting susceptible plants and contaminating them with highly carcinogenic mycotoxins, such as aflatoxins [1]. Once the fungus is established, formation of highly resistant structures termed sclerotia contribute to its survival under harsh environmental conditions [2]. Aflatoxins and other mycotoxins are thought to contaminate approximately one quarter of the world’s crops [3]. Economically, aflatoxin contamination leads to substantial losses each year mainly due to the necessary investments in the detection of infected crops and the removal of contaminated crops in developed countries. Both the U.S. Food and Drug Administration and the European Union have set limits on the amount of aflatoxins allowed in food and feed commodities of 20 parts per billion and 2 parts per billion respectively [4]. In developing countries lacking regulatory oversight for allowable levels of aflatoxin contamination in crops, adverse health impacts are also of concern due to ingestion of contaminated food or feed. This includes acute aflatoxicosis (aflatoxin poisoning) that can lead to jaundice, edema of the limbs, pain, vomiting, necrosis, and potentially acute liver failure or death [5–8]. Chronic aflatoxicosis can result in cancer (primarily liver cancer), immune suppression, stunted growth in children, and other pathological conditions [6, 8, 9]. Due to increasing climate change, outbreaks of aflatoxin contamination and those of other mycotoxins are predicted to become more prevalent worldwide [10]. Increased incidences of drought and higher temperatures can lead to conditions favoring A. flavus growth and aflatoxin production while weakening host plant defenses [11]. Furthermore, elevated carbon dioxide levels along with other environmental factors linked to climate change have been shown to cause increased expression of genes in the aflatoxin biosynthetic pathway [12].
Current approaches are insufficient to control crop colonization and aflatoxin contamination by A. flavus. It is possible that uncovering the regulatory mechanism governing aflatoxin production as well as those controlling A. flavus development and survival could provide novel genetic targets to be used in control strategies to decrease the detrimental effects caused by this fungus. Previous studies, particularly in Aspergillus, revealed that secondary metabolism, including the production of mycotoxins, and fungal development are genetically linked i.e. [13–17]. Examples of this coordinated regulatory mechanism are the putative methyltransferase LaeA and the global regulator VeA. These proteins, shown to interact with each other as part of a protein complex designated velvet [2, 18], are epigenetic regulators governing aflatoxin production, as well as conidiation and sclerotial formation in A. flavus [19–21]. Arginine methyltransferases (PRMTs) are epigenetic regulators that work through histone methylation. Arginine methylation of histones has been associated with transcriptional regulation, RNA processing and transport, signal transduction and DNA repair in mammals [22]. PRMTs transfer methyl groups from S-adenosylmethionine (SAM) to the guanidine nitrogen atoms of arginine [23]. This methylation results in a dimethylated arginine that can be in either an asymmetric or symmetric configuration [24]. These methylation patterns define two types of PRMTs: type I catalyze asymmetric dimethylation and type 2 catalyze symmetric dimethylation [24]. So far nine different PRMTs have been identified in humans, and homologs of three of these, PRMT1, PRMT3, and PRMT5, have been found to be conserved in other eukaryotes [24], including lower eukaryotes such as the yeast Saccharomyces cerevisiae [25] and the model filamentous fungus Aspergillus nidulans [26], where the genes encoding these proteins were designated rmtA, rmtB and rmtC [26]. Homologs of these PRMTs have not been previously characterized in A. flavus.
The present study focuses on elucidating the role of the PRMT1/rmtA homolog in A. flavus. Previous work showed that PRMT1/rmtA targets the amino-terminal tails of arginine 3 residue on the H4 histone inducing changes in chromatin structure in humans [27, 28] and in A. nidulans [23, 26] with a type I methylation pattern [26]. Several PRMT1/rmtA homologs have been further characterized in other fungi. In S. cerevisiae, mutations in Hmt1, the PRMT1/rmtA homolog, resulted in cold sensitive alleles [25]. Deletion of rmtA in A. nidulans causes growth reduction under conditions of oxidative stress [29]. In Fusarium graminearum, deletion of amt1, also a homolog of rmtA, lead to a reduction in vegetative growth, oxidative stress tolerance, virulence, and deoxynivalenol production [30]. Neurospora crassa, amt-1 was necessary to sustain normal growth rates [31]. Our current studies in A. flavus revealed an indispensable role of rmtA in proper regulation of secondary metabolism, specifically aflatoxin biosynthesis, as well as in developmental processes, affecting conidiation and production of sclerotia in this agriculturally important fungus. It is possible that alteration of epigenetic regulation involving PRMTs could be used in novel control approaches to reduce the detrimental effects of A. flavus and of other fungal species.
Materials and Methods
Sequence Search, Alignment and Phylogenetic Analysis
The deduced amino acid sequence encoded by A. flavus rmtA (AFL2G_09078) was obtained from the Broad Institute Aspergillus Comparative Database. BLAST searches were performed on NCBI (http://www.ncbi.nlm.nih.gov/) using the blastp search tools to obtain the homologous sequences and corresponding e-values. The search was carried out using the A. flavus RmtA deduced protein sequence as query. To compare similarity and identity of RmtA to other homologs, a Needle pairwise sequence alignment was performed (http://www.ebi.ac.uk/Tools/psa/emboss_needle/). MUSCLE sequence alignment (http://www.ebi.ac.uk/Tools/msa/muscle/) was carried out with A. flavus RmtA as well as its homologs from other eukaryotes. This was followed by shading using the Boxshade tool version 3.21 (http://www.ch.embnet.org/software/BOX_form.html).
Phylogenetic analysis was performed with two groups of species. The first group included different fungal species: Asperillus oryzae, Aspergillus terreus, Aspergillus fumigatus, Aspergillus kawachii, Aspergillus niger, Aspergillus nidulans, Neurospora crassa, Fusarium graminearum, Cyrptococcus neoformans, Schizosaccharomyces pombe, Saccharomyces cerevisiae, Candida albicans, Rhodosporidium toruloides, Puccinia graminis, Trichosporon asahii, and Coprinopsis cinerea. The second group consisted of eukaryotic model organisms: Schizosaccharomyces pombe, Saccharomyces cerevisiae, Aspergillus nidulans, Homo sapiens, Arabidopsis thaliana, Xenopus tropicalis, Danio rerio, Mus musculus, Drosophila melanogaster, and Caernorhabditis elegans.
The software MEGA v6.0 was used for sequence alignment and analysis [32]. MUSCLE settings were used for multiple sequence alignment. For generation of phylogenetic trees, a Maximum-likelihood model was used with a bootstrap value of 1000 (http://megasoftware.net/).
Strains and Growth Conditions
Aspergillus flavus CA14-wild-type (WT) (Δku70), CA14 (pyrG−, niaD−, Δku70) (SRRC collection # 1709), CA14-ΔrmtA (ΔrmtA::pyrG, Δku70), CA14-com-rmtA (ΔrmtA::pyrG, rmtA::niaD, Δku70), and CA14-OErmtA (gpdA(p)::rmtA::trpC(t)::pyrG, Δku70) strains were used in this study. All strains were grown on Potato Dextrose Broth (PDB) medium at 30°C in the dark, unless specified differently. Agar (10 g/L) was added in the case of solid medium (PDA). Strains were maintained as 30% glycerol stocks at -80°C.
Generation of the rmtA Deletion Strain (ΔrmtA)
An rmtA deletion cassette was generated by fusion PCR as previously described [33]. The 5’ and 3’ UTR fragments were first PCR amplified using primers Afl_rmtA_p1 and Afl_rmtA_p2, obtaining a 1.3 kb product corresponding to the 5’ UTR, and primers Afl_rmtA_p3 and Afl_rmtA_p4, obtaining a 1.6 kb fragment of the 3’UTR. For both reactions, A. flavus CA14 genomic DNA was used as template. These two DNA fragments were then PCR fused to another fragment corresponding to the pyrG marker from Aspergillus fumigatus. The intermediate fragment containing the marker was PCR amplified from plasmid p1439 using primers Afl_rmtA_p5 and Afl_rmtA_p6. Primer pair AFL_RMTA_F and AFL_RMTA_R was used for the final fusion PCR step. All the primers used in this study are listed in S1 Table. Fungal transformation was performed using A. flavus CA14 as the host strain as previous described [34]. Transformants were selected on Czapek Dox (CZ, Difco, Franklin Lakes, New Jersey, USA) plus sucrose as osmotic stabilizer without supplementation of uridine and uracil. Transformants were confirmed by Southern blot analysis. A selected deletion rmtA strain was then transformed with plasmid pSDS2.2, containing niaD A. fumigatus to obtain a prototroph.
Generation of the rmtA Complementation Strain
A complementation strain was obtained by transforming the A. flavus ΔrmtA mutant with the rmtA wild-type allele. The complementation vector was generated as follows: a DNA fragment contained the entire rmtA coding region and 5′ and 3′ UTRs was first PCR amplified with primers comp RMTA_flavus_F and comp RMTA_flavus_R (S1 Table) from CA14 genomic DNA. Then, the PCR product was digested with NotI and PstI and ligated to the pSD52.2 vector, previously digested with the same restriction enzymes. pSD52.2 contains the niaDA. fumigatus transformation marker. The resulting plasmid was designated pSD52.2-rmtA-com. This vector was then transformed into ΔrmtA, and the transformants were selected on CZ medium using nitrate as the sole nitrogen source. Complementation was confirmed by diagnostic PCR using OE_RMTA_F and OE_RMTA_R (S1 Table).
Generation of the rmtA Over-Expression Strain (OErmtA)
To generate the rmtA over-expression strain, the entire rmtA coding region was PCR amplified from CA14 genome using the OE_RMTA_F and OE_RMTA_R primers (S1 Table). The PCR product was then digested with AscI and NotI and ligated into the previously digested pTDS.1 plasmid, containing an A. nidulans gpdA promoter and trpC terminator, as well as the pyrGA. fumigatus marker. This resulted in the generation of plasmid pTDS.1rmtAOE. The vector was then transformed into CA14. Confirmation of plasmid integration in the transformants was performed by diagnostic PCR using primers OE_RMTA_F and OE_RMTA_R. A selected transformant was converted into a prototroph by transforming the strain with pSDS2.2.
Morphological Studies
Aspergillus flavus wild type, ΔrmtA, complementation and OErmtA strains were point-inoculated on 30 ml of PDA medium and incubated in the dark at 30°C. Fungal growth was measured as colony diameter (mm) each day. To quantify conidial and sclerotial production, 106 spores/ml were inoculated into 5 ml of melted PDA top agar and placed onto a 25 ml PDA solid medium. Cores (7 mm diameter) were collected to quantify conidia after 48 h and 72 h and 7 days, homogenized in water and counted using a hemocytometer (Hausser Scientific, Horsham, PA) under a Nikon Eclipse E-400 bright-field microscope (Nikon Inc., Melville, NY, USA). Sclerotial cores (16 mm diameter) were collected 24 days after inoculation, washed with 70% ethanol to eliminate conidiophores and counted using a Lieder stereo-zoom microscope. Experiments were performed in triplicate.
For sclerotial morphological analysis, strains were point-inoculated on 35 ml of PDA, on GMM [35] with 2% sorbitol, and on Wickerham medium (2 g yeast extract, 3 g peptone, 5 g corn steep solids, 2 g dextrose, 30 g sucrose, 2 g NaNO3, 1 g K2HPO4∙3H2O, 0.5 g MgSO4∙7H2O, 0.2 g KCl, 0.1 g FeSO4∙7H2O, 15 g agar per liter—pH 5.5) [36]. The strains were incubated at 30°C. Micrographs were obtained using a Leica MZ75 dissecting microscope attached to a Leica DC50LP camera (Leica Microsystems Inc., Buffalo Grove, IL, USA). Micrographs were taken from the cultures after an ethanol (70%) wash to remove conidiophores. The experiment was also performed with 3 replicates.
Aflatoxin Analysis
Aflatoxin was extracted from top-agar inoculated cultures (three cores—16 mm diameter) with 5 ml chloroform. The chloroform phase was then collected and allowed to evaporate overnight. Dried residues were resuspended in 300 μl of chloroform. Twenty-five microliters of each extracts were separated by thin layer chromatography (TLC) as previously described [37] on silica plate (Si250F, J.T. Baker) using chloroform:acetone (85:15,v/v) as solvent system. Then plates were allowed to air dry, sprayed with 12.5% AlCl3 solution in 95% ethanol and baked at 80°C for 10 minutes. Presence of aflatoxin was detected under ultraviolet light at a wavelength of 375 nm. The aflatoxin B1 standard was purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
Oxidative Stress Tolerance
PDA medium plates containing different concentrations of menadione (0 mM, 5 mM, 7.5 mM, 10 mM, 12.5 mM, and 15 mM) were point-inoculated with the A. flavus wild type, ΔrmtA, complementation and OE rmtA strains. Cultures were incubated for 48 h in the dark at 30°C.
Gene Expression Analysis
Petri dishes containing 25 ml of PDB (Potato Dextrose Broth) were inoculated with conidia (106 spores/ml) of A. flavus wild type, ΔrmtA, complementation and OErmtA strains. Cultures were incubated in liquid stationary conditions at 30°C in the dark. Total RNA was extracted from lyophilized mycelial samples using TRIsure (Bioline, Taunton, MA, USA) reagent according to the manufacturer instructions. Gene expression analysis was performed either by Northern blot or qRT-PCR. For Northern blots, probe templates of aflM/ver-1 were amplified by PCR from A. flavus genomic DNA using primers ver1-Nor-S and ver1-Nor-A and labeled with dCTPp32 (S1 Table). For qRT-PCR, five micrograms of total RNA was treated with RQ1 RNase-Free DNase (Promega. Madison, WI, USA). cDNA was synthesized with Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega, Madison, WI, USA). qRT-PCR was performed with the Applied Biosystems 7000 Real-Time PCR System using SYBR green dye for fluorescence detection. cDNA was normalized to A. flavus 18S ribosomal gene expression, and the relative expression levels were calculated using the 2-ΔΔCT method [38]. Primer pairs used are indicated in S1 Table.
Statistical Analysis
Statistical analysis was applied to analyze all of the quantitative data in this study utilizing ANOVA (analysis of variance), in conjunction with a Tukey's multiple comparison testusing a p-value of p < 0.05 for samples that are determine to be significantly different.
Results
RmtA Is Conserved in Other Eukaryotes
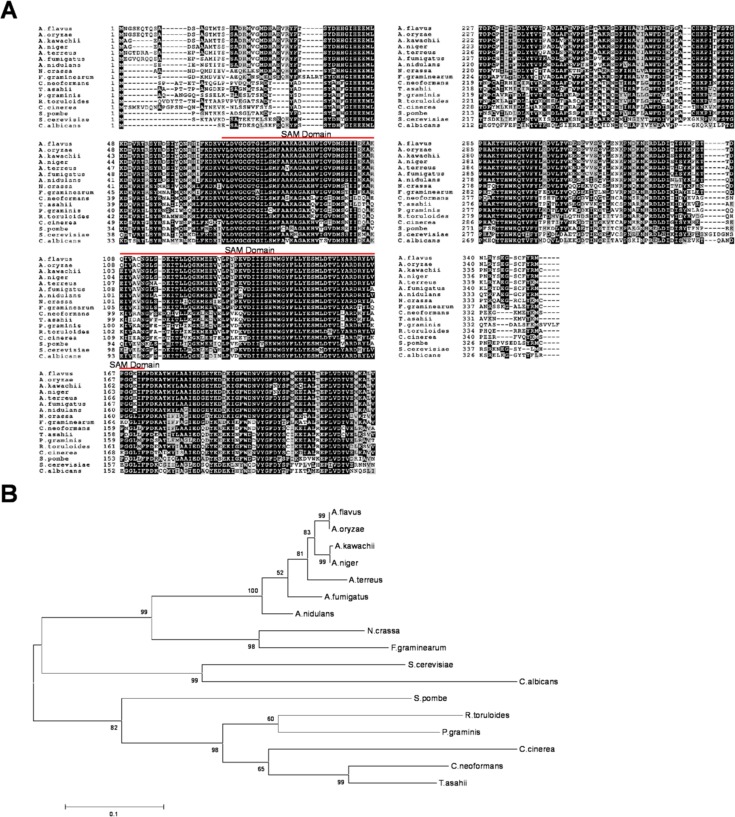
Comparative analysis of the A. flavus RmtA deduced amino acid sequence revealed significant identity (>50%) with putative homologs present in numerous fungal species (S2 Table). This trend was also identified among model eukaryotic species, with shared identity greater than 45% (S3 Table). Sequence alignment showed a highly conserved S-adenosyl methionine (SAM) binding domain among these putative homologs in other fungal species and other non-fungal eukaryotes (Fig 1A & S1A Fig). In addition, the RmtA phylogenetic tree was consistent with both fungal and other eukaryotes’ taxonomy (Fig 1B & S1B Fig) [39].
Fig 1. Multiple sequence alignment and phylogenetic analysis of RmtA and other fungal homologs.
(A) Sequences aligned using Muscle (http://www.ebi.ac.uk/Tools/msa/muscle/). Alignment was visualized with BoxShade v3.21 (http://ch.embnet.org/software/BOX_form.html). (B) Phylogenetic tree of RmtA homologs from different fungal species. Phylogenetic trees constructed using MEGA v6.0. Trees were generated with Maximum-Likelihood model with a bootstrap value of 1000.
Comparison of A. flavus RmtA, RmtB, and RmtC deduced amino acid sequences revealed to be distinct from each other (S2 Fig), while they are highly conserved with respect to their corresponding homologs in A. nidulans (S2B Fig).
rmtA Is Required for Normal Conidiation
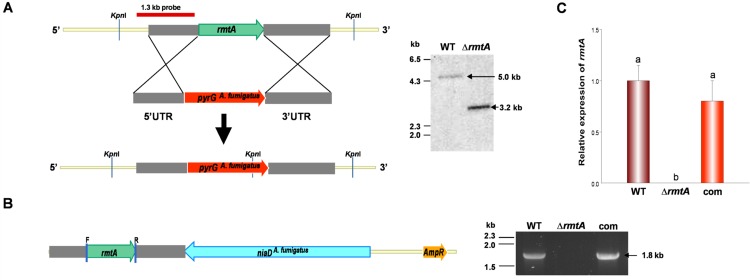
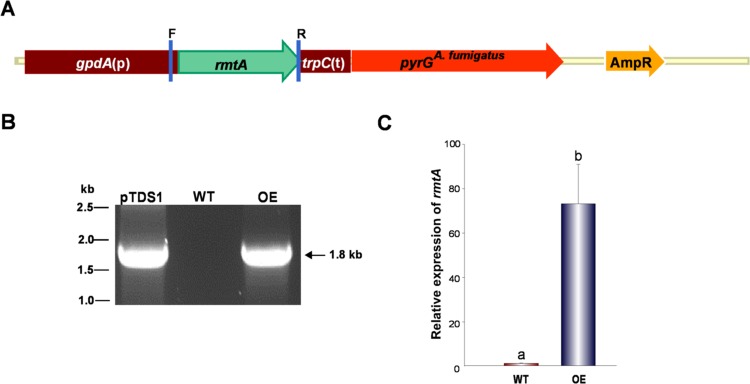
To determine the effects of rmtA on morphogenesis and other cellular processes, rmtA deletion and complementation strains were constructed. The deletion strain was confirmed by Southern blot analysis (Fig 2A). Genomic DNA from both wild-type and ΔrmtA strains was isolated and digested with KpnI. A 1.3 kb DNA fragment corresponding to the 5’ UTR region of rmtA was used to generate the radioactive probe utilized in this hybridization. The presence of a 3.2 kb band in the Southern blot analysis confirmed the rmtA deletion (Fig 2A). With respect to the complementation strain, diagnostic PCR was used to verify the integration of the wild-type allele in the ΔrmtA strain (Fig 2B). qRT-PCR was utilized to confirm the lack of rmtA expression in the rmtA mutant under conditions that allow transcription of this gene in the wild-type and complementation control strain (Fig 2C). Additionally, an over-expression strain was created as described in the Materials and Methods section. Verification of this strain was carried out by diagnostic PCR (Fig 3), obtaining the expected 1.8 kb PCR product. Over-expression of rmtA was also confirmed by qRT-PCR (Fig 3C). All the strains generated in this study presented similar growth rate compared to the wild-type strain (S3 Fig).
Fig 2. Construction of the rmtA deletion and complementation strains.
(A) Diagram showing the gene replacement strategy using the selection marker pyrG from A. fumigatus and Southern blot analysis. Recombination events between the flanking regions are indicated with crosses. KpnI sites are indicated in both the wild-type and modified loci. A 1.3 kb fragment was used as probe as indicated. On the right, Southern blot image confirming of the deletion of rmtA. Genomic DNA from the wild type (WT) and from a selected deletion mutant ΔrmtA was digested with KpnI. Expected 5.0 kb and 3.2 kb bands are shown for WT and ΔrmtA respectively. (B) Linearized representation of the plasmid containing the rmtA wild-type allele. The niaD gene from A. fumigatus was used as selection marker for fungal transformation. Primers OE_RMTA_F and OE_RMTA_R (S1 Table), used for confirmation of the strain, are labeled in this figure as F and R respectively. On the right, gel electrophoresis results showing the presence of a 1.8 kb PCR product, confirming the presence of the wild-type allele in the complementation strain. Wild type and ΔrmtA were used as positive and negative control, respectively. (C) Expression analysis of rmtA by qRT-PCR. Strains were inoculated in PDB (106 spores/ml), and cultures were grown for 48 h at 30°C. The error bars represent standard errors. Values were normalized to the expression levels in the wild type, considered as 1. Different letters on the columns indicate values that are statistically different (p < 0.05).
Fig 3. Confirmation of the OErmtA (OE).
(A) Linearized representation of the over-expression plasmid. The pyrG gene from A. fumigatus was used as a selection marker for fungal transformation. (B) Gel electrophoresis results showing the presence of a 1.8 kb PCR product, confirming the presence of the over-expression cassette. Plasmid pTRS.1rmtAOE and genomic DNA from the wild type were used as positive and negative control respectively. F and R represent primers OE_RMTA_F and OE_RMTA_R respectively. (C) Expression analysis of rmtA by qRT-PCR. Strains were inoculated in PDB (106 spores/ml), and cultures were grown for 48 h at 30°C. The error bars represent standard errors. Values were normalized to the expression levels in the wild type, considered as 1. Different letters on the columns indicate values that are statistically different (p < 0.05).
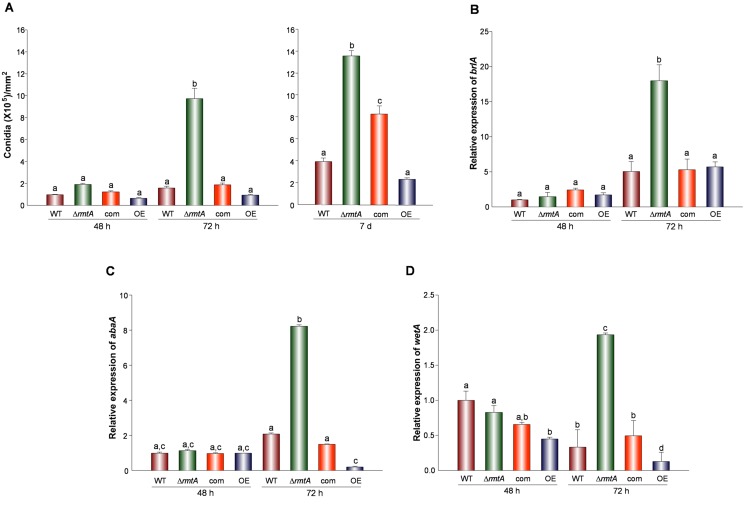
Our study revealed a significant increase (5-fold) in conidial production in the ΔrmtA strain compared to the wild-type (Fig 4). Complementation with the wild-type allele rescued wild-type phenotype. While hypercondiation was observed in the ΔrmtA, over-expression of rmtA resulted in a reduction in conidial production (Fig 4A). Gene expression analysis showed that transcription of brlA, abaA, and wetA, genetic components of a key central regulatory pathway necessary for the activation of conidiation (reviewed by Krijgsheld et al, 2013[40]) is regulated by rmtA. Deletion of rmtA resulted in a significant increase in expression of all three genes (Fig 4B, 4C & 4D), while forced over-expression of rmtA led to a decrease in abaA and wetA expression levels.
Fig 4. Effect of rmtA on conidiation.
(A) Quantification of conidia. Aspergillus flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains were grown on PDA medium for up to 7 days at 30°C. Seven millimeter cores were taken from each culture. Conidia were counted using a hemocytometer. Values represent the average of 3 replicates. Error bars represent standard error. (B, C, D) qRT-PCR expression analysis of brlA. abaA and wetA, respectively. Strains were inoculated in PDB stationary cultures (106 spores/ml), and were grown for 72 h at 30°C. The error bars represent standard errors. Values were normalized to the expression levels in the wild type, considered as 1. Different letters on the columns indicate values that are statistically different (p < 0.05).
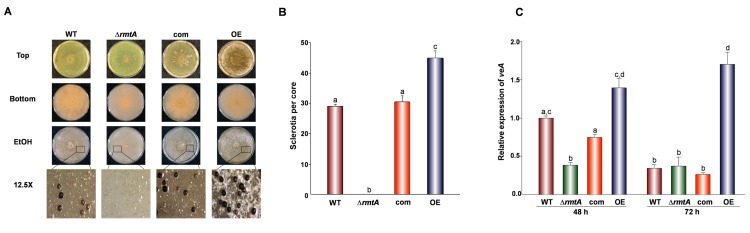
rmtA Affects Sclerotial Production
The ΔrmtA strain demonstrated a complete abolishment of sclerotial production when grown on PDA, a medium that allows production of these structures in the wild type (Fig 5). This pattern was observed even after 24 days of incubation. However, the over-expression presented a significant increase of sclerotia compared to the control strains. Similarly, on GMM with 2% sorbitol, another medium conducive to sclerotial production, the deletion rmtA strain did not produce any sclerotia (S4 Fig). Only on aged cultures growing on Wickerham medium, which is highly conducive to sclerotial production, a few sclerotia were produced by the deletion strain (S5 Fig). Interestingly, our study showed that rmtA positively influences the expression of the global regulator veA (Fig 5C), known to be necessary for sclerotial production in A. flavus (reviewed by Calvo and Cary, 2015 [2]). Our gene expression analysis revealed that at earlier time points veA transcription levels are reduced in ΔrmtA in comparison to wild-type. Furthermore, veA expression increases when expression of rmtA is abnormally increased in the OErmtA strain.
Fig 5. Effect of rmtA on sclerotial production on PDA.
(A) A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains were point-inoculated on PDA medium and incubated for 9 days in the dark at 30°C. Photographs of cultures were taken before and after spraying 70% ethanol to remove conidia in order to improve visualization of sclerotia. Micrographs were obtained approximately 1.5 cm away from the center of the plate using a Leica MZ75 dissecting microscope at 12.5X magnification. (B) Quantification of sclerotia. A. flavus wild type, (WT), ΔrmtA, complementation (com) and OE rmtA strains grown on PDA medium for 24 days at 30°C. Sixteen millimeter cores were collected 1 cm away from the center. Number of sclerotia in each core were counted under a Leica MZ75 dissecting microscope. The experiment included 3 replicates. Error bars represent standard error. (C) qRT-PCR expression analysis of veA. A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains were inoculated in PDB stationary cultures (106 spores/ml), and were grown for 48 and 72 h at 30°C. Error bars represent standard errors. Values were normalized to the expression levels in the wild type, considered as 1. Different letters on the columns indicate values that are statistically different (p < 0.05).
rmtA Is Required for Normal Aflatoxin B1 Biosynthesis and Production of Other Secondary Metabolites
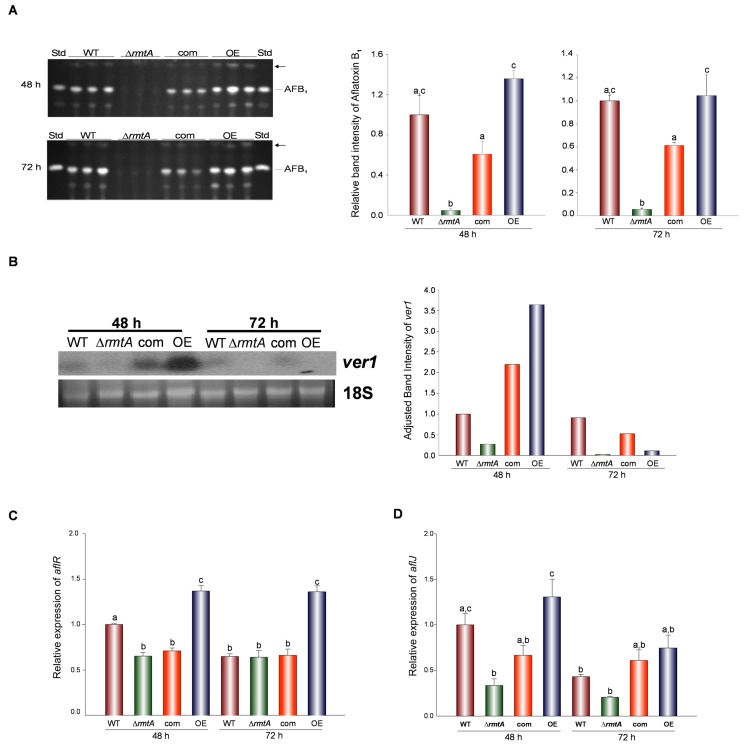
Our TLC analysis revealed that ΔrmtA presented a decrease in aflatoxin B1 production compared to the wild-type strain at both time points analyzed (48 h and 72 h) (Fig 6). However, OErmtA showed a slight increase in aflatoxin production with respect to the control strains. Additionally, our TLC analysis indicated that rmtA is necessary for the production of another metabolite (Fig 6A).
Fig 6. rmtA positively regulates Aflatoxin B1 production.
(A) TLC analysis of aflatoxin B1 produced by A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains growing at 30°C on PDA top-agar inoculated cultures for 48 h and 72 h. Aflatoxin standard (AFB1) was also included on either side of the plate. Arrow indicates an unknown metabolite whose synthesis is also affected by rmtA. Densitometry of aflatoxin performed using GelQuantNET software is shown. (B) Northern Blot analysis of ver1. All strains were grown in PDB stationary cultures (106 spores/ml) for 72 h at 30°C. 18S rRNA was used as loading control. Densitometry of Northern blot results is shown. (C & D) Expression analysis of aflR and aflJ by qRT-PCR respectively. The error bars represent standard errors. Different letters on the columns indicate values that are statistically different (p < 0.05).
To determine the effect of rmtA on the expression of genes involved in aflatoxin biosynthesis, transcription levels of the structural gene aflM/ver-1, commonly used as indicator of aflatoxin cluster activation, as well as expression levels of the regulatory genes aflR and aflS/aflJ [41], were examined at 48 h and 72 h after inoculation (Fig 6B, 6C and 6D). Northern blot analysis of aflM/ver-1 revealed that its expression was positively regulated by rmtA; while deletion of rmtA decreased expression of aflM/ver-1, over-expression of rmtA clearly enhanced expression of this structural gene at 48 h (Fig 6B). Our results also indicated that while deletion of rmtA did not affect aflR, an increase was observed in the over-expression strain (Fig 6C). Notably, deletion of rmtA resulted in a decrease in aflS/aflJ expression, while over-expression of rmtA increased it, particularly at 48 h (Fig 6D).
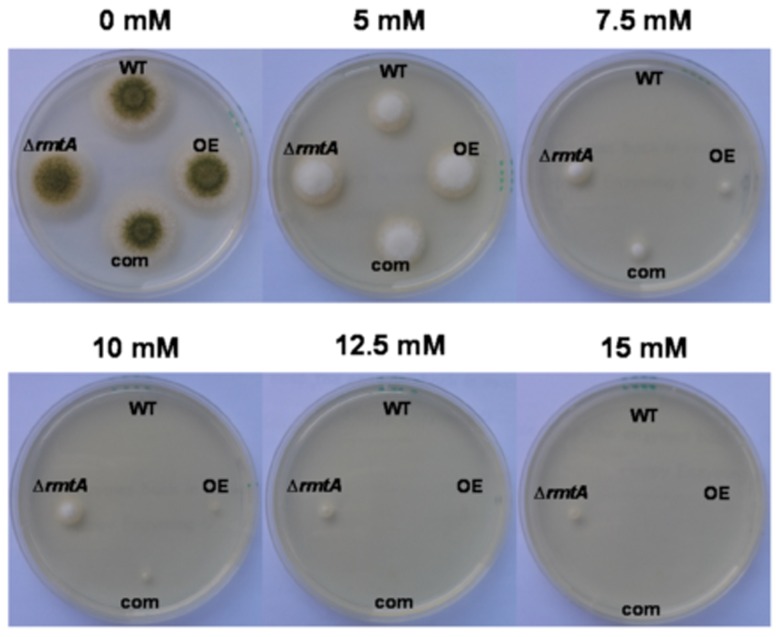
Altered Expression of rmtA Affects Oxidative Stress Tolerance
In order to evaluate the possible role of rmtA in oxidative stress response, A. flavus wild- type, ΔrmtA, complementation and OErmtA strains were tested on medium containing different concentrations of menadione (5 mM to 15 mM). Under these conditions, ΔrmtA presented an increased tolerance to oxidative stress with respect to the other strains (Fig 7).
Fig 7. Role of rmtA in oxidative stress tolerance.
A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains were point-inoculated on PDA containing a range of menadione concentrations (5 mM, 7.5 mM, 10 mM, 12.5 mM, and 15 mM). Cultures were incubated for 48 h at 30°C.
Discussion
In this study we investigated the role of rmtA, encoding a putative type I arginine methyltransferase, in development and secondary metabolism of the agriculturally important fungus A. flavus. Our in-silico analysis revealed that the deduced protein, RmtA, is highly conserved within filamentous fungi, as well as in a diversity of eukaryotic organisms, including humans. As in the case of A. flavus RmtA, all RmtA homologs analyzed contain the SAM binding domain. Although the role of RmtA in histone methylation was demonstrated in the phylogenetically closely related fungus A. nidulans [29] the only phenotype reported in this study was an increase of oxidative stress sensitivity. The present study shows that in A. flavus several cellular processes are regulated by rmtA. Based our results, while the protein sequence is highly conserved, the regulatory output appears to vary depending on the species. Previous studies showed that rmtA is necessary for growth in Neurospora crassa [31], in Fusarium graminearum [30] and in the basidiomycete Coprinopsis cinerea [42]. However, our study showed that absence of rmtA does not affect growth in A. flavus. Additionally, the role of rmtA on oxidative stress tolerance in A. flavus differs from that described in A. nidulans and F. graminearum. While deletion of rmtA in both of these fungi results in a hypersensitivity to oxidative stress [29, 30], absence of rmtA in A. flavus increases resistance to this environmental condition. It is possible that the epigenetic mechanism involving rmtA in these fungal species diverged over the course of evolution resulting in variation in its regulatory scope leading to different adaptations suited for each species niche.
Several studies have associated other methyltransferases and other histone modifiers with regulation of secondary metabolism in fungi, including Aspergillus species. For example the histone acetyltransferase EsaA was shown to regulate production of sterigmatocystin, penicillin, terrequinone, and orsellinic acid in A. nidulans [43]. Another methyltransferase, LlmF, was also shown to be a negative regulator of sterigmatocystin in this model fungus [44]. In A. flavus, the putative methyltransferase LaeA is required for the production of cyclopiazonic acid, kojic acid, oryzaechlorin, aflatrem as well as aflatoxin [20]. Another example is the KMT6 histone methyltransferase in Fusarium graminearum, which has been reported to regulate both the fusarin C and carotenoid clusters [45]. Based on these reports, we examined whether rmtA was involved in the production of aflatoxin in A. flavus. Interestingly, our study revealed that rmtA is a positive regulator of aflatoxin biosynthesis and associated aflatoxin cluster genes. Our experiments showed that expression of aflJ (also termed aflS [41]) was positively regulated by rmtA. aflJ is a regulatory gene in the aflatoxin gene cluster that encodes a protein that promotes the activation of early genes in this cluster [41]. AflJ protein has been demonstrated to interact with AflR, a well-characterized transcription factor necessary for aflatoxin cluster activation [41]. Over-expression of rmtA resulted in higher expression of not only aflJ but also of aflR.
Interestingly, the synthesis of another metabolite was also affected by the absence of rmtA. This suggests a broader regulatory scope of rmtA on secondary metabolism in A. flavus. A study of the rmtA homolog, amt1, in Fusarium graminearum showed this gene as a positive regulator of the synthesis of deoxynivalenol, a harmful compound that is produced during Fusarium head blight [30]. It is likely that this aspect of rmtA regulation, involving the control of biosynthesis of natural products, might be conserved in other fungal species.
In fungi secondary metabolism is genetically linked to morphological development [14–16, 46]. Our study indicates that rmtA not only controls secondary metabolism, but it is also a regulator of conidiation in A. flavus. Air-borne conidiospores, or conidia, are an efficient way of fungal dissemination [47], which is particularly relevant in A. flavus field infestations. In addition, A. flavus can cause aspergillosis, particularly in immunocompromised patients, where conidia constitute the main inoculum [48]. In our study, absence of rmtA resulted in hyperconidiating colonies. This increase in the production of conidia coincided with an increase in the expression of brlA, abaA, and wetA. In Aspergillus, brlA is a regulatory gene that encodes a C2H2 zinc finger transcription factor [49] that initiates a central regulatory pathway that governs maturation of conidiophores vesicles. BrlA activates other regulatory genes such as abaA, which acts as a transcriptional switch controlling wetA, which activates the expression of spore-specific gene [40, 49]. This, together with the results of our study, suggests that the developmental effect of rmtA on conidiation is, at least in part, mediated by brlA. In addition, abnormally high rmtA transcription levels in the over-expression strain resulted in a slight reduction in conidiation which can be explained by lower expression levels of abaA and wetA. This result further supports the role of rmtA as negative regulator of asexual development in A. flavus.
Besides the effect of rmtA on conidiation, rmtA also affected sclerotial production. Sclerotia are structures composed of a matrix of hardened mycelia that allow A. flavus to survive adverse environmental conditions [50]. They are vestiges of fruiting bodies that in most cases lost the capacity to produce sexual spores; although formation of ascospores within sclerotia, termed stromata, has been observed under laboratory conditions [51]. When conditions are favorable again sclerotia will produce hyphae to establish a new mycelium and/or generates conidiophores on their surface further contributing to disseminate the fungus [52]. Our study showed that deletion of rmtA strongly reduces or blocks sclerotial production, while over-expression of rmtA resulted in hyper-production of these resistant structures, indicating that rmtA is a positive regulator of sclerotial formation. Interestingly, our study show that rmtA positively affects the expression of veA, encoding a global regulator that forms part of the nuclear velvet protein complex [18]. veA has been shown to be essential for sclerotial production in A. flavus [19]. Based on our results it is likely that the effect of rmtA on sclerotia could be affected by veA. In addition, veA has been shown to regulate other cellular processes in fungi, including conidiation and secondary metabolism [2, 53–60]. Therefore, other roles of rmtA described in this study could also be veA-dependent, for example veA is a repressor of conidiation [58, 60], and it is possible that the observed negative effect of rmtA on conidiation, as well as its positive effect on secondary metabolism, could also be influenced by the effect of rmtA on veA expression in A. flavus. As in our study, functional association of VeA with other methyltransferases, such as LlmF, VipC, VapB, and LaeA, that affect development and secondary metabolism has been previously described [18, 44, 61, 62].
In conclusion, this study contributes to the elucidation of rmtA functions in A. flavus, revealing high conservation among its homologs in many eukaryotes. Despite this conservation, the regulatory role of rmtA varies among fungal species, suggesting that “rewiring” of this regulatory mechanism has occurred through evolution. This study also shows that morphogenesis is under rmtA regulation, influencing the developmental balance between conidiation and sclerotial formation in A. flavus; rmtA negatively regulates conidial production while it promotes sclerotial development. In addition, we also demonstrated that rmtA positively regulates secondary metabolism, controlling the production of aflatoxin as well as the synthesis of another unknown metabolite. Other cellular processes are also under rmtA regulation, including oxidative stress response. These facts indicate that rmtA is a global regulator in A. flavus. Furthermore, we found that rmtA control the expression of the master regulator veA, suggesting that rmtA regulatory output is functionally connected with veA. The findings in this study could contribute to set the bases of novel control strategies to reduce the negative impact caused by A. flavus and other detrimental fungal species.
Supporting Information
(A) Sequences aligned using Muscle (http://www.ebi.ac.uk/Tools/msa/muscle/). Alignment was visualized with BoxShade v3.21 (http://ch.embnet.org/software/BOX_form.html). (B) Phylogenetic tree of RmtA homologs from model organisms. Phylogenetic trees constructed using MEGA v6.0. Trees were generated with Maximum-Likelihood model with a bootstrap value of 1000.
(PDF)
(A) Sequences alignment of A. flavus RmtA, RmtB (EED57275.1), and RmtC (EED50528.1) using Muscle (http://www.ebi.ac.uk/Tools/msa/muscle/). Alignment was visualized with BoxShade v3.21 (http://ch.embnet.org/software/BOX_form.html). (B) Phylogenetic tree of RmtA, RmtB, and RmtC from A. flavus and A. nidulans. Accession numbers for A. nidulans RmtB and RmtC sequence are XP_660700.1 and XP_657738.1 respectively. The phylogenetic tree was constructed using MEGA v6.0, and it was generated with Maximum-Likelihood model with a bootstrap value of 1000.
(PDF)
(A) Images of point-inoculated cultures of A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains growing on PDA after 7 days of incubation at 30°C. (B) Quantification of colony growth as colony diameter of cultures in (A). Different letters on the columns indicate values that are statistically different (p < 0.05).
(PDF)
A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains were point-inoculated and grown on GMM-sorbitol medium for 7 days at 30°C. Plates were then sprayed with ETOH and micrographs were taken approximately 1.5 cm from center at 12.5X magnification using a Leica MZ75 dissecting microscope coupled with a Leica DC SOLP camera.
(PDF)
A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains were point-inoculated on Wickerham medium and incubated for 7 days (A) and 17 days (B) at 30°C. Plates were then sprayed with ethanol and micrographs of sclerotia were obtained approximately 1.5 cm from the center at 12.5X magnification using a Leica MZ75 dissecting microscope.
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
This work was supported by USDA grant 58-6435-4-015 and the Department of Biological Sciences at Northern Illinois University.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the United States Department of Agriculture grant 58-6435-4-015 AMC.
References
- 1.Fink-Gremmels J. Mycotoxins: Their implications for human and animal health. Vet. Q. 1999; 21: 115–120. [DOI] [PubMed] [Google Scholar]
- 2.Calvo AM, Cary JW. Association of fungal secondary metabolism and sclerotial biology. Front Microbiol. 2015. February; 6: 62 10.3389/fmicb.2015.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robens J, Cardwell K. The costs of mycotoxin management to the USA: management of aflatoxins in the United States. J Toxicol Toxin Rev. 2003; 22: 139–152. 10.1081/TXR-12002408 [DOI] [Google Scholar]
- 4.Commission Regulation (EC) No 1525/98, Off. J. Eur. Comm. 1998; 201: 43–46. [Google Scholar]
- 5.Lancaster MD, Jenkins FP, Phillip JM. Toxicity associated with certain samples of groundnuts. Nature. 1961; 192: 1095–1096. 10.1038/1921096a0 [DOI] [Google Scholar]
- 6.CDC (Center for Disease Control and Prevention), Outbreak of aflatoxin poisoning-eastern and central province, Kenya, January-July, 2004. MMWR Morb Mortal Weekly Report. 2004;53: 790–792. [PubMed] [Google Scholar]
- 7.Fung F, Clark RF. Health effects of mycotoxins: A toxicological overview. J. Toxicol. Clin. Toxicol, 2004; 42: 217–234. 10.1081/CLT-120030947 [DOI] [PubMed] [Google Scholar]
- 8.Lewis L, Onsong M, Njapau H, Schurz-Rogers H, Luber G, Kieszak S, et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in Eastern and Central Kenya. Enviorn Health Perspect. 2005; 113: 1763–1767. 10.1289/ehp.7998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mckean C, Tang L, Tang M, Billam M, Wang Z, Theodorakis CW, et al. Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food Chem Toxicol. 2006; 44: 868–76. 10.1016/j.fct.2005.11.011 [DOI] [PubMed] [Google Scholar]
- 10.Battilani P., Toscano P., Van der Fels-Klerx H.J., Moretti A., Camardo Leggieri M., Brera et al. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci Rep. 2016. April; 6: 24328 10.1038/srep24328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotty P, Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int J Food Microbiol. 2007; 119: 109–115. 10.1016/j.ijfoodmicro.2007.07.060 [DOI] [PubMed] [Google Scholar]
- 12.Medina A, Rodriguez A, Magan N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front Microbiol 2014. July; 5(348). 10.3389/fmicb.2014.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mapleston RA, Stone MJ, Williams DH. The evolutionary role of secondary metabolites. Gene. 1992; 115: 151–157. 10.1016/0378-1119(92)90553-2 [DOI] [PubMed] [Google Scholar]
- 14.Hicks J, Yu J, Keller N, Adams T. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA Galpha protein-dependent signaling pathway. EMBO J. 1997;16: 4916–4923. 10.1093/emboj/16.16.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo AM, Wilson RA, Bok JW, Keller NP. Relationship between secondary metabolism and fungal development. Microbiol Mol Biol Rev. 2002; 66: 447–459. 10.1128/MMBR.66.3.447-459.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayram Ö, Braus G. Coordination of secondary metabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol Rev. 2012; 36: 1–24. 10.1111/j.1574-6976.2011.00285.x [DOI] [PubMed] [Google Scholar]
- 17.Bayram O, Bayram OS, Ahmed YL, Maruyama J, Valerius O, Rizzoli SO, et al. The Aspergillus nidulans MAPK module ANSte11-STe50-Ste7-Fus3 controls development and secondary metabolism. PLoS Genet. 2012. July; 8(7): E1002816 10.1371/journal.pgen.1002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayram O, Krappmann S, Ni M, Bok JW, Helmstaedt K, Valerius O, et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008; 320: 1504–1506. 10.1126/science.1155888 [DOI] [PubMed] [Google Scholar]
- 19.Duran RM, Cary JW, Calvo AM. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl Microbiol Biotechnol. 2007; 73: 1158–1168. [DOI] [PubMed] [Google Scholar]
- 20.Kale S, Milde L, Trapp M, Frisvad J, Keller N, Bok J. Requirement of LaeA for secondary metabolism and sclerotial production in Aspergillus flavus. Fungal Genet Biol. 2008; 45: 1422–1429. 10.1016/j.fgb.2008.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duran RM, Cary JW, Calvo AM. The role of veA on Aspergillus flavus infection of peanuts, corn and cotton. Open Mycol Journ. 2009; 3: 27–36. 1874-4370/09 473. [Google Scholar]
- 22.Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what and why. Mol Cell. 2009; 33: 1–13. 10.1016/j.molcel.2008.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gary JD, Clarke S. RNA and protein interactions modulated by protein arginine methylation. Prog. Nucleic Acid Res Mol Biol. 1998; 61: 65–131. 10.1016/S0079-6603(08)60825-9 [DOI] [PubMed] [Google Scholar]
- 24.Bachand F. Protein arginine methyltransferases: from unicellular eukaryotes to humans. Eukaryot Cell. 2007; 6: 889–898. 10.1128/EC.00099-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride AE, Weiss VH, Kim HK, Hogle JM, Silver PA. Analysis of the yeast arginine methyltransferase Hmt1p/Rmt1p and its in vivo function. Cofactor binding and substrate interactions. J Biol Chem. 2000; 275: 3128−3136. 10.1074/jbc.275.5.3128 [DOI] [PubMed] [Google Scholar]
- 26.Trojer P, Dangl M, Bauer I, Graessle S, Loidl P, Brosch G. Histone methyltransferases in Aspergillus nidulans: evidence for a novel enzyme with a unique substrate specificity. Biochemistry. 2004; 43: 10834–10843. 10.1021/bi049626i [DOI] [PubMed] [Google Scholar]
- 27.Strahl BD, Briggs SD, Brame CJ, Caldwell J, Hunt DF, Stallcup MR, et al. Methylation of histone H4 at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr Biol. 2001; 11: 996–1000. 10.1016/S0960-9822(01)00294-9 [DOI] [PubMed] [Google Scholar]
- 28.Aletta JM, Cimato TR, Ettinger MJ. Protein methylation: a signal event in post-translational modification. Trends Biochem Sci. 1998; 23: 89–91. 10.1016/S0968-0004(98)01185-2 [DOI] [PubMed] [Google Scholar]
- 29.Bauer I, Graessle S, Loidl P, Hohenstein K, Brosch G. Novel insights into the functional role of three protein arginine methyltransferases in Aspergillus nidulans. Fungal Genet Biol. 2010; 47: 551–561. 10.1016/j.fgb.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 30.Wang G, Wang C, Hou R, Zhou X, Li GH, Zhang S, et al. The AMT1 arginine methyltransferase gene is important for plant infection and normal hyphal growth in Fusarium graminearum. PLoS One. 2012. May; 7(5): E38324 10.1371/journal.pone.0038324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldman D, Ziv C, Gorovits R, Efrat M, Yarden O, Freitag M. Neurospora crassa protein arginine methyl transferases are involved in growth and development and interact with the NDR kinase COT1. PLoS One. 2013. November; 8(11): E80756 10.1371/journal.pone.0080756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szewczyk E, Nayak T, Oakley C, Edgerton H, Xiong Y, Taheri-Talesh N, et al. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc. 2007; 1: 3111–3120. 10.1038/nprot.2006.405 [DOI] [PubMed] [Google Scholar]
- 34.Cary JW, Ehrlich KC, Bland JM, Montalbano BG. The aflatoxin biosynthesis cluster gene, aflX, encodes an oxidoreductase involved in the conversion of Versicolorin A to Demethylsterigmatocystin. Appl Environ Microbiol. 2006; 72: 1096–1101. 10.1128/AEM.72.2.1096-1101.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Käfer E. The anthranilate synthetase enzyme complex and the trifunctional trpC gene of Aspergillus. Can J Genet Cytol. 1977; 19: 723–38. 10.1139/g77-079 [DOI] [PubMed] [Google Scholar]
- 36.Chang PK, Scharfenstein LL, Mack B, Ehrlich KC. The deletion of the Aspergillus flavus orthologue of A. nidulans fluG reduces conidiation and promotes the production of sclerotia but does not abolish aflatoxin biosynthesis. Appl Environ Microbiol. 2012; 78: 7557–63. 10.1128/AEM.01241-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleveland TE, Bhatnagar D, Foell CJ, McCormick SP. Conversion of a new metabolite to aflatoxin B2 by Aspergillus parasiticus. Appl Environ Microbiol. 1987; 53: 2804–2807. 0099-2240/87/122804-04$02.00/0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) method. Methods. 2001; 25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 39.Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE et al. A higher-level phylogenetic classification of the Fungi. Mycol Res. 2007; 111:509–47. 10.1016/j.mycres.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 40.Krijgsheld P, Bleichrodt R, van Veluw GJ, Wang F, Müller WH, Dijksterhuis J, et al. Development in Aspergillus. Stud Mycol. 2013; 74: 1–29. 10.3114/sim0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrlich K, Mack B, Wei Q, Li P, Roze L, Dazzo F, et al. Association with AflR in endosomes reveals new functions for AflJ in Aflatoxin biosynthesis. Toxins. 2012. December; 4(12): 1582–1600. 10.3390/toxins4121582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakazawa T, Tatsuta Y, Fujita T, Nakahori K, Kamada T. Mutations in the Cc.rmt1 gene encoding a putative protein arginine methyltransferase alter developmental programs in the basidiomycete Coprinopsis cinerea. Curr Genet. 2010; 56: 361–367. 10.1007/s00294-010-0307-1 [DOI] [PubMed] [Google Scholar]
- 43.Soukup A, Chiang Y, Bok J, Reyes-Dominguez Y, Oakley B, Wang C, et al. Over-expression of the Aspergillus nidulans histone 4 acetyltransferase EsaA increases activation of secondary metabolite production. Mol Microbiol. 2012; 86: 314–330. 10.1111/j.1365-2958.2012.08195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer JM, Theisen JM, Duran RM, Grayburn WS, Calvo AM, Keller NP. Secondary metabolism and development is mediated by LlmF control of VeA subcellular localization in Aspergillus nidulans. PLoS Genet. 2013. January; 9(1): E1003193 10.1371/journal.pgen.1003193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connolly LR, Smith KM, Freitag M. The Fusarium graminearum histone H3 K27 methyltransferase KMT6 regulates development and expression of secondary metabolite gene clusters. PLoS Genet. 2013. October; 9(10): E1003916 10.1371/journal.pgen.1003916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calvo AM. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet Biol. 2008; 45: 1053–1061. 10.1016/j.fgb.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 47.Parks H, Yu J. Genetic control of asexual sporulation in filamentous fungi. Curr Opin Microbiol. 2012; 15: 669–677. 10.1016/j.mib.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 48.Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Dennning DW. Aspergillus flavus: human pathogen, allergen, and mycotoxin producer. Microbiology. 2007; 153: 1677–1692. 10.1099/mic.0.2007/007641-0 [DOI] [PubMed] [Google Scholar]
- 49.Adams T, Boylan M, Timberlake W. BrlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell. 1988; 54: 353–362. 10.1016/0092-8674(88)90198-5 [DOI] [PubMed] [Google Scholar]
- 50.Coley-Smith JR, Cooke RC. Survival and Germination of Fungal Sclerotia. Annu Rev Phytopathol 1971; 9: 65–92. 10.1146/annurev.py.09.090171.000433 [DOI] [Google Scholar]
- 51.Horn B, Sorenson R, Lamb M, Sobolev V, Olarte R, Worthington C, et al. Sexual reproduction in Aspergillus flavus sclerotia naturally produced in corn. Phytopathology. 2014; 104: 75–85. 10.1094/PHYTO-05-13-0129-R [DOI] [PubMed] [Google Scholar]
- 52.Cotty P. Aflatoxin and sclerotial production in Aspergillus flavus: Influences in pH. Phytopathology. 1988; 78: 1250–1253. 10.1094/Phyto-78-1250 [DOI] [Google Scholar]
- 53.Kato N, Brooks W, Calvo AM. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot Cell. 2003; 2: 1178–1186. 10.1128/EC.2.6.1178-1186.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calvo AM, Wilson RA, Bok JW, Keller NP. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl Environ Microbiol. 2004; 70: 4773–4739. 10.1128/AEM.70.8.4733-4739.2004 426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoff B, Kamerewerd J, Sigl C, Mitterbauer R, Zadra I, Kürnsteiner H, et al. Two components of a velvet-like complex control hyphal morphogenesis, conidiophore development, and penicillin biosynthesis in Pencillium chryosgenum. Eukaryot Cell. 2010; 9: 1236–50. 10.1128/EC.00077-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.López-Berges MS, Hera C, Sulyok M, Schäfer K, Capilla J, Guarro J, et al. The velvet complex governs mycotoxin production and virulence of Fusarium oxysporum on plant and mammalian hosts. Mol Microbiol. 2013; 87: 49–65. 10.1111/mmi.12082 [DOI] [PubMed] [Google Scholar]
- 57.Karimi Aghcheh R, Németh Z, Atanasova L, Fekete E, Paholcsek M, Sándor E, et al. The VELVET A orthologue VEL1 of Trichoderma reesei regulates fungal development and is essential for cellulose gene expression. PLoS One. 2014. November; 9(11): E112799 10.1371/journal.pone.0112799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H, Han K, Kim K, Han D, Jahng K, Chae K. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet Biol. 2002; 37: 72–80. 10.1016/s1087-1845(02)00029-4 [DOI] [PubMed] [Google Scholar]
- 59.Myung K, Li S, Butchko RA, Busman M, Proctor RH, Abbas HK, Calvo AM. FvVE1 regulates biosynthesis of the mycotoxins fumonisins and fusarins in Fusarium verticillioides. J Agric Food Chem. 2009; 57: 5089–94. 10.1021/jf900783u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calvo AM, Lohmar JM, Ibarra B, Satterlee T. Velvet regulation of fungal development In: Wendland J (ed) Mycota Series. Vol. I 3rd edn, Springer International Publishing, Switzerland; 2016. pp 475–497. [Google Scholar]
- 61.Sarikaya-Bayram O, Bayram O, Feussner K, Kim JH, Kim HS, Kaever A, et al. Membrane-bound methyltransferase complex VapA-VipC-VapB guides epigenetic control of fungal development. Dev Cell. 2014; 29:406–20. 10.1016/j.devcel.2014.03.020 [DOI] [PubMed] [Google Scholar]
- 62.Sarikaya-Bayram O, Palmer JM, Keller N, Braus GH, Bayram O. One Juliet and four Romeos: VeA and its methyltransferases. Front Microbiol. 2015. January; 6: 1 10.3389/fmicb.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Sequences aligned using Muscle (http://www.ebi.ac.uk/Tools/msa/muscle/). Alignment was visualized with BoxShade v3.21 (http://ch.embnet.org/software/BOX_form.html). (B) Phylogenetic tree of RmtA homologs from model organisms. Phylogenetic trees constructed using MEGA v6.0. Trees were generated with Maximum-Likelihood model with a bootstrap value of 1000.
(PDF)
(A) Sequences alignment of A. flavus RmtA, RmtB (EED57275.1), and RmtC (EED50528.1) using Muscle (http://www.ebi.ac.uk/Tools/msa/muscle/). Alignment was visualized with BoxShade v3.21 (http://ch.embnet.org/software/BOX_form.html). (B) Phylogenetic tree of RmtA, RmtB, and RmtC from A. flavus and A. nidulans. Accession numbers for A. nidulans RmtB and RmtC sequence are XP_660700.1 and XP_657738.1 respectively. The phylogenetic tree was constructed using MEGA v6.0, and it was generated with Maximum-Likelihood model with a bootstrap value of 1000.
(PDF)
(A) Images of point-inoculated cultures of A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains growing on PDA after 7 days of incubation at 30°C. (B) Quantification of colony growth as colony diameter of cultures in (A). Different letters on the columns indicate values that are statistically different (p < 0.05).
(PDF)
A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains were point-inoculated and grown on GMM-sorbitol medium for 7 days at 30°C. Plates were then sprayed with ETOH and micrographs were taken approximately 1.5 cm from center at 12.5X magnification using a Leica MZ75 dissecting microscope coupled with a Leica DC SOLP camera.
(PDF)
A. flavus wild type (WT), ΔrmtA, complementation (com) and OErmtA (OE) strains were point-inoculated on Wickerham medium and incubated for 7 days (A) and 17 days (B) at 30°C. Plates were then sprayed with ethanol and micrographs of sclerotia were obtained approximately 1.5 cm from the center at 12.5X magnification using a Leica MZ75 dissecting microscope.
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.