Abstract
Mutations in the calreticulin gene (CALR) were recently identified in approximately 70–80% of patients with JAK2-V617F-negative essential thrombocytosis and primary myelofibrosis. All frameshift mutations generate a recurring novel C-terminus. Here we provide evidence that mutant calreticulin does not accumulate efficiently in cells and is abnormally enriched in the nucleus and extracellular space compared to wildtype calreticulin. The main determinant of these findings is the loss of the calcium-binding and KDEL domains. Expression of type I mutant CALR in Ba/F3 cells confers minimal IL-3-independent growth. Interestingly, expression of type I and type II mutant CALR in a non-hematopoietic cell line does not directly activate JAK/STAT signaling compared to JAK2-V617F expression. These results led us to investigate paracrine mechanisms of JAK/STAT activation. Here we show that conditioned media from cells expressing type I mutant CALR exaggerate cytokine production from normal monocytes with or without treatment with a toll-like receptor agonist. These effects are not dependent on the novel C-terminus. These studies offer novel insights into the mechanism of JAK/STAT activation in patients with JAK2-V617F-negative essential thrombocytosis and primary myelofibrosis.
Keywords: calreticulin, essential thrombocytosis, primary myelofibrosis, JAK/STAT signaling, cytokines
Introduction
Recently, mutations in CALR have been identified in approximately 70–80% of patients with JAK2-V617F-negative ET and PMF.1,2 The majority are frameshift mutations of two types – type I (52bp deletion; c.1092_1143del) and type II (5bp insertion; c.1154_1155insTTGTC)1,2 – creating the same open reading frame encoding a novel C-terminus with variability in the number of acidic residues retained. The fact that the same JAK/STAT-activated signatures exist in JAK2V617-mutated versus JAK2-V617F-wildtype patient samples3 suggest that CALR has an important role in regulating cytokine production and chronic inflammation in the hematopoietic system.
Calreticulin is a highly conserved, calcium-binding protein chaperone primarily located in the endoplasmic reticulum (ER). The most established function of calreticulin is to aid the assembly and cell surface expression of MHC class I molecules mainly by enhancing the stability of proteins4,5 and to facilitate retrieval of proteins from the golgi-ER organelles.6 Some of the diverse functions of calreticulin have been attributed to its role in calcium homeostasis and regulation of calcium-dependent proteins or role as a protein chaperone via its lectin domains.7 The frameshift mutations found in ET and PMF patients is predicted to disrupt calreticulin’s role as an ER chaperone, especially at times of ER stress.8 The loss of these important functions of calreticulin may also contribute to ET and PMF disease pathogenesis. As of this report, the biochemical properties of mutant calreticulin and its mechanism of JAK/STAT activation remain largely uncharacterized.
Several publications reported the clinical characteristics of JAK2-V617F versus CALR mutant ET and PMF patients. It is noteworthy that CALR mutant PMF patients have a significantly better median overall survival compared with JAK2-V617F PMF patients, 15.9 years versus 5.9 years, respectively.9 This difference remains unexplained but hints at differential mechanisms of disease pathogenesis. In addition, several groups reported that CALR mutant ET patients have higher platelet counts than JAK2-V617F mutant patients.10–13 One group generated a mutant-specific CALR antibody and noted more intense mutant CALR expression in megakaryocytes compared to other cells in the bone marrow, suggesting that mutant CALR may have a more prominent, lineage-specific effect on megakaryocytes than does JAK2-V617F.14 Diverging clinical features have emerged for type I versus type II mutant CALR patients that could in theory be explained by the structural and functional differences of the exact start site of the frameshift mutation.15
Here we describe our studies investigating the biochemical and functional characteristics of mutant calreticulin. We provide evidence that a paracrine mechanism is at least one mechanism calreticulin induces monocyte hyperreactivity to toll-like receptor agonists leading to overproduction of multiple cytokines known to be elevated in ET and PMF patients.
Methods
Cells and cell culture conditions
293FT and HeLa cells were maintained in 10% FBS in DMEM medium supplemented with nonessential amino acids, L-glutamine, and penicillin-streptomycin. Ba/F3 cells were maintained in 10% FBS and 15% WEHI conditioned media containing IL-3 in RPMI-1640 medium supplemented with L-glutamine and penicillin-streptomycin. Cell viability was assessed by MTS assay (CellTiter 96 AQueous One Solution, Promega). Cells were transfected using Lipofectamine 2000 (Life technologies). All cells were grown in 5% CO2 humidified incubator at 37°C.
Human CD14+ cell cultures
Mononuclear cells were prepared from human blood using Ficoll-Paque PLUS followed by CD14+ selection using magnetic microbeads (StemCell Technologies). CD14+ monocytes were cultured at 50,000 cells/ml for 24-hours in RPMI 1640 medium (Life Technologies) with 10% fetal bovine serum and R848 (Enzo Life Sciences) or lipopolysaccharide (LPS, Sigma-Aldrich). Conditioned culture media generated from HeLa cells transfected with CALR cDNA or vector-control was added to CD14+ cell cultures. Conditioned medium was harvested and TNF-α was quantified by the Quantikine ELISA kit (R&D Systems). We also used the Cytokine Human Magnetic 30-Plex Panel (Life Technologies) on the Luminex platform. Blood from patients and healthy volunteers were collected after informed consent, in accordance with research studies approved by the Institutional Review Board.
DNA constructs and qRT-PCR analysis
Human wildtype CALR cDNA was purchased from GE Healthcare Dharmacon. Mutant CALR cDNAs were generated by In-Fusion cloning (Clontech). cDNAs were verified by Sanger sequencing. We used pCDH1-EF1-eGFP and pCDH1-EF1 vectors, which are modified mammalian lentiviral expression vectors originally from System Biosciences. We also used a p3XFlag-CMV vector. The 3XFlag is an approximately 2.4 kD epitope tag. Primers to amplify exogenous CALR sequences for qRT-PCR analysis include forward: cgattacaaggatgacgatga (includes Flag sequences) and reverse: tccagaaactgctccttgaa.
Antibodies, Western blot analysis, and 2D PAGE analysis
The antibodies used in this study are listed in the Supplemental Table 1. Whole-cell lysates were lysed in buffer containing 0.5% Triton X-100, 120 mM NaCl, 50 mM Tris (pH 8.0), 2 mM EDTA, 1 mM Na2VO4, and 1:300 protease Inhibitor cocktail (P8340; Sigma-Aldrich). Nuclear and cytoplasmic fractions were prepared essentially as described.16 2D PAGE analysis of lysates was performed with Invitrogen’s ZOOM system. Densitometric analysis was performed using ImageJ software (NIH).
Microscopy
Cells were viewed and analyzed on a Zeiss Axio Observer.Z1 microscope using a 63× objective (Carl Zeiss, Jena, Germany). For the z-stack imaging, 12 images with a 63× oil objective at 0.1-µm increments were obtained above and below focused nuclei. ImageJ was used to analyze colocalization and threshold-defined quantitation of signal. For confocal analysis, samples were imaged on a Zeiss LSM 710 laser-scanning confocal microscope using a 63× 1.4 NA PlanApo objective. No stain or no transfection controls were used to evaluate specificity of the staining and to determine the threshold of exposure time to capture images. The IncuCyte machine (Essen BioScience) was used for real-time evaluation of fluorescence for 48–72 hours in live HeLa cells transiently transfected.
Immunohistochemical staining of bone marrow samples
Immunohistochemical calreticulin staining was performed using a standard protocol.17 Reviewers were blinded to sample identifiers and were asked to rank staining intensity from lightest to darkest. Three different slide sets were stained at a different time or by a different person. Slides were scanned with an Aperio ScanScope AT microscope using a 20× objective (Vista) and staining was quantified with Aperio ImageScope software.17 The software quantifies the intensity of staining and percent staining within a cell based on software-defined thresholds and identification of nuclei.
Mutant calreticulin allele frequency determination from blood or bone marrow samples
Exon 9 of CALR was PCR-amplified for size-based fragment analysis by capillary electrophoresis to identify and quantify insertion or deletion mutations, which represent 100% of known pathogenic CALR mutations in myeloproliferative neoplasms.
Results
Structural features of mutant calreticulin impact steady state expression in cells
The novel C-terminus of mutant calreticulin is schematically depicted in Fig. 1a. The last 44 C-terminal amino acids are shared between the two most common mutations, designated as type I and type II.1 The acidic Ca2+ binding C-terminal domain of wildtype calreticulin is largely replaced by basic, hydrophobic, and small nonpolar residues (in blue, green, and orange, respectively). The terminal KDEL signal is eliminated. The type II mutant calreticulin retains more acidic residues (in red) than does the type I mutant calreticulin; this difference may account for some of the phenotypic differences between type I and type II mutant calreticulin. We truncated the entire novel C-terminus terminus (Δ367 and Δ385 mutants) or the hydrophobic-rich tail (MSPARPRTSCREACLQGWTEA, Δ391 and Δ410 mutants) to investigate the contribution of these regions to the function of mutant calreticulin (Fig. 1b). We consistently observed significantly reduced steady state expression of untagged or Flag-tagged versions of type I and type II mutant calreticulin in 293FT and HeLa cells compared to wildtype calreticulin (Fig. 1c). Type I mutant calreticulin is expressed at the lowest level, while the type II mutant calreticulin is expressed at an intermediate level and wildtype calreticulin is expressed at the highest level. These findings were also observed in the studies reported by Klampfl et al.1 Deleting the entire novel C-terminus improved steady state expression substantially, while deleting the hydrophobic tail modestly improved steady state expression (Fig. 1d). We also performed cycloheximide experiments to quantify calreticulin turnover and evaluated the accumulation of calreticulin using real-time, live cell imaging (Supplemental Fig. 1). These studies confirmed that there is increased mutant calreticulin turnover and reduced mutant calreticulin in cells compared to wildtype calreticulin. We quantified CALR mRNA expression in cells expressing wildtype or mutant calreticulin by qRT-PCR and observed that the mutant CALR mRNA levels were similar to (type II) or higher than (type I) wildtype (Fig. 1e). Therefore, the significantly reduced steady state expression of mutant calreticulin in cells is due to post-transcriptional mechanisms involving the loss of the acidic Ca2+ binding C-terminal domain and the gain of a novel C-terminus.
Fig. 1. Structural properties of wildtype and mutant calreticulin.
(a) Protein structure models depicting the overall folding of wildtype calreticulin, type I mutant calreticulin, and type II calreticulin. The C-terminal tails are color coded according to the Lesk color scheme. The single letter code for the amino acids represented is red: DE, blue: KR, green: CVILPFYMW, magenta: NQH, orange: GAST. The number of amino acids (aa), predicted molecular weight in kiloDaltons (kD), and theoretical isolectric point (pI) for wildtype, type I and type II are listed in the table (ExPASY program Compute pI/Mw tool, Swiss Institute of Bioinformatics). (b) Schematic representation of various calreticulin mutants generated in our studies. The KDEL golgi-ER retrieval and ER-retention signal is indicated in black. (c) Western blot of steady state protein expression of Flag (vector-control), Flag-tagged wildtype calreticulin, and various Flag-tagged calreticulin mutants overexpressed in 293FT cells with α-actin as a loading control. This pattern has been observed in 293FT and HeLa cells in more than 5 blots. Quantitation relative to wildtype calreticulin is tabulated, including standard deviation (sd) and P values. (d) Western blot comparing the effects of truncating the hydrophobic region (Δ391 and Δ410) or the novel C-terminus (Δ367 and Δ385) on steady state expression of type I and type II mutant calreticulin, respectively. (e) qRT-PCR analysis of exogenous CALR mRNA levels in HeLa cells. We designed primers that were specific to exogenous CALR mRNA and removed all contaminating DNA by DNase digestion. Data are expressed relative to wildtype CALR as an average relative % from four independent experiments. *P=0.002, **P=0.089, sd for WT is 20.8, for type I is 41.7, and for type II is 40.4.
Total calreticulin expression is reduced in primary bone marrow samples from type I mutant calreticulin MF patients
Next we aim to characterize calreticulin expression in blood and marrow samples from four patients with JAK2V617F-negative myelofibrosis harboring type I mutant CALR. Patient characteristics are summarized in Supplemental Fig. 2a. Calreticulin expression in blood mononuclear cells was evaluated by 2D PAGE. We first optimized the conditions of the 2D PAGE using lysates from 293FT cells overexpressing CALR constructs (Supplemental Fig. 1c). For the 2D PAGE analysis, patients were on ruxolitinib (a JAK1/2 inhibitor, Incyte Corporation) treatment at the time lysates were harvested but the mutant allele burden was not generally reduced (Supplemental Fig. 2b). In the patient samples (Fig. 2a), type I mutant calreticulin was not easily detectable, consistent with our studies in transfected 293FT and HeLa cells. The main form detected was wildtype calreticulin (based on pI shift as shown in the legend), albeit at reduced levels compared to normal controls. We performed immunohistochemical analysis of total calreticulin expression in bone marrow samples obtained before ruxolitinib treatment (Fig. 2b). With one minor exception (control #3 in slide set #3), all three blinded reviewers consistently judged that all normal control bone marrow samples expressed more calreticulin than any of the MF bone marrow samples (Fig. 2b, left table). The slightly greater variability observed among the reviewers for slide set #3 was attributed to suboptimal staining. Quantitative image analysis of set #2 confirmed lower calreticulin expression in MF patients compared to normal controls (Fig. 2b, right table). Aside from minor variations, these results generally coincided with the rank list of all three blinded reviewers. In Fig. 2c, we show an example of randomly selected, in-plane megakaryocytes outlined for quantification. Normal megakaryocytes expressed higher levels of calreticulin compared with megakaryocytes from CALR-mutant MF patients. Interestingly, we noted prominent variability in total calreticulin staining in megakaryocytes from CALR-mutant MF patients; for example, some megakaryocytes showed moderate staining while others exhibited minimal to no staining (P1 and P3 in Fig. 2c). This inter-cell heterogeneity was not appreciated in the normal controls. These results align with our cell line work showing that mutant calreticulin does not accumulate in cells as well as wildtype calreticulin. In the patients’ bone marrow samples, the overall reduced total calreticulin likely reflects the fact that the cells have one wildtype allele and one mutant allele.
Fig. 2. Calreticulin expression in bone marrow samples from type I mutant CALR MF patients.
(a) 2D PAGE analysis of lysates from blood mononuclear cells of a normal control and Type I mutant CALR MF patients (P1–P4). Blots were probed for calreticulin and α-actin. A key is provided. The dashed red circle indicates the location of wildtype calreticulin and the blue dashed circle indicates α-actin. The localization of these spots was confirmed using lysates from 293FT cells expressing or not expressing wildtype calreticulin (Supplemental Fig. 1c). (b) Immunohistochemical calreticulin staining of bone marrow biopsy specimens. Shown are three controls (C1–C3) and four patient samples (P1–P4). The table on the left shows the ranking of immunohistochemical staining intensity from lightest to darkest as performed by three blinded reviewers on three independently stained slide sets (Set #1–3). The table on the right shows Aperio ImageScope quantification of slides from Set #2 showing staining intensity as described in the online methods. The P value indicated in the table compares the controls and patient samples. (c) Aperio ImageScope quantification of calreticulin staining in megakaryocytes. Shown are representative images defining megakaryocytes (circled cells) and a table showing the quantification of staining intensity in normal and type I mutant CALR MF patient samples. The P value indicated in the table compares the controls and patient samples.
Mutant calreticulin is abnormally enriched in the nucleus and in the extracellular space due to loss of the Ca2+-binding and KDEL domains
We performed confocal microscopy to evaluate the subcellular localization of mutant calreticulin. We analyzed the expression of various Flag-tagged calreticulin types in HeLa cells with or without thapsigargin, an inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase that induces ER stress. Type I and type II mutant calreticulin remained largely in the ER and in the cytoplasm, similar to wildtype calreticulin despite the loss of the Ca2+-binding and KDEL domains (Fig. 3a). In some cells, we detected prominent nuclear staining for the type I and type II mutant calreticulin (white arrowheads) but not uniformly in all cells, suggesting that there may be other cellular factors contributing to its nuclear localization. With routine microscopy we observed similar results (Supplemental Fig. 3a). We analyzed colocalization between the Flag antibody signal and the calreticulin antibody signal using ImageJ and observed highly concordant staining (Supplemental Fig. 3b). We also evaluated the subcellular localization of the most intense signal (highest 90%) to stringently evaluate the staining pattern (Supplemental Fig. 3c). Additional confocal images with a side view of cells are provided in Supplemental Fig. 4. Microscopy studies are not optimal to quantify relative expression when cells are stained on different slides and do not provide information on secreted proteins. To quantify these findings using a different approach, we isolated nuclear and cytoplasmic fractions from HeLa cells expressing wildtype or mutant calreticulin and performed western blotting. These results were consistent with the overall findings in the microscopy studies. A greater proportion of type I and type II mutant calreticulin were in the nuclear fractions compared to wildtype calreticulin even though there was a lower total amount of type I and type II expressed at steady state in whole cell extracts (Fig. 3b). All comparisons are evaluated relative to wildtype calreticulin (Fig. 3c). The deletion mutants, Δ367 and Δ385, also displayed enhanced nuclear localization, suggesting that the novel C-terminus domain is not required for this phenotype (Supplemental Fig. 3d). With thapsigargin treatment, there was reduced amount of calreticulin in the nucleus and in the cytoplasm for all conditions (Supplemental Fig. 3d). We quantified the amount of calreticulin in conditioned media by western blotting to determine whether mutant calreticulin is abnormally secreted (Fig. 3d). We observed a truncated protein of approximately 42 kD, which is 7–8 kD smaller than the wildtype Flag-tagged calreticulin. The truncated protein is Flag-tagged and is the same size, regardless of the calreticulin version expressed, indicating that this cleaved product does not include the novel mutant C-terminus. We also deduced that the wildtype C-terminus is not required for proteolytic cleavage. A proteolytic cleavage site at amino acid 340 has been reported.18 Together, these results show that type I and type II mutant calreticulin are abnormally enriched in the nucleus and in the conditioned media compared to wildtype calreticulin. These findings were not dependent on the novel C-terminus and suggest the loss of the Ca2+ binding and KDEL domains are responsible for this phenotype.
Fig. 3. Microscopic analysis and subcellular fractionation of mutant calreticulin.
(a) Confocal microscopy of wildtype, type I and type II mutant calreticulin expression in HeLa cells, with and without 2µM thapsigargin. White arrowheads indicate cells showing intense nuclear staining in cells expressing type I or type II mutant calreticulin. Additional confocal images are provided in Supplemental Fig. 4. (b) Representative western blots from experiments fractionating cytoplasmic and nuclear fractions of HeLa cells transfected with Flag-calreticulin. α-actin and SP1 were used as loading controls. SP1, a predominantly nuclear protein, shows significant enrichment in the nuclear fractions similarly across all experimental conditions tested. (c) Quantification of western blots comparing protein levels of type I and type II mutant calreticulin, always in comparison to wildtype calreticulin, in whole cell lysates, and in cytoplasmic and nuclear extracts. Different amounts of protein were loaded for nuclear versus cytoplasmic fractions but the same amount for each calreticulin type. Fold enrichment in the nucleus is in comparison to whole cell lysate values. Data are the mean ± sd of quantitation values from three western blots per condition (whole cell lysate, cytoplasmic fraction, and nuclear fraction), taken relative to wildtype. *P = 0.06, **P = 0.04, relative to wildtype. (d) Western blot of cell culture media collected 24 (bottom panel) or 48 hours (top and bottom panel) after transfection of HeLa cells with Flag-tagged CALR cDNA constructs. Culture media (DMEM) and media from cells transfected with empty Flag vector were used as controls.
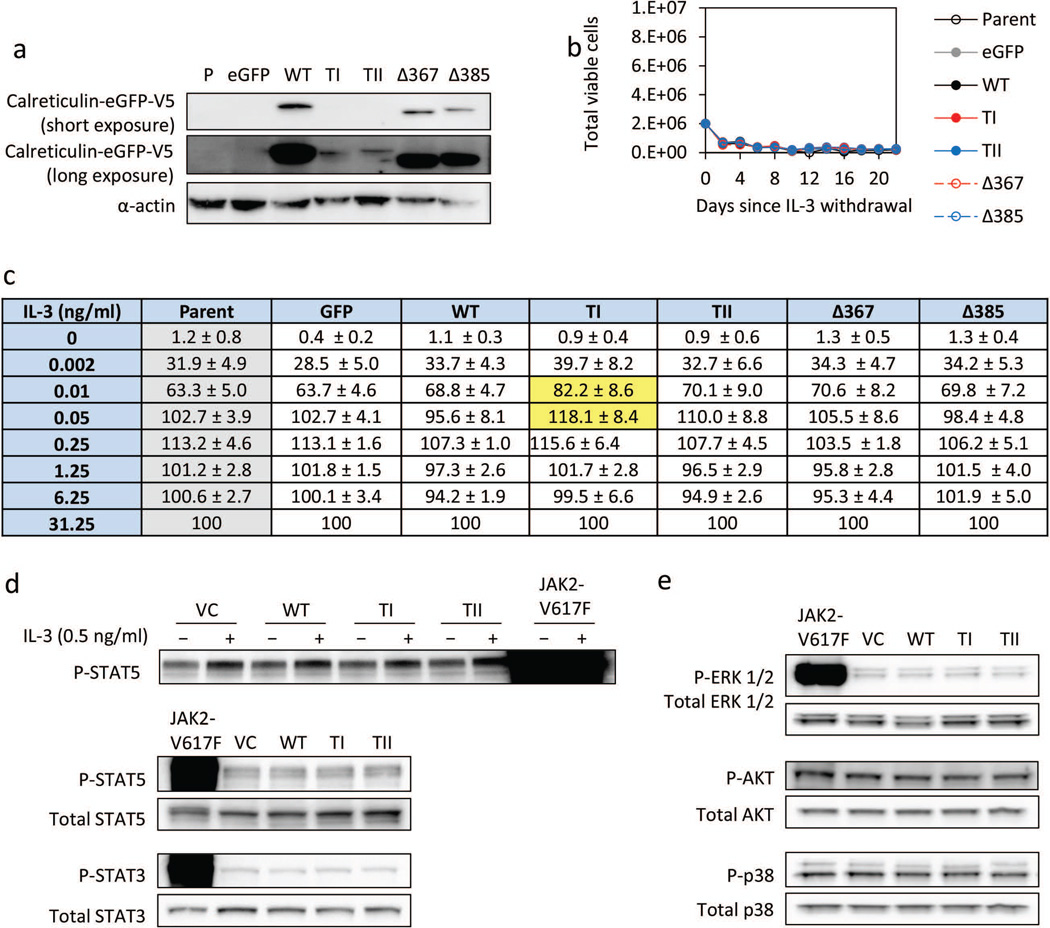
Expression of mutant calreticulin in Ba/F3 cells confers minimal IL-3-independent growth and JAK/STAT activation
We tested whether overexpression of mutant calreticulin confers IL-3-independent growth of Ba/F3 cells. We expressed GFP-tagged versions of wildtype or mutant calreticulin in Ba/F3 cells and established pure cell lines by sorting GFP-positive cells. The expression of calreticulin was confirmed by western blotting (Fig. 4a). There was a similar pattern of steady state level of expression in these Ba/F3 stable cell lines compared with the transient transfection experiments (Fig. 1c and Supplemental Fig. 1). Neither wildtype nor mutant calreticulin versions conferred IL-3 independent growth in our studies (Fig. 4b). These results contrast with the results reported by Klampfl et al., showing that overexpression of type I mutant calreticulin conferred IL-3-independent growth of Ba/F3 cells.1 To quantify more subtle differences in IL-3-independent growth of Ba/F3 cells overexpressing various types of calreticulin, we evaluated the growth of Ba/F3 cells in growth media supplemented with various doses of IL-3. Using this experimental approach, we consistently detected a slight but statistically significant growth advantage of cells expressing type I mutant calreticulin at low doses of IL-3, but did not observe a growth advantage of cells expressing the other types of calreticulin or with higher concentrations of IL-3 (Fig. 4c). In addition, we analyzed STAT5 activation in 293FT cells by western blotting and did not observe any major differences among the cells overexpressing various types of calreticulin, with or without IL-3 supplementation (Fig. 4d). JAK2-V617F overexpression in these experiments serves as a positive control and allows one to compare the relative degree of JAK/STAT activation by mutant calreticulin overexpression. We also examined activated STAT3 (Fig. 4d), ERK1/2, AKT, and p38 and did not observe any differences comparing wildtype versus mutant calreticulin (Fig. 4e). Together, these results suggest that mutant calreticulin overexpression confers minimal direct or cell-intrinsic JAK/STAT activation in a non-hematopoietic cell line.
Fig. 4. Evaluating IL-3-independent growth of Ba/F3 cells expressing mutant calreticulin.
(a) Western blot of calreticulin tagged with eGFP-V5 at the C-terminus in Ba/F3 cells with α-actin as a loading control. Two different exposures are included to show low levels of type I and type II expression. (b) Ba/F3 cell count following IL-3 withdrawal. The initial cell count was 2×106 per condition. Cultures were maintained for 3 weeks. Experiment was repeated three times. Shown is a representative experiment. (c) Relative % of total viable Ba/F3 cells expressing various CALR types three days after thorough IL-3 washout followed by addition of IL-3 at various concentrations. Each condition is normalized to its own 31.25 ng/ml IL-3 concentration using the MTS assay to quantify viable cells. Data are expressed as mean ± sd, averaging four experiments. Comparisons with P < 0.1, relative to parent Ba/F3 cell counts (gray column), are highlighted in yellow. P = 0.03 (0.01 ng/ml) and 0.08 (0.05 ng/ml). (d) Western blot showing phospho-STAT3 and phospho-STAT5 levels in 293FT cells overexpressing Flag-tagged CALR. Top panel shows cells with and without IL-3 treatment. Total STAT3 and total STAT5 were used as loading controls. (e) Western blot of 293FT cells overexpressing Flag-tagged CALR showing phospho- and total levels of ERK1/2, AKT, and p38.
Conditioned media from type I mutant calreticulin-expressing cells exaggerate cytokine production from monocytes
Since we observed low intracellular accumulation of mutant calreticulin and minimal IL-3-independent growth of Ba/F3 cells, we explored paracrine effects of cells expressing mutant calreticulin. We collected conditioned media from HeLa cells expressing Flag vector-control or various Flag-tagged calreticulin types and cultured normal CD14+ monocytes with or without R848, a toll-like receptor 7/8 agonist (TLR7/8). We first evaluated TNF-α, a pro-inflammatory cytokine known to be elevated in myeloproliferative neoplasms.19 Secreted TNF-α was quantified by ELISA. We observed that TNF-α production is significantly increased from monocytes cultured with conditioned media from cells expressing type I, type II, and Δ367 mutant calreticulin but not from vector-control, wildtype, or Δ385 mutant calreticulin (Fig. 5a). The raw values from three independent experiments are provided in Supplemental Fig. 5. With R848 treatment, these effects are grossly exaggerated. We then quantified TNF-α production from monocytes derived from a normal control and a type I mutant calreticulin MF patient with or without R848 and lipopolysaccharide (LPS, a TLR4 agonist) treatment. The type I mutant calreticulin-positive monocytes produced more TNF-α compared to the normal control monocytes at 5 and 10 µM R848 but not with the other conditions (Fig. 5b).
Fig. 5. Cytokine production in primary CD14+ monocytes cultured with conditioned media from mutant calreticulin-expressing cells.
(a) ELISA quantification of TNF-α produced by normal human CD14+ monocytes cultured with 7.5% or 15% conditioned media (CM) from HeLa cells expressing wildtype or mutant calreticulin for 24 hrs with or without 3µM R848. “3µM” is the equivalent amount of DMSO vehicle used for the 3µM R848 condition and not actually the concentration of DMSO. This strategy was applied in all the experiments. Each condition had three replicates. Vector-control (VC) is the empty 3XFlag vector. Data expressed as mean TNF-α level. Error bars represent sd. Shown is the results of one experiment. Two other independent experiments are presented with this experiment in Supplemental Fig. 5. The experimental conditions were modified between experiments so the absolute amount of TNF-α varied significantly but the overall results and the interpretation are similar. (b) ELISA quantification of TNF-α from CD14+ cells isolated from a normal donor and a type I mutant MF patient treated with DMSO, R848, or lipopolysaccharide (LPS) at the indicated concentrations. Each condition had three replicates. Shown are the results of one experiment, with data expressed as mean TNF-α level. Error bars represent sd. (c) Comprehensive evaluation of cytokines produced in the experiments presented in Fig. 5a and 5b using the Luminex platform. Standard curves for each cytokine evaluated met quality control assessment. We analyzed cytokine production from CD14+ monocytes cultured with 15% conditioned media from HeLa cells expressing the various calreticulin types (left panel). Shown is the heat map of relative cytokine production of the 18 cytokines that had values generally >5 pg/ml. We excluded from this analysis: FGF-Basic, G-CSF, IL-13, RANTES, Eotaxin, IL-4, IL-17, IL-5, IL-15, IFN-g, IL-2, IP-10 because either most or all the readings were close to or <5 pg/ml. The 1µM and 5µM DMSO (vehicle equivalent) were both suitable negative controls and displayed similar cytokine levels to the same controls in the normal versus type I mutant calreticulin patient monocyte studies (right panel). We selected for analysis the 5 µM R848 and 1 ng/ml LPS conditions. (d) Venn diagram illustrating the cytokines that were particularly interesting (defined in in Supplemental Fig. 6 and 7) in both types of experiments. Cytokines that displayed ≥ 2-fold induction are highlighted in bold.
We evaluated additional cytokines on the Luminex platform. Eighteen cytokines, chemokines, or growth factors had measureable levels above 5pg/ml. As shown in the heat map (Fig. 5c, left panel) and the graphs of the raw data for 18 cytokines/chemokines/growth factors (Supplemental Fig. 6), the conditioned media from type I and Δ367 mutant calreticulin-expressing cells concordantly induced a higher level of factors compared to vector-control, wildtype, type II and Δ385 mutant with R844 treatment. These factors included EGF, GM-CSF, IL-1B, IL-6, IL-10, IL-12, MIP-1A, MIP-1B, TNF-α, and VEGF. Most of these cytokines were also measurably higher than the vector-control and wildtype conditions with DMSO (control) treatment. The effects from the type I and Δ367 conditioned media clustered closer by hierarchal analysis than the type I and type II. Type II conditioned media uniquely induced high levels of IL-7 and MIG production from monocytes compared to the other conditions. When comparing monocytes derived from a type I mutant calreticulin MF patient versus a normal control, there was a greater than 2-fold production for GM-CSF, IL-10, IL-12, MCP-1, MIP-1A, and MIP-1B concordantly with R848 and LPS treatment (Fig. 5c, right panel; Supplemental Fig. 7). IFN-α and IL-1RA had greater than 1.5-fold induction but less than 2-fold induction. TNF-α production was increased in the type I mutant calreticulin-positive monocytes with R848 treatment but not with LPS treatment. Fig. 5d shows a Venn diagram illustrating the cytokines induced by type I mutant calreticulin in both the cell line work (Supplemental Fig. 6) and the primary patient samples (Supplemental Fig. 7) as defined in the figure legend.
Discussion
CALR is thought to function as a tumor suppressor gene with direct evidence that calreticulin and its N-terminal fragment, vasostatin, inhibits cell proliferation in various tumor types, inhibits angiogenesis in multiple tumor models, and promotes immunogenic cell death in the setting of chemotherapy.20–30 The recent discovery of frameshift mutations affecting the C-terminus suggests potential tumor-suppressor functions of the acidic Ca2+ binding domain and the KDEL signal at the C-terminus. Here we highlight the differences we observed between wildtype calreticulin and type I/II mutant calreticulin and the differences between type I and type II mutant calreticulin. The wildtype acidic Ca2+ binding domain at the C-terminus exists as an extended tail and its calcium binding capacity is an important factor in the secondary structure and thermostability of the protein.31 The type I and type II mutant calreticulin have largely lost this low-affinity but high-capacity Ca2+ binding domain. Previous studies show that reduced calcium concentration in the ER results in a less rigid protein conformation.32 This could explain our observation that mutant calreticulin poorly accumulates in cells and has increased protein turnover compared to wildtype calreticulin. The type II mutant calreticulin retains more acidic residues in the C-terminus than type I mutant and is overall intermediate in its steady state protein level compared to wildtype and type I mutant calreticulin. These results suggest that calcium binding capacity is an important determination of protein stability. However, this is not the only contributing factor because deletion of the entire novel C-terminus improved protein accumulation and reduced protein turnover. We also observed reduced total calreticulin staining in marrow samples from type I CALR MF patients compared to normal controls. Together, these findings suggest that the tumor-suppressor functions of calreticulin may be lost as a result of the frameshift mutations at the C-terminus that affect its protein accumulation in cells.
The mutant calreticulin that remained in cells did not show any marked changes in subcellular localization compared to wildtype calreticulin when evaluated by microscopy. This is in agreement with the findings reported by Klampfl et al. and Nangalia, et al.,1,2 However, there were some inter-cell heterogeneity and occasional cells with prominent nuclear localization of type I and type II mutant calreticulin. This inter-cell heterogeneity was also noted in the megakaryocytes from patients’ bone marrow samples. We took a more quantitative approach and observed that type I and type II mutant calreticulin are abnormally enriched in the nucleus compared to wildtype calreticulin, even though it is a small fraction of total mutant calreticulin expressed by cells. In addition, we detected a proteolytically cleaved, N-terminal fragment of calreticulin in conditioned media from cells expressing wildtype or mutant calreticulin. This secreted N-terminal fragment is the most abundant form of mutant calreticulin considering the relative amounts in the nucleus and in whole cell extracts. The presence of this N-terminal fragment in conditioned media from cells expressing the Δ367 and Δ385 mutants suggests that this phenotype is entirely due to the loss of acidic Ca2+ binding and KDEL domains, and not due to a gain-of-function of the novel C-terminus. These data illustrate a major shift in the compartmentalization of mutant calreticulin compared to wildtype calreticulin.
valuation of large clinical data sets revealed key clinicopathologic differences in terms of platelet dysfunction, megakaryocytic proliferation, and overall survival when comparing JAK2-V617F-mutated versus CALR-mutated patients.10–13 In our studies, we noted a minimal degree of IL-3-independent growth of Ba/F3 cells overexpressing type I mutant calreticulin (Fig. 4c). We did not observe complete IL-3 independence as reported by Klampfl et al.1 but this could be due to technical experimental differences. Type II mutant calreticulin overexpression in Ba/F3 cells did not induce IL-3 independent growth or any growth advantage with low levels of IL-3 compared with parent cells or cells expressing wildtype calreticulin. These results suggest that the worse prognosis associated with type II mutant when compared to type I mutant CALR patients33 may not be entirely explained by the same mechanism that confers IL-3-independent growth in the Ba/F3 assay. Based on our work, we propose that the structural differences between type I and type II mutant calreticulin12,34 may confer different pathways of disease pathogenesis. In addition, we directly compared mutant calreticulin and JAK2-V617F, which neither Klampfl, et al. nor Nangalia, et al. did, in their ability to activate JAK/STAT signaling in a non-hematopoietic cell line. These results revealed that there is minimal to no direct activation of the pathway by mutant calreticulin. Overall, these results point to indirect mechanisms, for example, the involvement of another cell type or intermediary protein (outside-in mechanism), leading to JAK/STAT activation.
Since we did not observe substantial, cell-intrinsic activation of JAK/STAT signaling by overexpressing calreticulin mutants in a non-hematopoietic cell line (Fig. 4d,e), we investigated potential paracrine effects from cells expressing mutant calreticulin. Cytokine production from monocytes was significantly higher when cultured with conditioned media from cells expressing type I or Δ367 mutant with or without a toll-like receptor agonist, compared to conditioned media from cells expressing wildtype calreticulin (Fig. 5c and Supplemental Fig. 6). Many of these cytokines are known to activate JAK/STAT signaling. The striking similarity of the cytokine profile produced by the Δ367 mutant and the type I mutant strongly suggests that the phenotype is predominately due to the loss of the wildtype C-terminus. However, the type I mutant behaves differently from the type II mutant, both clinically and biochemically, likely due to the small structural differences noted in a region that affects Ca2+ binding capacity. The paracrine effects are not likely due to the N-terminal calreticulin fragment since it is conserved among all mutants tested. Monocytes cultured in conditioned media from cells expressing type II had an entirely different cytokine profile. Some of the same cytokines in the conditioned media studies were also elevated in our studies with primary monocytes from a normal control and a type I MF patient (Fig. 5d). Because type I mutant-expressing monocytes are themselves hyperreactive to TLR agonists, these results suggest that both autocrine and paracrine effects are in play. Our work highlights mechanistic insights into cytokine production in JAK2-V617F-negative ET and PMF. We summarize our findings in a model comparing and contrasting wildtype and mutant calreticulin properties (Fig. 6). Additional studies are needed to confirm these findings in vivo, in additional patient samples, and to identify the secreted factor(s) that induce monocyte hyperreactivity. We propose that the secreted factor(s) may be full-length mutant calreticulin or a priming or signal amplifying cytokine. Our results suggest non-hematopoietic and hematopoietic cells expressing mutant calreticulin can secrete these factors. Extending these studies in megakaryocytes and stromal cells will also provide a more comprehensive understanding of the tumor microenvironment in MF.
Fig. 6. Model depicting the abnormal functions of mutant calreticulin.
The subcellular localization of wildtype and mutant calreticulin is depicted in this figure. We propose that a non-hematopoietic or hematopoietic cell expressing mutant calreticulin can induce this effect on monocytes. The wildtype N-domain and the lectin-like P-domain that gives calreticulin its protein chaperone properties are conserved in mutant calreticulin. These domains are depicted in gray. The wildtype acidic C-terminus domain is depicted in red and the KDEL signal is in black. The more basic C-terminus domain of mutant calreticulin (type I and type II) is depicted in blue. The numbered circles depict: 1. ER localization of wildtype and mutant calreticulin. 2. Nuclear localization of wildtype and mutant calreticulin. 3. Cytoplasmic localization of wildtype and mutant calreticulin. 4. Membrane localization of wildtype and mutant calreticulin. 5. Extracellular localization of N-terminal fragments of wildtype and mutant calreticulin. One is a smaller fragment also known as vasostatin (small gray circles). The other, observed in our studies, is a larger fragment generated by cleavage at amino acid #340 by proteases as previously reported (large gray circles).18 6. Secreted factor(s) induce hyperreactivity of monocytes with or without R848 (a toll-like receptor 7/8 agonist). Cytokines that were significantly elevated (see Fig. 5d and Supplemental Fig. 6 and 7 and the corresponding results section) in both the cell line work and the patient sample studies are indicated here. Highlighted in the yellow circled numbers are features that are abnormally enhanced in mutant calreticulin when compared to wildtype calreticulin.
Work to date has implicated a direct role for inflammatory cytokines in clonal myelopoeisis, clonal evolution, autoimmune disease, atherosclerotic disease, and secondary malignancies in myelofibrosis.19,35 Symptoms and disease burden correlate with elevated cytokines. IL-8, IL-2R, IL-12, and IL-15 were independent predictors of inferior survival in a large comprehensive study of cytokine profiling in PMF.36 Our work provides novel mechanistic insights into autocrine and paracrine mechanisms for amplification of cytokine production and JAK/STAT signaling in type I mutant CALR patients.
Supplementary Material
Acknowledgments
We acknowledge Jakki Martinez, Drs. Stefanie Kaech Petrie, Kevin Watanabe-Smith, and Julia Maxson for their advice and technical support and Drs. Brian Ruffell and Lisa Coussens for use of the macro and Aperio ScanScope AT microscope. This work was largely supported by grants supporting KTD, who receives support from the OHSU Knight Cancer Institute and the National Institutes of Health, National Heart, Lung and Blood Institute (1K08HL111280). This work was partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000128. LFN is supported by a grant from Office of Research in Women’s Health and the National Institute of Child Health and Human Development, Oregon BIRCWH Award Number 2K12HD043488. AA is supported by a National Cancer Institute Career Development Award (4 R00CA151670-03). BJD is supported by the Howard Hughes Medical Institute.
JWT, BJD, and KTD wish to disclose that they are investigators of an investigator-initiated clinical trial supported by Incyte Corporation.
Footnotes
Authorship
MRG, CAW, SHL, LFN, AA, JBD, TKC, HL, RR, GCB, and KTD designed and performed the research; MRG, LFN, AA, ET, RDP, GCB, JWT, BJD, and KTD contributed vital new reagents or analytical tools; MRG, CAW, SHL, LFN, AA, JBD, TKC, JE, ET, RR, MJC, RDP, GCB, JWT, BJD, and KTD analyzed data; and CAW, SHL, and KTD wrote the majority of the paper while the other co-authors reviewed the manuscript and provided comments.
Disclosure of Conflicts of Interest
While there is no perceived conflicts of interest related to the work described in this manuscript
References
- 1.Klampfl T, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 2.Nangalia J, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rampal R, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123:e123–e133. doi: 10.1182/blood-2014-02-554634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Cid N, et al. Modes of calreticulin recruitment to the major histocompatibility complex class I assembly pathway. J Biol Chem. 2010;285:4520–4535. doi: 10.1074/jbc.M109.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeffery E, Peters LR, Raghavan M. The polypeptide binding conformation of calreticulin facilitates its cell-surface expression under conditions of endoplasmic reticulum stress. J Biol Chem. 2011;286:2402–2415. doi: 10.1074/jbc.M110.180877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe C, et al. Calreticulin-dependent recycling in the early secretory pathway mediates optimal peptide loading of MHC class I molecules. EMBO J. 2009;28:3730–3744. doi: 10.1038/emboj.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. The Biochemical journal. 1999;344(Pt 2):281–292. [PMC free article] [PubMed] [Google Scholar]
- 8.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tefferi A, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507–2513. doi: 10.1182/blood-2014-05-579136. quiz 2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrikovics H, et al. Distinct clinical characteristics of myeloproliferative neoplasms with calreticulin mutations. Haematologica. 2014;99:1184–1190. doi: 10.3324/haematol.2014.107482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CC, et al. Frequencies, clinical characteristics, and outcome of somatic CALR mutations in JAK2-unmutated essential thrombocythemia. Annals of hematology. 2014;93:2029–2036. doi: 10.1007/s00277-014-2151-8. [DOI] [PubMed] [Google Scholar]
- 12.Tefferi A, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol. 2014;89:E121–E124. doi: 10.1002/ajh.23743. [DOI] [PubMed] [Google Scholar]
- 13.Wojtaszewska M, Iwola M, Lewandowski K. Frequency and Molecular Characteristics of Calreticulin Gene (CALR) Mutations in Patients with JAK2 -Negative Myeloproliferative Neoplasms. Acta haematologica. 2014;133:193–198. doi: 10.1159/000366263. [DOI] [PubMed] [Google Scholar]
- 14.Vannucchi AM, et al. Calreticulin mutation-specific immunostaining in myeloproliferative neoplasms: pathogenetic insight and diagnostic value. Leukemia. 2014;28:1811–1818. doi: 10.1038/leu.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shivarov V, Ivanova M, Tiu RV. Mutated calreticulin retains structurally disordered C terminus that cannot bind Ca(2+): some mechanistic and therapeutic implications. Blood cancer journal. 2014;4:e185. doi: 10.1038/bcj.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dao KH, et al. FANCL ubiquitinates beta-catenin and enhances its nuclear function. Blood. 2012;120:323–334. doi: 10.1182/blood-2011-11-388355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traer E, et al. Ponatinib overcomes FGF2-mediated resistance in CML patients without kinase domain mutations. Blood. 2014;123:1516–1524. doi: 10.1182/blood-2013-07-518381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hojrup P, Roepstorff P, Houen G. Human placental calreticulin characterization of domain structure and post-translational modifications. European journal of biochemistry / FEBS. 2001;268:2558–2565. doi: 10.1046/j.1432-1327.2001.02138.x. [DOI] [PubMed] [Google Scholar]
- 19.Hasselbalch HC. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine & growth factor reviews. 2013;24:133–145. doi: 10.1016/j.cytogfr.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Cai KX, et al. Suppression of lung tumor growth and metastasis in mice by adeno-associated virus-mediated expression of vasostatin. Clin Cancer Res. 2008;14:939–949. doi: 10.1158/1078-0432.CCR-07-1930. [DOI] [PubMed] [Google Scholar]
- 21.Li L, et al. Treatment of pancreatic carcinoma by adenoviral mediated gene transfer of vasostatin in mice. Gut. 2006;55:259–265. doi: 10.1136/gut.2005.064980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez NC, et al. Antiangiogenic and antitumor effects of Trypanosoma cruzi Calreticulin. PLoS neglected tropical diseases. 2010;4:e730. doi: 10.1371/journal.pntd.0000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma L, et al. Complete eradication of hepatocellular carcinomas by combined vasostatin gene therapy and B7H3-mediated immunotherapy. Journal of hepatology. 2007;46:98–106. doi: 10.1016/j.jhep.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 24.Mans S, Banz Y, Mueller BU, Pabst T. The angiogenesis inhibitor vasostatin is regulated by neutrophil elastase-dependent cleavage of calreticulin in AML patients. Blood. 2012;120:2690–2699. doi: 10.1182/blood-2012-02-412759. [DOI] [PubMed] [Google Scholar]
- 25.Peng XC, et al. Plasmid-encoding vasostatin inhibited the growth and metastasis of human hepatocellular carcinoma cells. Molecular and cellular biochemistry. 2014;395:265–272. doi: 10.1007/s11010-014-2135-y. [DOI] [PubMed] [Google Scholar]
- 26.Pike SE, et al. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J Exp Med. 1998;188:2349–2356. doi: 10.1084/jem.188.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pike SE, et al. Calreticulin and calreticulin fragments are endothelial cell inhibitors that suppress tumor growth. Blood. 1999;94:2461–2468. [PubMed] [Google Scholar]
- 28.Xiao F, et al. A gene therapy for cancer based on the angiogenesis inhibitor, vasostatin. Gene Ther. 2002;9:1207–1213. doi: 10.1038/sj.gt.3301788. [DOI] [PubMed] [Google Scholar]
- 29.Yao L, et al. Anti-tumor activities of the angiogenesis inhibitors interferon-inducible protein-10 and the calreticulin fragment vasostatin. Cancer immunology, immunotherapy : CII. 2002;51:358–366. doi: 10.1007/s00262-002-0294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao L, et al. Effective targeting of tumor vasculature by the angiogenesis inhibitors vasostatin and interleukin-12. Blood. 2000;96:1900–1905. [PubMed] [Google Scholar]
- 31.Wijeyesakere SJ, Gafni AA, Raghavan M. Calreticulin is a thermostable protein with distinct structural responses to different divalent cation environments. J Biol Chem. 2011;286:8771–8785. doi: 10.1074/jbc.M110.169193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villamil Giraldo AM, et al. The structure of calreticulin C-terminal domain is modulated by physiological variations of calcium concentration. J Biol Chem. 2010;285:4544–4553. doi: 10.1074/jbc.M109.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tefferi A, et al. The prognostic advantage of calreticulin mutations in myelofibrosis might be confined to type 1 or type 1-like CALR variants. Blood. 2014;124:2465–2466. doi: 10.1182/blood-2014-07-588426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tefferi A, et al. Type 1 vs type 2 calreticulin mutations in primary myelofibrosis: differences in phenotype and prognostic impact. Leukemia. 2014;28:1568–1570. doi: 10.1038/leu.2014.83. [DOI] [PubMed] [Google Scholar]
- 35.Hasselbalch HC. Perspectives on the impact of JAK-inhibitor therapy upon inflammation-mediated comorbidities in myelofibrosis and related neoplasms. Expert review of hematology. 2014;7:203–216. doi: 10.1586/17474086.2013.876356. [DOI] [PubMed] [Google Scholar]
- 36.Tefferi A, et al. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.