Abstract
Exposure to early life adversity is linked to impaired affective, cognitive, and behavioral functioning and increases risk for various psychiatric and medical conditions. Stress-induced increases in pro-inflammatory cytokines may be a biological mechanism of these effects. Few studies have examined cytokine levels in children experiencing early life adversity, and very little research has investigated cytokines or other markers of inflammation in saliva. In the present study, we examined salivary IL-1β and C-reactive protein (CRP) levels in relation to stress exposure in 40 children aged 3 to 5 years who were enrolled in a larger study of early life adversity. Childhood maltreatment status was assessed via review of child welfare records, and contextual stress exposure, traumatic life event history, and symptoms of psychopathology were assessed via caregiver interviews at a home visit. In a subsequent visit, salivary IL-1β and CRP were obtained before and after participation in four emotion-eliciting tasks. Number of past month contextual stressors, lifetime contextual stressors, and traumatic life events each demonstrated a significant main effect on IL-1β. Baseline IL-1β was positively associated with each of the significant main-effect adversities. Post-challenge IL-1β displayed positive associations with each adversity variable, but were not significant. CRP was not significantly associated with any of the adversity variables. Given evidence suggesting involvement of IL-1β in the neuropathology of psychiatric conditions, these results may have important implications for developmental outcomes.
Keywords: childhood maltreatment, cytokine, IL-1β, stress, preschool aged children
It is well established that exposure to childhood maltreatment and other adverse experiences undermine adaptive developmental outcomes in children (Appleyard, Egeland, van Dulmen, & Sroufe, 2005; Sameroff, Seifer, Baldwin, & Baldwin, 1993). Early adversity impairs affective, behavioral, cognitive and interpersonal functioning, and increases risk for psychiatric conditions including depressive, anxiety, and substance-use disorders (Brown & Anderson, 1991; Bryer, Nelson, Miller, & Krol, 1987; Burns et al., 2004). There is now increasing recognition that early environment also modifies risk for the development of several medical conditions, including hypertension, obesity, diabetes, heart disease, and pain syndromes (Price, Kao, Burgers, Carpenter, & Tyrka, 2013; Shonkoff & Garner, 2012). In fact, early stress appears to increase risk for early mortality, with one recent study showing that adults with six or more adverse experiences in childhood died nearly 20 years earlier than those without adversity (Brown et al., 2009).
Children in poverty are disproportionately exposed to adverse circumstances through direct and indirect mechanisms embedded within several levels of the environment including families, neighborhoods and communities, and the larger culture (Bronfenbrenner, 1977; Cicchetti & Lynch, 1993). Lack of resources directly impacts the physical environment, including the availability, stability, and quality of nourishment, shelter, neighborhood environment and schooling. Poverty also influences the social environment directly and indirectly, through effects on emotional stability and availability of caregivers and others in the community. Within families, children in poverty may be exposed to harsh or neglectful parenting and maltreatment, and parental mental illness, substance use, and incarceration. Within the immediate communities in which they live, children in poverty face neighborhood violence, a lack of employment options for their caregivers, low-quality childcare, and underfunded educational systems. The early-life health consequences of developing in poverty are well documented. For example, chronic diseases such as asthma and diabetes have long been known to be more prevalent and have disparate morbidity among impoverished individuals (Gaskin et al., 2013; Koinis-Mitchell et al., 2007). These wide-ranging effects of early adversity may result in part from constitutional changes in the inflammatory system.
Acute Stress Response and Inflammation
Exposure to acute stress or trauma initiates the inflammatory response, which involves increases in pro-inflammatory cytokines, as well as chemokines, adhesion molecules, and acute phase reactants. Cytokines are a large and diverse group of messenger proteins that act through intercellular signaling to regulate immune responses (Lacy & Stow, 2011; Robles, Glaser, & Kiecolt-Glaser, 2005). Pro-inflammatory cytokines play a vital role in mobilizing the immune response to infectious agents and injuries, and it is now clear that cytokines are also activated in response to acute psychological stress. Inflammatory proteins serve as part of an adaptive response that serves to minimize injury and promote healing in response to acute toxins, threats or injuries. However, excessive inflammation with prolonged and persistent elevation of pro-inflammatory cytokine levels is associated with major depression (MDD) and post-traumatic stress disorder (PTSD) in addition to other psychiatric and other medical conditions.
Inflammation and Psychopathology
A wealth of literature describes findings from clinical and epidemiologic samples showing that adults with MDD have elevated inflammatory responses or peripheral concentrations of cytokines, most commonly interleukin (IL)-6, IL-1β, and TNF-α, as well as the acute phase protein C-reactive protein (CRP). Meta-analyses have confirmed these associations (Dowlati et al., 2010; Hiles, Baker, de Malmanche, & Attia, 2012; Liu, Ho, & Mak, 2012). There is now also substantial evidence in adults that PTSD is associated with inflammation (Baker, Nievergelt, & O’Connor, 2012; Gola et al., 2013; O’Donovan et al., 2011; Pace et al., 2012). Few studies of children with psychiatric disorders have been conducted. Findings from a small number of studies of adolescent depression have been mixed (Mills, Scott, Wray, Cohen-Woods, & Baune, 2013), however a recent large study found that externalizing behavior at age 8 predicted elevated CRP at age 10, and age 8 internalizing and externalizing behaviors predicted elevated IL-6 at age 10 (Slopen, Kubzansky, & Koenen, 2013a).
Several lines of evidence implicate cytokine activity as a mechanism of these disorders. A recent meta-analysis found that IL-6 and CRP concentrations were significant predictors of the subsequent development of depressive symptoms (Valkanova, Ebmeier, & Allan, 2013). Inflammation is associated with decrements in learning and memory (Krishnadas et al., 2013; Nikas, 2013; Phillips et al., 2011; Teunissen et al., 2003; Wright et al., 2006; Yaffe et al., 2003), and therapeutic use of interferon for Hepatitis C can cause depression (Udina et al., 2012) Animal models show that peripheral administration of endotoxin or cytokines elicits “sickness behavior” including social withdrawal, and reduction of food intake and other activities (Dantzer, 2009). Cytokines do not passively cross the blood-brain barrier, but several direct and indirect routes for cytokines to act on the brain have been documented (Dantzer, 2009; Mills et al., 2013).
Role of Inflammatory Cytokines in Neuroplasticity
Further evidence supports a role for cytokine activity on the growth and development of neurons (Kohman & Rhodes, 2013). As with many physiological systems, there is some evidence that low levels of inflammatory cytokines may have salutary effects, while high levels may be deleterious. A number of studies have examined effects of cytokine exposure on neural progenitor cells. At relatively low concentrations, IL-6, IL-1β, and TNF-α induce neuronal differentiation and proliferation, but at high concentrations, pro-inflammatory cytokines reduce neurogenesis and cell survival (Araujo & Cotman, 1995; Barkho et al., 2006; Bernardino et al., 2008; Cacci, Claasen, & Kokaia, 2005; Monje, Toda, & Palmer, 2003; Zunszain et al., 2012). IL-1β may play a particularly important role in the brain (Dantzer, 2009). In animal models, central administration of IL-1β activates the HPA axis, reduces hippocampal brain-derived neurotrophic factor (BDNF), and impairs hippocampal-dependent learning (Koo & Duman, 2008). Furthermore, there is evidence from animal models that activation of the IL-1β receptor is necessary for stress to impair neurogenesis (Koo & Duman, 2008).
Childhood Adversity and Inflammation
There is currently a great deal of interest in the hypothesis that early stress exposure may lead to chronic inflammation. Several studies have shown that childhood adversity is linked to higher peripheral levels of inflammatory cytokines in adults with MDD, and there is some evidence for an effect of early stress in other conditions such as drug abuse, schizophrenia, and migraine (for a review, see Coelho, Viola, Walss-Bass, Brietzke, & Grassi-Oliveira, 2013).
Other work has examined associations of early experience with inflammatory markers in healthy subjects or those recruited from the community. This is a relatively new area of research and published findings are limited. Most of the studies on this topic have examined CRP in peripheral blood samples. CRP is an acute phase reactant produced by the liver in response to cytokines. In a large longitudinal birth-cohort study, Danese and colleagues (2007) found that childhood maltreatment was predictive of significantly higher plasma CRP levels in adulthood. Depression was associated with significantly elevated levels of CRP but childhood maltreatment accounted for much of this effect, and those with depression and maltreatment had the highest CRP levels (Danese et al., 2008). Additional large studies of adults have confirmed associations of childhood adversity or socioeconomic status and CRP (Appleton et al., 2012; Matthews, Chang, Thurston, & Bromberger, 2013; Pollitt et al., 2007) and CRP and IL-6 (Bertone-Johnson, Whitcomb, Missmer, Karlson, & Rich-Edwards, 2012; Rooks, Veledar, Goldberg, Bremner, & Vaccarino, 2012), however, two small studies of healthy adults did not find an effect of early stress on CRP levels (Carpenter, Gawuga, Tyrka, & Price, 2012; Hartwell et al., 2013).
Several studies have measured CRP in childhood and found positive associations with either adverse events (Slopen, Kubzansky, McLaughlin, & Koenen, 2013b) or socioeconomic adversity (Broyles et al., 2012; Dowd, Zajacova, & Aiello, 2010; Howe et al., 2010; McDade et al., 2005; Murasko, 2008). However, one study found that the link between CRP and adversity varied as a function of chronic interpersonal stress (Marin, Martin, Blackwell, Stetler, & Miller, 2007), another found an association of high SES and high CRP when those with very high CRP were included (Thomas, Cooper, Williams, Baker, & Davies, 2005), and other studies found no association of CRP with childhood economic status (Cook et al., 2000; Gimeno et al., 2008). Thus, it appears that the effect of childhood adversity that is seen on elevated levels of CRP in adulthood is more variable in studies of childhood CRP.
Only a few investigations have examined associations of early adversity with basal or induced cytokine levels in healthy participants. Stressful life events were associated with higher levels of plasma IL-6 in 10-year-old children (Slopen et al., 2013b) and TNF-α in children ages 5–10 (Dixon, Meng, Goldberg, Schneiderman, & Delamater, 2009), parental divorce or separation was linked to higher IL-4 levels (Herberth et al., 2008). Miller and Chen (2010) studied 135 female adolescents longitudinally over 1.5 years and found that those with harsh families had increasingly higher IL-6 responses to immune challenge but no effect on circulating IL-6 levels. In a study of adults, those with lower childhood SES had higher in vitro IL-6 responses to stimulation (Miller et al., 2009). Our group found that IL-6 response to a standardized psychosocial challenge task, was greater in healthy adults with a history of childhood maltreatment, although no difference in baseline IL-6 was observed (Carpenter et al., 2010). In a recent small study of healthy adults, Hartwell and colleagues (2013) found that reports of early trauma were correlated with basal levels of serum TNF-α, IL-6, and IL-1β.
Other than this small study of adults by Hartwell and colleagues, no studies have examined the relationship between childhood adversity and IL-1β concentrations, despite the important role of this cytokine in the inflammatory cascade, and evidence that it is implicated in the pathophysiology of MDD possibly due to inhibitory effects on neuroplasticity. Animal models of stress exposure show increases in IL-1β (e.g., Bailey, Kinsey, Padgett, Sheridan, & Leblebicioglu, 2009; Caso, Moro, Lorenzo, Lizasoain, & Leza, 2007; Nguyen et al., 1998; Porterfield, Gabella, Simmons, & Johnson, 2012; You et al., 2011), and in humans, there is evidence that IL-1β increases acutely in response to stress challenge, including cognitive, social, and sleep-deprivation paradigms (Brydon et al., 2005; Mastrolonardo, Alicino, Zefferino, Pasquini, & Picardi, 2007; Steptoe, Hamer, & Chida, 2007; Yamakawa et al., 2009).
None of the prior studies on this topic focused on the pre-school period, so it is not known whether stress-induced inflammation might begin during this early developmental stage. Blood sampling is very difficult with young children, and recent research has begun to examine inflammatory markers in saliva. Salivary cytokines are produced locally in the oral mucosa, however some studies have documented correlations of IL-1β and CRP in saliva and peripheral blood (Byrne et al., 2013; Megson, Fitzsimmons, Dharmapatni, & Bartold, 2010; Ouellet-Morin, Danese, Williams, & Arseneault, 2011; Riis et al., 2013). One study found that salivary IL-1β increased in response to psychosocial stress challenge (Mastrolonardo et al., 2007).
In the present study, we examined the hypothesis that salivary IL-1β and CRP would be elevated in association with stress exposure in a sample of pre-school-aged children.
Methods
Participants
Forty families who were enrolled in a larger study of child maltreatment and other adversities participated in this study. All families consented to examination of child welfare records to determine maltreatment status. Families with a maltreated child who remained at home with the caregiver (n=18) were identified from the local child welfare agency and an emergency maltreatment assessment service via record review as described below. Families with no documented episodes of maltreatment (n=22) were recruited at a low-income pediatric medical clinic during a well-child visit and at childcare centers.
Children ranged in age from 3 to 5 years (M = 50 months; SD = 9.6 months), were racially and ethnically diverse (15 White non-Hispanic, 11 Hispanic, 6 Black, 8 other races), and 22 were male. Most caregivers (n=36) were biological mothers. Nine caregivers had less than a high school degree, 15 completed high school, 11 some post-secondary education, and 5 had a bachelor’s degree. Twenty-one caregivers were unemployed and 38 of the families qualified for public assistance. Based on review of available medical records and parent report, children with chronic illness, medication use, obesity, and failure-to-thrive were excluded. Those with acute illness or medication use were included no less than 2 weeks following resolution of illness and medication use.
Child maltreatment status
Trained research staff coded child welfare records using the System for Coding Subtype and Severity of Maltreatment in Child Protective Records (Barnett et al., 1993). Five maltreatment subtypes and severity scores ranging from 1 (least severe) to 5 (most severe) were derived, and children with a case of moderate to severe levels of maltreatment (score of 3–5) within the prior 6 months were eligible for participation. Four children had substantiated cases of physical abuse, 4 sexual abuse, 4 physical neglect/failure to provide, 4 physical neglect/lack of supervision, and 10 emotional maltreatment.
Procedure
Families completed a series of two home visits and a battery of questionnaires in between visits. During the first visit, caregivers completed interviews on child stress exposure and symptoms of child psychopathology. The second home visit occurred in the afternoon. After a 15-minute period of free play, a baseline pre-challenge saliva sample was collected with a Salimetrics Children’s Swab (State College, PA). Children then participated in four emotion-eliciting tasks from the Lab-TAB Laboratory Temperament Assessment Battery (Lab-TAB) including two one-minute fear episodes (stranger approach and scary mask), a two-minute frustration episode (attractive toy in a transparent box) and a one-minute exuberance episode (pop-up snakes). After another period of free play, a post-challenge saliva sample was collected at the end of the visit, approximately 30 minutes after completion of the Lab-TAB.
Measures
Socioeconomic adversity
Three indicators of low socioeconomic status were obtained via questionnaire (parental education < high school degree, parental unemployment, and single parenthood) and summed to create a socioeconomic adversity variable.
Contextual stress interview
Caregivers completed a semi-structured interview developed in our laboratory to assess the child’s experience of contextual stressors in the past month and in the child’s lifetime. Categories were: death of a caregiver, separation from a caregiver, frequent change of residence or homelessness, inadequate food or clothing, and other events including witnessing neighborhood violence or parental arrest. Each domain was scored positive if at least one episode occurred, and domains were summed for past month and lifetime.
Traumatic life events and child symptoms
The Diagnostic Infant and Preschool Assessment (DIPA; Scheeringa, & Haslett, 2010) interview was conducted with caregivers to assess child experiences of traumatic life events and symptoms of PTSD and MDD. Interviews were conducted by trained clinical social workers and a PhD level psychologist, reviewed in a group supervision format, and scored based upon group consensus. Traumatic events in each domain were dichotomized (no trauma versus ≥ 1 trauma), then summed to create a scale for number of types of traumas experienced in the child’s lifetime. Physical and sexual abuse were not included because they were assessed as maltreatment (above). Possible scores ranged from 0 to 8.
Symptoms of PTSD and MDD experienced within the past month were summed. None of the children met DSM-IV criteria for PTSD or Major Depression, and only two children met Research Diagnostic Criteria for PTSD, thus diagnostic status was not considered.
Parenting stress
Caregivers completed the 36-item Parenting Stress Index Short Form (Abidin, 1995), and the total score was used to assess stress associated with parenting.
Inflammatory markers
The baseline saliva sample was assayed for IL-1β and CRP. IL-1β was also assayed in the post-challenge saliva sample (given evidence that it may increase in response to stress). Saliva samples were assayed in duplicate at the Salimetrics Laboratory (State College, PA) using high sensitivity enzyme immunoassays. For IL-1β the intra-assay coefficient of variation was <3% and the inter-assay coefficient of variation was <5%. For CRP, the intra-assay coefficient of variation was <3.9% and the inter-assay coefficient of variation was <7.5%. Values were log transformed and winsorized prior to data analysis to adjust for skewed distributions and outliers.
Results
Preliminary Analyses
Descriptive statistics are displayed in Table 1. Child age, gender, race and ethnicity were not associated with IL-1β at baseline, IL-1β following the challenge task, or CRP and were considered no further. A repeated measures general linear model (GLM) tested change in IL-1β over the challenge task. There was not a significant effect of time on IL-1β, F(1, 39) = .06, ns).
Table 1.
Descriptive statistics
| Mean | SD | Range | |
|---|---|---|---|
| Baseline IL-1β pg/mL | 93.0 | 68.6 | 16.4 – 336.0 |
| Post Challenge IL-1β pg/mL | 94.6 | 69.6 | 19.3 – 318.4 |
| CRP ng/mL | 3.0 | 1.7 | 1.2 – 6.84 |
| Socioeconomic Adversity | 1.5 | 1.0 | 0.0 – 3.0 |
| Contextual Stressors Past Month | 0.6 | 0.9 | 0.0 – 3.0 |
| Contextual Stressors Lifetime | 1.4 | 1.2 | 0.0 – 4.0 |
| Traumatic Events | 0.8 | 1.1 | 0.0 – 3.0 |
| Parenting Stress | 40.2 | 35.2 | 1.0 – 95.0 |
| PTSD Symptoms | 2.6 | 6.4 | 0.0 – 35.0 |
| MDD Symptoms | 1.0 | 2.8 | 0.0 – 15.0 |
Note: n=40
Associations of Adversity Variables with IL-1β
Associations of each adversity variable with IL-1β were tested with repeated measures GLMs. The number of contextual stressors in the past month (F(1, 38) = 6.07, p = .018) and in the child’s lifetime (F(1, 38) = 4.67, p = .037), and the number of traumas (F(1, 38) = 4.73, p = .036) each exerted a significant between-subjects effect on IL-1β. The socioeconomic adversity variable showed a trend-level main effect (F(1, 38) = 3.29, p = .078). There were no between-subjects effects of child maltreatment status (F(1, 38) = .48, ns) or parenting stress (F(1, 38) = .10, ns). None of the adversity measures showed within-subjects effects.
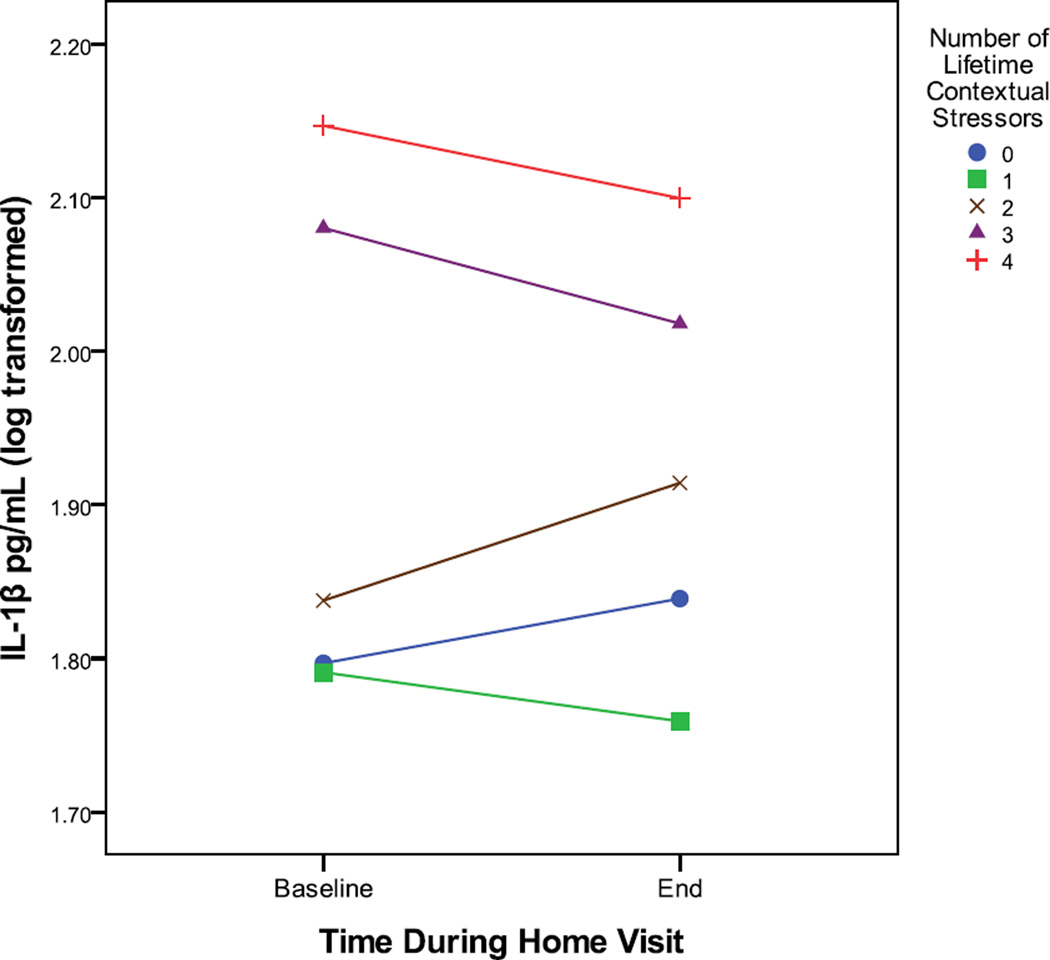
Correlations were used to examine the effects of the significant adversity measures on IL-1β at each time point. As shown in Table 2, baseline IL-1β was positively associated with each of the significant main-effect adversities. Post-challenge IL-1β showed positive correlations with each adversity variable but these did not reach significance. Figure 1 depicts the mean IL-1β values for each time point according to the number of lifetime contextual stressors.
Table 2.
Correlations of continuous adversity variables and IL-1β
| Baseline IL-1β | Post Challenge IL-1β | |
|---|---|---|
| Socioeconomic Adversity | .35* | .15 |
| Contextual Stressors Past Month | .45** | .21 |
| Contextual Stressors Lifetime | .32* | .27t |
| Traumatic Events | .36* | .24 |
Note: n=40.
p < .10.
p < .05.
p < .01
Figure 1.
Post Hoc Analysis of Differences in IL-1β Based Upon Specific Stress Experiences
In exploratory post-hoc analyses, several individual stressors were found to be associated with higher levels of baseline IL-1β at each time-point (Table 3).
Table 3.
Baseline IL-1β (log transformed) and individual adversity variables
| Experienced | Not Experienced |
||
|---|---|---|---|
| t | Mean(SD) | Mean(SD) | |
| Socioeconomic Adversity | |||
| Parental education | 1.64 | 1.9 (0.3) | 1.8 (0.3) |
| Parental unemployment (n=21) | 2.33* | 2.0 (0.3) | 1.7 (0.3) |
| Single parent family (n=15) | 0.52 | 1.9 (0.4) | 1.8 (0.3) |
| Contextual Stressors Past Month | |||
| Caregiver separation (n=10) | 1.66 | 2.0 (0.3) | 1.8 (0.3) |
| Food insecurity (n=3) | 2.01* | 2.2 (0.1) | 1.8 (0.3) |
| Other stressor (n=7) | 3.88** | 2.2 (0.2) | 1.8 (0.3) |
| Contextual Stressors Lifetime | |||
| Death of a caregiver (n=7) | −0.72 | 1.8 (0.3) | 1.9 (0.3) |
| Caregiver separation (n=16) | 2.05* | 2.0 (0.4) | 1.8 (0.3) |
| Housing/homelessness (n=6) | 0.67 | 1.9 (0.3) | 1.8 (0.3) |
| Food insecurity (n=4) | 6.80** | 2.2 (0.0) | 1.8 (0.3) |
| Other stressor (n=15) | 2.57** | 2.0 (0.3) | 1.8 (0.3) |
| Traumatic Life Events | |||
| Car accident (n=4) | 2.06* | 2.2 (0.33) | 1.8 (0.3) |
| Witnessing violence (n=7) | 3.37** | 2.2 (0.21) | 1.8 (0.3) |
| Accidental burning (n=4) | −0.43 | 1.8 (0.28) | 1.9 (0.3) |
| Hospitalization (n=9) | 2.10* | 2.1 (0.36) | 1.8 (0.3) |
| Other trauma (n=8) | −0.67 | 1.8 (0.33) | 1.9 (0.3) |
Note:
p < .05.
p < .01.
Parental education was dichotomized; n=24 had a high school degree or less education.
IL-1β and Child Symptoms
Repeated measures GLM was used to test for effects of child symptoms of PTSD and MDD on IL-1β over time. There were no between- or within-subjects effects.
CRP, Contextual Stressors and Child Symptoms
There were no associations of CRP at the single time point with any of the adversity variables or symptom measures.
Discussion
These findings indicate that salivary IL-1β is increased in association with adverse experiences in early childhood. To our knowledge, this is the first study to show an association of this important pro-inflammatory cytokine with stress exposure in children, and the first to examine any inflammatory markers in relation to stress exposure in children of preschool-age. As discussed above, evidence from animal and human studies supports a role of inflammatory cytokines in the development of MDD and PTSD as well as the broad-ranging health effects of stress-induced inflammation. Exposure to stress increases IL-1β in several brain regions including the hippocampus, and administration of IL-1β in the brain activates the HPA axis, reduces hippocampal BDNF, and impairs hippocampal-dependent learning, suggesting important effects on neuroplasticity (Kohman & Rhodes, 2013; Koo & Duman, 2008). Consistent with the hypothesis that IL-1β and other inflammatory proteins are involved in the neuropathology of psychiatric conditions, a study of adolescent suicides found increased activity of IL-1β, IL-6, and TNF-α in the prefrontal cortex (Pandey et al., 2012).
To our knowledge, this is the first study to examine childhood adversity in relation to salivary cytokine levels. Salivary cytokines are produced by oral mucosal cells and may not be indicative of systemic inflammation. However, some evidence indicates that there are at least modest correlations of some inflammatory proteins in saliva and peripheral blood in adolescents (Byrne et al., 2013; Riis et al., 2013), including IL-1 β (Riis et al., 2013) and in adults (Ouellet-Morin et al., 2011; Out, Hall, Granger, Page, & Woods, 2012; Williamson, Munro, Pickler, Grap, & Elswick, 2012). That IL-1β increased acutely in response to a psychosocial stress challenge (Mastrolonardo et al., 2007) indicates that this cytokine can be responsive to central nervous system activation and does not simply reflect the oral mucosal response to local infection or injury. Our findings of an association with childhood adversity provide further support for a brain-saliva connection. Unfortunately we did not have a measure of oral hygiene, so it is possible that the association with stress exposure is due to an effect of stress on oral hygiene and associated dental caries. However, that exposure to stressors was linked to salivary IL-1β in a sample in which nearly all families qualified for public assistance suggests that the findings are unlikely to be explained by an effect of poverty overall on oral hygiene. Tooth eruption has also been linked to elevations of salivary cytokines, however, most children have a full set of primary teeth by age 3 (American Dental Association, 2005) and permanent teeth do not begin to erupt until age 6–7 (American Dental Association, 2006), so this is unlikely to explain our findings.
Given the association of IL-1β with other adversities, it is somewhat surprising that we did not find an effect of documented childhood maltreatment in this preliminary study. In this sample, non-maltreated children were exposed to significant economic and other adversity, so effects of maltreatment may have been obscured by these other influences. In addition, the maltreated children included in this study remained in the home with their primary caregiver. This occurs when it is determined by the local child welfare agency that the environment can be made safe for the child, either by preventing further contact with the perpetrator, or by providing services to a caregiver who is considered to be able to maintain a safe and nurturing environment. Thus, the most severe and chronic forms of maltreatment would not be included in this study. In addition, the maltreated children were exposed to different types of abuse and neglect, and the nature of these experiences may be determinants of the biological sequelae (Cicchetti, 2013). Due to the small numbers of children with each maltreatment type, we were not able to assess this in this preliminary study. In addition, because the sample as a whole was impoverished and exposed to a variety of contextual stressors, it is also possible that undocumented cases of maltreatment, such as neglect, may have occurred in the non-maltreated group.
It is of note that while stress exposure was linked to IL-1β, neither IL-1β nor CRP was linked to psychiatric symptoms in this modest-sized sample. It is possible that IL-1β will be predictive of the future development of mood or anxiety symptoms. Indeed, a study by Miller and Cole (2012) revealed that among subjects with childhood adversity, but not those without adversity, high IL-6 forecasted depression 6 months later. We also did not see an increase in salivary IL-1β over our sampling period. In healthy adults, Mastrolonardo et al. (2007) did observe increases in salivary IL-1β from baseline to 10 minutes following a psychosocial stress test. In the present study, it is possible that the second saliva sample was not taken early enough to detect a rise in this cytokine or that the Lab-TAB vignettes were not sufficiently challenging to arouse a cytokine stress response.
Salivary CRP was not associated with adversity in our sample of preschool-aged children. This is in contrast to findings of elevated plasma or serum CRP in recent large studies of older children and adults with a history of childhood adversity or maltreatment, although findings for CRP in children are mixed with some showing no association as reviewed above. The effect in positive studies tends to be modest in size, so that larger sample sizes may be required. In addition, some evidence indicates that elevated CRP in association with early stress may be highest, or may only be evident, in those who also have depression (Danese et al., 2011; Danese et al., 2008; Miller & Cole, 2012) or behavioral or affect dysregulation (Appleton et al., 2012). The association of CRP with childhood adversity has been reported exclusively in plasma or serum, so it is possible that salivary CRP is not elevated in association with early stress, however, recent work suggests at least modest correlations between blood and saliva measures of CRP (Byrne et al., 2013; Ouellet-Morin et al., 2011). Alternatively, given that findings on the effects of adversity on CRP measured in childhood CRP have been variable, it is possible that consistent basal elevations of CRP do not occur until later in childhood.
Limitations of our study include the modest sample size and the lack of a contemporaneous blood sample to validate our findings with systemic measures of inflammation. In addition, both poverty and early adversity have been linked to obesity and adipocytes are one source of peripheral cytokines. Associations of BMI with childhood adversity account for at least some of the effects for CRP and TNF-α in peripheral blood (Dixon et al., 2009; Matthews et al., 2013; Slopen et al., 2013b). It is not clear whether obesity influences inflammatory proteins in saliva, however. We excluded obesity and failure-to-thrive when identified in the records or by caregivers during screening. BMI, available from chart review for 24 children, was not associated with salivary IL-1β or CRP in this study (data not shown), so this is unlikely to account for our findings. However, future work with larger samples is needed to determine whether obesity or adiposity influences saliva cytokines or CRP.
Strengths of this study include the careful measurement of adverse experiences including documented evidence of maltreatment from child welfare records, in-home assessments and a standardized challenge protocol, and the use of an at-risk, impoverished sample. In addition, we examined IL-1β over time during a stress challenge, but did not find stress-induced changes. Finally, we carefully excluded children with chronic illness and medication in order to avoid these confounds, and children with acute illness or medications were studied after resolution of the condition.
It important to note that our findings occurred in the context of poverty, and that among other stressors, unemployment and food insecurity were linked to increased levels of IL-1β. However, poor health outcomes among impoverished children are neither universal nor impervious to change. The majority of children developing in poverty do not have identifiable health problems during childhood or early adulthood. In addition to differential exposure to stressors, protective processes play a role. For example, Brody and colleagues (2013) found that among individuals developing in poverty during childhood, those with the least supportive environments have the highest allostatic load (as indexed by blood pressure, catecholamine levels, and body mass index) in late adolescence. Chen and colleagues (2011) found that among adults with a history of low childhood SES, those who reported high maternal warmth during childhood had lower inflammatory responses to immune challenge suggesting that supportive family environments may buffer these negative effects of environment.
Finally, interventions may produce change. For example, there is evidence that providing opportunities for individuals living in poverty to move to better neighborhoods reduces the risk of obesity (Ludwig et al., 2011). Although we are not aware of similar data regarding change in inflammatory markers, given the effects of stress on inflammation, improvements in social environments are likely to be beneficial. Medications that target inflammation, including nonsteroidal anti-inflammatory drugs (NSAIDS), TNF-alpha antagonists, and antibiotics are being examined as possible treatments for mood disorders (Tyrka et al., 2013). Emerging data indicate that exercise, weight loss, yoga, and meditation may have anti-inflammatory effects (e.g., Rosenkranz et al., 2013; Bhasin et al., 2013); these approaches may be particularly appropriate components of an intervention for children exposed to adversity.
Acknowledgments
This research was supported by grant R01 MH083704 awarded to the first author from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIMH. We thank the many research assistants who contributed to this project including Rebecca Berger, Ashley Clement, and Brittney Josefson. We also thank Hasbro Children’s Hospital, Rhode Island Head Start, and the Rhode Island Department of Children, Youth, and Families for assisting in recruitment of study participants.
References
- Abidin RR. Parenting Stress Index: Professional Manual. Odessa, Florida: Psychological Assessment Resources, Inc.; 1995. [Google Scholar]
- American Dental Association Division of Communications, Journal of the American Dental Association, & ADA Council on Scientific Affairs. Tooth eruption: The permanent teeth. The Journal of the American Dental Association. 2006;137(1):127. doi: 10.14219/jada.archive.2006.0031. [DOI] [PubMed] [Google Scholar]
- American Dental Association Division of Communications, Journal of the American Dental Association, & ADA Council on Scientific Affairs. Tooth eruption: The primary teeth. The Journal of the American Dental Association. 2005;136(11):1619. doi: 10.14219/jada.archive.2005.0095. [DOI] [PubMed] [Google Scholar]
- Appleton AA, Buka SL, McCormick MC, Koenen KC, Loucks EB, Kubzansky LD. The association between childhood emotional functioning and adulthood inflammation is modified by early-life socioeconomic status. Health Psychology. 2012;31(4):413–422. doi: 10.1037/a0027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard K, Egeland B, van Dulmen MH, Sroufe LA. When more is not better: The role of cumulative risk in child behavior outcomes. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2005;46(3):235–245. doi: 10.1111/j.1469-7610.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Araujo DM, Cotman CW. Differential effects of interleukin-1 beta and interleukin-2 on glia and hippocampal neurons in culture. International Journal of Developmental Neuroscience. 1995;13(3-4):201–212. doi: 10.1016/0736-5748(94)00072-b. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Kinsey SG, Padgett DA, Sheridan JF, Leblebicioglu B. Social stress enhances IL-1beta and TNF-alpha production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiology and Behavior. 2009;98(3):351–358. doi: 10.1016/j.physbeh.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Nievergelt CM, O’Connor DT. Biomarkers of PTSD: Neuropeptides and immune signaling. Neuropharmacology. 2012;62(2):663–673. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, et al. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells and Development. 2006;15(3):407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development and social policy. Norwood, NJ: Ablex; 1993. pp. 7–73. [Google Scholar]
- Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26(9):2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. American Journal of Preventive Medicine. 2012;43(6):611–620. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin MK, Dusek JA, Chang BH, Joseph MG, Denninger JW, Fricchione GL, Benson H, Libermann TA. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLOS ONE. 2013;8:1–13. doi: 10.1371/journal.pone.0062817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen YF, Kogan SM, Evans GW, Windle M, et al. Supportive family environments, genes that confer sensitivity, and allostatic load among rural African American emerging adults: A prospective analysis. Journal of Family Psychology. 2013;27(1):22–29. doi: 10.1037/a0027829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U. Doing your own thing--our undoing. Child Psychiatry and Human Development. 1977;8(1):3–10. doi: 10.1007/BF01463472. [DOI] [PubMed] [Google Scholar]
- Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, et al. Adverse childhood experiences and the risk of premature mortality. American Journal of Preventive Medicine. 2009;37(5):389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Brown GR, Anderson B. Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. American Journal of Psychiatry. 1991;148(1):55–61. doi: 10.1176/ajp.148.1.55. [DOI] [PubMed] [Google Scholar]
- Broyles ST, Staiano AE, Drazba KT, Gupta AK, Sothern M, Katzmarzyk PT. Elevated C-reactive protein in children from risky neighborhoods: Evidence for a stress pathway linking neighborhoods and inflammation in children. PLOS ONE. 2012;7(9):e45419. doi: 10.1371/journal.pone.0045419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Jia H, Mohamed-Ali V, Zachary I, Martin JF, et al. Psychological stress activates interleukin-1beta gene expression in human mononuclear cells. Brain, Behavior, and Immunity. 2005;19(6):540–546. doi: 10.1016/j.bbi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Bryer JB, Nelson BA, Miller JB, Krol PA. Childhood sexual and physical abuse as factors in adult psychiatric illness. American Journal of Psychiatry. 1987;144(11):1426–1430. doi: 10.1176/ajp.144.11.1426. [DOI] [PubMed] [Google Scholar]
- Burns BJ, Phillips SD, Wagner HR, Barth RP, Kolko DJ, Campbell Y, et al. Mental health need and access to mental health services by youths involved with child welfare: A national survey. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(8):960–970. doi: 10.1097/01.chi.0000127590.95585.65. [DOI] [PubMed] [Google Scholar]
- Byrne ML, O’Brien-Simpson NM, Reynolds EC, Walsh KA, Laughton K, Waloszek, et al. Acute phase protein and cytokine levels in serum and saliva: A comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain, Behavior, and Immunity. 2013;34:164–175. doi: 10.1016/j.bbi.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. Journal of Neuroscience Research. 2005;80(6):789–797. doi: 10.1002/jnr.20531. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Price LH. C-reactive protein, early life stress, and wellbeing in healthy adults. Acta Psychiatrica Scandinavica. 2012;126(6):402–410. doi: 10.1111/j.1600-0447.2012.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso JR, Moro MA, Lorenzo P, Lizasoain I, Leza JC. Involvement of IL-1beta in acute stress-induced worsening of cerebral ischaemia in rats. European Neuropsychopharmacology. 2007;17(9):600–607. doi: 10.1016/j.euroneuro.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. Annual research review: Resilient functioning in maltreated children--past, present, and future perspectives. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2013;54(4):402–422. doi: 10.1111/j.1469-7610.2012.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Toward an ecological/transactional model of community violence and child maltreatment: Consequences for children’s development. Psychiatry. 1993;56(1):96–118. doi: 10.1080/00332747.1993.11024624. [DOI] [PubMed] [Google Scholar]
- Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: A systematic review. Acta Psychiatrica Scandinavica. 2013 doi: 10.1111/acps.12217. [DOI] [PubMed] [Google Scholar]
- Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, et al. C-reactive protein concentration in children: Relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149(1):139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, et al. Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry. 2011;16(3):244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry. 2008;65(4):409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunology and Allergy Clinics of North America. 2009;29(2):247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, Meng H, Goldberg R, Schneiderman N, Delamater A. Stress and body mass index each contributes independently to tumor necrosis factor-alpha production in prepubescent Latino children. Journal of Pediatric Nursing. 2009;24(5):378–388. doi: 10.1016/j.pedn.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in U.S. children aged 3-16 years. American Journal of Preventive Medicine. 2010;39(4):314–320. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Thorpe RJ, Jr, McGinty EE, Bower K, Rohde C, Young JH, et al. Disparities in diabetes: The nexus of race, poverty, and place. American Journal of Public Health. 2013 doi: 10.2105/AJPH.2013.301420. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Ferrie JE, Elovainio M, Pulkki-Raback L, Keltikangas-Jarvinen L, Eklund C, et al. When do social inequalities in C-reactive protein start? A life course perspective from conception to adulthood in the Cardiovascular Risk in Young Finns Study. International Journal of Epidemiology. 2008;37(2):290–298. doi: 10.1093/ije/dym244. [DOI] [PubMed] [Google Scholar]
- Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Moran-Santa Maria MM, Twal WO, Shaftman S, DeSantis SM, McRae-Clark AL, et al. Association of elevated cytokines with childhood adversity in a sample of healthy adults. Journal of Psychiatric Research. 2013;47(5):604–610. doi: 10.1016/j.jpsychires.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberth G, Weber A, Roder S, Elvers HD, Kramer U, Schins RP, et al. Relation between stressful life events, neuropeptides and cytokines: Results from the LISA birth cohort study. Pediatric Allergy and Immunology. 2008;19(8):722–729. doi: 10.1111/j.1399-3038.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: Exploring the causes of heterogeneity. Brain, Behavior, and Immunity. 2012;26(7):1180–1188. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Howe LD, Galobardes B, Sattar N, Hingorani AD, Deanfield J, Ness AR, et al. Are there socioeconomic inequalities in cardiovascular risk factors in childhood, and are they mediated by adiposity? Findings from a prospective cohort study. International Journal of Obesity. 2010;34(7):1149–1159. doi: 10.1038/ijo.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS. Neurogenesis, inflammation and behavior. Brain, Behavior, and Immunity. 2013;27(1):22–32. doi: 10.1016/j.bbi.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinis-Mitchell D, McQuaid EL, Seifer R, Kopel SJ, Esteban C, Canino G, et al. Multiple urban and asthma-related risks and their association with asthma morbidity in children. Journal of Pediatric Psychology. 2007;32(5):582–595. doi: 10.1093/jpepsy/jsl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proceedings of the National Academy of Sciences. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, McLean J, Batty GD, Burns H, Deans KA, Ford I, et al. Socioeconomic deprivation and cortical morphology: Psychological, social, and biological determinants of ill health study. Psychosomatic Medicine. 2013;75(7):616–623. doi: 10.1097/PSY.0b013e3182a151a7. [DOI] [PubMed] [Google Scholar]
- Lacy P, Stow JL. Cytokine release from innate immune cells: Association with diverse membrane trafficking pathways. Blood. 2011;118(1):9–18. doi: 10.1182/blood-2010-08-265892. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. Journal of Affective Disorders. 2012;139(3):230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, et al. Neighborhoods, obesity, and diabetes--a randomized social experiment. New England Journal of Medicine. 2011;365(16):1509–1519. doi: 10.1056/NEJMsa1103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin TJ, Martin TM, Blackwell E, Stetler C, Miller GE. Differentiating the impact of episodic and chronic stressors on hypothalamic-pituitary-adrenocortical axis regulation in young women. Health Psychology. 2007;26(4):447–455. doi: 10.1037/0278-6133.26.4.447. [DOI] [PubMed] [Google Scholar]
- Mastrolonardo M, Alicino D, Zefferino R, Pasquini P, Picardi A. Effect of psychological stress on salivary interleukin-1beta in psoriasis. Archives of Medical Research. 2007;38(2):206–211. doi: 10.1016/j.arcmed.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Chang YF, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: Role of obesity. Brain, Behavior, and Immunity. 2013 doi: 10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Leonard WR, Burhop J, Reyes-Garcia V, Vadez V, Huanca T, et al. Predictors of C-reactive protein in Tsimane’ 2 to 15 year-olds in lowland Bolivia. American Journal of Physical Anthropology. 2005;128(4):906–913. doi: 10.1002/ajpa.20222. [DOI] [PubMed] [Google Scholar]
- Megson E, Fitzsimmons T, Dharmapatni K, Bartold PM. C-reactive protein in gingival crevicular fluid may be indicative of systemic inflammation. Journal of Clinical Periodontology. 2010;37(9):797–804. doi: 10.1111/j.1600-051X.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21(6):848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. 2012;72(1):34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT. Research review: The role of cytokines in depression in adolescents: A systematic review. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2013;54(8):816–835. doi: 10.1111/jcpp.12080. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Murasko JE. Male-female differences in the association between socioeconomic status and atherosclerotic risk in adolescents. Social Science and Medicine. 2008;67(11):1889–1897. doi: 10.1016/j.socscimed.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, et al. Exposure to acute stress induces brain interleukin-1beta protein in the rat. Journal of Neuroscience. 1998;18(6):2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikas JB. Inflammation and immune system activation in aging: A mathematical approach. Scientific Reports. 2013;3:3254. doi: 10.1038/srep03254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, et al. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Disease Markers. 2011;30(2-3):123–132. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Danese A, Williams B, Arseneault L. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain, Behavior, and Immunity. 2011;25(4):640–646. doi: 10.1016/j.bbi.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Out D, Hall RJ, Granger DA, Page GG, Woods SJ. Assessing salivary C-reactive protein: Longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain, Behavior, and Immunity. 2012;26(4):543–551. doi: 10.1016/j.bbi.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain, Behavior, and Immunity. 2012;26(1):13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, et al. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. Journal of Psychiatric Research. 2012;46(1):57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Batty GD, van Zanten JJ, Mortensen LH, Deary IJ, Calvin CM, et al. Cognitive ability in early adulthood is associated with systemic inflammation in middle age: the Vietnam experience study. Brain, Behavior, and Immunity. 2011;25(2):298–301. doi: 10.1016/j.bbi.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European Journal of Epidemiology. 2007;22(1):55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Porterfield VM, Gabella KM, Simmons MA, Johnson JD. Repeated stressor exposure regionally enhances beta-adrenergic receptor-mediated brain IL-1beta production. Brain, Behavior, and Immunity. 2012;26(8):1249–1255. doi: 10.1016/j.bbi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: An overview. Biological Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Out D, Dorn LD, Beal SJ, Denson LA, Pabst S, et al. Salivary cytokines in healthy adolescent girls: Intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Developmental Psychobiology. 2013 doi: 10.1002/dev.21149. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Glaser R, Kiecolt-Glaser JK. Out of balance: A new look at chronic stress, depression, and immunity. Current Directions in Psychological Science. 2005;14(2):111–115. [Google Scholar]
- Rooks C, Veledar E, Goldberg J, Bremner JD, Vaccarino V. Early trauma and inflammation: Role of familial factors in a study of twins. Psychosomatic Medicine. 2012;74(2):146–152. doi: 10.1097/PSY.0b013e318240a7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behavior and Immunity. 27:174–184. doi: 10.1016/j.bbi.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameroff AJ, Seifer R, Baldwin A, Baldwin C. Stability of intelligence from preschool to adolescence: The influence of social and family risk factors. Child Development. 1993;64(1):80–97. doi: 10.1111/j.1467-8624.1993.tb02896.x. [DOI] [PubMed] [Google Scholar]
- Scheeringa MS, Haslett N. The reliability and criterion validity of the Diagnostic Infant and Preschool Assessment: A new diagnostic instrument for young children. Child Psychiatry and Human Development. 2010;41(3):299–312. doi: 10.1007/s10578-009-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS. Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, & Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, Koenen KC. Internalizing and externalizing behaviors predict elevated inflammatory markers in childhood. Psychoneuroendocrinology. 2013a;38(12):2854–2862. doi: 10.1016/j.psyneuen.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology. 2013b;38(2):188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Teunissen CE, van Boxtel MP, Bosma H, Bosmans E, Delanghe J, De Bruijn C, et al. Inflammation markers in relation to cognition in a healthy aging population. Journal of Neuroimmunology. 2003;134(1-2):142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- Thomas NE, Cooper SM, Williams SR, Baker JS, Davies B. Fibrinogen, homocyst(e)ine, and C-reactive protein concentrations relative to sex and socioeconomic status in British young people. American Journal of Human Biology. 2005;17(6):809–813. doi: 10.1002/ajhb.20447. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatrica Scandinavica. 2013;128:434–437. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, Forns X, et al. Interferon-induced depression in chronic hepatitis C: A systematic review and meta-analysis. Journal of Clinical Psychiatry. 2012;73(8):1128–1138. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. Journal of Affective Disorders. 2013;150(3):736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Williamson S, Munro C, Pickler R, Grap MJ, Elswick RK., Jr Comparison of biomarkers in blood and saliva in healthy adults. Nursing Research and Practice. 2012;2012:246178. doi: 10.1155/2012/246178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CB, Sacco RL, Rundek T, Delman J, Rabbani L, Elkind M. Interleukin-6 is associated with cognitive function: The Northern Manhattan Study. Journal of Stroke and Cerebrovascular Diseases. 2006;15(1):34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Yamakawa K, Matsunaga M, Isowa T, Kimura K, Kasugai K, Yoneda M, et al. Transient responses of inflammatory cytokines in acute stress. Biological Psychology. 2009;82(1):25–32. doi: 10.1016/j.biopsycho.2009.05.001. [DOI] [PubMed] [Google Scholar]
- You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, et al. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: Involvement in depression. Behavioural Brain Research. 2011;225(1):135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, et al. Interleukin-1beta: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]