Abstract
Background
Hemeproteins such as free myoglobin can undergo autoxidation and catalyze lipid peroxidation, increasing oxidative stress. Creatine phosphokinase (CPK) elevation is a marker for free myoglobin after myocyte damage. Since oxidative injury is a key mechanism of injury-related organ dysfunction, we hypothesized that serum CPK levels correlate with mortality and need for and duration of inotropic support, i.e. shock, among critically injured patients.
Methods
We conducted a retrospective review of 17,847 patients admitted to a single Trauma Intensive Care Unit over 9 years. 2,583 patients with serum CPK levels were included in the analysis. Patient data were collected continuously into an electronic ICU repository. Univariate analysis was accomplished using Spearman correlation and the Mann Whitney U test. Propensity score adjustment models accounting for potential confounders were used to assess the independent effect of CPK level on mortality, need for inotropic support, and duration of inotropic support.
Results
Median CPK was significantly higher in patients who died (916 [IQR 332, 2472] vs. 711 [253, 1971], p= 0.004) and in those who required inotropic medications (950 [353, 2525] vs. 469 [188, 1220], p< 0.001). After adjusting for propensity score and potential confounders the odds of mortality increased by 1.10 (95% CI 1.02– 1.19, p= 0.020) and the odds of inotropic medication use increased by 1.30 (95% CI 1.22–1.38, p <0.001) per natural log unit increase in CPK. There was a significant association between CPK level and duration of inotropic support (Spearman’s rho .237, p< 0.001) that remained significant in a propensity score adjusted model.
Conclusion
In critically injured patients, elevated serum CPK level is independently associated with mortality, need for inotropic medication, and duration of inotropic support. This study is the first to evaluate the relationship of CPK level and mortality in addition to surrogate measures of shock in a population of critically injured patients. If these associations are verified prospectively, there may be a role for treatment with hemeprotein reductants, such as Paracetamol, to mitigate the effects of shock and end-organ dysfunction.
Keywords: shock, mortality, hemeprotein-mediated, creatine phosphokinase, oxidative injury
BACKGROUND
Trauma is consistently the leading cause of death among patients between the ages of 1 and 44 (National Vital Statistics System, National Center for Health Statistics, CDC). The presence of shock after critical injury is associated with worse outcomes including organ failure and infection in those who survive [1]. Insults related to shock and traumatic injury result in inflammation and increased oxidant stress exacerbating tissue and organ injury [2,3]. Interventions aimed at ameliorating this process have the potential to improve outcomes in critically injured patients.
Hemeprotein-mediated oxidative injury is emerging as an important mechanism contributing to end-organ damage. Examples of this mechanism include myocardial injury related to ischemia/reperfusion, cerebral vasospasm after subarachnoid hemorrhage, and rhabdomyolysis-induced renal failure [4]. Myocyte injury resulting in ATP depletion results in myoglobin redox cycling and culminates in the production of hydroxyl and ferryl protoporphyrin radicals capable of inducing lipid peroxidation in patients with renal failure related to rhabdomyolysis [5–7]. Although the release of myoglobin (Mb) ultimately results in oxidative damage [5,8], creatine phosphokinase (CPK) is released in parallel and is the preferred measure to follow clinically [9]. Due to this fact, CPK elevation may serve as a marker for this injury pattern in critically ill trauma patients.
Despite the fact that CPK elevation results from multiple conditions related to trauma [10,11], it remains an under-recognized phenomenon in critically injured patients. One study conducted at a center that monitors CPK levels regularly on all critically injured patients found that 85% had abnormal levels [12].
Because oxidative stress has been linked to worse outcomes including organ dysfunction in critically ill surgical patients, we wanted to investigate a potential source of oxidant injury. Hemoprotein-mediated oxidative injury related to myocyte damage may contribute to the oxidative burden in critically ill trauma patients, but has not been previously investigated. To support further research endeavors into this potential source of injury we elected to look at a convenience sample of patients in order to obtain preliminary data that would support the prospective evaluation of this concept. If this mechanism significantly contributes to organ dysfunction related to oxidative injury, this process has the potential to be prevented by hemoprotein reductants such as Paracetamol. As a starting point in the initial investigation of this process, we conducted a retrospective analysis to test the hypothesis that increased serum CPK levels in critically injured patients are associated with the need for and the duration of inotropic support, a surrogate measure of shock, and mortality.
METHODS
Study Setting
This study was approved by the Vanderbilt University Institutional Review Board (no. 130484). Vanderbilt University Medical Center (VUMC) is an academic level I trauma center that provides trauma care for a catchment area of approximately 65,000 square miles. There are approximately 3800 trauma related admissions per year, including around 800 patients admitted to the trauma intensive care unit (ICU). The 14 bed trauma ICU is located within a 31 bed integrated Acute and Sub-Acute care unit.
Data Collection
VUMC maintains a fully implemented electronic medical record for all patients including orders, charting, and documentation. Information such as age, gender, ventilator use, injury severity scoring, hospital diagnoses, infectious complications, inotropic medication utilization, hospital and ICU length of stay, and other data are captured for each patient admitted to the ICU in real-time and collected into a secure electronic data repository. We also incorporated data recorded into our trauma registry. This data was retrieved electronically in a password-protected manner and de-identified for subsequent analysis.
We retrospectively analyzed data from 17,847 critically injured patients admitted to the trauma ICU over a 9 year period between December 2004 and November 2012. We performed our analysis on a convenience sample of patients (n= 2,583) with serum CPK levels drawn during the time of their hospitalization.
Outcome Measures
Serum CPK levels were obtained at the discretion of the primary care team and were recorded if they were drawn from admission through the day of discharge. CPK levels at our institution are typically obtained in patients to rule in/out rhabdomyolysis, myocardial infarction, compartment syndrome, and significant crush injury. We evaluated CPK levels recorded throughout hospitalization in order to capture elevations related to both the initial injury pattern as well as complications of injury such as those related to intervention, compartment syndrome, infection, and electrolyte abnormality. If multiple CPK levels were recorded for a single patient, the peak level was utilized for further analysis as it likely represents the maximum extent of injury or exposure to pro-oxidant myoglobin. A CPK level of greater than 300 U/L was considered to be elevated based on the upper limit of normal specified by our clinical laboratory.
Information regarding the administration of inotropic medication and duration of inotropic support was documented in the electronic medical record and captured into the electronic database. We chose inotropic support as a measure of shock because information regarding the use of inotropic medication is readily available and reflects a more accurate picture of ongoing shock and instability. Other measures of shock such as blood pressure and heart rate are not continuously captured and we do not specifically tailor resuscitation based on lab values such as lactate and base deficit. Inotropic medications included dopamine, epinephrine, norepinephrine, phenylephrine, and vasopressin. The general indication for inotropic support at our institution is persistent hypotension (systolic blood pressure <90 mmHg) refractory to fluid and blood resuscitation. Therapy is guided based on invasive measures of systemic pressure provided by arterial lines or pulmonary artery catheters.
Mortality data was also collected. If a patient did not die during the hospitalization encompassing their ICU admission they were considered to be alive for further analysis. If a patient was admitted more than once to the ICU during the nine year study window then only data from the initial episode of care was used for analysis.
Statistical analysis
Since data are not normally distributed, continuous variables are reported as median values with interquartile range (IQR). Differences between patients with measured CPK levels and those without were compared using χ2 statistics for categorical variables and the Mann-Whitney U test for continuous variables. Univariate analysis was accomplished using Spearman correlation, the Mann Whitney U test, and the Kruskal-Wallis test where appropriate. CPK is obtained on the basis of clinical suspicion in patients who are likely to have elevations as a result of injury or insult. Because increasing CPK level may be associated with factors directly related to shock and mortality, we attempted to minimize potential confounding and selection biases by developing a propensity score for CPK level. The propensity score for CPK level was determined using a linear regression model that included 14 covariates chosen a priori based on their known or potential effects on CPK level and possible causal relationship to outcome measures. These covariates included age, gender, race, injury severity score (ISS), acute renal failure, cardiac disease (ischemic heart disease, pericardial/endocardial disease, and cardiac arrest), compartment syndrome, crush injury, delirium tremens, malignant hyperthermia, myocyte infection/inflammation, rhabdomyolysis, seizure activity, and thyroid disorders. CPK level was natural log-transformed in all models to provide normality in regression residuals. The primary analyses evaluated hospital mortality and need for inotropic support in relation to serum CPK level utilizing multivariable logistic regression models and the independent effect of CPK level on duration of inotropic support using Poisson regression. The covariates included in the final models were age, gender, race (White vs. non-White), ISS, and propensity score. Missing data for ISS in regression models were imputed using multiple imputation methods [13]. All statistical tests were two-tailed with p < 0.05 set as significant. SPSS version 20 (IBM Corporation, Armonk, NY) was used for analysis.
RESULTS
Patient characteristics
Of 17,847 critically injured patients admitted to the trauma ICU, 2,583 had at least one CPK level recorded during their hospital stay and constituted the study population. Patients with CPK levels were older, had a higher expected mortality, longer duration of mechanical ventilation, longer hospital and ICU length of stay, and higher in-hospital mortality than patients without CPK levels admitted during the same time period. A median of 1 (IQR 1, 3) CPK measurement was obtained among the study population. The median peak plasma CPK level in the study population was 754 U/L (IQR 269–2024). The median time to peak CPK level was 1 day (IQR 0, 3). Patients with one or more of the diagnoses (n= 419) included in the model used to calculate propensity score had significantly higher CPK levels (1129 U/L [IQR 327–3629] vs. 702 U/L [IQR 257–1796], p <0.001). These diagnoses included acute renal failure, cardiac disease (ischemic heart disease, pericardial/endocardial disease, and cardiac arrest), compartment syndrome, crush injury, delirium tremens, malignant hyperthermia, myocyte infection/inflammation, rhabdomyolysis, seizure activity, and thyroid disorders. Based on trauma registry diagnostic data, patients with compartment syndrome and crush injuries comprised less than 5% of patients included in the analysis. Descriptive data are shown in Table 1.
Table 1.
Baseline Characteristics and Descriptive Data (n= 2,583)
| Characteristic | Median (IQR) or n (%) |
|---|---|
| Age, yr | 57 (44, 70) |
| Male | 1,855 (72) |
| ISS | 22 (14, 33) |
| Initial GCS | 15 (4, 15) |
| Blunt mechanism | 2,381 (92) |
| ICU LOS | 4 (2, 9) |
| Hospital LOS | 8 (4, 15) |
| Ventilated | 1,636 (63) |
| Ventilator time, days | 2 (0, 7) |
| Require inotropic support | 1,653 (64) |
| Duration of inotropic support, days | 1 (0, 2) |
| Received blood product | 1,489 (58) |
| Hospital mortality | 396 (15) |
IQR= interquartile range; ISS= injury severity score; GCS= Glasgow Coma Scale; ICU= intensive care unit; LOS= length of stay
Mortality
Plasma CPK levels were significantly higher in non-survivors (916 U/L [IQR 332–2472) vs. 711 U/L [IQR 253–1971], p= 0.004) (Table 2). Utilizing multivariable logistic regression analysis, we sought to determine the independent effect of CPK level on in-hospital mortality. The unadjusted odds of mortality was 1.11 (95% CI 1.03–1.19, p= 0.005) per natural log unit increase in CPK. The propensity score-adjusted odds of mortality after controlling for age, gender, race (White vs. non-White), and ISS was 1.10 (95% CI 1.02–1.19, p= 0.020) per natural log unit increase in CPK (Table 3).
Table 2.
Univariate Analysis of Primary Outcome Measures (n= 2,583)
| Variable | CPK | p | |
|---|---|---|---|
| Mortality | |||
| Yes | 916 (332, 2,472) | 0.004 | |
| No | 711 (253, 1,971) | ||
| Inotropic Support | |||
| Yes | 950 (353, 2,525) | <0.001 | |
| No | 469 (188, 1,220) |
Note: Values shown are median (interquartile range). CPK= creatine phosphokinase
Table 3.
Association Between Peak CPK Level, Mortality, and Inotropic Requirement
| Mortality: | |||
| Model | OR (95% CI) | p | |
| Unadjusted | 1.106 (1.032–1.187) | 0.005 | |
| Adjusted† | 1.101 (1.015–1.194) | 0.020 | |
| Inotropic Support: | |||
| Model | OR (95% CI) | p | |
| Unadjusted | 1.348 (1.272–1.428) | <0.001 | |
| Adjusted† | 1.297 (1.216–1.383) | <0.001 | |
| Duration of Inotropic Support: |
|||
| Model | RR (95% CI) | p | |
| Unadjusted | 1.170 (1.149–1.191) | <0.001 | |
| Adjusted† | 1.087 (1.072–1.101) | <0.001 | |
Adjusted for propensity, age, gender, race (White vs. non-White), and ISS
OR= odds ratio; RR= relative risk; CI= confidence interval; ISS= injury severity score
Inotropic Requirement
In patients who required inotropic support, norepinephrine and vasopressin drips were utilized in greater than 50% of cases, while dopamine was used in less than 2% of cases overall. In our study population, a median of 1 inotropic medication (IQR 1, 2) was used in a given day. Patients who required inotropic support had significantly higher CPK levels (950 U/L [IQR 353–2525] vs. 469 U/L [IQR 188–1220], p< 0.001) (Table 2). Multivariate logistic regression analysis was performed to examine the association between CPK level and need for inotropic support. The unadjusted OR for inotropic medication use was 1.35 (95% CI 1.27–1.43, p<0.001). A propensity score adjustment model controlling for age, gender, race (White vs. non-White), and ISS was utilized to determine that the adjusted odds of inotropic medication use increased by 1.30 (95% CI 1.22–1.38, p<0.001) per natural log unit increase in CPK (Table 3).
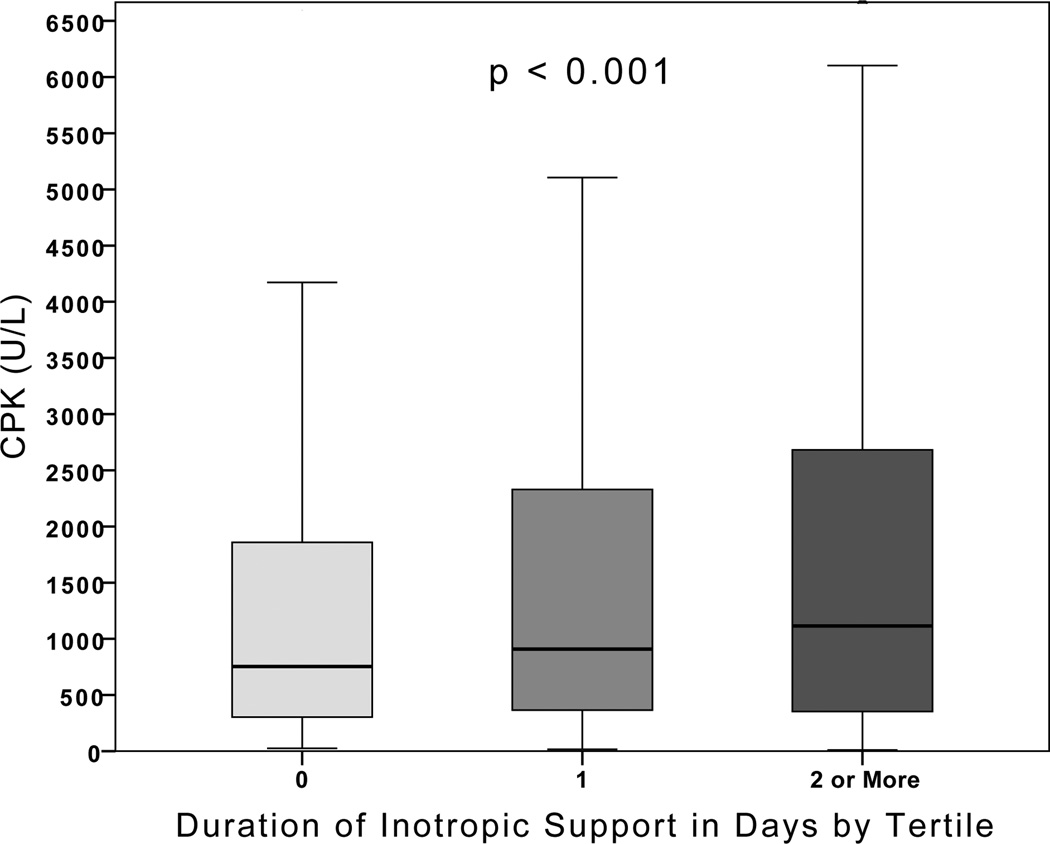
There was a significant association between peak CPK level and duration of inotropic support (Spearman’s rho 0.237, p<0.001). From the lowest tertile of inotrope duration measured as days on inotropic support to the highest, there was a significant increase in CPK level (p< 0.001, Figure 1). Poisson regression analysis was used to examine this association further by assessing the independent effect of CPK level on duration of inotropic support in days. The unadjusted relative risk for increased duration of inotropic support was 1.17 (95% CI 1.15–1.19, p<0.001). After propensity score adjustment and controlling for age, gender, race (White vs. non-White), and ISS the mean duration of inotropic support increased by 9% for every one natural log unit increase in CPK (Relative Risk: 1.09, 95% CI 1.07–1.10, p<0.001) (Table 3).
Figure 1.
Duration of Inotropic Support in Days by Tertile
In a subset analysis we evaluated the correlation of inotropic medication use with other known measures of shock such as systolic blood pressure, base deficit, and lactate on presentation, the need for blood transfusion, and number of blood products transfused. We found that all of these variables were significantly correlated with the need for inotropic support (p <0.001).
Clinical outcomes
Of the study population 72% (n= 1,872) had elevated CPK levels (>300 U/L). These patients were more likely to be ventilated (67% vs. 54%), receive blood products (62% vs. 45%), and had a higher ISS (24 [IQR 16, 34] vs. 20 [IQR 13, 29]) (all p<0.001). Hospital stay was significantly longer in patients with elevated CPK (8 days [IQR 4–16] vs. 7 days [IQR 3–15], p<0.001). Patients with elevated CPK also had a longer ICU length of stay (5 days [IQR 2–10] vs. 3 days [IQR 1–8], p<0.001) and increased mortality (16.6% vs. 12.1%, p= 0.005).
DISCUSSION
Among critically injured adults admitted to the trauma ICU there was a significant association between CPK level and mortality as well as need for inotropic medication and duration of inotropic support even after propensity score adjustment and controlling for potential confounders. To our knowledge, this study is the first to evaluate the relationship of CPK level and mortality in addition to surrogate measures of shock in a population of critically injured patients.
Disease states causing ATP depletion disrupt myocyte integrity causing leakage of both CPK and myoglobin into the interstitial fluid space, resulting in oxidative injury [14]. Myoglobin redox cycling contributes to end organ failure through hemeprotein-mediated oxidative injury in patients with rhabdomyolysis, but several authors suggest that CPK is a more reliable marker in assessing the presence and intensity of muscle damage [9,15]. CPK elevation therefore may signify increased oxidative burden and appears to be a common occurrence in critically injured patients [12,16]. In the current study 73% of patients had elevated CPK levels.
CPK elevation has been previously associated with shock as a result of intravascular volume depletion and death in relation to electrolyte disturbances and malignant cardiac arrhythmias in patients with complications of severe rhabdomyolysis [17]. In the current study, the lowest abnormal CPK level among non-survivors and among those requiring inotropic support was 301 U/L and median CPK levels were well below five times greater than the upper limit of normal. This finding further suggests that CPK level may be associated with mortality and inotropic medication use as a surrogate measure of shock independent of whether or not patients meet the clinical criteria for rhabdomyolysis.
It is clear that shock in the setting of traumatic injury is associated with poor outcomes including the development of organ failure and increased mortality [18]. Aggressive management strategies aimed at minimizing and preventing factors that contribute to shock and organ dysfunction should be implemented and may lessen the burden of disease attributed to traumatic injury. If the associations determined in this study are prospectively validated, treatment with hemeprotein reductants may provide improved outcomes in critically injured patients with CPK elevation. Paracetamol inhibits myoglobin redox cycling and has shown promise at therapeutic doses in animal studies and in critically ill patients [5,19].
Limitations to this study include the retrospective design using data collected into an electronic data repository. In addition, a significant limitation is the introduction of selection bias by including only patients with documented CPK levels. CPK is not a routine lab measurement and is obtained on the basis of clinical suspicion. We attempted to minimize this bias by including a propensity score adjusted model in analyses of our primary outcome measures, but realize that this would not address hidden biases within our study. However, due to the novelty of this concept and the relative absence of data related to the association of CPK level with clinical outcomes other than in trauma patients with rhabdomyolysis, we believe this to be appropriate starting point. We plan to use this preliminary data to justify the costs associated with evaluating this relationship further in a prospective manner. Additionally, we relied on CPK as a surrogate marker of ferryl myoglobin-induced oxidative injury and did not directly evaluate measures of oxidative stress. In future prospective analysis we plan to evaluate F2-isoprostane levels, byproducts of ferryl myoglobin, and CPK levels concomitantly in relation to clinical outcomes. Further prospective analysis is required to determine causation, and evaluate the utility of CPK level as a prognostic indicator of clinical outcomes.
In conclusion, elevated serum CPK levels are independently associated with mortality, need for inotropic medication, and duration of inotropic support. Due to the complexity of disease in the trauma population and the costs inherent in the prospective analysis of mechanisms related to oxidative injury, these data provide a starting point to justify further evaluation. These associations should be verified prospectively and may provide insight into who may benefit from therapies aimed at reducing the burden of hemeprotein-mediated oxidative injury.
Supplementary Material
Contributor Information
Kaushik Mukherjee, Email: kaushik.mukherjee@vanderbilt.edu.
Patrick R. Norris, Email: patrick.norris@vanderbilt.edu.
Ayumi Shintani, Email: ayumi.shintani@vanderbilt.edu.
Lorraine B. Ware, Email: lorraine.ware@vanderbilt.edu.
L. Jackson Roberts, Email: jack.roberts@vanderbilt.edu.
Addison K. May, Email: addison.may@vanderbilt.edu.
REFERENCES
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006 Jun;60(6 Suppl):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Gando S, Sawamura A, Hayakawa M. Trauma, shock, and disseminated intravascular coagulation: lessons from the classical literature. Ann Surg. 2011 Jul 1;254(1):10–19. doi: 10.1097/SLA.0b013e31821221b1. [DOI] [PubMed] [Google Scholar]
- 3.Crimi EE, Sica VV, Williams-Ignarro SS, Zhang HH, Slutsky ASA, Ignarro LJL, Napoli CC. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006 Feb 1;40(3):9–9. doi: 10.1016/j.freeradbiomed.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 4.Alayash AIA, Patel RPR, Cashon RER. Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid Redox Signal. 2001 Apr 1;3(2):313–327. doi: 10.1089/152308601300185250. [DOI] [PubMed] [Google Scholar]
- 5.Boutaud OO, Moore KPK, Reeder BJB, Harry DD, Howie AJA, Wang SS, Carney CKC, Masterson TST, Amin TT, Wright DWD, Wilson MTM, Oates JAJ, Roberts LJL. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci U S A. 2010 Feb 9;107(6):2699–2704. doi: 10.1073/pnas.0910174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt S, Reeder B, Wilson M, Harvey S, Morrow JD, Roberts LJ, Moore K. Increased lipid peroxidation in patients with rhabdomyolysis. Lancet. 1999 Apr 10;353(9160):1241–1241. doi: 10.1016/S0140-6736(98)05768-7. [DOI] [PubMed] [Google Scholar]
- 7.Holt SGS, Moore KPK. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001 May 1;27(5):803–811. doi: 10.1007/s001340100878. [DOI] [PubMed] [Google Scholar]
- 8.Boutaud O, Roberts LJI. Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free Radic Biol Med. 2011;51(5):1062–1067. doi: 10.1016/j.freeradbiomed.2010.10.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009 Jul 2;361(1):62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 10.Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. 2000 doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 11.Slater MS, Mullins RJ. Rhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients: a review. J. Am. Coll. Surg. 1998 Jun;186(6):693–716. doi: 10.1016/s1072-7515(98)00089-1. [DOI] [PubMed] [Google Scholar]
- 12.Brown CVR, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004 Jun;56(6):1191–1196. doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic … - Frank E. Harrell - Google Books. 2001 [Google Scholar]
- 14.Morykwas MJ, Howell H, Bleyer AJ, Molnar JA, Argenta LC. The effect of externally applied subatmospheric pressure on serum myoglobin levels after a prolonged crush/ischemia injury. J Trauma. 2002 Sep;53(3):537–540. doi: 10.1097/00005373-200209000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Beetham RR. Biochemical investigation of suspected rhabdomyolysis. Ann Clin Biochem. 2000 Sep 1;37(Pt 5):581–587. doi: 10.1258/0004563001899870. [DOI] [PubMed] [Google Scholar]
- 16.Smith WA, Hardcastle TC. A crushing experience: The spectrum and outcome of soft tissue injury and myonephropathic syndrome at an Urban South African University Hospital. African Journal of Emergency Medicine. 2011 Mar;1(1):17–24. [Google Scholar]
- 17.Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009 Oct;67(9):272–283. [PubMed] [Google Scholar]
- 18.Zenati MS, Billiar TR, Townsend RN, Peitzman AB, Harbrecht BG. A brief episode of hypotension increases mortality in critically ill trauma patients. J Trauma. 2002 Aug;53(2):232–236. doi: 10.1097/00005373-200208000-00007. –discussion236–7. [DOI] [PubMed] [Google Scholar]
- 19.Janz DR, Bastarache JA, Peterson JF, Sills G, Wickersham N, May AK, Roberts LJ, Ware LB. Association Between Cell-Free Hemoglobin, Acetaminophen, and Mortality in Patients With Sepsis: An Observational Study. Crit. Care Med. 2013 Jan 10; doi: 10.1097/CCM.0b013e3182741a54. :–. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.