Abstract
Purpose
The pathophysiology of vision loss in persons with diabetic retinopathy (DR) is complex and incompletely defined. We hypothesized that retinal pigment epithelium (RPE) and rod and cone photoreceptor dysfunction, as measured by dark adaptometry, would increase with severity of DR, and that pan-retinal photocoagulation (PRP) would exacerbate this dysfunction.
Methods
Dark adaptation (DA) was measured in subjects with diabetes mellitus and healthy controls. Dark adaptation was measured at 5° superior to the fovea following a flash bleach, and the data were analyzed to yield cone and rod sensitivity curves. Retinal layer thicknesses were quantified using spectral-domain optical coherence tomography (OCT).
Results
The sample consisted of 23 controls and 73 diabetic subjects. Subjects with moderate nonproliferative diabetic retinopathy (NPDR) exhibited significant impairment of rod recovery rate compared with control subjects (P = 0.04). Cone sensitivity was impaired in subjects with proliferative diabetic retinopathy (PDR) (type 1 diabetes mellitus [T1DM]: P = 0.0047; type 2 diabetes mellitus [T2DM]: P < 0.001). Subjects with untreated PDR compared with subjects treated with PRP exhibited similar rod recovery rates and cone sensitivities. Thinner RPE as assessed by OCT was associated with slower rod recovery and lower cone sensitivity, and thinner photoreceptor inner segment/outer segment layer was associated with lower cone sensitivity.
Conclusions
The results suggest that RPE and photoreceptor cell dysfunction, as assessed by cone sensitivity level and rod- and RPE-mediated dark adaptation, progresses with worsening DR, and rod recovery dysfunction occurs earlier than cone dysfunction. Function was preserved following PRP. The findings suggest multiple defects in retinoid function and provide potential points to improve visual function in persons with PDR.
Keywords: diabetic retinopathy, dark adaptation, pan-retinal photocoagulation
Diabetic retinopathy (DR) is a major cause of visual impairment and blindness in developed and developing countries, affecting approximately 93 million persons worldwide.1 Of these, 21 million have macular edema and 17 million have proliferative DR (PDR).1 These late-stage forms of DR are treated with pan-retinal laser photocoagulation (PRP) or vascular endothelial growth factor inhibition when visual acuity is threatened.2 However, the pathophysiologic mechanisms that lead to impaired vision remain poorly defined. Diabetic retinopathy has traditionally been described as a vascular complication of diabetes, but it causes dysfunction of the entire neurovascular unit,3 including retinal microvessel leakage and occlusion, impaired glial cell metabolic homeostasis, and inflammatory responses involving microglial cells, leading to depressed retinal neuronal cell function and survival.4,5
Clinical studies using electroretinography (ERG) have shown impaired ganglion cell function beginning after 6 months of diabetes.6 Likewise, persons with nonproliferative DR (NPDR) perform significantly worse on frequency doubling perimetry (FDP), again revealing ganglion cell dysfunction.4 Likewise, ERG components specific to photoreceptor, Müller, and bipolar cell function show significant deterioration after 10 years of disease.6,7 Histologic examination of rat and mouse cone cells also reveals morphologic changes in photoreceptor and second-order neurons after only 3 months of diabetes.8,9 Reports of photostress recovery time, which tests the retinal pigment epithelium (RPE) cells and cone photoreceptors, present mixed findings. Two studies have found impairment in the advanced stages of NPDR,10,11 and one report noted that photostress impairment predicted progression to PDR within 3 years.10 However, other studies of photostress recovery time have not found significant changes in subjects with DR or PDR, except in persons who have undergone PRP for PDR.12,13
Considering these findings, the inner retinal (ganglion, amacrine, and biopolar cells) function appears more impaired in early-stage DR compared with outer retinal (photoreceptor and RPE cells) function, although the evidence is incomplete and there is likely variation in the progression of neuronal dysfunction. Although there is strong evidence that both DR and PRP impair retinal neuronal function,12 we have an incomplete understanding of the effects of these processes on outer retinal function as disease progresses. One strategy to better describe outer retinal function in diabetes is through dark adaptation (DA) testing, which measures rod and cone sensitivity levels as well as rod and cone recovery rates from a bleaching light stimulus. These parameters can reflect photoreceptor and RPE function, and have been used to demonstrate outer retinal dysfunction in various outer retinal diseases, including retinitis pigmentosa and age-related macular degeneration (AMD).14,15 However, the evidence regarding DA and DR is mixed on whether diabetic subjects have impaired DA.
We sought to better characterize macular outer retinal function in subjects with diabetes by obtaining DA responses in normal control subjects and diabetic subjects with mild to severe DR, as well as those who had undergone PRP. In keeping with the evidence presented here, we hypothesized that DA responses, representing photoreceptor, RPE, and Müller cell function, would become progressively impaired with increasing severity of DR.
Methods
This study was conducted at the University of Michigan W. K. Kellogg Eye Center. Participants were recruited from the clinics and through the University of Michigan Clinical Studies website from August 2012 through June 2014. Informed consent was obtained from all subjects before participation in the study. The research was approved by the University of Michigan Medical School Institutional Review Board, adhered to the tenets of the Declaration of Helsinki, and was compliant with the Health Insurance Portability and Accountability Act.
Subjects
We recruited four groups of patients: (1) adults with diabetes mellitus (DM) and NPDR (NPDR group); (2) adults with DM and PDR (pre-PRP group); (3) adults with diabetes and PDR with history of PRP (post-PRP group); and (4) healthy adults (control group). All subjects were tested once. Inclusion criteria for the NPDR group were type 1 DM as defined by the American Diabetes Association diagnostic criteria; duration of DM ≥ 5 years; age 18 to 65 years; and best-corrected visual acuity (BCVA) ≥ 20/30. Inclusion criteria for the pre-PRP and post-PRP groups were the same as for the NPDR group except for (1) either type 1 or type 2 DM (T1 or T2); (2) best-corrected visual acuity ≥ 20/80; and (3) evidence of active PDR on dilated fundus examination (pre-PRP) or history of PRP administered at least 6 months before enrollment (post-PRP). Inclusion criteria for the control group were the same as for the NPDR group except that they could not have DM. Exclusion criteria for all subjects included any eye disease other than DR, including clinically significant macular edema; prior kidney, pancreas, or heart transplant; malignancy (with the exception of basal cell carcinoma); neurologic disease; history of substance abuse; blood pressure > 180/100 mm Hg; pregnant or nursing; and refractive error > ±6.00 diopters (spherical equivalent). Fluorescein angiography was not obtained in subjects, so macular ischemia cannot be conclusively ruled out, but most patients had excellent visual acuity, so macular ischemia is unlikely.
Baseline Evaluation
One study eye was chosen from each patient. If both eyes met the eligibility criteria, the eye with the better visual acuity was examined. If both eyes had the same acuity, the right eye was chosen. All subjects underwent ophthalmologic examination including refraction and measurement of BCVA using the Electronic Visual Acuity Tester (EVA; Jaeb Center for Health Research, Tampa, FL, USA) with E-ETDRS protocol. A blood sample was obtained from each participant to measure hemoglobin A1C (HbA1C).
Fundus photographs, either 7-field 30° monoscopic images using Zeiss FF 450plus fundus camera (Carl Zeiss Meditec, Dublin, CA, USA) or wide-field images using an Optos camera (Optos, Dunfermline, UK), were obtained in each study eye. A masked retinal specialist (TWG) graded the photographs and assigned the degree of retinopathy, using the Early Treatment Diabetic Retinopathy Disease Severity Scale.16
Optical Coherence Tomography
The Spectralis optical coherence tomography (OCT) instrument (Heidelberg Engineering, Heidelberg, Germany) was used to obtained a 20° × 20° cube scan centered on the fovea (97 sections, 512 A-scans in each B-scan, and 3.87-μm axial pixel pitch). Macular cube scans were analyzed in the inner-superior regions defined by Early Treatment Diabetic Retinopathy Study, as those corresponded with the retinal location of the DA stimulus. Within each of these areas, the thickness of the nerve fiber layer (NFL), ganglion cell layer + inner plexiform layer (GCL+IPL), inner nuclear layer (INL), outer plexiform layer + outer nuclear layer (OPL+ONL), inner segment/outer segment layer (IS/OS), and RPE was calculated (Fig. 1). Our naming convention follows the definition of the layer thicknesses based on the boundaries delineated in Chiu et al.17 The semiautomated protocol used to quantify the thicknesses of these layers has been described in previous publications.12 We have reported the reliability of our software for segmentation of normal eyes17 and eyes with significant diabetic pathology18,19 in previous publications in detail. Considering the manual correction of automatically segmented layer by an expert grader, and that the observed pathology in this study is significantly less than that in our previous work,18,19 we expect that the reliability of our results is close to that reported in our study of segmentation of normal eyes.17
Figure 1.
Representative OCT B-scan. This B-scan shows the delineation of retinal layer boundaries performed by our semiautomated protocol. NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segment; OS, outer segment; RPE, retinal pigment epithelium.
Dark Adaptation
Dark adaptation responses were obtained in all subjects using the AdaptDx dark adaptometer (MacuLogix, Inc., Hummelstown, PA, USA) using the protocol described by Jackson et al.4 Subjects' eyes were dilated with 1% tropicamide and 2.5% phenylephrine hydrochloride. Briefly, at the beginning of the test, subjects were bleached with a 2-ms 5.8 × 10^4 cd m−2 s−1 flash, equivalent to 82% bleaching level for rods, after which sensitivity recovery was measured. The stimulus light was a 505-nm, 2° diameter circular test spot located 5° superior to the fovea, a point that is not ablated by PRP. The zero log unit stimulus intensity was 5 scotopic cd/m2. Thresholds were measured approximately every 30 seconds. Thresholds were repeatedly measured until the subject's sensitivity consistently exceeded below 5 × 10−3 cd/m2. These settings were chosen to test macular outer retinal function, including cone, rod, and RPE cells. The resulting DA curve was characterized by three parameters: cone sensitivity level, rod recovery rate, and rod intercept time, which were our principal dependent variables (Fig. 2). The cone sensitivity level is the threshold of the cone photoreceptors derived from the cone plateau of the DA curve. The rod recovery rate is the slope of the second component of rod-mediated DA.20 The rod intercept is the time required for sensitivity recovery to reach a criterion sensitivity level of 5 × 10−3 cd/m2, a level within the second half of the second component of rod-mediated DA, and is derived by regression analysis of the second half of the second slope of rod-mediated DA. Because the rod intercept is expressed in minutes, it is more intuitive to understand than a slope and has been found useful in 12 peer-reviewed published experimental studies and proof-of-concept clinical trials in patients with DR and AMD.4,12,14,21–29
Figure 2.
A normal dark adaptation response. The normal 58-year-old subject is bleached with a 2-ms 5.8 × 10^4 cd m−2 s−1, 505-nm flash with the AdaptDx. Following bleaching, sensitivity recovery is tested with a 2° circular test region 5° superior to the fovea (black dots). Nonlinear regression was used to fit an exponential-linear model to the dark adaptation function (line). The model's parameters included cone plateau (1.97 log units), rod recovery rate (0.31 log units/min), and rod intercept time (9.0 minutes).
Statistical Analysis
Nonlinear regression was used to fit an exponential-linear model to the DA function to estimate the cone sensitivity level and rod recovery rate.30 Nonlinear regression was performed with IGOR PRO 6 (Wavemetrics, Lake Oswego, OR, USA). Data analysis was performed using STATA (2009, Stata Statistical Software Release 11; StataCorp LP, College Station, TX, USA). Means and standard deviations were calculated for continuous variables. Analysis of variance (ANOVA) was used to examine for differences in continuous variables between different stages of DR. Pairwise t-tests were used to compare cone sensitivity and rod recovery rates between groups. Linear regression models were constructed for cone sensitivity and rod recovery rate to examine the effects of age, HbA1C, diabetes type, and retinal layer thickness, in addition to retinopathy stage. Statistical significance was defined as a P value < 0.05.
Results
The cohort consisted of 23 control subjects and 73 diabetic subjects, of whom 12 had type 2 diabetes mellitus and 61 had type 1 diabetes mellitus (Table 1). This relatively high proportion of subjects with type 1 diabetes reflects the profiles of patients who volunteer for research studies in our institution. Seven responses were partially or completely excluded from analysis because of fixation errors or because the study parameters could not be calculated. There were significant differences in age and HbA1C between subjects in different retinopathy categories. All groups had significantly higher HbA1C than the control group. The post-PRP group was significantly older than the control group, while the groups with no retinopathy, mild NPDR, and moderate NPDR were significantly younger than the control group.
Table 1.
Subject Characteristics

Results of DA testing were analyzed by diabetes type and retinopathy status so that impairment could be examined as DR severity progressed. Analysis of variance revealed significant differences in all three major outcomes based on retinopathy category: cone sensitivity level (P < 0.001), rod recovery rate (P = 0.002), and rod intercept (P = 0.006) (Table 2). Compared to control subjects, cone sensitivity level was significantly impaired in subjects with PDR, both in those with T1DM (controls mean: 2.1 log units; T1DM PDR mean: 1.9 log units; P = 0.0047) and T2DM (controls mean: 2.1 log units; T2DM PDR mean: 1.6 log units; P < 0.001) (Fig. 3). Although subjects in the PDR group with T2DM had significantly worse cone sensitivity compared to subjects with T1DM by pairwise testing (T2DM PDR mean: 1.6 log units; T1DM PDR mean: 1.9; P = 0.01), this effect was not present in our multivariable regression model outlined below. Beginning at the level of moderate NPDR, diabetic subjects had significantly impaired rod recovery rates compared to controls (controls mean: 0.29 log units/min; moderate NPDR mean: 0.19 log units/min; P = 0.04) (Fig. 4). Rod intercept time was significantly slower in subjects with PDR and PRP compared to controls except for the T1DM PDR group (Table 2). There was no significant difference between PDR and PRP groups for any outcome. Comparison to reports of the rod intercept time in subjects with AMD shows that the impairment seen in our diabetic subjects was modest: Subjects with advanced DR had rod intercept times similar to subjects with borderline AMD, with far less impairment than in subjects with moderate or advanced AMD, though the subjects with AMD were much older than our subjects.29
Table 2.
Dark Adaptation Results

Figure 3.
Cone plateau levels in relation to retinopathy status. No Ret, no retinopathy; NPDR, nonproliferative diabetic retinopathy; Mod NPDR, moderate nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PRP, pan-retinal photocoagulation. Bars represent means, and whiskers represent 95% confidence intervals. Cone sensitivity level was significantly impaired beginning in subjects with proliferative diabetic retinopathy compared to control subjects: T1DM (P = 0.0047); T2DM (P < 0.001). There was no significant difference between PDR and PRP groups. In pairwise testing, subjects in the PDR T2DM group had significantly worse cone sensitivity compared to T1DM PDR subjects (P = 0.01); however, this effect was not present in our multivariable regression model. Source code from UCLA Statistical Consulting Group.47
Figure 4.
Rod recovery rates by retinopathy status. Bars represent means, and whiskers represent 95% confidence intervals. For rod recovery rate, there was a significant difference between control group and moderate NPDR (P value = 0.04), and between control group and both the PDR and post-PRP groups except for PDR (T1DM). There was no significant difference between PDR and PRP groups or between T1DM and T2DM. Source code from UCLA Statistical Consulting Group.47
To investigate the effects of factors other than retinopathy status, linear multivariable regression models were constructed for cone sensitivity and rod recovery rate (Tables 3, 4). For both models, the independent variables were DR category (reference group: no retinopathy, including diabetic subjects with no retinopathy and control subjects), diabetes type (reference group: control subjects), age, and HbA1C. As above, more advanced retinopathy was found to be significantly associated with impairment of both rod recovery and cone sensitivity. The results indicate that all retinopathy categories were associated with impaired rod recovery, with all categories having a significant association except post-PRP. Increasing age was also significantly associated with impaired rod recovery. For cone plateau, the results show that retinopathy categories PDR and post-PRP were significantly associated with impairment; HbA1C and diabetes type were not significantly associated with either outcome. Considering these findings, our results suggest that impaired rod recovery rate begins early in DR; our model finds significant impairment in subjects with moderate NPDR, and impairment in cone sensitivity begins in more advanced DR.
Table 3.
Results of Multivariable Linear Regression of Rod Recovery Rate
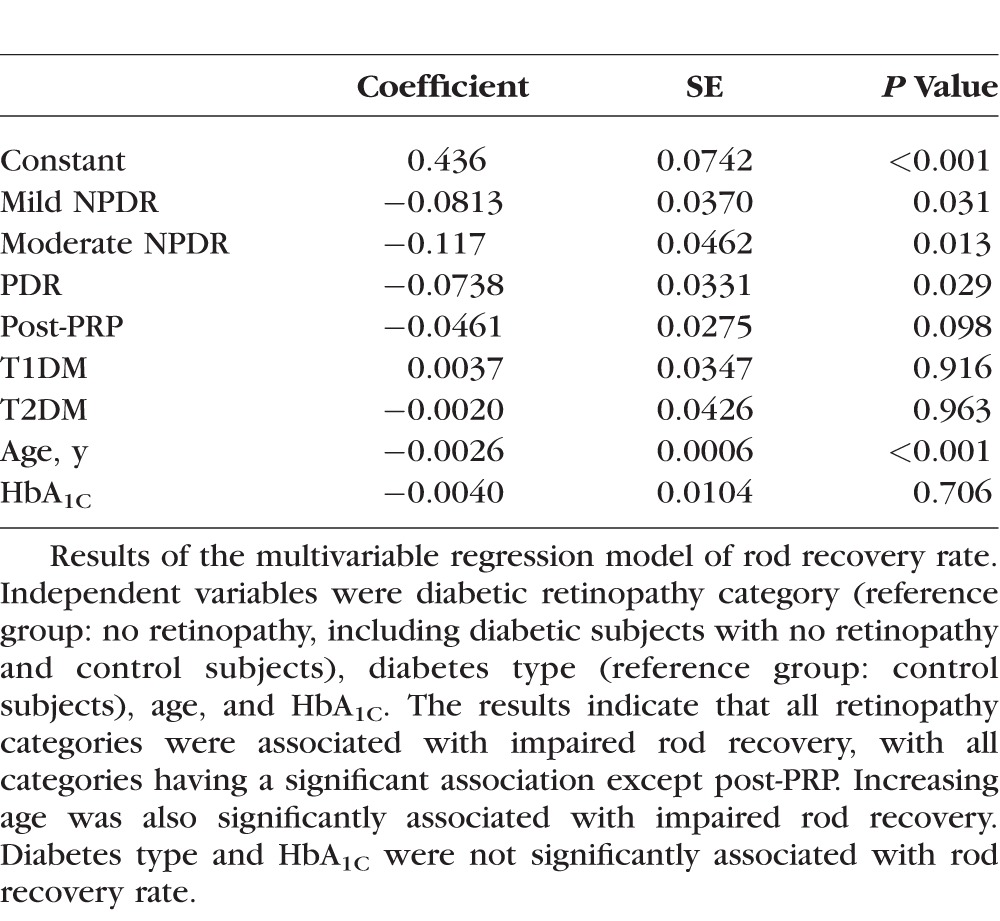
Table 4.
Results of Multivariable Linear Regression of Cone Sensitivity Level

Boynton et al.12 previously showed that thinning of the retina was associated with loss of retinal function in patients with DR. There were significant differences in NFL, IS/OS, and RPE thicknesses between subjects in different retinopathy categories (Table 5). The mean NFL thickness was 2 to 10 μm thicker in subjects with advanced DR compared to control subjects, and the mean IS/OS and RPE thickness was 2 to 4 μm thinner in subjects with advanced DR compared to controls. These data were used to examine for correlations between retinal function and retinal layer thickness (Tables 6, 7). Lower cone sensitivity was associated with thinner RPE, IS/OS layer, and GCL+IPL. Slower rod recovery rate was associated with thinner RPE and GCL+IPL, but not IS/OS layer. Both lower cone sensitivity and slower rod recovery rates were associated with thicker NFL. These findings suggest that reduction in cone sensitivity and rod recovery is associated with altered retinal structure, especially with thinner outer retinal layers, which are believed to be the sites of processes important to cone sensitivity and rod recovery, as discussed below.
Table 5.
Thickness of Retinal Layers
Table 6.
Results of Simple Linear Regressions of Cone Sensitivity Level and Retinal Layer Thickness

Table 7.
Results of Simple Linear Regressions of Rod Recovery Rate and Retinal Layer Thickness

Discussion
The goal of this study was to improve the understanding of outer retinal macular function in diabetic subjects by using DA responses from subjects at different stages of retinopathy. By using DA testing in diabetic subjects, predominately with T1DM, with mild to advanced DR, this study investigated RPE and photoreceptor dysfunction at different levels of DR. Our testing included three parameters with which to examine retinal function: cone sensitivity level, rod recovery rate, and rod intercept time. We focus on cone sensitivity and rod recovery, as those parameters are more easily correlated with specific biochemical processes. Improved understanding of the pathogenesis of outer retinal function in persons with DR could help identify therapeutic targets for these patients. To the best of our knowledge, this is one of the first studies to apply multiple tests of photoreceptor/RPE function in persons with DR.
Importantly, the rod recovery rate is thought to derive from the visual retinoid cycle, which allows us to interpret our results as indicating the function of that process. Our results show clear deterioration of both rod recovery rate and cone sensitivity in diabetic subjects compared to control subjects, representing outer retinal dysfunction. In our model, every stage of DR was significantly associated with impaired rod recovery when compared to no retinopathy, except for post-PRP. The finding that retinopathy status post-PRP is not significantly associated with greater impairment of rod recovery is unexpected and could reflect that many of the post-PRP subjects in our cohort managed their diabetes well, as evidenced by this group having the lowest HbA1C of all the diabetic groups. In comparison, the model of cone sensitivity, which is less well understood, revealed impairment to begin later in DR; PDR and post-PRP status were significantly associated with impairment, but no earlier stages of DR were. Potential explanations for the earlier impairment of rod recovery will be discussed below.
In addition to retinopathy category, our model also found that increasing age was significantly associated with slowed rod recovery. The effect of aging on the rod intercept between young adults and old adults (mean age difference of 50 years) is 0.5 minutes.29 The effects found in the PRP groups are much larger than reported aging effects. Surprisingly, HbA1C was not significantly associated with either cone sensitivity or rod recovery, possibly because HbA1C impacted retinopathy status, which was also included in the model. Moreover, the post-PRP group had the lowest mean HbA1C of the diabetic groups, an unexpected finding that negated the overall trend of HbA1C increasing as DR progressed and function worsened.
Further, our results suggest that retinal layer thickness is correlated with cone sensitivity and rod recovery rate. Retinal pigment epithelium was thinner in subjects with reduced cone sensitivity and rod recovery rate, and subjects with low cone sensitivity also had a thin IS/OS layer. The correlation of thin outer retinal layers with impaired DA responses corroborates the association between impaired outer retinal function and altered outer retinal structure. Further, GCL+IPL was thinner in subjects with reduced cone sensitivity and rod recovery rate, reflecting the structural impact of DR on the entire retina. Our data also suggest that NFL is thicker in subjects with impaired DA responses, an unexpected finding that requires further investigation, but may reflect gliosis.12
As mentioned above, the visual retinoid cycle is thought to be an important factor in determining rod recovery rates. The retinoid cycle describes how the vitamin A derivative, 11-cis retinal, is used and regenerated in photoreceptor and RPE cells (Fig. 5). When light is absorbed in the rod and cone photoreceptor cells, 11-cis retinal bound to opsin is isomerized to all-trans retinal, which initiates the visual response.31 The all-trans retinal is subsequently transported to RPE cells, where it is converted back to 11-cis retinal, which is then transported back to photoreceptor cells, where it can again bind to opsin and absorb light.31 In cone cells, there is evidence that Müller cells also play a minor role in regenerating 11-cis retinal, providing an additional pathway for the visual cycle.32
Figure 5.

Key steps in the retinoid cycle of vision. Enzymes (red) and binding proteins (blue) involved in 11-cis-retinal regeneration are found in both photoreceptor and RPE cells. Metabolic transformations occurring in the RPE take place in the smooth endoplasmic reticulum, where key enzymes of the visual cycle are located. PC, phosphotidylcholine. Addendum to original legend: Lecithin retinol acyltransferase, retinal G-protein coupled receptor, RPE65, and opsin are decreased in diabetic rat retinas.36,37 Thus, we hypothesize that reduced expression of these visual cycle proteins contributed to the reduced cone sensitivity and rod recovery rate in our diabetic subjects. Reprinted from Kiser PD, Golczak M, Palczewski K. Chem Rev. 2014;114:194–232,48 an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for noncommercial purposes. Copyright 2013 American Chemical Society.
This framework of the visual cycle has been used to describe the biological underpinnings of rod recovery rates. Within a DA response, several parameters can be measured, including cone recovery rate from bleaching, cone sensitivity, rod recovery rate from bleach, and rod sensitivity. Each of these parameters is described in depth by Lamb and Pugh.33 They state that both the cone and rod recovery rates are determined by the rate at which 11-cis retinal is regenerated and delivered to the photoreceptors.33 Rod and cone recovery rates are impaired in subjects with genetic mutations of proteins involved in the visual cycle, notably RPE65 and 11-cis-RDH,33 giving further evidence for the connection between the visual cycle and DA rates.
Given reports of diabetic subjects with impaired recovery rates even with normal visual acuity,34,35 it seems plausible that DR affects the visual cycle, though the exact mechanism is unclear. In other retinal diseases that cause recovery rate impairment, such as AMD, some researchers have suggested that deposits of protein and lipid (drusen) between the RPE and choroid could impair transport of nutrients into the RPE, possibly affecting the visual cycle by causing vitamin A deficiency.33 There is no evidence of a similar process in diabetes, but diabetes impairs the transcription of genes involved in the visual cycle, which could cause phenotypic changes similar to the genetic mutations discussed above. Of note, expression of visual cycle proteins lecithin retinol acyltransferase, retinal G-protein coupled receptor, RPE65, and opsin, is decreased in diabetic rat retinas.36,37 It is possible that reduced expression of these visual cycle proteins in the RPE and photoreceptors contributed to the reduced cone sensitivity and rod recovery rate in our diabetic subjects. However, this hypothesis is untested and requires further investigation.
While it seems that a link between diabetes and proteins involved in the visual cycle has been established, the published evidence regarding rod and cone recovery rates in diabetic subjects is mixed. Multiple studies have reported that diabetic subjects have impaired rod recovery rates28,34,35,38; others have not found a significant difference between diabetic subjects and controls.39–41 Additionally, because of methodologic inconsistencies between reports, it is difficult to draw firm conclusions about the effect of DR on rod recovery rates, including the stage of DR at which rod recovery impairment begins. For instance, many of the reports cited here have classified DR using a previous scheme, lacked control subjects, or did not stratify findings by DR stage. There are also important differences in the dark adaptometers and testing protocols used. Most other reports used bleaches lasting several minutes, while our study, using the AdaptDx adaptometer, had a flash bleach. This short but intense bleach makes it difficult to assess cone recovery rate, though we were able to consistently describe cone sensitivity level and rod recovery rates. Further, the location and size of the test stimulus vary between reports. One study using the Goldmann-Weekers adaptometer used a 1.25° stimulus 15° temporal to the fovea,39 while our test stimulus was set to 1.7°, though it projected to a point 5° above the fovea. Retinal ischemic damage is known to occur earlier in DR at the peripheral location used with the Goldmann-Weekers adaptometer, likely making this location more sensitive to retinal neuronal dysfunction.42 The principal advantage of our parafoveal location is that both rod and cone cells are in high concentration more centrally, which allowed us to assess both of their functions using one location.43 In contrast, while rod cell density remains roughly constant from 5° to 15°, cone cell concentration is markedly reduced at 15° compared to 5°, making cone cell function more difficult to assess peripherally.43 Further, macular function is the most important to overall vision and function, so we believe that the function at this location is an important representation of visual function.
Taken together, multiple differences in the reports of rod recovery rates in diabetic subjects hinder meaningful comparisons between studies. We submit that our study is an important contribution to the evidence regarding rod recovery rates in diabetic subjects, because it includes subjects with all stages of DR and tests them with a uniform protocol. Our finding that moderate NPDR is significantly associated with rod recovery impairment is consistent with previous findings, and suggests that rod and RPE dysfunction begins before PDR and thus before most clinicians treat DR. Of note, clinically significant macular edema was an exclusion criterion for participation in this study, so the results reveal changes in retinal function and structure independent of retinal thickening, the most common indication for treatment.
Likewise, the evidence is mixed regarding cone sensitivities, and there are important methodologic differences among the published studies similar to those described for studies regarding rod recovery rates. Few studies report on cone sensitivity, and those that do have tested only subjects at a certain stage of DR. Reports of subjects with mild NPDR have not found impairment in cone sensitivity,39,40 but significant differences were reported between controls and subjects who had received PRP.41 By studying subjects with all stages of DR at a parafoveal, cone-dense location, our study adds important information to the literature of cone sensitivity dysfunction in diabetic subjects, suggesting that impairment begins relatively late in the course of DR, at the PDR stage. It is unclear why cone sensitivity is preserved longer than rod recovery in the progression of DR. That rod recovery is rod and RPE mediated and that cone sensitivity appears to be mostly cone mediated, as will be discussed below, is one possible explanation and needs to be further investigated.
Unlike the situation with rod recovery rates, the biochemical processes that determine cone sensitivities are not well understood. It is unlikely that the regeneration of 11-cis-retinal determines sensitivity, as with the rod recovery rate. Rather, cone and rod sensitivities represent the minimum stimulus required for a visual signal to be stronger than the background noise in the retina.33,44 The length of rod and cone cell outer segments, which contain high concentrations of opsin, has been suggested as one feature that affects sensitivity in animals.45 The proposed importance of the length of the photoreceptors is supported by the positive correlation between IS/OS thickness and cone sensitivity: Subjects with thinner IS/OS had lower cone sensitivity. Despite these reports, there is no consensus on what determines cone and rod sensitivities, so it is difficult to hypothesize what might be driving the sensitivity impairment in the diabetic subjects in our cohort.
Dark adaptation provides several parameters with which outer retinal function can be assessed. There are other tests of outer retinal function, including ERG and photostress, and reports of those tests in diabetic subjects present mixed evidence, with some finding impairment beginning in NPDR and others reporting that impairment begins in PDR, or even only after PRP. In our cohort of subjects with predominately T1DM, our findings suggest that early-stage DR is associated with impaired rod recovery and that cone sensitivity impairment begins in PDR. However, the fact that cone sensitivity as assessed by DA remains intact until PDR does not necessarily mean that no damage occurs earlier; histopathology, OCT, and adaptive optics may provide evidence of early defects. Previous research using ERG has demonstrated cone sensitivity changes at earlier stages of DR.46 In a previously published report of OCT results in the PDR and PRP subjects in our cohort, we found that the RPE was significantly thinner in PDR subjects compared to the RPE in control subjects, and the photoreceptor layer was significantly thinner in subjects who had undergone PRP.12 Further structural and histopathologic evidence is needed in subjects at all stages of DR to understand the progression of this outer retinal thinning, which could represent cellular damage and be associated with dysfunction. The functional data described here, along with further work describing retinal function in DR, will aid future searches for therapeutic targets in DR.
Acknowledgments
The authors thank Debra Thompson and Daniel Green for helpful insights.
Supported by EY20582 and DK094292, The Taubman Institute, a Research to Prevent Blindness Physician-Scientist Award (TWG), and EY022691 (SF). GRJ is an employee and shareholder of MacuLogix, Inc.
Disclosure: J.C. Bavinger, None; G.E. Dunbar, None; M.S. Stem, None; T.S. Blachley, None; L. Kwark, None; S. Farsiu, None; G.R. Jackson, MacuLogix (I, E, S), P; T.W. Gardner, None
References
- 1. Yau JW,, Rogers SL,, Kawasaki R,, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans JR,, Michelessi M,, Virgili G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2014: CD011234. [DOI] [PMC free article] [PubMed]
- 3. Gardner TW,, Abcouwer SF,, Barber AJ,, Jackson GR. An integrated approach to diabetic retinopathy research. Arch Ophthalmol. 2011; 129: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackson GR,, Scott IU,, Quillen DA,, Walter LE,, Gardner TW. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. Br J Ophthalmol. 2012; 96: 699–703. [DOI] [PubMed] [Google Scholar]
- 5. Abcouwer SF,, Gardner TW. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment: neuroretinal adaptation in diabetic retinopathy. Ann N Y Acad Sci U S A. 2014; 1311: 174–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parisi V,, Uccioli L. Visual electrophysiological responses in persons with type 1 diabetes. Diabetes Metab Res Rev. 2001; 17: 12–18. [DOI] [PubMed] [Google Scholar]
- 7. Mortlock KE,, Chiti Z,, Drasdo N,, Owens DR,, North RV. Silent substitution S-cone electroretinogram in subjects with diabetes mellitus. Ophthalmic Physiol Opt. 2005; 25: 392–399. [DOI] [PubMed] [Google Scholar]
- 8. Énzsöly A,, Szabó A,, Kántor O,, et al. Pathologic alterations of the outer retina in streptozotocin-induced diabetes. Invest Ophthalmol Vis Sci. 2014; 55: 3686–3699. [DOI] [PubMed] [Google Scholar]
- 9. Hombrebueno JR,, Chen M,, Penalva RG,, Xu H. Loss of synaptic connectivity particularly in second order neurons is a key feature of diabetic retinal neuropathy in the Ins2Akita mouse. PLoS One. 2014; 9: e97970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frost-Larsen K,, Larsen H-W. Nyctometry—a new screening method for selection of patients with simple diabetic retinopathy who are at risk of developing proliferative retinopathy. Acta Ophthalmol (Copenh). 1983; 61: 353–361. [DOI] [PubMed] [Google Scholar]
- 11. Midena E,, Segato T,, Giuliano M,, Zucchetto M. Macular recovery function (nyctometry) in diabetics without and with early retinopathy. Br J Ophthalmol. 1990; 74: 106–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boynton GE,, Stem MS,, Kwark L,, Jackson GR,, Farsiu S,, Gardner TW. Multimodal characterization of proliferative diabetic retinopathy reveals alterations in outer retinal function and structure. Ophthalmology. 2015; 122: 957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu G,, Weiter JJ,, Santos S,, Ginsburg L,, Villalobos R. The macular photostress test in diabetic retinopathy and age-related macular degeneration. Arch Ophthalmol. 1990; 108: 1556–1558. [DOI] [PubMed] [Google Scholar]
- 14. Jackson GR,, Scott IU,, Kim IK,, Quillen DA,, Iannaccone A,, Edwards JG. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014; 55: 1427–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alexander KR,, Fishman GA. Prolonged rod dark adaptation in retinitis pigmentosa. Br J Ophthalmol. 1984; 68: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson C,, Ferris FL,, Klein RE,, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003; 110: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 17. Chiu S,, Li X,, Nicholas P,, Toth C,, Izatt J,, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010; 18: 19413–19428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee JY,, Chiu SJ,, Srinivasan PP,, et al. Fully automatic software for retinal thickness in eyes with diabetic macular edema from images acquired by cirrus and spectralis systems. Invest Ophthalmol Vis Sci. 2013; 54: 7595–7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiu SJ,, Allingham MJ,, Mettu PS,, Cousins SW,, Izatt JA,, Farsiu S. Kernel regression based segmentation of optical coherence tomography images with diabetic macular edema. Biomed Opt Express. 2015; 6: 1172–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leibrock CS,, Reuter T,, Lamb TD. Molecular basis of dark adaptation in rod photoreceptors. Eye. 1998; 12: 511–520. [DOI] [PubMed] [Google Scholar]
- 21. Owsley C,, Huisingh C,, Clark M,, Jackson G,, McGwin G. Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health [published online ahead of print August 19 2015]. Curr Eye Res. doi:http://dx.doi.org/10.3109/02713683.2015.1011282. [DOI] [PMC free article] [PubMed]
- 22. Flamendorf J,, Agrón E,, Wong W,, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015; 122: 2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scott IU,, Jackson GR,, Quillen DA,, et al. Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in patients with severe nonproliferative or non–high-risk proliferative diabetic retinopathy: a randomized clinical rrial. JAMA Ophthalmol. 2014; 132: 535–543. [DOI] [PubMed] [Google Scholar]
- 24. Owsley C,, Huisingh C,, Jackson GR,, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest Ophthalmol Vis Sci. 2014; 55: 4776–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson G,, Clark M,, Scott I,, Walter L,, Quillen D,, Brigell M. Twelve-month natural history of dark adaptation in patients with AMD. Optom Vis Sci. 2014; 91: 925–931. [DOI] [PubMed] [Google Scholar]
- 26. Holfort SK,, Nørgaard K,, Jackson GR,, et al. Retinal function in relation to improved glycaemic control in type 1 diabetes. Diabetologia. 2011; 54: 1853–1861. [DOI] [PubMed] [Google Scholar]
- 27. Clark ME,, McGwin G,, Neely D,, et al. Association between retinal thickness measured by spectral-domain optical coherence tomography (OCT) and rod-mediated dark adaptation in non-exudative age-related maculopathy. Br J Ophthalmol. 2011; 95: 1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holfort SK,, Jackson GR,, Larsen M. Dark adaptation during transient hyperglycemia in type 2 diabetes. Exp Eye Res. 2010; 91: 710–714. [DOI] [PubMed] [Google Scholar]
- 29. Jackson GR,, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. J Ocul Biol Dis Infor. 2008; 1: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGwin GJ,, Jackson GR,, Owsley C. Using nonlinear regression to estimate parameters of dark adaptation. Behav Res Methods Instrum Comput. 1999; 31: 712–717. [DOI] [PubMed] [Google Scholar]
- 31. Jacobson SG,, Aleman TS,, Cideciyan AV,, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci U S A. 2007; 104: 15123–15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J-S,, Kefalov VJ. The cone-specific visual cycle. Prog Retin Eye Res. 2011; 30: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamb TD,, Pugh EN. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004; 23: 307–380. [DOI] [PubMed] [Google Scholar]
- 34. Amemiya T. Dark adaptation in diabetics. Ophthalmologica. 1977; 174: 322–326. [DOI] [PubMed] [Google Scholar]
- 35. Zetterström B,, Gjötterberg M. Photocoagulation in diabetic retinopathy with special reference to its effect on dark adaptation. Acta Ophthalmol (Copenh). 1973; 51: 512–519. [DOI] [PubMed] [Google Scholar]
- 36. Kirwin SJ,, Kanaly ST,, Hansen CR,, Cairns BJ,, Ren M,, Edelman JL. Retinal gene expression and visually evoked behavior in diabetic long evans rats. Invest Ophthalmol Vis Sci. 2011; 52: 7654–7663. [DOI] [PubMed] [Google Scholar]
- 37. Kirwin SJ,, Kanaly ST,, Linke NA,, Edelman JL. Strain-dependent increases in retinal inflammatory proteins and photoreceptor FGF-2 expression in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2009; 50: 5396–5404. [DOI] [PubMed] [Google Scholar]
- 38. Henson DB,, North RV. Dark adaptation in diabetes mellitus. Br J Ophthalmol. 1979; 63: 539–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greenstein VC,, Thomas SR,, Blaustein H,, Koenig K,, Carr RE. Effects of early diabetic retinopathy on rod system sensitivity. Optom Vis Sci. 1993; 70: 18–23. [DOI] [PubMed] [Google Scholar]
- 40. Kurtenback A,, Mayser HM,, Jagle H,, Fritsche A,, Zrenner E. Hyperoxia hyperglycemia, and photoreceptor sensitivity in normal and diabetic subjects. Vis Neurosci. 2006; 23: 651–661. [DOI] [PubMed] [Google Scholar]
- 41. Mäntyjärvi M. Colour vision and dark adaptation in diabetic patients after photocoagulation. Acta Ophthalmol (Copenh). 1989; 67: 113–118. [DOI] [PubMed] [Google Scholar]
- 42. Dodo Y,, Murakami T,, Uji A,, Yoshitake S,, Yoshimura N. Disorganized retinal lamellar structures in nonperfused areas of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015; 56: 2012–2020. [DOI] [PubMed] [Google Scholar]
- 43. Curcio CA,, Sloan KR,, Kalina RE,, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990; 292: 497–523. [DOI] [PubMed] [Google Scholar]
- 44. Koenig D,, Hofer H. The absolute threshold of cone vision. J Vis. 2011; 11 (1): 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Battelle B-A,, LaVail MM. Rhodopsin content and rod outer segment length in albino rat eyes: modification by dark adaptation. Exp Eye Res. 1978; 26: 487–497. [DOI] [PubMed] [Google Scholar]
- 46. Holopigian K,, Greenstein VC,, Seiple W,, Hood DC,, Carr RE. Evidence for photoreceptor changes in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997; 38: 2355–2365. [PubMed] [Google Scholar]
- 47. UCLA Statistical Consulting Group. Stata FAQ: how can I make a bar graph with error bars? Available at: http://www.ats.ucla.edu/stat/stata/faq/barcap.htm. Accessed May 5 2015.
- 48. Kiser PD,, Golczak M,, Palczewski K. Chemistry of the retinoid (visual) cycle. Chem Rev. 2014; 114: 194–232. [DOI] [PMC free article] [PubMed] [Google Scholar]







