Abstract
Biliary atresia is a severe cholangiopathy of early infancy that destroys extrahepatic bile ducts and disrupts bile flow. With a poorly defined disease pathogenesis, treatment consists of the surgical removal of duct remnants followed by hepatoportoenterostomy. Although this approach can improve the short-term outcome, the liver disease progresses to end-stage cirrhosis in most children. Further improvement in outcome will require a greater understanding of the mechanisms of biliary injury and fibrosis. Here, we review progress in the field, which has been fuelled by collaborative studies in larger patient cohorts and the development of cell culture and animal model systems to directly test hypotheses. Advances include the identification of phenotypic subgroups and stages of disease based on clinical, pathological and molecular features. Stronger evidence exists for viruses, toxins and gene sequence variations in the aetiology of biliary atresia, triggering a proinflammatory response that injures the duct epithelium and produces a rapidly progressive cholangiopathy. The immune response also activates the expression of type 2 cytokines that promote epithelial cell proliferation and extracellular matrix production by nonparenchymal cells. These advances provide insight into phenotype variability and might be relevant to the design of personalized trials to block progression of liver disease.
Introduction
Biliary atresia, a multifaceted liver disease of complex pathogenesis, has devastating consequences to child health with rapid progression to end-stage cirrhosis if not treated in a timely fashion. Departing from solely descriptive studies, the past decade has seen a proliferation of mechanistic experiments that use in vitro and in vivo model systems to directly test disease-related hypotheses. Here, we review how these studies advance our understanding of disease pathogenesis by providing unique insights into how an insult from one or more environmental factors triggers an immune response, which is modified by genetic and developmental factors to target the bile duct epithelium and produce the clinical spectrum of biliary atresia.
Biliary atresia results from an inflammatory and fibrosing obstruction of extrahepatic bile ducts. Along with the general term of ‘neonatal hepatitis’, biliary atresia is a leading cause of neonatal cholestasis;1–4 as a single disease, biliary atresia is the number one indication for paediatric liver transplantation worldwide.5 By the time of diagnosis, extrahepatic bile ducts are completely obstructed. At the tissue level, segmental or wholesale loss of the epithelial lining of extrahepatic bile ducts exists, with extensive fibrosis and occasional foci of inflammation. By contrast, intrahepatic bile ducts are typically hyper-plastic, embedded in portal tracts that contain variable inflammation and fibrosis, and are surrounded by lobules with features of cholestasis and variable degrees of giant multinucleated hepatocytes.6
Epidemiology
The disease occurs on all continents, with variable geographical frequency ranging from 1 in 5,000 in Taiwan to an estimated 1 in 15,000 in the USA and 1 in 19,000 live births per year in the Netherlands.7–11 A seasonal clustering of the disease has been reported, with rates three times higher in infants born between December and March,11,12 but the lack of seasonality in a large study of 119 Japanese infants underscores the variable geographical frequency of disease.13 Biliary atresia has a slight female predominance (1.25:1), especially in patients who also have splenic malformations and the incidence is substantially higher among nonwhite infants.11,14 An association with advanced maternal age, increased parity and a tendency for early foetal losses has been found.11 Biliary atresia has rare familial recurrence, with twin studies showing that most sets are discordant for the disease.15–19
Disease hallmarks and outcomes
The clinical hallmarks of the disease are simple and reproducible: pathological jaundice with direct or conjugated hyperbilirubinemia; acholic stools; variable levels of hepato splenomegaly; and the onset of symptoms restricted to the first few months of life. The progression to end-stage cirrhosis when the disease is untreated is consistent. The initial goal of clinical management is to arrive at the diagnosis promptly so that surgical intervention can remove the atretic biliary remnants and create a Rouxen-Y intestinal conduit for bile drainage, also known as the Kasai hepatoportoenterostomy.20 Unfortunately, an unacceptable 40–50% of patients still do not have improved bile drainage after the hepatoportoenterostomy;14,21–23 even when they do, the majority of the patients require liver transplantation for prolonged survival.5
Precise phenotyping of disease
Jaundice and acholic stools are uniformly present in infants with biliary atresia. However, increasing evidence supports the existence of specific clinical forms and variants based on the presence of nonhepatic malformations, time of disease onset and morphological and molecular analysis of the hepatobiliary system (Table 1).24–26
Table 1.
Clinical phenotypes of biliary atresia*
| Phenotype | Incidence and disease features | Pathogenesis |
|---|---|---|
| Perinatal form | ~80% of patients, isolated malformations, jaundice-free period after birth | Type 1 cytokine response |
| Embryonic form | 7-10% of patients, BASM, laterality defects, early onset of jaundice | Gene mutations and variants |
| Cystic variant | 8% of patients, might co-exist with BASM, poor response when HPE performed after 70 days of age | Type 2 cytokine response |
| CMV-associated | Variable incidence based on geography and methodological detection, poor response to HPE | CMV infection |
Key features and a proposal for the main mechanisms of pathogenesis. Abbreviations: BASM, biliary atresia splenic malformation; CMV, cytomegalovirus; HPE, hepatoportoenterostomy.
Perinatal or nonsyndromic biliary atresia
Most infants fall into this clinical form, which is commonly referred to as ‘perinatal’ based on the isolation of viruses in affected livers and on the detection of serum bilirubin levels (either conjugated or unconjugated) in the first 1–2 days of life in infants later diagnosed with biliary atresia.27 However, in the absence of reproducible data demonstrating a perinatal insult, this group of patients might be more appropriately referred to as ‘nonsyndromic’. A subgroup of these patients might have single or multiple nonhepatic malformations such as cardiovascular abnormalities and intestinal malrotation.14,28–30 Coexisting malformations do not necessarily worsen the hepatobiliary disease,30 but need proper attention to prevent complications and improve clinical outcome.
Embryonic biliary atresia
About 10% of affected infants have an earlier onset of jaundice, often present at birth and have nonhepatic congenital malformations.14,28–30 This group of patients is also referred to as having the congenital, fetal or prenatal form of biliary atresia. Consistent with an early onset of bile duct injury, extrahepatic bile ducts might be absent (that is, no sign of a fibrous cord at the time of exploratory laparotomy). Splenic abnormalities (as plenia, double spleen and polysplenia) have been reported in 8–12% of infants with the embryonic form of disease, which occurs in isolation or in combination with one or more additional defects (for example, preduodenal portal vein, interrupted inferior vena cava, intestinal mal rotation) in a variant known as biliary atresia splenic malformation (BASM) syndrome.14,28 Infants with BASM have a higher association with maternal diabetes mellitus than those with the nonsyndromic form and might have a worse outcome after hepatoportoenterostomy.
Cystic biliary atresia
The presence of a cystic malformation near the site of the common bile duct obstruction, constitutes an anatomic variant often referred to as cystic biliary atresia, which might be associated with improved bile drainage after hepatoportoenterostomy.31 Some of the infants with biliary cysts are detected prenatally during routine ultrasonography; jaundice and acholic stools might present soon after birth or after an asymptomatic period of 1–3 months of life. A review of a large cohort of infants with biliary atresia reported the presence of biliary cysts in ~8% of patients.32 Infants with this cystic variant were younger at presentation in this cohort, but a delay in performing a portoenterostomy beyond 70 days of age was associated with poor long-term survival with the native liver. In addition, some of the infants with the cystic variant of biliary atresia shared features of BASM. Detection of prenatal cystic changes in some patients suggests an intra-uterine onset of disease; perhaps also suggesting that the mechanisms of intrauterine tissue injury differs from the other clinical forms. A promin ent type 2 T helper (TH2)-cell-mediated inflammatory response has been associated with cystic biliary atresia in mice.33 Mice engineered to have a prominent TH2 response, in a model of rotavirus-induced biliary atresia, was associated with an increased frequency of cystic changes in extrahepatic bile ducts.33
Cytomegalovirus-associated biliary atresia
Reports of an association between cytomegalovirus detection and poor biliary drainage after hepatoportoenterostomy could be of particular relevance in designing clinical care protocols and future trials. One study of disease aetiology in 85 Chinese patients identified cytomegalovirus DNA and/or protein in the livers of 51 infants,34 and a second study of a smaller cohort showed a low percentage of patients with normalization of bilirubin levels after hepatoportoenterostomy.35 Poor bile drainage and highest risk of death were also linked to the presence of cytomegalovirus IgM antibodies in a cohort of infants at King's College Hospital, London, UK, who had a 10% survival with the native liver at 2 years of age.36 In a larger cohort of 260 patients, these investigators also found that infants with cytomegalovirus-associated biliary atresia had the highest aspartate aminotransferase:platelet ratio index when compared with patients with the cystic, BASM and perinatal forms of biliary atresia.37 However, geographical variations in cytomegalovirus-associated biliary atresia exist.38,39 Some of the inconsistencies between studies might result from methodological differences used to study the patient population. For example, using an assay that tests lymphocyte activation to cytomegalovirus proteins identified a positive response in 56% of infants in the USA, even when no other biochemical markers of cytomegalovirus infection were present.40 Altogether, efforts to precisely define the status of cytomegalovirus infection in patient cohorts are justified by this data, as it might trigger unique pathogenic mechanisms and/or constitute a severity factor influencing the clinical outcome in biliary atresia.
Staging the disease at diagnosis
Several clinical reports suggest that an age <60 days at the time of hepatoportoenterostomy is associated with improved bile flow and long-term outcome.41 This association implies that a younger patient will have an earlier phase of disease and perhaps a lesser degree of liver injury. However, age alone is not a uniform predictor of outcome as demonstrated by the poor survival with the native liver of a cohort of young infants with biliary atresia after hepatoportoenterostomy,42 suggesting that other factors influence the clinical course of disease, such as the coexistence of severe cardiovascular disease, cytomegalovirus infection, and the type and extent of liver pathology at diagnosis. Prominent histological findings of inflammation in the liver at diagnosis has been reported to correlate with a poor response to hepatoportoenterostomy.43 If one assumes that prominent inflammation reflects the severity of tissue injury this association would make sense, as would the poor outcome despite surgery. Alternatively, if one assumes that prominent inflammation reflects an early phase of tissue involvement or injury this association would be unexpected and an improved outcome after surgical intervention would be expected. Our lack of understanding of the relationship between histopathology and clinical response is underscored by other studies reporting a poor outcome in children with advanced hepatic fibrosis,44,45 or the lack of a correlation between fibrosis and clinical outcome.46
Seeking additional insight into the relationship between the degree of liver injury with age at diagnosis and the clinical course after hepatoportoenterostomy, Moyer et al.26 scored liver biopsy samples from infants for inflammation and fibrosis at the time of diagnosis. Only 19% and 11% of the biopsies revealed high scores for inflammation and fibrosis, respectively. The remaining 70% had mixed histological features (lower scores for inflammation and fibrosis). None of these histo logical groups correlated with age at diagnosis, clinical par ameters, or 2-year survival with the native liver. Reasoning that morphological quantification methods might be limited by sampling artefacts or variability in injury patterns, the investigators generated gene expression signatures of biopsy fragments from the same patients. By differential profiling of gene expression and prediction analysis models, most of the individuals were grouped into inflammation (36%) or fibrosis (55%), with only 9% of the specimens displaying mixed molec ular profiling. Infants with an inflammatory signature were younger than those with fibrosis; however, age alone could not predict molecular grouping based on the observation that several infants younger than 8 weeks were classified into the fibrosis group. The clinical relevance of the molecular classification was supported by a decreased survival without transplantation at 2 years of age in infants with the fibrosis signature.26 If these studies are validated in new prospective cohorts, molecular staging (with or without histological staging) of liver disease should be considered when designing new clinical trials.
Pathogenic mechanisms of disease
Studies into the epidemiology, pathology and clinical forms of biliary atresia implicate numerous factors in the pathogenesis of disease, including: defects in embryo-genesis; abnormal fetal or prenatal circulation; genetic factors; environmental toxins; viral infection; abnormal inflammatory response; autoimmunity; and susceptibility factors.47,48 These seemingly different factors can be grouped into the broadly defined categories of abnormal morphogenesis, environmental factors and inflammatory dysregulation, which could interplay to produce a particular phenotype of biliary atresia (Box 1).
Defective embryogenesis
Developmental defects of the liver and biliary tract might be genetically driven or might result from an insult that disrupts biological circuits critical to organogenesis. Genetic mutations and/or variants relevant to embryogenesis might be linked to biliary atresia pathogenesis, as suggested by BASM and laterality defects (anomalies of left-right determination of visceral organs), among others. Disruption of complex molecular circuits during vulnerable periods of embryogenesis could also result from an insult at a precise time in embryogenesis, with a potential for reprogramming cellular differentiation and an abnormal developmental outcome. Although no specific insult during embryogenesis has yet been linked directly to biliary atresia, abnormal cell fate in the developing bile duct has been proposed to produce ductal plate malformations that persist postnatally in some patients.49 The ductal plate is a bilayered tubular structure surrounded by thick mesenchyme that is formed near the portal vein between 11 and 13 weeks of gestation.50 The plate gradually disappears, except for a focal area where it forms a lumen and gives rise to intrahepatic bile ducts;51 its postnatal persistence raises the possibility that abnormal mesenchymal and cholangiocyte signals halt remodelling of hilar ducts. Although ductal plate malformation has been described in infants with the embryonic form of biliary atresia, a study of the morphology of several portal tracts from each of eight infants with the perinatal form of biliary atresia and six with BASM identified ductal plate malformation in only 10% of the portal tracts, with no predilection to either clinical forms of the disease.52
Abnormal fetal or prenatal circulation
Potential defects in prenatal circulation have been implicated in disease pathogenesis based on the presence of anatomical variants of hepatic artery in some patients with biliary atresia, the findings of arterial hyperplasia or hypertrophy in liver specimens and on the importance of arterial blood flow for integrity of bile ducts.53,54 Although limited data support this mechanism, it is possible that arterial changes might be driven by primary growth signals, or represent a response to a diseased micro-environment that might be rich in growth-promoting signals that favour angiogenesis.55,56
Genetic factors
The contribution of genetic defects to the development of biliary atresia is suggested by the association of nonhepatic abnormalities such as polysplenia or asplenia, cardio vascular defects, intestinal malrotation and a spectrum of laterality defects.57 Experimentally, the inactivation or overexpression of the genes Invs, Hes1, Hnf6 (also known as Onecut1), Hnf1b, Foxf1, Foxa1 or Foxa2, Sox17, Lgr4 and Pdx1 in mice have been shown to disrupt normal embryogenesis of the extrahepatic biliary system, causing a spectrum of anatomical malformations of the gallbladder and extrahepatic bile ducts, including hypoplasia and agenesis (reviewed elsewhere).58 Gene mutations causally linked to biliary atresia in humans, however, remain elusive. For example, mutations in INVS were not identified in children with laterality defects and biliary atresia.59 Promising observations have linked mutations in CFC1, a different laterality gene,60,61 but they have not been validated in a large cohort. Studies investigating gene sequence variants as susceptibility factors for biliary atresia are garnering interest, such as the reports of single nucleotide polymorphisms (SNPs) in JAG1, CD14, MIF, ITGB2, ADIPOQ and VEGF.62–67
A genome-wide association study (GWAS) of 35 patients identified one common heterozygous deletion of chromosome 2 (2q37.3), which included GPC1 in two unrelated patients. GPC1 encodes glypican-1, a glycoprotein located in the apical membrane of cholangiocytes mediating intercellular signalling via the Hedgehog pathway. Diseased livers had abnormal localization of glypican-1, and morpholino-mediated knockdown of gpc1 led to developmental defects in zebrafish bile ducts.68 Interestingly, the potential contribution of Hedgehog pathway was also implied by results from immunostaining and gene expression studies in human liver samples, which suggested aberrant hepatic Hedgehog signalling in biliary atresia.69 A different GWAS in a larger cohort of 324 Chinese patients identified a high prevalence of the SNP rs17095355 on 10q24, upstream of ADD3, which encodes the F-actin binding protein adducin 3.70 This association was replicated in Thai (n = 124) and US (n = 171) patient cohorts.71,72 Although the biological relevance of this SNP is not yet known, the differential hepatic expression of ADD3 in affected livers suggest that SNPs in ADD3 might serve as a susceptibility factor or disease modifier.72 Further progress in the field will require comprehensive, whole-genome mutation surveys in a patient population that is precisely phenotyped and meets stringent criteria for adequate statistical analyses, followed by proof-of-principle experiments to explore the biological relevance of SNPs.
Environmental toxins
Without clear evidence of disease heritability, there remains the possibility that genetic and developmental defects might predispose infants to biliary atresia by triggering an abnormal response that targets the bile ducts upon exposure to detrimental environmental factors. The potential contribution of environmental toxins to pathogenesis of disease was suggested by the occurrence of two biliary atresia-like endemics in lambs and calves in New South Wales, Australia in 1964 and 1988.73 In a remarkable effort, an investigative team from Philadelphia, USA, imported two species of Dysphania plants from pastures on which the affected animals had grazed and screened chemical extracts purified from the plants using a zebrafish bile excretion assay. They purified a phytosterol, which they named biliatresone, which selectively destroyed extrahepatic bile ducts in zebrafish larvae in a dose-dependent and time-dependent manner. Furthermore, culture of 3D cholangiocyte spheroids with biliatresone induced lumen occlusion and loss of cellular polarity.74 Interestingly, a zebrafish mutant known as ductbend displayed heightened sensitivity to biliatresone. Genetic mapping experiments localized the ductbend locus to chromosome 22, in a region that has conserved synteny to two independent susceptibility loci for biliary atresia in humans (10q24.2 and 16p13.3), thus potentially linking an environmental toxin to a genetic susceptibility loci.75
Viral infection
Microbial agents, especially viruses, have been implicated in the aetiology of biliary atresia. Since initially suggested by Benjamin Landing in 1974,76 several viruses have been detected directly in injured livers and biliary remnants or indirectly by the presence of serological markers of infection in patients with biliary atresia. The viruses include cytomegalovirus, human papillomavirus, human herpes virus 6, Epstein–Barr virus, reovirus and rotavirus in specific groups of patients. The identification of individual viruses in affected tissues has been inconsistent in different populations (as reviewed elsewhere77). One of the factors that might limit the identification of viruses includes the variability in methodologies used to detect infection. For example cytomegalovirus infection is detected by measuring serum levels of anti-cytomegalovirus IgM antibodies, lymphocyte activation and molecular footprinting of an immune response to viral infection.38,40,78,79 Reovirus infection is detected by measuring serum levels of anti-reovirus type 3 IgG and IgM antibodies.80–83 Another potential factor limiting the detection of viral infection is the ability of the neonatal immune system to clear the virus. In an experimental mouse model of biliary atresia, rotavirus was effectively cleared from the hepatobiliary system by the second week of infection.84
Abnormal inflammation and autoimmunity
Among all proposed factors linked to the pathogenesis of biliary atresia, the immune system is central, as evidenced by the infiltration of the liver by inflammatory cells, the overexpression of cytokines and/or chemokines at the time of diagnosis and mechanistic experiments in disease models.25,85–88
Experimental models of biliary injury
At least four experimental systems have been used to study biliary atresia. The first, serving primarily as an observational model of toxin-induced bile duct injury, is represented by young lambs and calves in New South Wales, Australia, which were reported to have a disease similar to biliary atresia.73 The animals have not yet been used in experiment-driven manipulation to study the disease. Second, sea lampreys have been shown to undergo loss of the gallbladder and bile ducts during normal metamorphosis and have been used as a model of ‘programmed atresia’.89,90 Third, zebrafish have been increasingly used to study mutation-induced and toxin-induced defects in the development of intrahepatic and extrahepatic bile ducts.90,91
Finally, mice in the first postnatal week have been used as experimental models.92–94 Newborn BALB/c mice infected with Rhesus rotavirus type A (RRV) constitutes a model that recapitulates several features of human disease. In this model, RRV infection in the first 2 days of life reproducibly triggers hepatobiliary injury and cholestasis within a week of infection.95,96 Some of the key features shared with the human disease include: a time-restricted susceptibility of bile duct injury to the early postnatal period; acholic stools; bile duct proliferation and portal inflammation; and the type 1 rich inflammatory response in the liver and bile ducts.25,84,97–101 The model provides opportunities for direct examination of extrahepatic bile ducts to investigate different stages of injury and progression to lumenal obstruction, tasks that cannot be performed in humans because of the advanced fibrosis in biliary remnants at diagnosis. Although, the model is a unique system to study the role of the immune system in epithelial injury and duct obstruction, it is not suitable to study how biliary injury results in progressive liver fibrosis because of the high mortality before the development of advanced fibrosis or cirrhosis at 2 weeks of age.
Data from diseased human livers
In general, the cellular and molecular profiles of the hepatic microenvironment at the time of diagnosis are largely proinflammatory and profibrotic. For example, CD4+ and CD8+ T cells populate affected livers and are associated with the overexpression of activation markers, such as IFN-γ, IL-2, the IL-2 receptor CD25, TNF and the transferrin receptor CD71.102–106 Cholangiocyte py knosis and necrosis have been associated with infiltration of mononuclear cells in the walls of interlobular bile ducts, duct walls at the porta hepatis and remnants of extra-hepatic bile ducts.107–109 In vitro studies using T cells show that patients with biliary atresia have oligoclonal expansion of CD4+ and CD8+ T cells,110 providing more direct evidence that tissue lymphocytes share common biological programmes. Despite the limited evidence for disease specificity, these technically challenging experiments add functional relevance of T cells in disease pathology and set the stage for future studies investigating their relationship to molecular epitopes on cholangiocytes and mechanisms of autoimmunity.
A proinflammatory molecular profile was reported in a large-scale gene expression analysis of liver biopsies from infants with biliary atresia.111 The approach identified a genetic footprint in which genes involved in the type 1 T helper (TH1) cell response were activated at early stages of biliary atresia, with simultaneous but transient suppression of markers of humoral immunity.111 Other studies reported increased number and activation of Kupffer cells and the expression of the MHC class II antigen HLA-DRA in the livers of infants with biliary atresia.104,112,113 The aberrant expression of HLA-DRA by cholangiocytes led to the proposal that they might have antigen-presenting properties, but testing this hypothesis in the laboratory found no evidence that virus-infected cholangiocytes induced lymphocyte proliferation.114 This type of experiment lends strong support for the need to complement observations from studies in human tissues with mechanistic studies using cell and animal models.
Mechanisms of epithelial injury
Studies of the cellular localization of RRV and the temporal dynamics of inflammatory cells in BALB/c mice have provided insight into early mechanisms of epithelial injury. Following infection, RRV can be detected in cholangiocytes of intrahepatic and extrahepatic bile ducts, macrophages and dendritic cells.84,114–117 Infection of cholangiocytes is cytopathic and largely dependent on the expression of α2β1-integrin.116 In culture, cholangiocytes infected by RRV express variable levels of cytokines and chemokines,114,118 but at levels that are below the threshold to induce chemotaxis.119
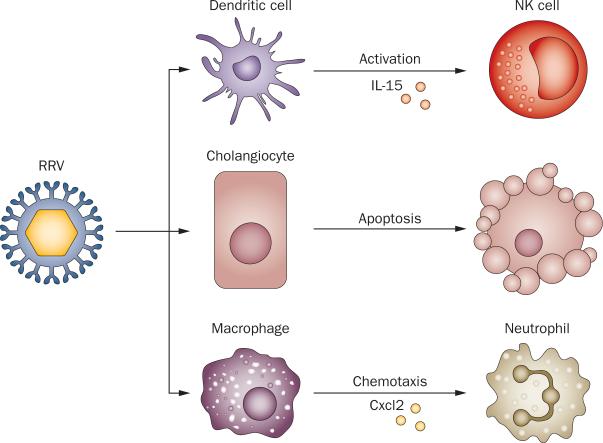
Signals that trigger epithelial injury seem to derive from two other cell types that are targeted by RRV, macrophages and dendritic cells. RRV has been detected in the mouse hepatic macrophage cell line RAW264.7 resulting in the secretion of Mip2 and/or Cxcl2 and the induction of neutrophil chemotaxis.119 Notably, deficiency of Cxcr2, one of the receptors that recognize Cxcl1, Cxcl2 and Cxcl5 (the murine orthologs of IL-8) decreases epithelial injury and inflammation of liver and extrahepatic bile ducts.25 Studies of dendritic cells after RRV infection found plasma cytoid dendritic cells (pDCs) as the most abundant dendritic cell population in early phases of infection; they are targeted by the virus and produce IL-15.120 The importance of pDCs is supported by evidence of decreased epithelial injury and reduced incidence of bile duct obstruction in mice that are depleted of pDCs after RRV infection. A similarly milder phenotype was observed by antibody-mediated inhibition of IL-15, a potent activator of natural killer cells,121,122 suggesting that the expression of pDC-derived cytokines represents an important triggering event that targets bile ducts (Figure 1).120
Figure 1.
Cellular targets and molecular events after RRV infection. When administered into BALB/c mice in the first 2 postnatal days, RRV infects cholangiocytes, inducing apoptosis and necrosis. RRV also targets hepatic DCs, which activate NK cells via Il-15 and hepatic macrophages, which secrete Cxcl2 that attracts neutrophils. Abbreviations: Cxcl2, C-X-C motif chemokine 2; NK, natural killer cell; RRV, Rhesus rotavirus type A.
An even more dramatic effect on the prevention of epithelial injury and biliary obstruction was produced by depletion of natural killer cells.101 In these experiments, investigators first found that incubation of hepatic natural killer cells isolated from RRV-infected mice lyzed cholangiocytes in a contact-dependent fashion, with no lysis when the activating Nkg2d receptor (also known as Klrk1) was blocked on natural killer cells. Consistent with these in vitro studies, the depletion of natural killer cells or antibody blockade of Nkg2d immediately after birth prevented the development of jaundice in newborn mice infected with RRV.101 Detailed histological analysis of extrahepatic bile ducts revealed intact epithelium; without duct injury or obstruction, newborn mice grew into adulthood. Interestingly, this lack of duct injury or obstruction occurred despite the persistence of the virus in the liver, which suggested that RRV alone is not sufficient to produce biliary obstruction, but requires natural killer cells to breach the continuity of the duct epithelium and initiate a cascade of events that produce the atresia phenotype (Figure 2).
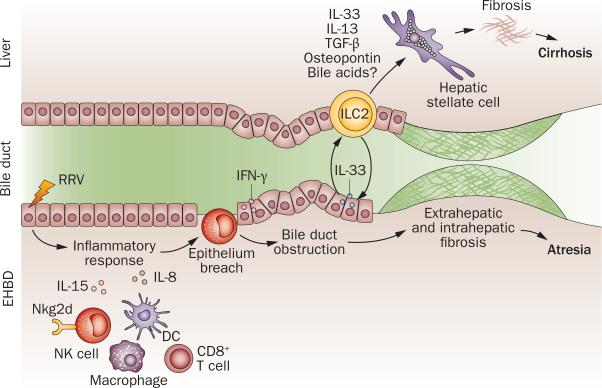
Figure 2.
Biological processes and effectors of injury in biliary atresia. The release of IL-15 by DCs after RRV infection or toxin activates NK cells and CD8+ T cells. Targeting of cholangiocytes by infection or toxin activates inflammatory cells and the release of IL-15, perforin and granzymes, which injures the epithelium. IFN-γ expression is linked to an amplification of epithelial injury and duct obstruction by prominent infiltration of lymphocytes and myeloid cells. The release of IL-33 and IL-13 by ILC2 promotes epithelial repair in bile ducts, and is linked to fibrosis in the liver (and perhaps of extrahepatic ducts). Abbreviations: DC, dendritic cell; EHBD, extrahepatic bile duct; ILC2, innate lymphocyte cell type 2; M, macrophage; N, neutrophil; NK, natural killer cell; RRV, Rhesus rotavirus type A.
Mechanisms of duct obstruction
The first studies that directly explored disease pathogenesis at the mechanistic level, focused on the cellular and molecular constituents of bile duct obstruction.123 The studies tested the hypothesis that IFN-γ is a key cytokine in the development of biliary atresia. Using newborn mice carry ing an inactivating mutation in the Ifng gene, infection with RRV caused transient cholestasis, but extrahepatic bile ducts were free of obstruction and newborn mice had improved survival.84 These findings seem to be unique for IFN-γ based on other studies that showed no improvement in the experimental phenotype if mice lacked IL-12 or underwent antibody depletion of TNF.124,125
One of the main sources of IFN-γ expression is hepatic CD4+ T cells, yet the depletion of these cells was not found to affect the inflammatory obstruction of extrahepatic bile ducts after RRV infection.100 Instead, the loss of CD8+ T cells prevented duct obstruction after rotavirus infection though enabling the development of cholangitis.100 The expression of perforin and granzymes by cytotoxic T cells and natural killer cells seem to be key effector molecules.126 The similarities between the phenotypes produced by the loss of CD8+ T cells or Ifnγ expression suggest that both factors work in parallel to promote duct obstruction (Figure 2).84,100 Although these data pointed to key roles of the TH1 cell response in pathogenesis of experimental biliary atresia, the administration of RRV into newborn mice engineered to lack a TH1 cell response by the inactivation of Stat1 gene unexpectedly resulted in the full obstructive phenotype, in addition to focal cystic dilatations of extrahepatic bile ducts.33 In Stat1-knockout mice, RRV induced a prominent TH2 lymphoid and type 2 myeloid responses, with IL-13 responsible for the obstructive phenotype.
Type 2 immunity and epithelial integrity
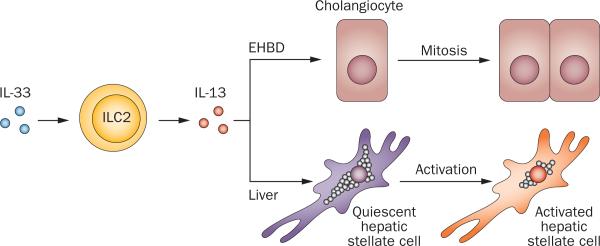
The unexpected obstruction of bile ducts in mice overexpressing type 2 cytokines suggested that the presence of an epithelial injury and an inflammatory response are sufficient to occlude the duct lumen and disrupt bile flow, even when the epithelial injury is functionally diverse such as in type 1 and 2 immune responses. Notably, these responses have also been documented in serum (at the protein level) and liver samples (as gene expression) at the time of diagnosis.33 The cohort of patients could have been divided into subgroups that share similar molecular profiles,33 in a fashion akin to the staging of liver disease by gene expression signatures.26 The molecular profiles might reflect a transition from a proinflammatory type 1 immune response to a fibrosis-related type 2 immune response.127 IL-33, one of the type 2 cytokines with alarmin properties, exerts a potent proliferative response in cholangiocytes of extrahepatic bile ducts and promotes epithelial integrity after RRV infection.128 Additional experiments revealed that IL-33 promotes cholangiocyte proliferation by inducing type 2 innate lymphoid cells (ILC2s) to release IL-13, thus identifying a new paracrine system in which an IL-33–ILC2s–IL-13 axis works in concert to regulate proliferation in neighbouring bile duct epithelial cells.128 Interestingly, in the liver, IL-33 activates hepatic stellate cells and promotes hepatic fibrosis, raising the possibility that this paracrine system could be a contributor to the progression of fibrosis characteristic of biliary atresia (Figure 3).129
Figure 3.
Epithelial and fibrogenic response induced by IL-33. The presence of IL-33 activates type 2 innate lymphoid cells (ILC2) to release IL-13, which induces cholangiocyte proliferation in the neighbouring epithelium of extrahepatic bile ducts. In the liver, the IL-33–ILC2–IL-13 circuit activates the profibrogenic phenotype of hepatic stellate cells. Abbreviation: EHBD, extrahepatic bile duct.
Susceptibility factors
The biological basis for the time-restricted onset of biliary atresia is not fully understood. One possibility is a genetic predisposition caused by SNPs in CFC1 and other genes (see earlier); however, the lack of congenital malformations in the majority of patients suggests that other biological processes might be susceptibility factors. Among such processes is an inflammatory response triggered by the presence of rare maternal cells in the liver of affected infants (maternal chimerism).130,131 Evidence for this reaction to maternal cells is largely circumstantial at this time. Another susceptibility factor is an imbalanced response by the immune system to an exogenous insult at a vulnerable time of postnatal development.
The mouse model of biliary atresia has a unique susceptibility to disease in early postnatal life. Specific targeting of cholangiocytes by RRV has been linked to the expression of α2β1-integrin, which influenced viral attachment and biliary injury.116 Another important susceptibility factor are regulatory T (TREG) cells, which have an important immunomodulatory function; their absence leads to an array of autoimmune phenotypes. In the RRV mouse model, the number of TREG cells in the first 3 days of life is very low, even after RRV challenge,132,133 and their abundance correlates inversely with the degree of liver and biliary injury induced by RRV infection.133,134 Mechanistically, TREG cells regulate the activation of hepatic T cells by CD86-dependent co-stimulation with dendritic cells.134 How these TREG cells modulate susceptibility of biliary injury in humans remains poorly defined.
A working model of disease
Previous models of pathogenesis broadly linked disease phenotype to the interplay between environmental factors and developmentally regulated tissue responses that target the biliary system and disrupt bile flow. Contemporary studies that more precisely characterize the clinical features, quantify the cellular and molecular composition of affected tissues and explore mechanisms of bile duct injury enable a revised proposal that includes a more-refined model of disease.
An inclusive model of disease begins with the exposure to environmental toxins or viruses (Figure 2). Experimentally, biliatresone targets cholangiocytes, while rotavirus infects cholangiocytes, macrophages and dendritic cells. Disease susceptibility probably involves sequence variants in candidate genes (for example, ADD3), functional immaturity of TREG cells and other developmental factors. Gene variants might be particularly important for pathogenesis in children with BASM and/or laterality defects.
Injured or infected cholangiocytes and cells of the innate immune system produce chemoattractants to myeloid cells (for example, Cxcl2 in mice or IL-8 in humans) and engage natural killer cells to amplify cholangiocyte injury and break the continuity of the biliary epithelium. Key effector molecules in early phases of epithelial injury in mice include Il-15, Nkg2d, Rae1, and Cxcl2 (or IL-8 in humans). What follows is a prominent response of the adaptive immune system, with the activation of CD8+ T cells and expression of IFN-γ, perforin and granzymes, which targets the epithelium; together with other inflammatory cells, a luminal plug is formed that stops bile flow. Each one of these effectors of injury in experimental atresia has been validated in patients’ livers at the time of diagnosis. The production of autoantibodies by B cells targeting enolase and other cholangiocyte epitopes might contribute to early epithelial injury or to the ongoing in trahepatic biliary disease after portoenterostomy.135
Less well known are the factors responsible for progressive biliary fibrosis, but emerging data point to the existence of a more fibrogenic type 2 immune response driven by ILC2s, hepatic stellate cells and the expression of IL-33, IL-13, TGF-β1, osteopontin and related extracellular matrix molecules.136–138 Other factors that could contribute to this phenotype include the toxicity of retained bile acids and other components of the inflammatory response, such as IL-17 and related cytokines; however, these factors remained largely unexplored (Figure 2).
Conclusions
Research on biliary atresia has entered a new phase of discoveries, fuelled largely by careful phenotyping of patients and by mechanistic experiments using model systems. Moving forward, investigation will be facilitated by the access to large cohorts via multicentre studies, such as the European consortium of Biliary Atresia and Related Diseases139 or the Childhood Liver Disease Research Network (ChiLDReN).140
The systematic use of complementary approaches to detect biomarkers of infection will aid the search for a viral aetiology of biliary atresia. Testing for cytomegalo-virus in multicentre cohorts might be particularly rele vant based on its preliminary relationship to poor outcome after hepatoportoenterostomy. As for environmental toxins, the field awaits greater knowledge on the cytotoxic or cytopathic effects of biliatresone, its effects on the immune system and whether it is detected in tissues from affected patients.
Using larger cohorts in GWAS or exome sequencing experiments will increase the power of gene association studies in biliary atresia. On the basis of the low incidence of the disease, the inclusion of trios (the patients and parents) in sequencing experiments might narrow the number of sequence variants and aid discoveries on genetic susceptibility.
The combination of human and mouse studies has identified several inflammatory pathways that can serve as therapeutic targets. However, blocking individual pathways requires preclinical testing in a model that more closely recapitulates the progressive liver disease after hepatoportoenterostomy. One area of particular interest might be the IL-33–ILC2–IL-13 circuit based on its role in promoting experimental fibrosis. Future studies will investigate whether the disruption of this circuit suppresses the inflammation-induced fibrosis and lowers the rate of progression to cirrhosis.
Prospective validation studies, perhaps using ‘disease modelling’ strategies, are important to better define the clinical relevance of different phenotypic forms, treatment responses and progression of liver disease. The same is true for staging of liver disease at diagnosis and the identification of cytomegalovirus-positive patient cohorts. These studies will enable patient-based investigations that more consistently implicate pathogenic mechanisms with clinical phenotypes and speed up the discovery of disease biomarkers.
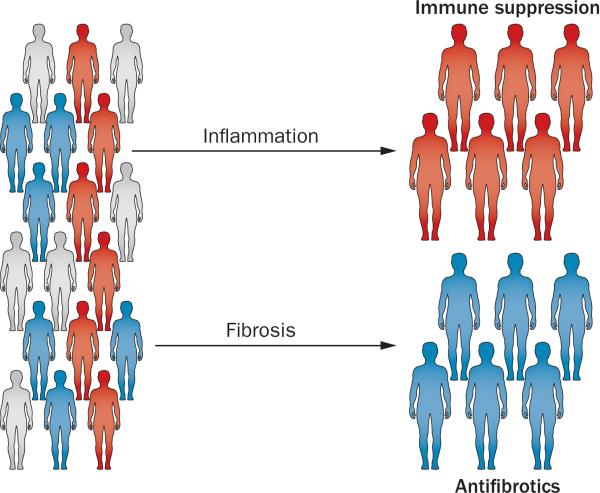
If the different clinical phenotypes and stages of liver disease are validated, they will form a strong rationale for personalized clinical trials. Despite the predominant inflammatory signature uncovered in clinical and preclinical studies, the use of corticosteroids in patients with biliary atresia has not been shown to reliably improve biliary drainage after hepatoportoenterostomy.141 In the prospective, placebo-controlled, double blind START trial (steroid in biliary atresia randomized trial, conducted by ChiLDReN),142 high-dose cortico steroids after hepatoportoenterostomy did not significantly increase the proportion of patients achieving biliary drainage when compared to placebo (58.6% versus 48.6%, respectively; P = 0.43), or transplant-free survival (58.7% versus 59.4%, respectively; P = 0.99).142 Interestingly, an open-label corticosteroid trial that included patients younger than 70 days at the time of hepatoportoenterostomy and excluding specific phenotypes (BASM and patients positive for anti-cytomegalovirus IgM antibodies) showed improved short-term bile drainage.143 This type of open-label trial and the emerging evidence discussed here, point to the possibility of future trials that match therapy with a predominant biological disease process, thus enabling the inclusion of patients that will benefit from targeted therapies and minimizing unwarranted exposure to drug-induced toxicity (Figure 4).
Figure 4.
Designing clinical trials based on biological stages of liver disease. Histopathology and molecular methods to stage the liver disease might identify predominantly inflammation (red), fibrosis (blue) and mixed (grey) subgroups of patients at diagnosis. Matching of the disease stages with specific therapies in future trials could yield improved efficacy and prevent unnecessary exposure of patients to drug toxicity.
Key points.
■ Biliary atresia is an inflammatory and fibrosing cholangiopathy of infancy caused by viruses, environmental toxins and targeted epithelial injury that obstructs extrahepatic bile ducts and rapidly progresses to end-stage cirrhosis
■ Precise clinical phenotyping classifies patients into nonsyndromic and embryonic forms, and into cyst-associated and cytomegalovirus-associated variants, which might have different disease pathologies
■ Liver tissue scoring for inflammation and fibrosis by histological features and gene expression profiles identifies different stages of disease at diagnosis
■ Analyses of human livers and models of experimental biliary atresia suggest that a type 1 immune response has a key role in the pathogenesis of bile duct injury and obstruction
■ The expression of type 2 cytokines promote cholangiocyte proliferation in extrahepatic bile ducts and hepatic fibrosis
■ Clinical, histological and molecular variability of disease at presentation form a strong rationale for future trials that take into consideration the predominant biological features of affected infants
Box 1 | Biological categories and pathogenic factors.
Abnormal morphogenesis
Defective embryogenesis
■ BASM
■ Laterality defects
■ Ductal plate malformation
Abnormal prenatal circulation
■ Vascular abnormalities
■ Arterial hyperplasia or hypertrophy
Genetic factors
■ Nonhepatic malformations
■ SNPs in: CFC1; JAG1; CD14; MIF; ITGB2; ADIPOQ; VEGF; GPC1; and ADD3
Environmental factors
Toxins
■ Biliatresone (from Dysphania plants)
Viruses
■ Cytomegalovirus
■ Reovirus
■ Rotavirus
■ Human papillomavirus
■ Human herpes virus 6
■ Epstein–Barr virus
Immune dysregulation
Abnormal inflammatory response
■ Activation of dendritic cells
■ T cells
■ Natural killer cells
■ Macrophages or Kupffer cells
■ Expression of IFN-γ, IL-2, CD25, TNF, IL-15, NKG2D, perforin and granzymes, IL-8
Autoimmunity
■ HLA-DRA
■ Oligoclonal expansion of T cells
■ Anti-enolase antibodies
Susceptibility factors
■ Gene SNPs
■ Microchimerism
■ TREG cells
■ α2β1-integrins
Abbreviations: ADD3, adducin 3; BASM, biliary atresia splenic malformation; CFC1, cripto, FRL-1, cryptic family 1; GPC1, glypican 1; HLA-DRA, major histocompatibility complex, class II, DR alpha; ITGB2, integrin, beta 2; JAG1, jagged 1; MIF, macrophage migration inhibitory factor; NKG2D, killer cell lectin-like receptor subfamily K, member 1; SNP, single nucleotide polymorphism; TREG cell, regulatory T cell; VEGF, vascular endothelial growth factor.
Review criteria.
This Review presents a perspective of how recent patient-based and laboratory-based studies advance the understanding of pathogenic mechanisms of biliary atresia. To select the studies, we performed a MEDLINE search combining the medical subject headings of “biliary atresia” and “children,” published in the English literature as original articles up to 30 September 2014.
Acknowledgements
The authors are supported by the NIH grants DK64008 and DK83781 to J.A.B. and DK95001 to A.M. J.A.B. is the Cincinnati Principal Investigator of the NIDDK-funded Childhood Liver Disease and Research Network (NIH grant DK62497).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
J.A.B. and A.A. contributed equally to researching data for the manuscript. All authors contributed equally to the discussion of content, writing and reviewing and editing the manuscript before submission.
References
- 1.Dehghani SM, Efazati N, Shahramian I, Haghighat M, Imanieh MH. Evaluation of cholestasis in Iranian infants less than three months of age. Gastroenterol. Hepatol. Bed Bench. 2015;8:42–48. [PMC free article] [PubMed] [Google Scholar]
- 2.Hoerning A, et al. Diversity of disorders causing neonatal cholestasis—the experience of a tertiary pediatric center in Germany. Front. Pediatr. 2014;2:65. doi: 10.3389/fped.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee WS, Chai PF, Boey CM, Looi LM. Aetiology and outcome of neonatal cholestasis in Malaysia. Singapore Med. J. 2010;51:434–439. [PubMed] [Google Scholar]
- 4.Stormon MO, Dorney SF, Kamath KR, O’Loughlin EV, Gaskin KJ. The changing pattern of diagnosis of infantile cholestasis. J. Paediatr. Child Health. 2001;37:47–50. doi: 10.1046/j.1440-1754.2001.00613.x. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber RA, Kleinman RE. Biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2002;35(Suppl. 1):S11–S16. doi: 10.1097/00005176-200207001-00005. [DOI] [PubMed] [Google Scholar]
- 6.Russo P, et al. Design and validation of the biliary atresia research consortium histologic assessment system for cholestasis in infancy. Clin. Gastroenterol. Hepatol. 2011;9:357–362. doi: 10.1016/j.cgh.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chardot C, et al. Epidemiology of biliary atresia in France: a national study 1986–1996. J. Hepatol. 1999;31:1006–1013. doi: 10.1016/s0168-8278(99)80312-2. [DOI] [PubMed] [Google Scholar]
- 8.Houwen RH, et al. Time-space distribution of extrahepatic biliary atresia in the Netherlands and West Germany. Surg. Inf. Child. 1988;43:68–71. doi: 10.1055/s-2008-1043419. [DOI] [PubMed] [Google Scholar]
- 9.Hsiao CH, et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology. 2008;47:1233–1240. doi: 10.1002/hep.22182. [DOI] [PubMed] [Google Scholar]
- 10.McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet. 2000;355:25–29. doi: 10.1016/S0140-6736(99)03492-3. [DOI] [PubMed] [Google Scholar]
- 11.Yoon PW, Bresee JS, Olney RS, James LM, Khoury MJ. Epidemiology of biliary atresia: a population-based study. Pediatrics. 1997;99:376–382. doi: 10.1542/peds.99.3.376. [DOI] [PubMed] [Google Scholar]
- 12.Strickland AD, Shannon K. Studies in the etiology of extrahepatic biliary atresia: time-space clustering. J. Pediatr. 1982;100:749–753. doi: 10.1016/s0022-3476(82)80576-3. [DOI] [PubMed] [Google Scholar]
- 13.Wada H, et al. Insignificant seasonal and geographical variation in incidence of biliary atresia in Japan: a regional survey of over 20 years. J. Pediatr. Surg. 2007;42:2090–2092. doi: 10.1016/j.jpedsurg.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Shneider BL, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J. Pediatr. 2006;148:467–474. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 15.Fallon SC, Chang S, Finegold MJ, Karpen SJ, Brandt ML. Discordant presentation of biliary atresia in premature monozygotic twins. J. Pediatr. Gastroenterol. Nutr. 2013;57:e22–e23. doi: 10.1097/MPG.0b013e31826a1044. [DOI] [PubMed] [Google Scholar]
- 16.Hyams JS, Glaser JH, Leichtner AM, Morecki R. Discordance for biliary atresia in two sets of monozygotic twins. J. Pediatr. 1985;107:420–422. doi: 10.1016/s0022-3476(85)80524-2. [DOI] [PubMed] [Google Scholar]
- 17.Lachaux A, et al. Familial extrahepatic biliary atresia. J. Pediatr. Gastroenterol. Nutr. 1988;7:280–283. doi: 10.1097/00005176-198803000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Smith BM, Laberge JM, Schreiber R, Weber AM, Blanchard H. Familial biliary atresia in three siblings including twins. J. Pediatr. Surg. 1991;26:1331–1333. doi: 10.1016/0022-3468(91)90613-x. [DOI] [PubMed] [Google Scholar]
- 19.Strickland AD, Shannon K, Coln CD. Biliary atresia in two sets of twins. J. Pediatr. 1985;107:418–420. doi: 10.1016/s0022-3476(85)80523-0. [DOI] [PubMed] [Google Scholar]
- 20.Kasai M, Suzuki S. A new operation for “non-correctable” biliary atresia, hepatic portoenterostomy. Shujutsu. 1959;13:733–739. [Google Scholar]
- 21.Chardot C, et al. Improving outcomes of biliary atresia: French national series 1986–2009. J. Hepatol. 2013;58:1209–1217. doi: 10.1016/j.jhep.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber RA, et al. Biliary atresia: the Canadian experience. J. Pediatr. 2007;151:659–665. doi: 10.1016/j.jpeds.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 23.Superina R, et al. The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann. Surg. 2011;254:577–585. doi: 10.1097/SLA.0b013e3182300950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohi R. Biliary atresia: a surgical perspective. Clin. Liver Dis. 2000;4:779–804. doi: 10.1016/s1089-3261(05)70141-0. [DOI] [PubMed] [Google Scholar]
- 25.Bessho K, Bezerra JA. Biliary atresia: will blocking inflammation tame the disease? Annu. Rev. Med. 2011;62:171–185. doi: 10.1146/annurev-med-042909-093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moyer K, et al. Staging of biliary atresia at diagnosis by molecular profiling of the liver. Genome Med. 2010;2:33. doi: 10.1186/gm154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harpavat S, Finegold MJ, Karpen SJ. Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics. 2011;128:e1428–e1433. doi: 10.1542/peds.2011-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davenport M, Savage M, Mowat AP, Howard ER. Biliary atresia splenic malformation syndrome: an etiologic and prognostic subgroup. Surgery. 1993;113:662–668. [PubMed] [Google Scholar]
- 29.Davenport M, et al. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J. Pediatr. 2006;149:393–400. doi: 10.1016/j.jpeds.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz KB, et al. Extrahepatic anomalies in infants with biliary atresia: results of a large prospective North American multicenter study. Hepatology. 2013;58:1724–1731. doi: 10.1002/hep.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muise AM, et al. Biliary atresia with choledochal cyst: implications for classification. Clin. Gastroenterol. Hepatol. 2006;4:1411–1414. doi: 10.1016/j.cgh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Davenport M, Caponcelli E, Livesey E, Hadzic N, Howard E. Surgical outcome in biliary atresia: etiology affects the influence of age at surgery. Ann. Surg. 2008;247:694–698. doi: 10.1097/SLA.0b013e3181638627. [DOI] [PubMed] [Google Scholar]
- 33.Li J, et al. Th2 signals induce epithelial injury in mice and are compatible with the biliary atresia phenotype. J. Clin. Invest. 2011;121:4245–4256. doi: 10.1172/JCI57728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, et al. The perinatal infection of cytomegalovirus is an important etiology for biliary atresia in China. Clin. Pediatr. (Phila) 2012;51:109–113. doi: 10.1177/0009922811406264. [DOI] [PubMed] [Google Scholar]
- 35.Shen C, Zheng S, Wang W, Xiao XM. Relationship between prognosis of biliary atresia and infection of cytomegalovirus. World J. Pediatr. 2008;4:123–126. doi: 10.1007/s12519-008-0024-8. [DOI] [PubMed] [Google Scholar]
- 36.Davenport M, Grieve A. Maximizing Kasai portoenterostomy in the treatment of biliary atresia: medical and surgical options. South African Med. J. 2012;102:865–867. doi: 10.7196/samj.6120. [DOI] [PubMed] [Google Scholar]
- 37.Davenport M. Biliary atresia: clinical aspects. Semin. Pediatr. Surg. 2012;21:175–184. doi: 10.1053/j.sempedsurg.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Fischler B, Svensson JF, Nemeth A. Early cytomegalovirus infection and the long-term outcome of biliary atresia. Acta Paediatr. 2009;98:1600–1602. doi: 10.1111/j.1651-2227.2009.01416.x. [DOI] [PubMed] [Google Scholar]
- 39.Schukfeh N, Al-Gamrah A, Petersen C, Kuebler JF. Detection of hepatotropic viruses has no impact on the prognosis after Kasai procedure. J. Pediatr. Surg. 2012;47:1828–1832. doi: 10.1016/j.jpedsurg.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Brindley SM, et al. Cytomegalovirus-specific T-cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology. 2012;55:1130–1138. doi: 10.1002/hep.24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sokol RJ, et al. Screening and outcomes in biliary atresia: summary of a national institutes of health workshop. Hepatology. 2007;46:566–581. doi: 10.1002/hep.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volpert D, et al. Outcome of early hepatic portoenterostomy for biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2001;32:265–269. doi: 10.1097/00005176-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Azarow KS, Phillips MJ, Sandler AD, Hagerstrand I, Superina RA. Biliary atresia: should all patients undergo a portoenterostomy? J. Pediatr. Surg. 1997;32:168–172. doi: 10.1016/s0022-3468(97)90173-1. [DOI] [PubMed] [Google Scholar]
- 44.Pape L, Olsson K, Petersen C, von Wasilewski R, Melter M. Prognostic value of computerized quantification of liver fibrosis in children with biliary atresia. Liver Transpl. 2009;15:876–882. doi: 10.1002/lt.21711. [DOI] [PubMed] [Google Scholar]
- 45.Weerasooriya VS, White FV, Shepherd RW. Hepatic fibrosis and survival in biliary atresia. J. Pediatr. 2004;144:123–125. doi: 10.1016/j.jpeds.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 46.Santos JL, et al. The extent of biliary proliferation in liver biopsies from patients with biliary atresia at portoenterostomy is associated with the postoperative prognosis. J. Pediatr. Surg. 2009;44:695–701. doi: 10.1016/j.jpedsurg.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Balistreri WF, et al. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682–1692. doi: 10.1002/hep.510230652. [DOI] [PubMed] [Google Scholar]
- 48.Bezerra JA. The next challenge in pediatric cholestasis: deciphering the pathogenesis of biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2006;43(Suppl. 1):S23–29. doi: 10.1097/01.mpg.0000228197.28056.2f. [DOI] [PubMed] [Google Scholar]
- 49.Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 50.Tan CE, Davenport M, Driver M, Howard ER. Does the morphology of the extrahepatic biliary remnants in biliary atresia influence survival? A review of 205 cases. J. Pediatr. Surg. 1994;29:1459–1464. doi: 10.1016/0022-3468(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 51.Takashima Y, Terada M, Kawabata M, Suzuki A. Dynamic three-dimensional morphogenesis of intrahepatic bile ducts in mouse liver development. Hepatology. 2014;61:1003–1011. doi: 10.1002/hep.27436. [DOI] [PubMed] [Google Scholar]
- 52.Pacheco MC, Campbell KM, Bove KE. Ductal plate malformation-like arrays in early explants after a Kasai procedure are independent of splenic malformation complex (heterotaxy). Pediatr. Dev. Pathol. 2009;12:355–360. doi: 10.2350/09-01-0598-OA.1. [DOI] [PubMed] [Google Scholar]
- 53.dos Santos JL, da Silveira TR, da Silva VD, Cerski CT, Wagner MB. Medial thickening of hepatic artery branches in biliary atresia. A morphometric study. J. Pediatr. Surg. 2005;40:637–642. doi: 10.1016/j.jpedsurg.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Ho CW, et al. The pathogenesis of biliary atresia: a morphological study of the hepatobiliary system and the hepatic artery. J. Pediatr. Gastroenterol. Nutr. 1993;16:53–60. doi: 10.1097/00005176-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 55.de Souza AF, et al. Angiopoietin 1 and angiopoietin 2 are associated with medial thickening of hepatic arterial branches in biliary atresia. Pediatr. Res. 2014;75:22–28. doi: 10.1038/pr.2013.177. [DOI] [PubMed] [Google Scholar]
- 56.Edom PT, Meurer L, da Silveira TR, Matte U, dos Santos JL. Immunolocalization of VEGF A and its receptors, VEGFR1 and VEGFR2, in the liver from patients with biliary atresia. Appl. Immunohist. Mol. Morphol. 2011;19:360–368. doi: 10.1097/PAI.0b013e3182028a8e. [DOI] [PubMed] [Google Scholar]
- 57.Carmi R, Magee CA, Neill CA, Karrer FM. Extrahepatic biliary atresia and associated anomalies: etiologic heterogeneity suggested by distinctive patterns of associations. Am. J. Med. Genet. 1993;45:683–693. doi: 10.1002/ajmg.1320450606. [DOI] [PubMed] [Google Scholar]
- 58.Yokoyama T, et al. Reversal of left-right asymmetry: a situs inversus mutation. Science. 1993;260:679–682. doi: 10.1126/science.8480178. [DOI] [PubMed] [Google Scholar]
- 59.Schon P, et al. Identification, genomic organization, chromosomal mapping and mutation analysis of the human INV gene, the ortholog of a murine gene implicated in left-right axis development and biliary atresia. Human Genetics. 2002;110:157–165. doi: 10.1007/s00439-001-0655-5. [DOI] [PubMed] [Google Scholar]
- 60.Davit-Spraul A, Baussan C, Hermeziu B, Bernard O, Jacquemin E. CFC1 gene involvement in biliary atresia with polysplenia syndrome. J. Pediatr. Gastroenterol. Nutr. 2008;46:111–112. doi: 10.1097/01.mpg.0000304465.60788.f4. [DOI] [PubMed] [Google Scholar]
- 61.Jacquemin E, Cresteil D, Raynaud N, Hadchouel M. CFC1 gene mutation and biliary atresia with polysplenia syndrome. J. Pediatr. Gastroenterol. Hepatol. Nutr. 2002;34:326–327. doi: 10.1097/00005176-200203000-00026. [DOI] [PubMed] [Google Scholar]
- 62.Arikan C, et al. Polymorphisms of the ICAM-1 gene are associated with biliary atresia. Dig. Dis. Sci. 2008;53:2000–2004. doi: 10.1007/s10620-007-9914-1. [DOI] [PubMed] [Google Scholar]
- 63.Shih HH, et al. Promoter polymorphism of the CD14 endotoxin receptor gene is associated with biliary atresia and idiopathic neonatal cholestasis. Pediatrics. 2005;116:437–441. doi: 10.1542/peds.2004-1900. [DOI] [PubMed] [Google Scholar]
- 64.Kohsaka T, et al. The significance of human jagged 1 mutations detected in severe cases of extrahepatic biliary atresia. Hepatology. 2002;36:904–912. doi: 10.1053/jhep.2002.35820. [DOI] [PubMed] [Google Scholar]
- 65.Lee HC, et al. Genetic variation in the vascular endothelial growth factor gene is associated with biliary atresia. J. Clin. Gastroenterol. 2010;44:135–139. doi: 10.1097/MCG.0b013e3181b152c2. [DOI] [PubMed] [Google Scholar]
- 66.Udomsinprasert W, et al. +276 G/T single nucleotide polymorphism of the adiponectin gene is associated with the susceptibility to biliary atresia. World J. Pediatr. 2012;8:328–334. doi: 10.1007/s12519-012-0377-x. [DOI] [PubMed] [Google Scholar]
- 67.Zhao R, et al. Polymorphism of ITGB2 gene 3’-UTR+145C/A is associated with biliary atresia. Digestion. 2013;88:65–71. doi: 10.1159/000352025. [DOI] [PubMed] [Google Scholar]
- 68.Cui S, et al. Evidence from human and zebrafish that GPC1 is a biliary atresia susceptibility gene. Gastroenterology. 2013;144:1107–1115. doi: 10.1053/j.gastro.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Omenetti A, et al. Hedgehog activity, epithelialmesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology. 2011;53:1246–1258. doi: 10.1002/hep.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Barcelo MM, et al. Genome-wide association study identifies a susceptibility locus for biliary atresia on 10q24.2. Hum. Mol. Genet. 2010;19:2917–2925. doi: 10.1093/hmg/ddq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaewkiattiyot S, Honsawek S, Vejchapipat P, Chongsrisawat V, Poovorawan Y. Association of X-prolyl aminopeptidase 1 rs17095355 polymorphism with biliary atresia in Thai children. Hepatol. Res. 2011;41:1249–1252. doi: 10.1111/j.1872-034X.2011.00870.x. [DOI] [PubMed] [Google Scholar]
- 72.Tsai EA, et al. Replication of a GWAS signal in a Caucasian population implicates ADD3 in susceptibility to biliary atresia. Hum. Genet. 2014;133:235–243. doi: 10.1007/s00439-013-1368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harper P, Plant JW, Unger DB. Congenital biliary atresia and jaundice in lambs and calves. Aust. Vet. J. 1990;67:18–22. doi: 10.1111/j.1751-0813.1990.tb07385.x. [DOI] [PubMed] [Google Scholar]
- 74.Waisbourd-Zinman O, et al. A novel toxin responsible for outbreaks of biliary atresia in livestock causes lumen obstruction in a cholangiocyte spheroid model [abstract 104]. Hepatology. 2014;60(Suppl.1):249A. [Google Scholar]
- 75.Zhao X, et al. The zebrafish duct bend mutation is a sensitizer to toxin-induced biliary atresia and a potential homologue of a human biliary atresia modifier [abstract 156]. Hepatology. 2014;60(Suppl. 1):274A. [Google Scholar]
- 76.Landing BH. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochal cyst—the concept of infantile obstructive cholangiopathy. Prog. Pediatr. Surg. 1974;6:113–139. [PubMed] [Google Scholar]
- 77.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin. Liver. Dis. 2007;27:233–242. doi: 10.1055/s-2007-985068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jevon GP, Dimmick JE. Biliary atresia and cytomegalovirus infection: a DNA study. Pediatr. Dev. Pathol. 1999;2:11–14. doi: 10.1007/s100249900083. [DOI] [PubMed] [Google Scholar]
- 79.Al-Masri AN, et al. Expression of the interferon-induced Mx proteins in biliary atresia. J. Pediatr. Surg. 2006;41:1139–1143. doi: 10.1016/j.jpedsurg.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 80.Glaser JH, Balistreri WF, Morecki R. Role of reovirus type 3 in persistent infantile cholestasis. J. Pediatr. 1984;105:912–915. doi: 10.1016/s0022-3476(84)80076-1. [DOI] [PubMed] [Google Scholar]
- 81.Morecki R, Glaser JH, Johnson AB, Kress Y. Detection of reovirus type 3 in the porta hepatis of an infant with extrahepatic biliary atresia: ultrastructural and immunocytochemical study. Hepatology. 1984;4:1137–1142. doi: 10.1002/hep.1840040608. [DOI] [PubMed] [Google Scholar]
- 82.Richardson SC, Bishop RF, Smith AL. Reovirus serotype 3 infection in infants with extrahepatic biliary atresia or neonatal hepatitis. J. Gastroenterol. Hepatol. 1994;9:264–268. doi: 10.1111/j.1440-1746.1994.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 83.Tyler KL, et al. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology. 1998;27:1475–1482. doi: 10.1002/hep.510270603. [DOI] [PubMed] [Google Scholar]
- 84.Shivakumar P, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-γ in experimental biliary atresia. J. Clin. Invest. 2004;114:322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feldman AG, Mack CL. Biliary atresia: cellular dynamics and immune dysregulation. Semin. Pediatr. Surg. 2012;21:192–200. doi: 10.1053/j.sempedsurg.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 87.Mack CL, Feldman AG, Sokol RJ. Clues to the etiology of bile duct injury in biliary atresia. Semin. Liver. Dis. 2012;32:307–316. doi: 10.1055/s-0032-1329899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petersen C, Davenport M. Aetiology of biliary atresia: what is actually known? Orphanet. J. Rare. Dis. 2013;8:128. doi: 10.1186/1750-1172-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cai SY, et al. Adult sea lamprey tolerates biliary atresia by altering bile salt composition and renal excretion. Hepatology. 2013;57:2418–2426. doi: 10.1002/hep.26161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yeoh EJ, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. [comment]. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 91.Cui H, et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 92.Bangaru B, Morecki R, Glaser JH, Gartner LM, Horwitz MS. Comparative studies of biliary atresia in the human newborn and reovirus-induced cholangitis in weanling mice. Lab. Invest. 1980;43:456–462. [PubMed] [Google Scholar]
- 93.Szavay PO, Leonhardt J, Czech-Schmidt G, Petersen C. The role of reovirus type 3 infection in an established murine model for biliary atresia. Eur. J. Pediatr. Surg. 2002;12:248–250. doi: 10.1055/s-2002-34477. [DOI] [PubMed] [Google Scholar]
- 94.Wilson GA, Morrison LA, Fields BN. Association of the reovirus S1 gene with serotype 3-induced biliary atresia in mice. J. Virol. 1994;68:6458–6465. doi: 10.1128/jvi.68.10.6458-6465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petersen C, et al. New aspects in a murine model for extrahepatic biliary atresia. J. Pediatr. Surg. 1997;32:1190–1195. doi: 10.1016/s0022-3468(97)90680-1. [DOI] [PubMed] [Google Scholar]
- 96.Riepenhoff-Talty M, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr. Res. 1993;33:394–399. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 97.Carvalho E, et al. Analysis of the Biliary Transcriptome in Experimental Biliary Atresia. Gastroenterology. 2005;129:713–717. doi: 10.1016/j.gastro.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 98.Leonhardt J, et al. Gene expression profile of the infective murine model for biliary atresia. Pediatr. Surg. Int. 2006;22:84–89. doi: 10.1007/s00383-005-1589-0. [DOI] [PubMed] [Google Scholar]
- 99.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ TH1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin. Immunol. 2005;115:200–209. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shivakumar P, et al. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology. 2007;133:268–277. doi: 10.1053/j.gastro.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J. Clin. Invest. 2009;119:2281–2290. doi: 10.1172/JCI38879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahmed AF, et al. CD8+ T cells infiltrating into bile ducts in biliary atresia do not appear to function as cytotoxic T cells: a clinicopathological analysis. J. Pathol. 2001;193:383–389. doi: 10.1002/1096-9896(2000)9999:9999<::aid-path793>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 103.Broome U, Nemeth A, Hultcrantz R, Scheynius A. Different expression of HLA-DR and ICAM-1 in livers from patients with biliary atresia and Byler's disease. J. Hepatol. 1997;26:857–862. doi: 10.1016/s0168-8278(97)80253-x. [DOI] [PubMed] [Google Scholar]
- 104.Davenport M, et al. Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J. Pediatr. Surg. 2001;36:1017–1025. doi: 10.1053/jpsu.2001.24730. [DOI] [PubMed] [Google Scholar]
- 105.Dillon PW, Belchis D, Minnick K, Tracy T. Differential expression of the major histocompatibility antigens and ICAM-1 on bile duct epithelial cells in biliary atresia. Tohoku J. Exp. Med. 1997;181:33–40. doi: 10.1620/tjem.181.33. [DOI] [PubMed] [Google Scholar]
- 106.Mack CL, et al. Biliary atresia is associated with CD4+ TH1 cell-mediated portal tract inflammation. Pediatr. Res. 2004;56:79–87. doi: 10.1203/01.PDR.0000130480.51066.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bill AH, Haas JE, Foster GL. Biliary atresia: histopathologic observations and reflections upon its natural history. J. Pediatr. Surg. 1977;12:977–982. doi: 10.1016/0022-3468(77)90609-1. [DOI] [PubMed] [Google Scholar]
- 108.Gosseye S, Otte JB, De Meyer R, Maldague P. A histological study of extrahepatic biliary atresia. Acta Paediatr. Belg. 1977;30:85–90. [PubMed] [Google Scholar]
- 109.Ohya T, Fujimoto T, Shimomura H, Miyano T. Degeneration of intrahepatic bile duct with lymphocyte infiltration into biliary epithelial cells in biliary atresia. J. Pediatr. Surg. 1995;30:515–518. doi: 10.1016/0022-3468(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 110.Mack CL, et al. Oligoclonal expansions of CD4+ and CD8+ T-cells in the target organ of patients with biliary atresia. Gastroenterology. 2007;133:278–287. doi: 10.1053/j.gastro.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bezerra JA, et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1563–1659. doi: 10.1016/S0140-6736(02)11603-5. [DOI] [PubMed] [Google Scholar]
- 112.Tracy TF, Jr, Dillon P, Fox ES, Minnick K, Vogler C. The inflammatory response in pediatric biliary disease: macrophage phenotype and distribution. J. Pediatr. Surg. 1996;31:121–125. doi: 10.1016/s0022-3468(96)90333-4. discussion 125–126. [DOI] [PubMed] [Google Scholar]
- 113.Urushihara N, et al. Elevation of serum interleukin-18 levels and activation of Kupffer cells in biliary atresia. J. Pediatr. Surg. 2000;35:446–449. doi: 10.1016/s0022-3468(00)90211-2. [DOI] [PubMed] [Google Scholar]
- 114.Barnes BH, et al. Cholangiocytes as immune modulators in rotavirus-induced murine biliary atresia. Liver Int. 2009;29:1253–1261. doi: 10.1111/j.1478-3231.2008.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Allen SR, et al. Effect of rotavirus strain on the murine model of biliary atresia. J. Virol. 2007;81:1671–1679. doi: 10.1128/JVI.02094-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jafri M, et al. Cholangiocyte expression of α2β1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G16–G26. doi: 10.1152/ajpgi.00442.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Erickson N, et al. Temporal-spatial activation of apoptosis and epithelial injury in murine experimental biliary atresia. Hepatology. 2008;47:1567–1577. doi: 10.1002/hep.22229. [DOI] [PubMed] [Google Scholar]
- 118.Jafri M, Donnelly B, Bondoc A, Allen S, Tiao G. Cholangiocyte secretion of chemokines in experimental biliary atresia. J. Pediatr. Surg. 2009;44:500–507. doi: 10.1016/j.jpedsurg.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohanty SK, Ivantes CA, Mourya R, Pacheco C, Bezerra JA. Macrophages are targeted by rotavirus in experimental biliary atresia and induce neutrophil chemotaxis by mip2/cxcl2. Pediatr. Res. 2010;67:345–351. doi: 10.1203/PDR.0b013e3181d22a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saxena V, et al. Dendritic cells regulate natural killer cell activation and epithelial injury in experimental biliary atresia. Sci. Transl. Med. 2011;3:102ra194. doi: 10.1126/scitranslmed.3002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuwajima S, et al. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat. Immunol. 2006;7:740–746. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 122.Sun CM, Fiette L, Tanguy M, Leclerc C, Lo-Man R. Ontogeny and innate properties of neonatal dendritic cells. Blood. 2003;102:585–591. doi: 10.1182/blood-2002-09-2966. [DOI] [PubMed] [Google Scholar]
- 123.Shivakumar P, Bezerra JA. Biliary atresia and Th1 function: linking lymphocytes and bile ducts: commentary on the article by Mack. et al. on page 79. Pediatr. Res. 2004;56:9–10. doi: 10.1203/01.PDR.0000129655.02381.F0. [DOI] [PubMed] [Google Scholar]
- 124.Mohanty SK, Shivakumar P, Sabla G, Bezerra JA. Loss of interleukin-12 modifies the pro-inflammatory response but does not prevent duct obstruction in experimental biliary atresia. BMC Gastroenterol. 2006;6:14. doi: 10.1186/1471-230X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tucker RM, Hendrickson RJ, Mukaida N, Gill RG, Mack CL. Progressive biliary destruction is independent of a functional tumor necrosis factor-alpha pathway in a rhesus rotavirus-induced murine model of biliary atresia. Viral Immunol. 2007;20:34–43. doi: 10.1089/vim.2006.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shivakumar P, Mourya R, Bezerra JA. Perforin and granzymes work in synergy to mediate cholangiocyte injury in experimental biliary atresia. J. Hepatol. 2014;60:370–376. doi: 10.1016/j.jhep.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 128.Li J, et al. Biliary repair and carcinogenesis are mediated by IL 33 dependent cholangiocyte proliferation. J. Clin. Invest. 2014;124:3241–3251. doi: 10.1172/JCI73742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marvie P, et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J. Cell. Mol. Med. 2010;14:1726–1739. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hayashida M, et al. The evidence of maternal microchimerism in biliary atresia using fluorescent in situ hybridization. J. Pediatr. Surg. 2007;42:2097–2101. doi: 10.1016/j.jpedsurg.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 131.Muraji T, et al. Maternal microchimerism in underlying pathogenesis of biliary atresia: quantification and phenotypes of maternal cells in the liver. Pediatrics. 2008;121:517–521. doi: 10.1542/peds.2007-0568. [DOI] [PubMed] [Google Scholar]
- 132.Miethke AG, et al. Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J. Hepatol. 2010;52:718–726. doi: 10.1016/j.jhep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tucker RM, Feldman AG, Fenner EK, Mack CL. Regulatory T cells inhibit TH1 cell-mediated bile duct injury in murine biliary atresia. J. Hepatol. 2013;59:790–796. doi: 10.1016/j.jhep.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lages CS, Simmons J, Chougnet CA, Miethke AG. Regulatory T cells control the CD8 adaptive immune response at the time of ductal obstruction in experimental biliary atresia. Hepatology. 2012;56:219–227. doi: 10.1002/hep.25662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lu BR, Brindley SM, Tucker RM, Lambert CL, Mack CL. alpha-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology. 2010;139:1753–1761. doi: 10.1053/j.gastro.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McHedlidze T, et al. Interleukin 33 dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Syn WK, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.European consortium of Biliary Atresia and Related Diseases. [online], http://www.bard-online.com.
- 140.Childhood Liver Disease Research Network. 2015 [online], http://www.childrennetwork.org.
- 141.Sarkhy A, Schreiber RA, Milner RA, Barker CC. Does adjuvant steroid therapy post-Kasai portoenterostomy improve outcome of biliary atresia? Systematic review and meta-analysis. Can. J. Gastroenterol. 2011;25:440–444. doi: 10.1155/2011/125610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bezerra JA, et al. Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. JAMA. 2014;311:1750–1759. doi: 10.1001/jama.2014.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Davenport M, Parsons C, Tizzard S, Hadzic N. Steroids in biliary atresia: single surgeon, single centre, prospective study. J. Hepatol. 2013;59:1054–1058. doi: 10.1016/j.jhep.2013.06.012. [DOI] [PubMed] [Google Scholar]