Abstract
Objective
Metabolomics has the potential to reveal novel pathways involved in the pathogenesis of preterm birth (PTB). The objective of this study was to investigate if the cervico-vaginal (CV) metabolome was different in asymptomatic women destined to have a PTB compared to term birth.
Study Design
A nested case-control study was performed using CV fluid collected from a larger prospective cohort. CV fluid was collected between 20-24 weeks (V1) and 24-28 weeks (V2). The metabolome was compared between women with a spontaneous PTB (n=10) to women who delivered at term (n=10). Samples were extracted and prepared for analysis using a standard extraction solvent method (Metabolon®, Durham, NC). Global biochemical profiles were determined using GC/MS and UPLC MS/MS. ANOVA was used to detect differences in biochemical compounds between the groups. A false discovery rate was estimated to account for multiple comparisons.
Result
A total of 313 biochemicals were identified in CV fluid. 82 biochemicals were different in the CV fluid at V1 in those destined to have a PTB compared to term birth while 48 were different at V2 (Table 1). Amino acid, carbohydrate, and peptide metabolites were distinct between women with and without PTB.
Conclusion
These data suggest that the CV space is metabolically active during pregnancy. Changes in the CV metabolome may be observed weeks, if not months, prior to any clinical symptoms. Understanding the CV metabolome may hold promise for unraveling the pathogenesis of PTB and may provide novel biomarkers to identify women most at risk.
Introduction
Preterm birth (PTB) is among the leading cause of neonatal morbidity and mortality accounting for 11.2% of live births in the US as of 2012.1 Studies have shown that women with a short cervix are at an increased risk for PTB,2-4 yet most cases of PTB are not preceded by a sonographic short cervix. The sonographic short cervix could be considered a phenotype of premature cervical modeling, but in fact, the immunologic, biochemical, molecular, and cellular processes involved in premature cervical remodeling have not been fully elucidated.5-9 While there is some emerging evidence regarding the cervico-vaginal (CV) microbiota and PTB,10,11 how the CV microbiota might alter cervical remodeling has not been studied. While clinical data support the role of infection and/or inflammation in the pathogenesis of preterm birth12 and understanding that cervical remodeling must occur at any gestational age for parturition to occur, the interaction of these biological and molecular events to induce spontaneous preterm birth have not been elucidated.
Metabolomics, the study of metabolites less than 1 kilodalton (kDa) necessary for structure and function of an organism at the cellular level, is able to provide information on multiple biological pathways acting in concert.13 It is a powerful tool of fundamental discovery that relays information from the host, the microbiota, and the environment to give a comprehensive view of a biological system in both healthy and diseased states. Metabolomics has the potential to uncover novel mechanistic pathways involved in premature cervical remodeling and PTB.
The importance of the metabolome may be best conceptualized by recent advances in understanding the role of gut microbiota in the development of colorectal cancer in patients with inflammatory bowel disease (IBD).14 While it is well known that patients with IBD are at an increased risk for colorectal cancer,15 it seems that the bacterial metabolites, more than the bacterial composition itself, may protect from or predispose towards cancer within the gastrointestinal tract.16 Metabolomic analysis has revealed the actions of the microbiota may result in the production of tumor-promoting metabolites, such as acetylaldehyde and nitrosamine, and may mediate tumor suppressive effects through inactivation of carcinogens.17,18 Furthermore, metabolomics have led to the discovery of novel targets for therapeutics in preventing cell proliferation in cancer.13 Examination of the metabolome has the potential to 1) reveal novel mechanistic pathways involved in the pathophysiology of disease and 2) provide therapeutic targets to prevent disease.
Metabolomics in pregnancy is in its infancy. Existing studies have demonstrated the ability to test the metabolome in maternal plasma, amniotic fluid, placenta, cord blood, and urine. These studies have explored the metabolome among women with pre-eclampsia, gestational diabetes, fetal growth restriction, and fetal malformations and have shown key differences in metabolites between cases and controls.19-23 Despite these studies, the CV fluid metabolome and how it might be altered in women with preterm birth has not been studied. Investigating the metabolome in CV fluid may provide insight into the cellular metabolism as it relates to the host and/or existing microbiota involved with premature cervical remodeling. Therefore, the objective of this study was to investigate if the CV fluid metabolome was different in asymptomatic women destined to have a PTB compared to term birth.
Materials and Methods
Study Population
A nested case-control study was performed from a prospective cohort. The “PREDICT” study was performed at a single, urban tertiary care institution between August 2011 and November 2012.24,25 This study was approved by the Institutional Review Board at the University of Pennsylvania. Women with a singleton pregnancy who were at high risk for preterm birth based on a prior history of preterm birth or second trimester loss, previous cervical surgery without a subsequent full term birth, and/or uterine anomaly were approached for recruitment into the study. Women who were receiving systemic steroids or immunosuppressive therapy, had HIV, lupus, pre-gestational diabetes mellitus, rheumatoid arthritis, Crohn's disease, ulcerative colitis, cancer or an organ transplant were excluded. Furthermore, women with activity in the vagina in the 24 hours prior to specimen collection (including sexual intercourse, trans-vaginal ultrasound, digital cervical exam, or vaginal lubricants), had active vaginal bleeding, evidence of rupture of membranes, and/or cervical dilation greater than or equal to 3 cm at the time of enrollment were excluded. Pertinent demographic, clinical, and obstetric data were abstracted from the medical record as previously described.24 Women with spontaneous preterm birth at less than 37 weeks of gestation were identified (n=10). Controls were selected at random from women having deliveries at > 38 weeks of gestation without evidence of chorioamnionitis. Cervico-vaginal biospecimens were collected at two time points: visit 1 (V1) occurred at 20 weeks to 23 weeks 6 days of gestation and visit 2 (V2) occurred at 24 weeks to 27 weeks 6 days of gestation. All cases of preterm birth were reviewed and confirmed that they were spontaneous preterm births.
Biospecimen Collection
CV fluid was collected following insertion of a sterile speculum using a sterile cotton-tipped swab. Samples were collected in 0.5 mL phosphate-buffered saline, immediately placed in liquid nitrogen, and stored at −80°C for future use.
Metabolomics Analysis
Metabolomics analysis was performed by Metabolon®, Inc. (Research Triangle Park, NC) as previously described.26,27 Samples were accessioned into the Metabolon® Laboratory Information Management System (LIMS) and assigned a unique identifier. The samples (and all derived aliquots) were bar-coded and tracked by the LIMS. All samples were maintained at −80°C until processed. The samples were prepared using the MicroLab STAR system from Hamilton Company. In order to remove the protein fraction while maximizing recovery of small molecules, the samples were prepared using a proprietary series of organic and aqueous extractions. The resulting extract was divided into two fractions: one for analysis by Ultra-Performance Liquid Chromatography/tandem mass spectrometry (UPLC-MS/MS) and one for analysis by Gas Chromatography/Mass Spectrometry (GC/MS). Samples were placed briefly on TurboVap (Zymark) to remove the organic solvent. Each sample was then frozen and underwent vacuum desiccation. Samples were then prepared for either LC/MS or GC/MS.
For quality assurance/quality control purposes, a selection of quality control compounds was added to every sample, including those that are being examined. These compounds were carefully chosen so as not to interfere with the measurement of the endogenous compounds. The quality control samples are primarily used to evaluate the control process for each study as well as aiding in data curation.
Liquid Chromatography/Mass Spectrometry
The liquid chromatography/mass spectrometry (LC/MS) portion of the platform was based on a Waters ACQUITY UPLC and Thermo-Finigan LTQ mass spectrometer, which consisted of an electrospray ionization source and linear ion trap mass analyzer. The sample extract was split into two aliquots, dried, and then reconstituted in acidic or basic LC-compatible solvents, each of which contained 11 or more injection standards at fixed concentrations. One aliquot was analyzed using acidic positive ion optimized conditions and the other using basic negative ion optimized conditions in two independent injections, using separate dedicated columns. Extracts reconstituted in acidic conditions were gradient eluted using water and methanol both containing 0.1% formic acid, while the basic extracts, which also used water/methanol, contained 6.5 mM ammonium bicarbonate. The MS analysis alternated between MS and data-dependent MS2 scans using dynamic exclusion, and the scan range was from 80-1000 mass to charge ratio (m/z). Raw data files were archived and extracted as described below.
Gas Chromatography/Mass Spectrometry
The samples destined for gas chromatography/mass spectrometry (GC/MS) analysis were re-dried under vacuum desiccation for a minimum of 24 hours prior to being derivatized under dried nitrogen using bistrimethyl-silyl-triflouroacetamide (BSTFA). The GC column was 5% phenyl and the temperature ramp was from 40° to 300°C in a 16 minute period. Samples were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization. The instrument was tuned and calibrated for mass resolution and mass accuracy on a daily basis. The information output from the raw data files was automatically extracted as discussed below.
Bioinformatics
The informatics system consisted of four major components, the LIMS, the data extraction and peak-identification software, data processing tools for quality control and compound identification, and a collection of information interpretation and visualization tools for use by data analysts. The hardware and software foundations for these informatics components were the LAN backbone, and a database server running Oracle 10.2.0.1 Enterprise Edition.
Data Extraction and Compound Identification
Raw data was extracted, peak-identified and quality control processed using Metabolon's® hardware and software.28 Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. Metabolon® maintains a library based on authenticated standards that contains the retention time/index, mass to charge ratio (m/z), and chromatographic data (including MS/MS spectral data) on all molecules present in the library. Furthermore, biochemical identifications are based on three criteria: retention index within a narrow window of the proposed identification, nominal mass match to the library +/−0.4 amu, and the MS/MS forward and reverse scores between the experimental data and authentic standards. The MS/MS scores are based on a comparison of the ions present in the experimental spectrum to the ions present in the library spectrum. While there may be similarities between these molecules based on one of these factors, the use of all three data points can be utilized to distinguish and differentiate biochemicals. More than 3500 commercially available purified standard compounds have been acquired and registered into LIMS for distribution to both the LC-MS and GC-MS platforms for determination of their analytical characteristics.
Statistical analyses
Analysis of variance (ANOVA) was used to identify biochemicals that differed significantly between the groups following log transformation and imputation of missing values, if any, with the minimum observed value for each compound. The data were analyzed both normalized to protein and non-normalized and results were similar between the groups. However, due to the significant differences in protein hydrolysis between cases and controls, the data presented here are those results which were not normalized to protein content. Two-way ANOVA with repeated measures was used to identify biochemicals exhibiting significant interaction and main effects for the experimental parameters of status and time. The data were analyzed in two ways to account for changes between groups and changes that occurred over time within the same subject. ANOVA was used when comparing metabolites between groups across a single time point (where each subject was unique) whereas two-way ANOVA with repeated measures was used to take into account metabolites that changed over time within each subject (accounting for within subject characteristics). Only metabolites with a p value of less than 0.05 were considered significant. Additionally, as this was a study of discovery, metabolites that approached significance (i.e. had an alpha level between 0.05 and 0.1) were explored.
An estimate of the false discovery rate (q-value) was calculated to account for multiple comparisons that occur in metabolomics studies.29 When analyzing numerous compounds, statistical significance may be found due to random chance. The q-value describes the false-discovery rate; a low q-value (q≤0.10) is indicative of high confidence in a result.
Random forest (RF) is a supervised classification technique reporting on the consensus of a large number of decision trees. In this study, the term and preterm birth cohorts at both time points were classified in order to: 1) assess the capacity to distinguish birth cohorts on the basis of global metabolic profiles and 2) identify biochemicals important to the classification. An accuracy of 25% is expected by random chance when comparing four groups, so the RF classification accuracy of 32% is better than random chance. Additionally, according to the “confusion matrix,” when the RF model misclassified samples it tended to pick the wrong time point (i.e., V1 vs V2) but the correct birth cohort (i.e., term vs. PTB), so the model may be even more accurate than the 32% accuracy belies.
Statistical Analysis for Clinical Data
Categorical variables were compared between groups by Chi-square (χ2) or Fisher's exact test, where appropriate. Continuous variables were compared by the Student t test or the Mann-Whitney U test, depending on the distribution of the data. Spearman's correlation coefficients were calculated to determine correlations between metabolites and cervical length less than 2.9 cm (median). A p-value less than 0.05 was considered statistically significant. Clinical data were analyzed using STATA (v11.0; StataCorp, Inc., College Station,TX).
Results
Demographics
Clinical and obstetric data are listed in Table 1. Women with a preterm delivery were similar in age, race, gestational age at V1 and V2, and cervical length at V1 compared to women with a term delivery. Not surprisingly, women who ultimately delivered preterm had a significantly higher number of prior PTB, significantly lower cervical length at V2 and gestational age at delivery as compared to term birth (Table 1).
Table 1.
Clinical and demographic characteristics of women with PTB and term birth.
| PTB | Term | p-valuec | |
|---|---|---|---|
| Age, yearsa | 23.5 (17-38) | 31.5 (26-35) | 0.22 |
| Raceb | 0.8 | ||
| White | 8 (20) | 7 (70) | |
| Black | 1 (10) | 2 (20) | |
| Asian | 1 (10) | 1 (10) | |
| Prior PTBb | 8 (80) | 2 (20) | 0.02 |
| Gestational age at V1, weeksa | 20 4/7 (20-23) | 20 3/7 (19 6/7 -23 3/7) | 0.7 |
| Gestational age at V2, weeksa | 26 3/7 (24 1/7-27 5/7) | 26 (24 6/7-27 4/7) | 1.0 |
| Cervical length at V1, cma | 3.3 (2.1-3.5) | 3.7 (2-4.8) | 0.07 |
| Cervical length at V2, cma | 2.9 (1.6-3.6) | 3.8 (2.4-4.7) | 0.03 |
| Gestational age at delivery, weeksa | 33 4/7 (26-36) | 40 (39-41) | <0.001 |
median (range)
n (%)
p<0.05 considered statistically significant
Cervicovaginal Metabolome
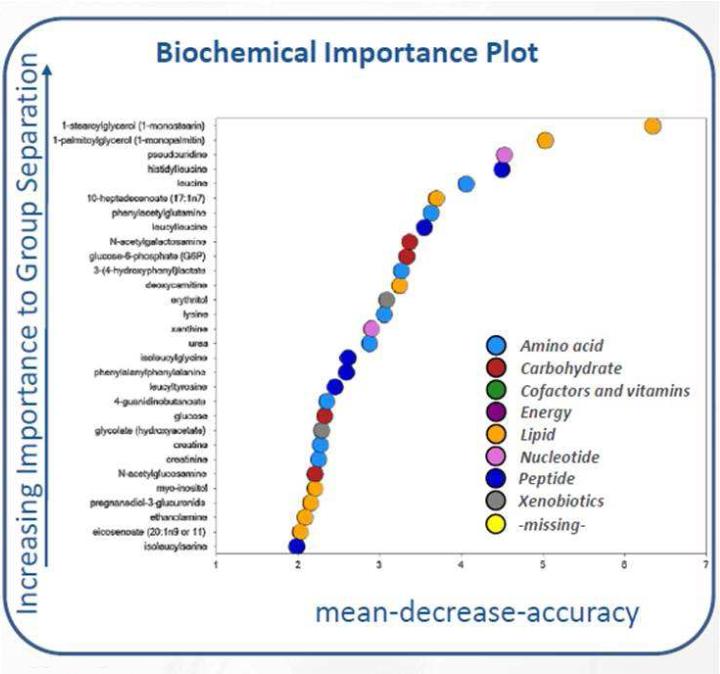
A total of 313 biochemicals were identified in CV fluid. Significant differences were noted in the CV fluid metabolome between women destined to have a PTB compared to term birth. Overall, women who had a term delivery had significant changes in carbohydrate and lipid metabolism between the 2nd and 3rd trimester. In women with a preterm delivery, significant changes in amino acid metabolism were noted between the 2nd and 3rd trimester. Of note, the significant change in carbohydrate metabolism observed among women with a term delivery from visit 1 to visit 2 does not occur in women with PTB. At visit 1, women with a preterm delivery had significant changes in amino acid and peptide metabolism as compared to women with a term delivery. Changes in multiple metabolic pathways were noted at visit 2; women with a preterm delivery had significant changes in amino acid, peptide, carbohydrate, and lipid metabolism as compared to women with a term delivery (Table 2). A random forest plot depicting classes of metabolites that were important in distinguishing between preterm and term delivery can be found in Figure 1.
Table 2.
| Metabolic Pathway | Term V2 | PTB V2 | PTB V1 | PTB V2 |
|---|---|---|---|---|
| Term V1 | PTB V1 | Term V1 | Term V2 | |
| Amino Acid | 0 | 5 | 6 | 3 |
| Peptide | 1 | 0 | 22 | 4 |
| Carbohydrate | 9 | 0 | 3 | 3 |
| Energy | 0 | 0 | 1 | 0 |
| Lipid | 3 | 0 | 3 | 3 |
| Nucleotide | 0 | 0 | 2 | 0 |
| Cofactors and Vitamins | 1 | 0 | 1 | 1 |
| Xenobiotics | 2 | 2 | 2 | 1 |
| Total | 16 | 7 | 40 | 15 |
significantly different pathways between comparison groups with p<0.05
Total number of pathways that are significantly different (either increased or decreased) between comparison groups
Figure 1.
Random Forest plot depicting classes of metabolites (along the y-axis) that were important in distinguishing between preterm and term births. Monoacylglycerols (lipid metabolism), protein hydrolysis markers (dipeptides), and amino acid sugars (carbohydrate metabolism) were among the metabolites that were most important in distinguishing the two groups.
A more in-depth look into the metabolome among women with preterm and term delivery reveals distinct differences in metabolites. In women delivering at term, carbohydrate and lipid metabolism appear to be down-regulated between the 2nd and 3rd trimester. Specifically for carbohydrate metabolism, glucose, glucose-6-phosphate, xylulose, maltohexose, N-acetylglucosamine, and N-acetylgalactosamine were significantly decreased by the third trimester (0.2, 0.14, 0.59, 0.35, 0.45, and 0.32-fold change, respectively, Table 3) in women who had a term delivery. Additionally, an increase in methyl-4-hydroxybenzoate, an anti-microbial agent, was noted among women with term delivery between the 2nd and 3rd trimester (8.8 fold, p<0.1). Similarly, important contributors of lipid metabolism were significantly decreased in the third trimester among women who delivered at term (myo-inositol, 1-palmitoylglycerol (1-monopalmitin), dehydroisoandrosterone sulfate (DHEA-S), Table 3). In contrast, components of lipid and carbohydrate metabolism were significantly up regulated at visit 2 among women with preterm delivery as compared to term delivery (palmitoleate 1.4 fold, 10-heptadecenoate 1.6 fold, 1-palmitylglycerol 2.24 fold, glucose 3.7 fold, glucose-6-phosphate 3 fold, Table 3). At visit 1, a significant down regulation of dipeptides was observed among women with preterm delivery as compared to term delivery. Over half of the 100 dipeptides detected in CV fluid were significantly reduced among women who had a preterm delivery (Table 3). At visit 2, a significant increase in n-acetylneuraminate (4.9 fold) was observed among women with preterm delivery as compared to term delivery (Table 3). Finally, none of the metabolites with significant fold change between preterm and term delivery correlated with cervical length <2.9 cm (data not shown).
Table 3.
| Metabolic Pathway | Sub-Pathway | Biochemical Name | Term V2 | PTB V2 | PTB V1 | PTB V2 |
|---|---|---|---|---|---|---|
| Term V1 | PTB V1 | Term V1 | Term V2 | |||
| Amino Acid | Lysine Metabolism | lysine | 0.48 | |||
| Phenylalanine and Tyrosine Metabolism | phenylacetylglutamine | 0.19 | ||||
| 3-(4-hydroxyphenyl)lactate | 0.36 | |||||
| p-cresol sulfate | 0.34 | |||||
| Tryptophan Metabolism | tryptamine | 1.66 | ||||
| indolelactate | 0.24 | |||||
| 3-indoxyl sulfate | 0.12 | |||||
| Methionine, Cysteine, SAM and Taurine Metabolism | cystine | 0.25 | ||||
| Urea cycle; Arginine and Proline Metabolism | urea | 0.55 | ||||
| ornithine | 0.07 | |||||
| Creatine Metabolism | creatinine | 0.65 | 0.6 | |||
| Guanidino and Acetamido Metabolism | 4-guanidinobutanoate | 0.66 | ||||
| Glutathione Metabolism | 5-oxoproline | 0.44 | ||||
| Peptide | Dipeptide | dimethylarginine (SDMA + ADMA) | 0.37 | |||
| alanylphenylalanine | 0.4 | |||||
| arginylproline | 0.33 | |||||
| glycylisoleucine | 0.42 | |||||
| glycylleucine | 0.4 | |||||
| isoleucylalanine | 0.47 | |||||
| isoleucylglutamate | 0.41 | |||||
| isoleucylglycine | 0.4 | 0.48 | ||||
| isoleucylserine | 0.37 | |||||
| isoleucylthreonine | 0.31 | 0.41 | ||||
| leucylglutamate | 0.44 | |||||
| leucylleucine | 0.49 | |||||
| phenylalanylisoleucine | 0.52 | |||||
| phenylalanylleucine | 0.45 | |||||
| phenylalanylphenylalanine | 0.66 | 0.64 | ||||
| threonylproline | 0.59 | |||||
| tyrosylglutamine | 0.37 | |||||
| tyrosylleucine | 0.41 | |||||
| valylalanine | 0.37 | |||||
| valylaspartate | 0.34 | |||||
| valylglycine | 0.34 | |||||
| valylleucine | 0.33 | |||||
| valylphenylalanine | 0.36 | |||||
| valylthreonine | 0.49 | |||||
| Carbohydrate | Glycolysis, Gluconeogenesis, and Pyruvate Metabolism | glucose | 0.2 | 3.73 | ||
| glucose-6-phosphate (G6P) | 0.14 | 3.04 | ||||
| Isobar: fructose 1,6-diphosphate, glucose 1,6-diphosphate, myo-inositol 1,4 or 1,3-diphosphate | 0.4 | |||||
| Pentose Metabolism | xylulose | 0.59 | ||||
| xylose | 0.57 | |||||
| Glycogen Metabolism | maltohexaose | 0.35 | ||||
| maltopentaose | 0.31 | |||||
| maltose | 0.1 | |||||
| Aminosugar Metabolism | N-acetylglucosamine | 0.45 | 0.43 | |||
| N-acetylgalactosamine | 0.32 | 0.62 | ||||
| N-acetylneuraminate | 4.9 | |||||
| Energy | Oxidative Phosphorylation | phosphate | 0.93 | |||
| Lipid | Long Chain Fatty Acid | palmitoleate (16:1n7) | 1.4 | |||
| 10-heptadecenoate (17:1n7) | 1.56 | |||||
| Inositol Metabolism | myo-inositol | 0.47 | 0.42 | |||
| Lysolipid | 1-stearoylglycerophosphoserine* | 0.45 | ||||
| Monoacylglycerol | 1-palmitoylglycerol (1-monopalmitin) | 0.44 | 0.64 | 2.24 | ||
| Steroid | dehydroisoandrosterone sulfate (DHEA-S) | 0.67 | ||||
| Nucleotide | Purine Metabolism, (Hypo)Xanthine/Inosine containing | xanthine | 5.88 | |||
| Pyrimidine Metabolism, Cytidine containing | cytosine | 0.25 | ||||
| Cofactors and Vitamins | Nicotinate and Nicotinamide Metabolism | nicotinamide | 0.43 | 2.12 | ||
| nicotinamide adenine dinucleotide reduced (NADH) | 2.22 | |||||
| Xenobiotics | Benzoate Metabolism | 3-hydroxyhippurate | 0.36 | |||
| catechol sulfate | 0.32 | |||||
| Food Component/Plant | erythritol | 0.84 | 0.85 | |||
| Chemical | 2-pyrrolidinone | 1.21 | 1.21 | |||
| glycolate (hydroxyacetate) | 0.66 |
significantly different pathways between comparison groups with p<0.05
green text indicates a decrease in fold change between groups, whereas red indicates an increase in fold change.
Comment
Novel to this study, we demonstrate that the cervico-vaginal space is metabolically active during pregnancy and that the CV metabolome is different in women destined to have a preterm birth. Important findings from this study include: 1) normal, term delivery is associated with metabolic changes in the CV space between the 2nd and 3rd trimester: 2) these metabolic changes (specifically down-regulation in the carbohydrate pathway) does not appear to occur in women who are destined to have a preterm birth; 3) women destined to have a preterm birth can be identified by differences in several metabolic processes compared to women who deliver at term.
Out of 3500 standardized metabolites that have been identified in other biological spaces, 313 were identified in the cervicovaginal space. There have been no other high-throughput metabolomic studies of the CV space and therefore it is difficult to compare our findings to the non-pregnant population. Of note, many different classes of metabolites have been identified in the gut metabolome, which may represent the different bacteria that reside in these respective spaces. Similar number of metabolites have been found in other studies involving the gut, however, there is a wide range.28
Three investigators have examined the metabolome and its association with preterm birth, two in amniotic fluid,30,31 and one in CV fluid .32 Both studies of amniotic fluid demonstrated alterations in xenobiotic metabolism among women with PTB.30,31 Only one other study has examined the metabolome in CV fluid.32 While these authors found that in the setting of a short cervix, the CV metabolome distinguished women with term and preterm birth, they did not report the distinct metabolic pathways that differed between the two groups.32 Our study is unique in that, using a metabolomics approach, it provides the first comprehensive, detailed description of metabolites that are present in the cervico-vaginal space that were distinct in women who ultimately had a PTB. While we would not expect the metabolome in amniotic fluid to mirror the CV space, it is notable that these studies found a metabolomic profile that was associated with inflammation. Therefore, these studies provide validity in a metabolomic approach which may serve to advance our understanding of preterm birth. The study herein further demonstrates the strength of a metabolomic approach.
The significant down regulation of carbohydrate metabolism observed in women who had a term delivery is notably absent among women with a preterm delivery. These metabolic changes could represent contributions from the host or the microbiota. Epithelial cells store and metabolize glycogen, which is deposited in large amounts during highly estrogenic states and is metabolized to lactic acid, hence contributing to the acidic pH found in a “healthy” genital tract33 Microbiota use glycogen as a substrate and is thought to support lactobacilli colonization,34,35 which has been associated with a reduction in adverse pregnancy outcomes.36 This significant down regulation in women who had a term delivery, together with the increase in the anti-microbial agent methyl-4-hydroxybenzoate, may represent a stable vaginal microbiome that is not observed in women destined for preterm birth. Alternatively, down regulation of carbohydrate metabolism in women destined to have a term delivery may represent a ‘quiescent state’ in the CV space that is necessary for the cervix to maintain integrity; the lack of down regulation of these carbohydrate pathways in women destined to deliver preterm may indicate that this quiescent state is never reached and hence there is active energy utilization that may be synonymous with premature cervical remodeling. Interestingly, n-acetylneuraminate or sialic acid was significantly increased in the 3rd trimester among women with preterm birth as compared to term birth. Sialic acid is a ubiquitous cell-signaling molecule with important roles in immunity.37 Specifically, sialic acid is thought to play a role in viral entry into the cell.38 Its notable increase among women with preterm birth may represent an enhanced affinity for infection at the cellular level.
Over 100 dipeptides were detected in the CV fluid and nearly half were decreased among women with PTB in the 2nd trimester as compared to term. Of note, the average total protein among the PTB and term group differed more at V1 (605 μg/ml vs. 745 μg/ml, respectively) than V2 (504 μg/ml vs. 543 μg/ml, respectively) suggesting that the decreased dipeptides might reflect a reduced level of protein hydrolysis among the women with PTB. Changes in the expression of proteases and protease inhibitors have been detected days before spontaneous labor.39,40 Therefore, it is possible that the decrease in dipeptides represents alterations in proteases and their inhibitors among women destined to have a PTB.
While these data demonstrate promise for study of the CV metabolome and understanding the pathogenesis of preterm birth, our sample size was small. Though we did not find a correlation between metabolites and a shortened cervical length, our study might have been underpowered to reveal an association between metabolic processes and sonographic short cervix. Alternatively, the lack of correlation may suggest that the cellular activity of the CV space is more biologically relevant in understanding and predicting preterm birth than short cervix. However, larger studies are needed before such conclusions can be made. Another limitation of our study is that the cases had significantly more prior PTB and that the metabolic changes observed may be unique to these women and not mechanistically involved in having a PTB in this pregnancy. These results will need to be validated in a larger sample size in different populations. Finally, the cohorts were not matched to factors such as age, race, or parity, which may have biased the results. Noting these limitations, the results of this study provide a new paradigm in which to conceptualize the pathogenesis of preterm birth. With our discovery-based approach, this study provides an unbiased assessment of the CV metabolic profiles in asymptomatic women destined to have a preterm birth.
These findings demonstrate the biological importance of the CV metabolome to pregnancy. Notably, these findings suggest unique metabolic profiles in women who ultimately have a term or preterm birth. As such, these data challenge the current paradigm that preterm labor is simply labor occurring earlier in gestation.41 The identification of distinct metabolites in the CV fluid metabolome among women who ultimately have a PTB compared to term birth unveiled important metabolic pathways that are likely involved in the pathogenesis of spontaneous preterm birth. Undoubtedly, future work will need to reveal the contribution of the host and microbiota to these metabolic changes and will need to elucidate the physiological changes that are resulting in the metabolic differences in women who ultimately have a preterm birth. Investigating the CV metabolome holds great promise for unraveling mechanisms involved in term and preterm parturition.
Supplementary Material
Acknowledgments
FUNDING: Maternal and Child Health Research Fund; R01NR014784
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as ORAL CONCURRENT abstract #11 at the 35th Annual Meeting for the Society for Maternal Fetal Medicine in San Diego, CA, Feb. 2nd to 7th, 2015.
The authors have no conflict of interest to disclose.
References
- 1. http://www.marchofdimes.org/peristats/Peristats.aspx. May 30, 2014.
- 2.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. The New England journal of medicine. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. The New England journal of medicine. 2007;357:462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 4.Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction (Cambridge, England) 2007;134:327–40. doi: 10.1530/REP-07-0032. [DOI] [PubMed] [Google Scholar]
- 6.Timmons BC, Mitchell SM, Gilpin C, Mahendroo MS. Dynamic changes in the cervical epithelial tight junction complex and differentiation occur during cervical ripening and parturition. Endocrinology. 2007;148:1278–87. doi: 10.1210/en.2006-0851. [DOI] [PubMed] [Google Scholar]
- 7.Nold C, Anton L, Brown A, Elovitz M. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: a possible mechanism of premature cervical remodeling and preterm birth. American journal of obstetrics and gynecology. 2012;206:208, e1–7. doi: 10.1016/j.ajog.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. Journal of immunology (Baltimore, Md : 1950) 2009;182:2700–7. doi: 10.4049/jimmunol.0803138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? American journal of obstetrics and gynecology. 2008;198:314, e1–8. doi: 10.1016/j.ajog.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Hassan SS, Gajer P, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014;2:18. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman RW, Fukushima M, Jiang H, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reproductive sciences (Thousand Oaks, Calif) 2014;21:32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevin DC, Kuehne A, Zamboni N, Sauer U. Biological insights through nontargeted metabolomics. Current opinion in biotechnology. 2014;34C:1–8. doi: 10.1016/j.copbio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nature reviews Microbiology. 2014;12:661–72. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 15.Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in Crohn's disease: a meta-analysis of population-based cohort studies. The American journal of gastroenterology. 2005;100:2724–9. doi: 10.1111/j.1572-0241.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwabe RF, Jobin C. The microbiome and cancer. Nature reviews Cancer. 2013;13:800–12. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanhaecke L, Knize MG, Noppe H, De Brabander H, Verstraete W, Van de Wiele T. Intestinal bacteria metabolize the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine following consumption of a single cooked chicken meal in humans. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2008;46:140–8. doi: 10.1016/j.fct.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Kassie F, Rabot S, Kundi M, Chabicovsky M, Qin HM, Knasmuller S. Intestinal microflora plays a crucial role in the genotoxicity of the cooked food mutagen 2-amino-3-methylimidazo [4,5-f]quinoline. Carcinogenesis. 2001;22:1721–5. doi: 10.1093/carcin/22.10.1721. [DOI] [PubMed] [Google Scholar]
- 19.Bahado-Singh RO, Akolekar R, Mandal R, et al. Metabolomics and first-trimester prediction of early-onset preeclampsia. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25:1840–7. doi: 10.3109/14767058.2012.680254. [DOI] [PubMed] [Google Scholar]
- 20.Graca G, Goodfellow BJ, Barros AS, et al. UPLC-MS metabolic profiling of second trimester amniotic fluid and maternal urine and comparison with NMR spectral profiling for the identification of pregnancy disorder biomarkers. Molecular bioSystems. 2012;8:1243–54. doi: 10.1039/c2mb05424h. [DOI] [PubMed] [Google Scholar]
- 21.Dunn WB, Brown M, Worton SA, et al. Changes in the metabolic footprint of placental explant-conditioned culture medium identifies metabolic disturbances related to hypoxia and pre-eclampsia. Placenta. 2009;30:974–80. doi: 10.1016/j.placenta.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Ivorra C, Garcia-Vicent C, Chaves FJ, Monleon D, Morales JM, Lurbe E. Metabolomic profiling in blood from umbilical cords of low birth weight newborns. Journal of translational medicine. 2012;10:142. doi: 10.1186/1479-5876-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanos V, Atzori L, Makarenko K, Melis GB, Ferrazzi E. Metabolomics application in maternal-fetal medicine. BioMed research international. 2013;2013:720514. doi: 10.1155/2013/720514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastek JA, Hirshberg A, Chandrasekaran S, et al. Biomarkers and cervical length to predict spontaneous preterm birth in asymptomatic high-risk women. Obstetrics and gynecology. 2013;122:283–9. doi: 10.1097/AOG.0b013e31829ab714. [DOI] [PubMed] [Google Scholar]
- 25.Elovitz MA, Brown AG, Anton L, Gilstrop M, Heiser L, Bastek J. Distinct cervical microRNA profiles are present in women destined to have a preterm birth. American journal of obstetrics and gynecology. 2014;210:221, e1–11. doi: 10.1016/j.ajog.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 26.Iams JD. Clinical practice. Prevention of preterm parturition. The New England journal of medicine. 2014;370:254–61. doi: 10.1056/NEJMcp1103640. [DOI] [PubMed] [Google Scholar]
- 27.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical chemistry. 2009;81:6656–67. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 28.Russell WR, Hoyles L, Flint HJ, Dumas ME. Colonic bacterial metabolites and human health. Current opinion in microbiology. 2013;16:246–54. doi: 10.1016/j.mib.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Nieman DC, Shanely RA, Gillitt ND, Pappan KL, Lila MA. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. Journal of proteome research. 2013;12:4577–84. doi: 10.1021/pr400717j. [DOI] [PubMed] [Google Scholar]
- 30.Menon R, Jones J, Gunst PR, et al. Amniotic fluid metabolomic analysis in spontaneous preterm birth. Reproductive sciences (Thousand Oaks, Calif) 2014;21:791–803. doi: 10.1177/1933719113518987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero R, Mazaki-Tovi S, Vaisbuch E, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23:1344–59. doi: 10.3109/14767058.2010.482618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auray-Blais CRE, Gagnon R, Berthiaume M, Pasquier JC. Metabolomics and preterm birth: What biomarkers in cervicovaginal secretions are predictive of high-risk pregnant women? International Journal of Mass Spectrometry. 2011;307:33–8. [Google Scholar]
- 33.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Human reproduction (Oxford, England) 2001;16:1809–13. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 34.Mirmonsef P, Hotton AL, Gilbert D, et al. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PloS one. 2014;9:e102467. doi: 10.1371/journal.pone.0102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spear GT, French AL, Gilbert D, et al. Human alpha-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. The Journal of infectious diseases. 2014;210:1019–28. doi: 10.1093/infdis/jiu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC microbiology. 2009;9:116. doi: 10.1186/1471-2180-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Annals of the New York Academy of Sciences. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. The Journal of biological chemistry. 2013;288:12067–79. doi: 10.1074/jbc.M113.453654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heng YJ, Di Quinzio MK, Liong S, Permezel M, Rice GE, Georgiou HM. Temporal investigation of matrix metalloproteinases and their inhibitors in human cervicovaginal fluid in late pregnancy and labor. Reproductive sciences (Thousand Oaks, Calif) 2012;19:55–63. doi: 10.1177/1933719111413299. [DOI] [PubMed] [Google Scholar]
- 40.Heng YJ, Di Quinzio MK, Permezel M, Rice GE, Georgiou HM. Cystatin A protease inhibitor and cysteine proteases in human cervicovaginal fluid in term pregnancy and labor. American journal of obstetrics and gynecology. 2011;204:254, e1–7. doi: 10.1016/j.ajog.2010.10.912. [DOI] [PubMed] [Google Scholar]
- 41.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science (New York, NY) 2014;345:760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.