SUMMARY
Objectives
Anterior temporal lobectomy is curative for many patients with disabling medically refractory temporal lobe epilepsy, but carries an inherent risk of disabling verbal memory loss. Although accurate prediction of iatrogenic memory loss is becoming increasingly possible, it remains unclear how much weight such predictions should have in surgical decision making. Here we aim to create a framework that facilitates a systematic and integrated assessment of the relative risks and benefits of surgery versus medical management for patients with left temporal lobe epilepsy.
Methods
We constructed a Markov decision model to evaluate the probabilistic outcomes and associated health utilities associated with choosing to undergo a left anterior temporal lobectomy versus continuing with medical management for patients with medically refractory left temporal lobe epilepsy. Three base-cases were considered, representing a spectrum of surgical candidates encountered in practice, with varying degrees of epilepsy-related disability and potential for decreased quality of life in response to post-surgical verbal memory deficits.
Results
For patients with moderately severe seizures and moderate risk of verbal memory loss, medical management was the preferred decision, with increased quality-adjusted life expectancy. However, the preferred choice was sensitive to clinically meaningful changes in several parameters, including quality of life impact of verbal memory decline, quality of life with seizures, mortality rate with medical management, probability of remission following surgery, and probability of remission with medical management.
Significance
Our decision model suggests that for patients with left temporal lobe epilepsy, quantitative assessment of risk and benefit should guide recommendation of therapy. In particular, risk for and potential impact of verbal memory decline should be carefully weighed against the degree of disability conferred by continued seizures on a patient-by-patient basis.
Keywords: Anterior temporal lobectomy, Verbal memory decline, Quality of life, Markov process
Epilepsy accounts for 1% of life-years lost due to disability worldwide, and affects 0.5–1% of the world’s population.1 Although seizure medications are effective in preventing seizures in most patients, up to 40% of patients remain pharmacoresistant.2 Temporal lobe epilepsy, most commonly due to mesial temporal sclerosis, accounts for the majority of cases of medically refractory epilepsy.3–5 Anterior temporal lobectomy (ATL) is an effective treatment for individuals with medically refractory temporal lobe epilepsy (TLE),6,7 producing median long-term seizure remission rates of 66%.8
The decision to perform epilepsy surgery is based on a careful risk–benefit analysis, weighing the probability of cure and anticipated gain in quality of life (QOL) over the patient’s remaining lifetime against the risks of surgery. A recent decision analysis concluded that ATL provides significant gains in quality-adjusted life expectancy compared to medical management in patients with medically refractory epilepsy.9 This analysis is consistent with the findings of the Quality Standard Subcommittee of the American Academy of Neurology (AAN), who systemically reviewed 25 studies of TLE and recommended that patients with medically refractory epilepsy be referred to an epilepsy surgery center for consideration of surgery.1
However, previous studies have not specifically considered how seizure lateralization should be factored into epilepsy surgical decision making. While resection of the hippocampus and parahippocampus as part of standard right ATL generally produces minimal measurable postoperative neurocognitive deficits in patients with right TLE, 30–60% of patients who undergo left ATL (L-ATL) experience verbal memory decline.10–18 Thus, whether an individual has left or right TLE is an essential consideration when evaluating patients for ATL.
A number of metrics are currently used to preoperatively predict verbal memory decline following L-ATL. Left hippocampal sclerosis,18,19 neuropsychological test performance,10,11,13,14,16,18,20 age of onset,13,20–22 and functional magnetic resonance imaging (fMRI) protocols11 can predict verbal memory decline with high sensitivity and specificity. However, despite improvements in our ability to predict verbal memory decline, physicians lack two essential tools to make systematic use of these predictions: (1) quantitative methods for translating verbal memory loss as measured by neurocognitive test scores into decreases in QOL, similar to methods that have been developed for neurologic deficits following stroke23,24; and (2) an explicit decision analytic framework that weighs the disutility due to memory loss against the potential gains in seizure control offered by surgery.
We hypothesized that supplying these two missing tools to explicitly account for the risk of verbal memory decline might alter the surgery versus medical management decision, depending on left-sided versus right-sided seizure focus. To test this hypothesis we considered as base-cases three representative cases from our group that were considered for L-ATL with differing levels of disability due to epilepsy, levels of predicted memory loss, and expected levels of disability should the predicted memory loss occur. We constructed a Markov decision model to quantify the quality-adjusted life expectancy of patients with medically refractory left TLE following medical management or L-ATL and accompanying verbal memory decline. For the purposes of our model, we assigned plausible disutility values to the base-cases ranging from 0.4 to 0.1, comparable to those seen with mildly to moderately disabling neuropsychological deficits following stroke or dementia (Table S1). Our results demonstrate the importance of appropriately weighing potential memory loss in decisions regarding L-ATL, while simultaneously highlighting a critical need for further work to develop a standardized tool to assign individualized utility values to predicted verbal memory decline for use in the shared decision making process.25
METHODS
Model structure
We adapted a previously published Markov decision model9 by introducing a probability of verbal memory loss and a corresponding explicit penalty (percent reduction in QOL) associated with verbal memory decline following L-ATL. Our model considered the decision between medical management or L-ATL and accompanying verbal memory decline (Fig.1). Potential surgical complications included mortality, transient complications, or long-term complications. During each yearly cycle, patients could relapse and develop recurrent seizures, enter remission and become seizure-free, or die. Mortality transition probabilities were taken from published mortality rates due to seizure relapse or remission.26,27 Age-specific mortality rates were obtained from U.S. life tables.28 The complete decision tree is shown in Fig. S1.
Figure 1.
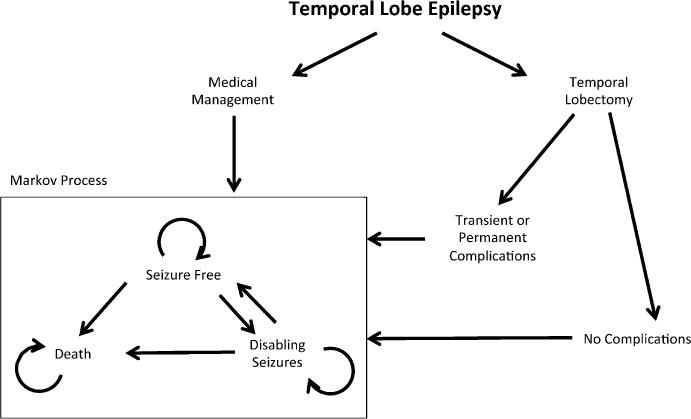
Markov transition diagram. Decision analysis tree constructed to analyze the preferred therapeutic decision between medical management or L-ATL for patients with medically refractory left temporal lobe epilepsy. Potential surgical complications include transient or long-term complications, and result in seizure freedom, disabling seizures, or death. For each decision, patients enter a Markov process that results in seizure relapse and development of recurrent seizures, seizure remission, or death. Appropriate probabilities and quality of life scores are listed in Table 1.
Epilepsia © ILAE
Model assumptions and parameters
Model parameters (Table 1) were adapted from an analysis performed by the Quality Standards Subcommittee of the AAN that used one randomized trial and 24 observational studies to determine the efficacy and safety of ATL,1 and previously published preference-based QOL values acquired from patient interviews.9 For patients with right TLE, base-case assumptions were adapted from Choi et al., with the exception of the QOL assigned to living with continued disabling seizures following uncomplicated epilepsy surgery. For this QOL we used the modified value of 0.75 so that it would equal the preference-based QOL score associated with disabling seizures preceding surgery (rather than assigning a slightly higher QOL to living with disabling seizures following surgery), reasoning that the chronic state of disabling seizures carries similar QOL, independent of treatment pathway.
Table 1.
Base-case values and ranges tested in sensitivity analyses
| Base-case assumptions and ranges for sensitivity analysis, %
| ||
|---|---|---|
| Events | Medical | Surgical |
| Seizure-free during first year | 8 (0–16) | 71.9 (69.5–74.3) |
| Relapse if seizure-free at end of year, from years 1 to 5 | 25.4 (10.9–46.2) | 5.6 (2.9–8.3) |
| Relapse if seizure-free at end of year, after 5 years | 25.4 (10.9–46.2) | 4.2 (1.6–6.8) |
| Seizure remission if not seizure-free at end of year, from years 1 to 5 | 4.7 (3–7) | 5.9 (0.9–11) |
| Seizure remission if not seizure-free at end of year, after 5 years | 1.6 (1–2.3) | 2.0 (0.2–7.2) |
|
| ||
| Surgical complications, %
| ||
| Death | 0.3 (0–0.75) | |
| Permanent deficit (other than verbal memory loss) | 4.0 (2.0–6.0) | |
| Transient complication | 8.0 (6.0–10) | |
|
| ||
| Standardized mortality ratios
| ||
| Seizure-free | 1.11 (0.63–1.94) | |
| Not seizure-free, surgery | 5.64 (3.49–9.09) | |
| Not seizure-free, medical management | 5.40 (3.96–7.37) | |
|
| ||
| Verbal memory decline, %
| ||
| Predicted verbal memory decline accurate | 100 (60–100) | |
|
| ||
| Quality of life scores for model health states
| ||
| Free of disabling seizures | ||
| Medical management | 0.96 (0.84–1.0) | |
| Surgery, without complications | 0.97 (0.87–1.0) | |
| Surgery, permanent complication | 0.77 (0.32–1.0) | |
| Surgery, transient complication | 0.96 (0.84–1.0) | |
| Not free of disabling seizures | ||
| Medical management | 0.75 (0.38–1.0) | |
| Surgery, without complications | 0.75 (0.41–1.0) | |
| Surgery, permanent complication | 0.66 (0.19–1.0) | |
| Surgery, transient complication | 0.75 (0.38–1.0) | |
| Transient surgical complication, days of life deducted | 1.5 (0–25.0) | |
|
| ||
| Quality of life penalty for memory loss
| ||
| Severe | 0.4 | |
| Moderate | 0.25 | |
| Mild | 0.1 | |
To account for the decline in QOL associated with verbal memory decline, we introduced a QOL penalty factor that decreases the utility of surgical outcome states by a certain percentage. This penalty factor is intended to represent impact of memory loss predicted based on routine presurgical evaluations, typically appraisal of the age of epilepsy onset,13,20–22 preoperative neuropsychological test profile,10,11,13,14,16,18,20 and either memory asymmetry score from Wada testing21,29,30 or predicted memory decline based on fMRI evaluation.11 For our base-cases the probability that the predicted degree of memory decline in fact occurs was assumed to be 100%. This assumption corresponds to a risk-averse patient perspective, that is, it assumes that a patient will or should be counseled to proceed with L-ATL only if the analysis favors this decision, even when the predicted postoperative memory deficit is considered guaranteed to occur. Nevertheless, to account for uncertainty, we also performed sensitivity analysis on the probability that the predicted degree of verbal memory decline actually occurs (Fig. S2).
Base-cases
To study the relative importance of verbal memory decline associated with L-ATL, we considered three archetypal base-cases abstracted from actual case histories seen in our clinical practice (Table 2). Each case had epilepsy refractory to at least two antiseizure medications and had undergone a standard presurgical evaluation: clinical history, MRI and positron emission tomography (PET) neuroimaging, video electroencephalography (EEG) monitoring, EEG recording of several typical seizures, neuropsychological testing, and fMRI confirming the diagnosis of seizures of lesional left anterior medial temporal lobe origin. The three patients differed in their relative levels of disability due to epilepsy and predicted verbal memory loss. QOL penalties associated with verbal memory loss were chosen to approximately match previously reported QOL values for comparable neuropsychological deficits following stroke23,24 or dementia31 (Table S1). The cases were as follows:
Table 2.
Expected outcomes of decision analysis for base-cases
| Base-case | Age (years) | QOL for not free of disabling seizures | QOL penalty for memory loss | Decision |
|---|---|---|---|---|
| R-ATL | 35 | 0.75 | 0 | Surgery |
| Patient 1 | 41 | 0.6 | 0.1 | Surgery |
| Patient 2 | 39 | 0.9 | 0.4 | Medication |
| Patient 3 | 33 | 0.8 | 0.25 | Medication |
Patient 1 (severely disabled, minimal memory risk)
A 41-year-old left-handed woman with medically refractory epilepsy, with onset of epilepsy at age 12, with one to three complex partial seizures each week and one to two secondary generalized convulsions each month. Her seizures had cost her the ability to drive and hold employment, and she complained of severe fatigue related to medication side effects and frequent seizures; hence she considered her epilepsy extremely disabling. Neuropsychological testing revealed significant left temporal lobe dysfunction at baseline (i.e., delayed retention of story at the second percentile; verbal list learning at the first percentile). Wada testing was not interpreted because she became obtunded following the injections. Based on her very poor baseline verbal memory test scores, her risk of substantial verbal memory loss should she undergo an L-ATL was predicted to be minimal.
The QOL assigned to her life with seizures was set to 0.6 (severe disability), and the QOL penalty factor for memory loss in this case was set to 0.1 (mild penalty), representing an anticipated 10% decline in QOL due to verbal memory loss.
Patient 2 (mildly disabled, high memory risk)
A 39-year-old right-handed woman with medically refractory epilepsy, with onset of epilepsy at age 37. Her seizures only rarely caused appreciable impairment of consciousness, were typically brief (<10 s), and generally occurred once or twice monthly. She was unable to drive, but she was able to use public transportation for all of her needs, including traveling to work as an accountant. A significant decline in memory was considered likely to end her career. Based on her late age of seizure onset, intact verbal memory test scores (i.e., delayed retention of story at the 37th percentile; verbal list learning at the 66th percentile), and Wada evidence of left-sided language and bilateral memory (Wada Memory Asymmetry = 1), the risk of verbal memory loss should she undergo L-ATL was predicted to be substantial. The QOL assigned to living with seizures was set to 0.9 (mild disability), and the QOL penalty factor for memory loss in this case was set to 0.4, representing a 40% decline in QOL due to verbal memory loss (severe penalty).
Patient 3 (moderately disabled, moderate memory risk)
A 33-year-old right -handed man with medically refractory epilepsy, with onset at age 11, with complex partial seizures lasting 1–3 min occurring every 2–3 days, and occasional secondarily generalized seizures. His seizures increasingly limited his ability to participate in the care of his two young children, although he remained employed as a salesman. Based on his intermediate neuropsychological test scores (i.e., delayed retention of story at the 16th percentile; verbal list learning at the 47th percentile), bilateral language lateralization on fMRI (Laterality Index = 0.2), the risk of verbal memory loss should he undergo L-ATL was predicted to be intermediate. The QOL assigned to living with seizures was set to 0.8 (moderate disability), and the QOL penalty factor for memory loss in this case was set to 0.25, representing a 25% decline in QOL due to verbal memory loss.
Analyses
We constructed a Markov decision model for our base-case analyses. We verified that our decision model successfully reproduces results from a previous decision analysis,9 when the QOL penalty of verbal memory loss is equal to zero, representing the case of a typical R-ATL. Using the baseline values from Choi et al. of a medically refractory 35-year-old patient with TLE, this analysis confirmed that surgery is the preferred decision. We also repeated key sensitivity analyses from Choi et al. to further verify that the model faithfully reproduces prior results in the absence of QOL penalties for memory loss. Sensitivity analyses were performed, in which values for surgical mortality rate, standard mortality ratio (SMR) with seizures and medical management, and QOL of being seizure-free were varied from 0 to 1. In all cases, values that would change the preferred decision faithfully recapitulated previous findings (Fig. S3). All analyses were performed using TreeAge Pro (TreeAge Software Inc, Williamstown, MA, U.S.A.).
RESULTS
Patient 1 was predicted to have minimal impact of verbal memory loss following surgery but had severely disabling seizures. We considered this a situation in which surgery would generally be the preferred decision on subjective clinical grounds, and our decision model correspondingly favored the surgical approach, predicting 20.76 quality-adjusted life-years (QALYs) for surgery compared to 14.46 QALYs for medical management.
In contrast, verbal memory decline posed a significant risk to patient 2, who experienced only minimal seizure-related disability. We considered this a situation in which medical management would generally be preferred, and our decision model indeed favored medical management, yielding 22.08 QALYs for medical management compared to 16.70 QALYs for surgery.
Patient 3 was believed to be an intermediate candidate on clinical grounds due to the uncertainty regarding optimal management, given his moderate disability and moderate risk of verbal memory loss. In agreement with this clinical impression, our decision model predicts that medical management and surgical management produce similar quality-adjusted life expectancies, with 23.82 QALYs for medical management versus 23.10 QALYs for surgery.
One-way sensitivity analysis
To investigate the extent to which variation of parameters would affect the preferred decision when taking into account verbal memory decline, we performed one-way sensitivity analyses for each base-case (Fig.2). For patient 1, for medical management to be the preferred decision, key parameter values would have to be extended to extreme values: surgical mortality ≤30.6%, SMR for disabling seizures with medical management <1.835, or annual probability of seizure remission with medical management >24.2%.
Figure 2.
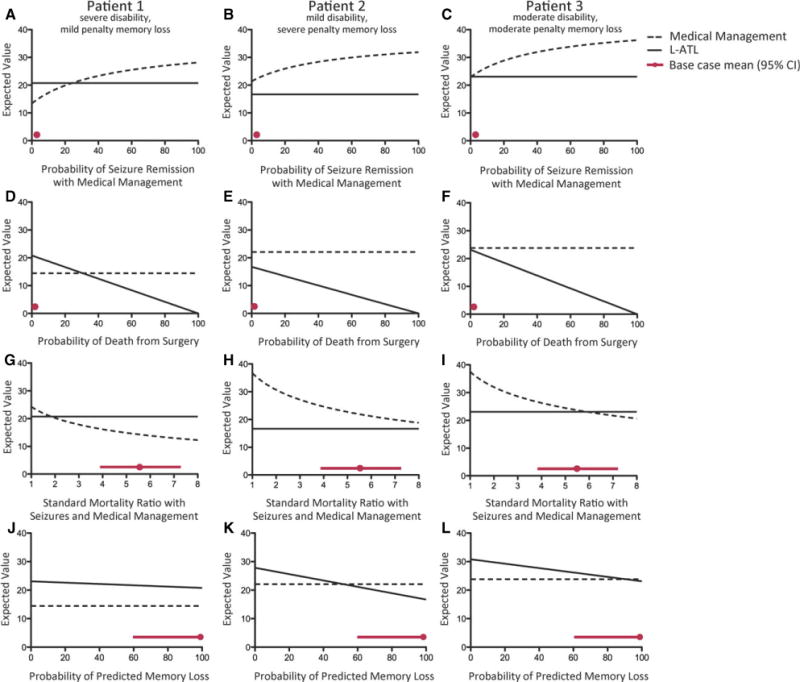
One-way sensitivity analyses. Each line represents the quality-adjusted life-years (QALYs) associated with L-ATL (solid) or medical management (dotted) for three different base-case patients (Table 2). Red circles represent the base-case value for each variable, and red lines indicate 95% confidence intervals (CIs). (A–C) As the probability of remission with medical medication increases, the benefit of medical management increases. The base-case is 1.6% (with 95% CI 1.0–2.3%); for patient 1 the threshold is 24.2% (above which medical management is preferred), for patient 2 medical management is always preferred, and for patient 3 the threshold is 0.4% (above which medical management is preferred). (D–F) As the probability of surgical mortality increases, the benefit of medical management increases. The base-case is 0.3% (0–0.75%); for patient 1 the threshold is 30.6% (above which medical management is preferred), and for patients 2 and 3, medical management is always preferred. (G–I) As the SMR of seizures with medical management increases, the benefit of medical management decreases. The base-case is 5.40 (3.96–7.37); for patient 1 the threshold is 1.835 (below which medical management is preferred), for patient 2 medical management is always preferred, and for patient 3 the threshold is 5.905 (below which medical management is preferred). (J–L) As the probability of predicted verbal memory increases, the benefit of surgery decreases. The base-case threshold is 100% (for patient 1 L-ATL is always preferred), for patient 2 the threshold is 51.7% (above which medical management is preferred), and for patient 3 the threshold is 90.7% (above which medical management is preferred).
Epilepsia © ILAE
Conversely, for patient 2, no plausible values of surgical mortality, SMR for disabling seizures with medical management, or annual probability of seizure remission with medical management caused surgery to become preferred. If the probability of predicted verbal memory decline fell to <51.7%, surgery would be the preferred decision.
One-way sensitivity analyses performed with patient 3 revealed that many threshold values for which the preferred strategy was changed fell within the 95% CI associated with each variable. In particular, in order for surgery to be preferred, SMR for disabling seizures with medical management must exceed 5.905, the annual probability of seizure-remission with medical management must fall to <0.4%, or the probability of predicted verbal memory decline must fall to <90.7%.
Two-way sensitivity analysis
Because of the significant impact the QOL penalty of verbal memory decline had on the preferred clinical decision for left TLE, we performed two-way sensitivity analysis to determine the threshold QOL values for which verbal memory decline would change the decision to pursue L-ATL. For these two-way sensitivity analyses, we varied the QOL penalty of verbal memory decline in concert with the QOL of living with seizures, the probability of becoming seizure-free following L-ATL, SMR for disabling seizures with medical management, or annual probability of seizure remission with medical management (Fig.3). Whenever the QOL of living with seizures was not varied, we used the default base-case value of 0.75.
Figure 3.
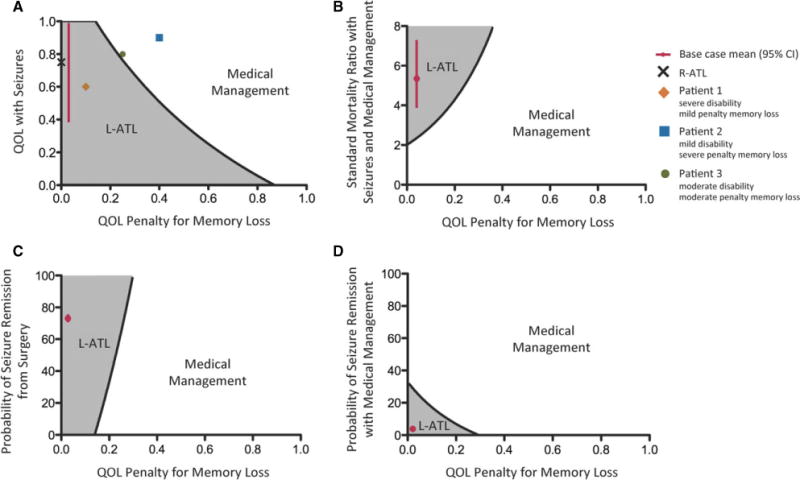
Two-way sensitivity analyses. Gray areas represent values for which surgery is preferred, and white areas represent values for which medical management is preferred. Overlaid points represent the base-case values, including a prototypical patient with right temporal lobe epilepsy (black cross), and our three patient base-cases with left temporal lobe epilepsy (1, orange diamond; 2, blue square; and 3, green circle). Red circles represent the base-case value for each variable, and red lines indicate 95% CIs. QOL penalty of memory loss was varied with the following variables: QOL with seizures (A), SMR of seizures with medical management (B), probability of remission following surgery (C), and the probability of remission with medical management (D). For two-way sensitivity analyses with a constant QOL with seizures (B–D), a utility value of 0.75 was used.
Epilepsia © ILAE
When comparing the decision to undergo surgery or medical management, considering both QOL with seizures and the QOL penalty of verbal memory decline, it becomes apparent that for patients with both moderately disabling seizures and a moderate QOL penalty of verbal memory decline, the therapeutic decision is no longer straightforward. For patient 3, a decline in the QOL associated with disabling seizures to 0.754 would result in surgery being the preferred decision. Two-way sensitivity analyses demonstrate that minimal changes in the QOL penalty of memory loss result in large changes for the threshold values of the probability of becoming seizure-free following L-ATL or SMR for disabling seizures with medical management. These findings demonstrate that for patients with left TLE, the impact of verbal memory loss must be carefully taken into account when considering whether to undergo L-ATL.
DISCUSSION
Anterior temporal lobectomy is an effective treatment option for patients with medically refractory TLE.7 However, L-ATL poses significant risk of verbal memory decline following surgery,32 and thus whether a patient has left or right TLE has a significant impact on surgical decision making. Our decision model demonstrates that for patients with left TLE and a moderate predicted impact of verbal memory decline, medical management is the preferred decision by a small margin. This decision is sensitive to minor changes in the magnitude of the QOL penalty for verbal memory decline, QOL with seizures, mortality rate of continued medical management, probability of remission following surgery, probability of remission with medical management, and probability of predicted memory decline occurring. These findings suggest that the decision to pursue L-ATL must involve a delicate case-by-case risk–benefit analysis.
A previous decision analysis demonstrated that for patients with medically refractory TLE and no significant risk for loss of QOL due to verbal memory loss, ATL provides significant gains in life expectancy and quality-adjusted life expectancy compared to medical management.9 Our model reproduces these findings for patients with right TLE and therefore no significant risk of verbal memory decline, and for patients with severely disabling left TLE and minimal predicted loss of QOL due to verbal memory decline. However, in patients with less severe seizures or significant predicted verbal memory decline, surgery and medical management showed similar overall risk–benefit profiles. These findings demonstrate that the decision to pursue surgery or medical management for patients with left TLE requires careful consideration of the potential loss in QOL due to verbal memory decline as well as the gains in QOL that might result from seizure freedom.
Several independent measures are available to assess the risk for verbal memory decline following L-ATL. The absence of left hippocampal sclerosis on MRI has been reported to show modest association with worse verbal memory decline following surgery.18,19 High preoperative neuropsychological test performance is strongly predictive of worse verbal memory decline following L-ATL,10,11,13,14,16,18,20 as is later age of onset.13,20–22 More recently, fMRI protocols originally developed to measure language lateralization have demonstrated significant power to predict verbal memory decline following L-ATL similar to Wada testing, without the associated risk of an invasive procedure. When used in combination with neuropsychological test scores and age of epilepsy onset, fMRI has been found capable of predicting verbal memory decline for individual patients with 70–90% sensitivity and 73–100% specificity.11
Despite the substantial resources devoted toward assessing the risk of memory loss in preoperative evaluations for L-ATL, the use of these predictions in surgical decision making remains largely subjective and informal. Our findings highlight the benefit of formal utility-based QOL evaluation tools for verbal memory function that can be used preoperatively to aid in the decision whether to pursue L-ATL. Currently, verbal memory decline is measured by decline in neuropsychological test scores, which do not necessarily translate directly into impact on QOL, making comparisons with other surgical risks difficult.10–13,16,18,33 Postoperative patient surveys have demonstrated that verbal memory decline resulted in a decline in Quality of Life in Epilepsy Inventory (QOLIE-89) scores for patients not in seizure remission following surgery, but verbal memory decline did not affect QOLIE-89 scores if the patients experienced seizure remission.33 Our model is comparable with these findings, as the QOL penalty of verbal memory loss must exceed 0.227 (moderate to severe) for QOL following successful surgery to fall below preoperative QOL (Fig. S4). Patients who underwent R-ATL had greater increases in QOL than those who underwent L-ATL, as measured by psychiatric interviews, Symptom Checklist-90 (SCL-90), and QOLIE-31.34 However, surveys such as QOLIE are not utility based,35 and therefore cannot be employed for balancing the benefits of seizure remission against the risks of memory loss. In addition, the QOLIE survey was designed to measure the overall health-related QOL of epilepsy patients, not the specific impact of verbal memory decline on QOL.36
Our model relies on and emphasizes the need for QOL measurements that consider not only the magnitude of potential verbal memory decline, but also the QOL impact of verbal memory decline on a personalized basis for individual patients. The most significant concerns of epilepsy surgery candidates are often improved social and occupational outcomes from surgery, even over cognitive improvement.37 Thus, when predicting impact of verbal memory decline, consideration must be given to the importance of verbal memory in patients’ social and occupational lives. A tool that takes into account both expected magnitude of memory loss and its corresponding impact on a patient’s individualized QOL would personalize and facilitate more meaningful use of preoperative risk assessment data.
In addition to the need for a better instrument to estimate the QOL impact of verbal memory decline, our model has important limitations. As with all decision analyses, the strength of our conclusions rests on the accuracy of the parameters in our model (Table 1), many of which contain uncertainties.1,9 In particular, the utility values for QOL with disabling epilepsy that we adopted from previously published work are based on a small sample size of patients with epilepsy.9 However, we have used sensitivity analyses to determine at what values the preferred decision changes, and have noted when these values are not extreme, as is the case for patient 3. Our model does not consider the possibility of eventual verbal memory decline as part of the natural history of TLE for patients undergoing medical management, an important area of uncertainty and ongoing research.38–40 At present this possibility must be considered on an individualized patient basis: As the rate of verbal memory declines with medical management increases, so would the preference to pursue L-ATL. It is important to emphasize that the utilities assigned to our base-cases cannot be directly applied to all surgical subjects. Rather, applying our framework requires determining individualized QOL scores for living with seizures and QOL penalties for verbal memory decline. The QOL penalty for postoperative verbal memory decline must account for both the magnitude of predicted verbal memory decline as determined by preoperative evaluation as well as the likely impact on a patient’s social and occupational life. Finally, measurable verbal memory loss does not occur in all patients who undergo L-ATL.10–18 Our model best applies to patients with left TLE for whom presurgical testing does predict significant verbal memory loss and some consequent level of disability, and for risk-averse patients inclined to proceed with L-ATL only if the risk–benefit balance in the “worst case scenario” for predicted memory decline still favors surgery.
Our results provide a structured framework for deliberating about whether to offer epilepsy surgery versus continued medical management to patients with medically refractory left TLE, and for counseling patients facing this decision. In cases of right TLE, or when verbal memory decline is of minimal concern, our findings support previous models that strongly recommend surgery. However, for patients with left TLE, our model suggests that the optimal decision depends critically on quantifying and balancing the patient-specific impact of continued seizures against the competing risks of surgery and predicted magnitude of verbal memory decline. Further research is needed to develop utility-based neuropsychological assessment tools that will help patients and physicians use the decision framework presented herein to decide when the risk of verbal memory loss is outweighed by the potential benefits of a left anterior temporal lobectomy.
Supplementary Material
Figure S1. Diagram of the Markov model analyzed in this work.
Figure S2. Sensitivity analysis for the probability that the predicted memory loss occurs.
Figure S3. Sensitivity analyses for right anterior temporal lobectomy (no risk of memory loss).
Figure S4. QOL penalty for memory loss following successful surgery.
Table S1. Comparison of utility values for verbal memory loss with other deficits.
Biographies

Elliot Akama-Garren is an undergraduate at the Massachusetts Institute of Technology.

Dr. Bianchi is a Sleep Neurologist and teaches a Decision Analysis course at Harvard Medical School.
Footnotes
DISCLOSURE
This was an unfunded study. None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Engel J, Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 2.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia. 2000;41:342–351. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 3.Engel J., Jr Etiology as a risk factor for medically refractory epilepsy: a case for early surgical intervention. Neurology. 1998;51:1243–1244. doi: 10.1212/wnl.51.5.1243. [DOI] [PubMed] [Google Scholar]
- 4.Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Coordination Active du Réseau Observatoire Longitudinal de l’ Epilepsie. Epilepsia. 2001;42:464–475. doi: 10.1046/j.1528-1157.2001.31400.x. [DOI] [PubMed] [Google Scholar]
- 5.Semah F, Picot MC, Adam C, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- 6.Hauser WA, Hesdorffer DC. Epilepsy: Frequency, causes, and consequences. New York: Demos; 1990. p. 400. [Google Scholar]
- 7.Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 8.Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 9.Choi H, Sell RL, Lenert L, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300:2497–2505. doi: 10.1001/jama.2008.771. [DOI] [PubMed] [Google Scholar]
- 10.Baxendale S, Thompson P, Harkness W, et al. Predicting memory decline following epilepsy surgery: a multivariate approach. Epilepsia. 2006;47:1887–1894. doi: 10.1111/j.1528-1167.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 11.Binder JR, Sabsevitz DS, Swanson SJ, et al. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49:1377–1394. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chelune GJ, Naugle RI, Lüders H, et al. Individual change after epilepsy surgery: practice effects and base-rate information. Neuropsychology. 1993;7:41–52. [Google Scholar]
- 13.Gleissner U, Helmstaedter C, Schramm J, et al. Memory outcome after selective amygdalohippocampectomy in patients with temporal lobe epilepsy: one-year follow-up. Epilepsia. 2004;45:960–962. doi: 10.1111/j.0013-9580.2004.42203.x. [DOI] [PubMed] [Google Scholar]
- 14.Helmstaedter C, Elger CE. Cognitive consequences of two-thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia. 1996;37:171–180. doi: 10.1111/j.1528-1157.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee TMC, Yip JTH, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43:283–291. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- 16.Lineweaver TT, Morris HH, Naugle RI, et al. Evaluating the contributions of state-of-the-art assessment techniques to predicting memory outcome after unilateral anterior temporal lobectomy. Epilepsia. 2006;47:1895–1903. doi: 10.1111/j.1528-1167.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 17.Sabsevitz DS, Swanson SJ, Morris GL, et al. Memory outcome after left anterior temporal lobectomy in patients with expected and reversed Wada memory asymmetry scores. Epilepsia. 2001;42:1408–1415. doi: 10.1046/j.1528-1157.2001.38500.x. [DOI] [PubMed] [Google Scholar]
- 18.Stroup E, Langfitt J, Berg M, et al. Predicting verbal memory decline following anterior temporal lobectomy (ATL) Neurology. 2003;60:1266–1273. doi: 10.1212/01.wnl.0000058765.33878.0d. [DOI] [PubMed] [Google Scholar]
- 19.Seidenberg M, Hermann B, Wyler AR, et al. Neuropsychological outcome following anterior temporal lobectomy in patients with and without the syndrome of mesial temporal lobe epilepsy. Neuropsychology. 1998;12:303–316. doi: 10.1037//0894-4105.12.2.303. [DOI] [PubMed] [Google Scholar]
- 20.Hermann BP, Seidenberg M, Haltiner A, et al. Relationship of age at onset, chronologic age, and adequacy of preoperative performance to verbal memory change after anterior temporal lobectomy. Epilepsia. 1995;36:137–145. doi: 10.1111/j.1528-1157.1995.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 21.Jokeit H, Ebner A, Holthausen H, et al. Individual prediction of change in delayed recall of prose passages after left-sided anterior temporal lobectomy. Neurology. 1997;49:481–487. doi: 10.1212/wnl.49.2.481. [DOI] [PubMed] [Google Scholar]
- 22.Saykin AJ, Gur RC, Sussman NM, et al. Memory deficits before and after temporal lobectomy: effect of laterality and age of onset. Brain Cogn. 1989;9:191–200. doi: 10.1016/0278-2626(89)90029-8. [DOI] [PubMed] [Google Scholar]
- 23.Eckman MH, Rosand J, Knudsen KA, et al. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis Stroke. 2003;34:1710–1716. doi: 10.1161/01.STR.0000078311.18928.16. [DOI] [PubMed] [Google Scholar]
- 24.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156:1829–1836. [PubMed] [Google Scholar]
- 25.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44:681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 26.Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia. 2002;43:170–174. doi: 10.1046/j.1528-1157.2002.33800.x. [DOI] [PubMed] [Google Scholar]
- 27.Sperling MR, Feldman H, Kinman J, et al. Seizure control and mortality in epilepsy. Ann Neurol. 1999;46:45–50. doi: 10.1002/1531-8249(199907)46:1<45::aid-ana8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 28.Arias E. United States life tables, 2009. Natl Vital Stat Rep. 2014;62:1–40. [PubMed] [Google Scholar]
- 29.Lee GP, Park YD, Westerveld M, et al. Wada memory performance predicts seizure outcome after epilepsy surgery in children. Epilepsia. 2003;44:936–943. doi: 10.1046/j.1528-1157.2003.05003.x. [DOI] [PubMed] [Google Scholar]
- 30.Loring DW, Meador KJ, Lee GP, et al. Wada memory asymmetries predict verbal memory decline after anterior temporal lobectomy. Neurology. 1995;45:1329–1333. doi: 10.1212/wnl.45.7.1329. [DOI] [PubMed] [Google Scholar]
- 31.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin RC, Kretzmer T, Palmer C, et al. Risk to verbal memory following anterior temporal lobectomy in patients with severe left-sided hippocampal sclerosis. Arch Neurol. 2002;59:1895–1901. doi: 10.1001/archneur.59.12.1895. [DOI] [PubMed] [Google Scholar]
- 33.Langfitt JT, Westerveld M, Hamberger MJ, et al. Worsening of quality of life after epilepsy surgery: effect of seizures and memory decline. Neurology. 2007;68:1988–1994. doi: 10.1212/01.wnl.0000264000.11511.30. [DOI] [PubMed] [Google Scholar]
- 34.Cunha I, Oliveira J. Quality of life after surgery for temporal lobe epilepsy: a 5-year follow-up. Epilepsy Behav. 2010;17:506–510. doi: 10.1016/j.yebeh.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Langfitt JT, Vickrey BG, McDermott MP, et al. Validity and responsiveness of generic preference-based HRQOL instruments in chronic epilepsy. Qual Life Res. 2006;15:899–914. doi: 10.1007/s11136-005-5231-3. [DOI] [PubMed] [Google Scholar]
- 36.Cramer JA, Perrine K, Devinsky O, et al. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia. 1998;39:81–88. doi: 10.1111/j.1528-1157.1998.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 37.Taylor DC, McMackin D, Staunton H, et al. Patients’ aims for epilepsy surgery: desires beyond seizure freedom. Epilepsia. 2001;42:629–633. doi: 10.1046/j.1528-1157.2001.34400.x. [DOI] [PubMed] [Google Scholar]
- 38.Helmstaedter C, Kurthen M, Lux S, et al. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425–432. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- 39.Hermann BP, Seidenberg M, Dow C, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60:80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- 40.Oyegbile TO, Dow C, Jones J, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Diagram of the Markov model analyzed in this work.
Figure S2. Sensitivity analysis for the probability that the predicted memory loss occurs.
Figure S3. Sensitivity analyses for right anterior temporal lobectomy (no risk of memory loss).
Figure S4. QOL penalty for memory loss following successful surgery.
Table S1. Comparison of utility values for verbal memory loss with other deficits.


