Abstract
Background
The introduction of 7-valent pneumococcal conjugate vaccine (PCV7) into the US childhood immunization schedule in 2000 has substantially reduced vaccine-serotype invasive pneumococcal disease (IPD) in both young children and unvaccinated older children and adults. All-cause pneumonia hospitalizations also markedly declined in young children by 2004. Because of concern about increases in disease caused by non-vaccine serotypes, we assessed whether the pneumonia reduction in young children was sustained through 2009 and whether pneumonia hospitalizations in older age groups also declined.
Methods
Annual all-cause pneumonia hospitalization rates were estimated using the Nationwide Inpatient Sample. Pneumonia hospitalizations were defined by pneumonia listed first or listed in another position if sepsis, meningitis or empyema was the first listed diagnosis. Average annual rates in pre-PCV7 (1997–1999) and late PCV7 years (2007–2009) were used to estimate annual declines in pneumonia hospitalizations.
Results
Annual pneumonia hospitalization rates declined by 551.1 (95% confidence interval 445.1–657.1) per 100,000 children aged <2 years, translating to 47,172 fewer hospitalizations annually compared to expected based on pre-PCV7 rates. The decline of 1300.8 (984.0–1617.6) pneumonia hospitalizations per 100,000 adults aged ≥85 years translated to 73,243 fewer hospitalizations annually. Pneumonia hospitalizations declined by 8.4 (0.6–16.2), 85.3 (7.0–163.6), and 359.8 (199.6–520.0) per 100,000 adults aged 18–39, 65–74 and 75–84 years, respectively. Overall, we estimated an age-adjusted annual reduction of 54.8 (41.1–68.5) per 100,000 or 168,182 fewer pneumonia hospitalizations annually.
Conclusions
Declines in childhood pneumonia were sustained during the decade since PCV7 introduction. Substantial reductions in pneumonia hospitalizations in adults were also observed.
Introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) into the US infant immunization schedule in 2000 resulted in major reductions in the incidence of invasive pneumococcal disease (IPD) in all age groups.1,2 The marked decline of IPD in unvaccinated as well as vaccinated individuals is attributable to PCV7 indirect or “herd” protection. By preventing the acquisition and carriage of vaccine serotypes in the nasopharynx of vaccinated children, PCV7 interfered with this key step in the pathogenesis of pneumococcal disease and reduced transmission of vaccine serotypes.3–6
Pneumococcal pneumonia accounts for 20%–60% of community-acquired pneumonia.7 A reduction in pneumonia was an expected outcome of PCV7 vaccination, as the major US pre-licensure trial reported 30% efficacy against radiographically-defined pneumonia.8 Our previous time series analysis estimated a 39% (95% confidence interval [CI] 22%–52%) reduction in all-cause pneumonia hospitalizations among US children aged <2 years by 2004, associated with PCV7 introduction. More modest declines in other age groups were statistically significant only in adults aged 18–39 years.9 In a model based on hospital discharge data coupled with vaccine uptake information from ten States, Simonsen et al estimated that through 2006, the PCV7 vaccination program had prevented 800,000 US pneumococcal pneumonia hospitalizations.10
Along with the decline in pneumococcal disease attributed to PCV7, however, there was an increase in disease caused by non-vaccine serotypes, in particular serotype 19A.1 There is concern that this “serotype replacement” could have eroded some of the gains from PCV7 introduction.11–13 We aimed to determine whether the early observed reductions in all-cause pneumonia hospitalizations were sustained through the first decade of PCV7 use. We performed a comprehensive evaluation of US pneumonia hospitalizations following PCV7 introduction, prior to the switch to the 13-valent pneumococcal conjugate vaccine (PCV13) in 2010.
METHODS
Data sources
The Agency for Healthcare Research and Quality (AHRQ) collects discharge diagnoses for a 20% sample of US hospitals. This Nationwide Inpatient Sample (NIS)14 is the largest all-payer US inpatient care database, with data from about 8 million hospitalizations annually. The sampling design includes non-Federal community hospitals as the primary sampling units, and all discharges from sampled hospitals. In 2009, the sample included 1050 hospitals in 44 states. Stratification and weighting variables enable calculation of national estimates and trends, accounting for the complex sampling design and the expanded sampling framework over time. Up to 15 discharge diagnoses are coded using the International Classification of Diseases, Ninth revision Clinical Modification (ICD-9-CM) with the first-listed diagnosis regarded as the primary reason for hospitalizations.
Definitions of pneumonia hospitalizations
Pneumonia hospitalizations were defined by International Classification of Diseases 9–Clinical Modification codes. All-cause pneumonia hospitalizations had a first-listed discharge diagnosis of pneumonia (480.xx-486.xx or 487.0) or a first-listed discharge diagnosis of meningitis (321.xx, 013.0.x, 003.21, 036.0, 036.1, 047, 047.0, 047.1, 047.8, 047.9, 049.1, 053.0, 054.72, 072.1, 091.81, 094.2, 098.82, 100.81, 112.83, 114.2, 115.01, 115.11, 115.91, 130.0, 320, 320.0, 320.1, 320.2, 320.3, 320.7, 320.81, 320.82, 320.89, 320.8, 320.9, 322, 322.0, 322.9), septicemia (038.1x, 038.4x, 003.1, 020.2, 022.3, 031.2, 036.2, 038, 038.0, 038.2, 038.3, 038.8, 038.9, 054.5, 785.52, 790.7, 995.91, 995.92) or empyema (510.xx) and a pneumonia diagnosis in another diagnosis field. Pneumococcal pneumonia hospitalizations met the all-cause pneumonia definition, and also had a specific code for pneumococcal infection or a code indicating lobar pneumonia (481.xx, 038.2, 041.2, or 320.1).15
Statistical analysis
NIS data from 1997 to 2009 were used to estimate the annual number of pneumonia hospitalizations for children aged <2, 2–4, and 5–17 years and adults 18–39, 40–64, 65–74, 75–84, and ≥85 years. Annual total and age-specific rates were obtained by dividing the annual number of pneumonia hospitalizations by annual populations from the US Census Bureau and expressed as hospitalizations per 100,000 persons.
We combined three years prior to PCV7 introduction (1997–1999) as baseline years, since no declines in all-cause pneumonia were observed over these years.9 PCV7 was licensed in the US in February, 2000 and vaccine uptake increased rapidly after June 2000 when the US government purchased PCV7 for the Vaccines for Children Program.9 Thus 2000 was considered a transition year and not included in analyses grouped by year. We estimated average annual pneumonia rates for three pre-specified time periods 1997–1999 (pre-PCV7 years), 2001–2006 (early PCV7 years), and 2007–2009 (late PCV7 years). We compared hospitalization rates in late PCV7 years with pre-PCV7 rates and estimated age-specific rate differences and percent declines in annual pneumonia hospitalizations. The choice of 2007–2009 allowed comparison of the most recent 3-year period with the 3-year baseline. The average annual rate was calculated for each time period, with the variance being the sum of each year’s variance divided by the number of years squared. Rate differences were the differences in the average annual rates. The variance of the rate difference was the sum of the average rate variances and the 95% confidence interval was calculated using a normal approximation. Relative rate declines were derived from the rate difference estimates. Age-specific average annual rate differences were applied to the 2009 US population to estimate the absolute annual reductions in pneumonia hospitalizations by 2009.
Role of funding source and human subject considerations
The Centers for Disease Control and Prevention (CDC) funded this research in part and CDC investigators participated in the study design, data review, and preparation for publication. This study was considered exempt research by the Vanderbilt Institutional Review Board.
RESULTS
From 1997 to 2009 there were 17,892,085 all-cause pneumonia hospitalizations representing 4.1% of all US non-birth hospitalizations, including 7.2% of such hospitalizations for children and 3.9% of all hospitalizations for adults. Pneumonia was the first listed diagnosis for 90%, sepsis for 9%, and meningitis and empyema for <1% each.
Pneumonia hospitalization rates
Annual US pneumonia hospitalization rates were highest at the extremes of age in all time periods, with rates for children aged <2 years similar to those of adults aged 65–74 years, around 1000 per 100,000. Rates for persons aged ≥75 years were 2 to 5-fold higher. Pneumonia hospitalizations declined progressively at the extremes of age from pre-PCV7 years to early PCV7 and late PCV7 years (Figure 1).
Fig. 1.
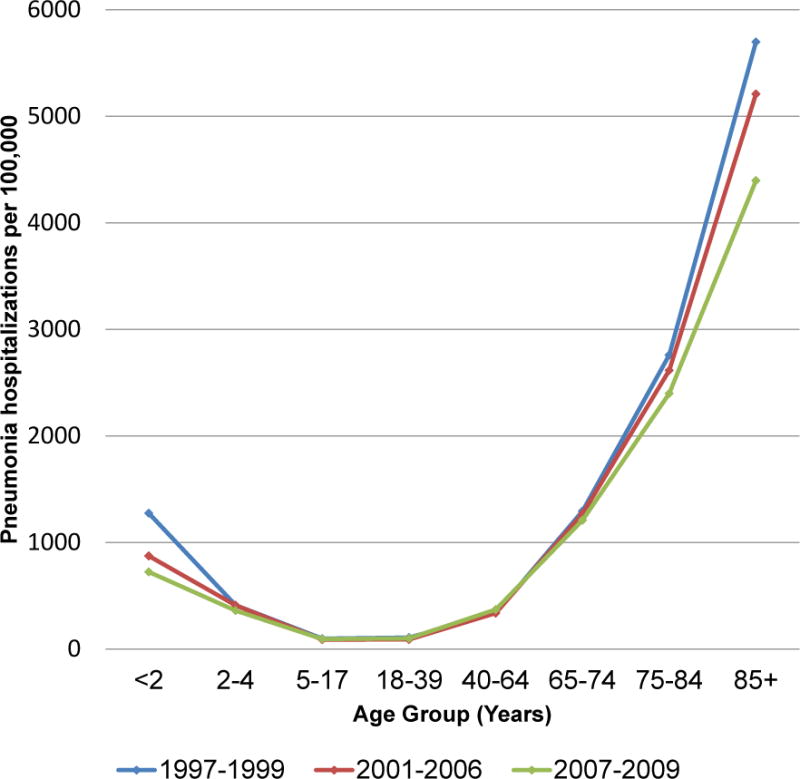
US age-specific average annual rates of all-cause pneumonia hospitalizations per 100,000, for three periods: 1997–1999 (pre-PCV7), 2001–2006 (early PCV7), and 2007–2009 (late PCV7).
Pneumonia hospitalization rates in children aged <2 years declined substantially following PCV7 introduction in 2000. Pneumonia rates in older children were much lower and stable throughout (Figure 2a). Most of the decline in young children occurred quickly following PCV7 introduction. The decline in pneumonia hospitalizations was sustained through late PCV7 years, and rates both for children aged <2 and 2–4 years were lower in late than in pre-PCV7 years. Rates for children aged 5–17 years were the lowest of all age groups and changed little over the study period (Figure 2b).
Fig. 2.
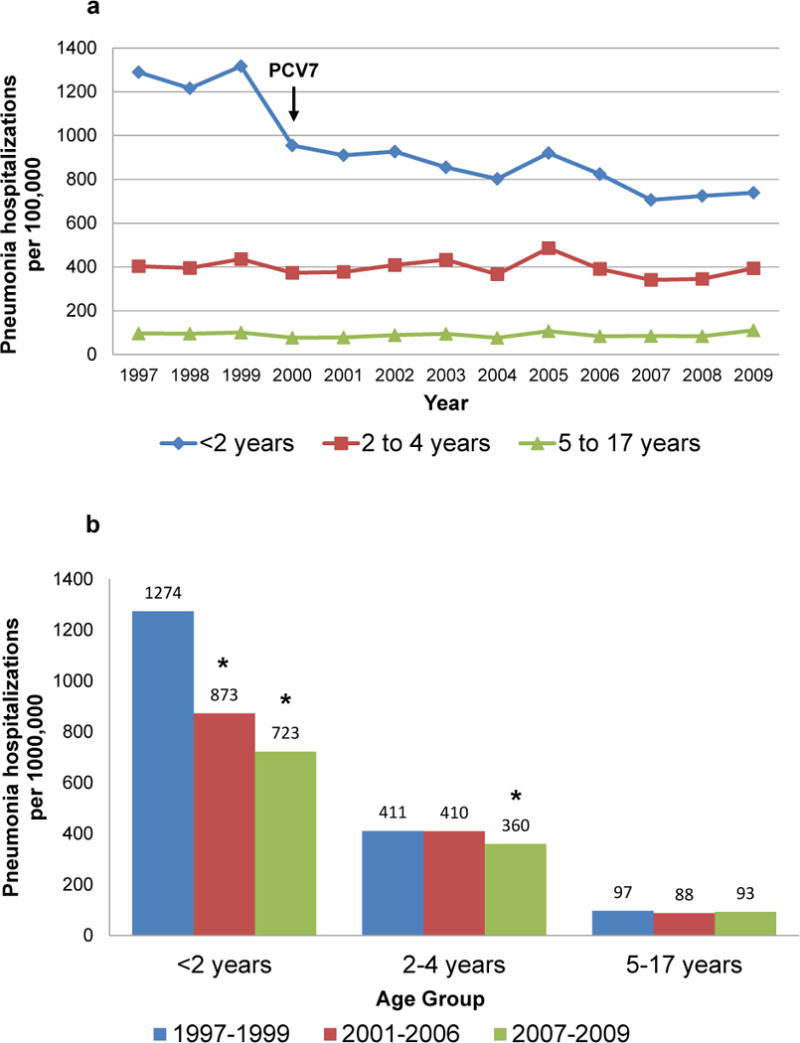
All-cause pneumonia hospitalizations per 100,000 US children, 2a. Annual rates, 1997–2009; 2b. Average annual rates, 1997–1999, 2001–2006, 2007–2009; the year of 7-valent pneumococcal conjugate vaccine (PCV7) introduction, 2000 is not included. * p<.05 for rate decline from pre-PCV7 years
Pneumonia hospitalization rates in most adult age groups also appeared to decline beginning in 2000 (Figure 3a). For adults aged ≥65 years, pneumonia hospitalization rates progressively declined, and rates in late PCV7 years were lower than those in pre-PCV7 years. Small declines were observed early following PCV7 introduction for adults aged 18–39 years and rates in late PCV7 years remained significantly lower than those in pre-PCV7 years; however, rates actually increased modestly in adults aged 40–64 years (Figure 3b).
Fig. 3.
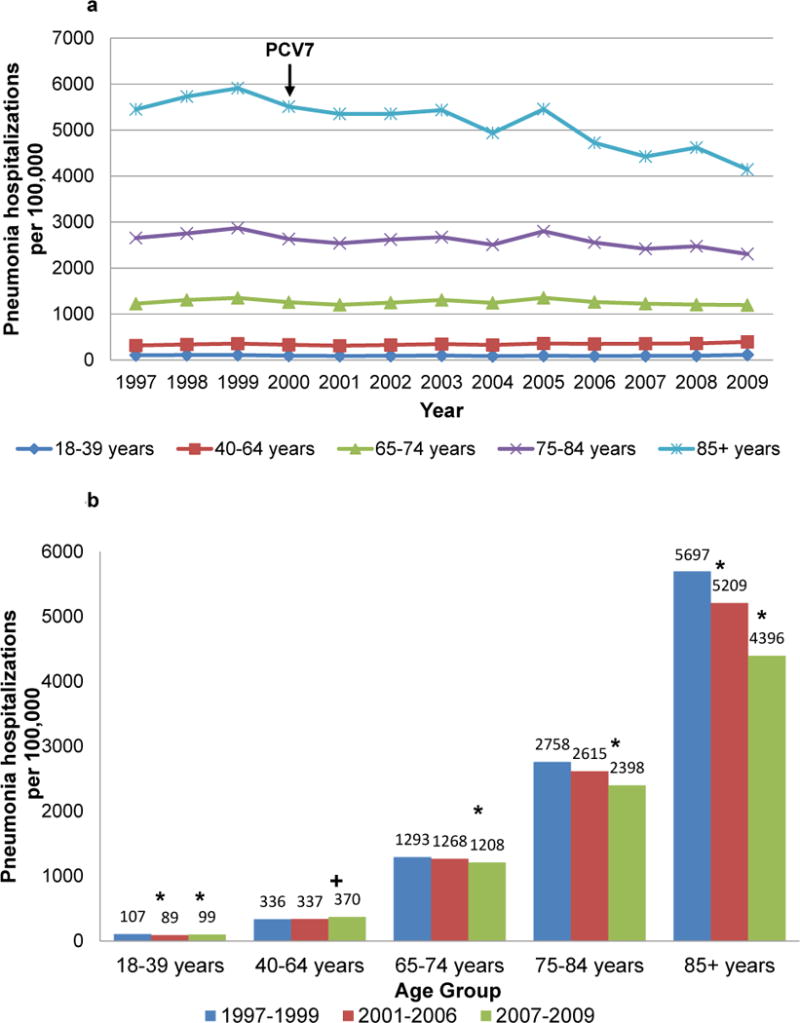
All-cause pneumonia hospitalizations per 100,000 US adults, 3a. Annual rates, 1997–2009; 3b. Average annual rates, 1997–1999, 2001–2006, 2007–2009; the year of 7-valent pneumococcal conjugate vaccine (PCV7) introduction, 2000 is not included. * p<.05 for rate decline from pre-PCV7 years; +p<.05 for rate increase from pre-PCV7 years
Comparison of late PCV7 (2007–2009) with pre-PCV7 (1997–1999) years
In both the pre-PCV7 and late PCV7 years, length of stay increased with increasing age (Table 1), but was consistently shorter in late PCV7 years for all age groups. The proportion of pneumonias coded as pneumococcal was smaller at the extremes of age in both time periods, but declined in all age groups over time. In-hospital mortality was lowest in those aged 2–4 years (0.2%) and increased with increasing age. Over time, in-hospital mortality remained similar or declined at the extremes of age; whereas, mortality increased in young adults.
Table 1.
All cause pneumonia rates and characteristics Pre-PCV7 (1997–1999) versus late PCV7 (2007–2009)
| Pneumonia rates and characteristics | Time period | Age Group (years)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| <2 | 2–4 | 5–17 | 18–39 | 40–64 | 65–74 | 75–84 | 85+ | ||
|
|
|||||||||
| Average annual hospitalizations per 100,000 | Pre-PCV7 | 1274 (1185–1364) |
411 (383–439) |
97 (91–104) |
107 (102–113) |
336 (321–351) |
1293 (1239–1348) |
2758 (2643–2873) |
5697 (5457–5937) |
|
| |||||||||
| Late PCV7 | 723 (666–780) |
360 (329–391) |
93 (85–101) |
99 (93–104) |
370 (351–388) |
1208 (1152–1264) |
2398 (2287–2510) |
4396 (4189–4603) |
|
|
|
|||||||||
| Median (IQR) stay in days | Pre-PCV7 | 2.3 (1.4–3.6) |
2.0 (1.2–3.1) |
2.3 (1.4–3.8) |
3.2 (1.9–5.4) |
4.0 (2.4–6.6) |
4.6 (2.8–7.5) |
4.9 (3.0–7.9) |
5.2 (3.2–8.2) |
|
| |||||||||
| Late PCV7 | 1.9 (1.1–3.2) |
1.8 (1.1–2.9) |
2.0 (1.2–3.6) |
3.0 (1.7–5.7) |
3.8 (2.1–6.9) |
4.2 (2.4–7.4) |
4.3 (2.6–7.5) |
4.4 (2.6–7.2) |
|
|
|
|||||||||
| % Coded as pneumococcal | Pre-PCV7 | 2.2 (1.9–2.5) |
3.2 (2.8–3.5) |
4.2 (3.9–4.6) |
8.4 (7.6–9.2) |
7.4 (7.0–7.8) |
5.6 (5.4–5.8) |
4.6 (4.5–4.8) |
3.7 (3.6–3.9) |
|
| |||||||||
| Late PCV7 | 1.5 (1.3–1.7) |
2.7 (2.3–3) |
2.9 (2.6–3.2) |
4.6 (4.4–4.8) |
4.8 (4.6–4.9) |
3.5 (3.3–3.6) |
2.6 (2.5–2.7) |
2.2 (2.1–2.4) |
|
|
|
|||||||||
| % In-hospital death | Pre-PCV7 | 0.2 (0.2–0.3) |
0.2 (0.1–0.3) |
0.4 (0.3–0.5) |
1.8 (1.6–1.9) |
4.3 (4.2–4.5) |
6.8 (6.7–7.0) |
9.3 (9.2–9.4) |
13.0 (12.8–13.2) |
|
| |||||||||
| Late PCV7 | 0.2 (0.1–0.2) |
0.1 (0.1–0.2) |
0.4 (0.3–0.5) |
2.2 (2–2.4) |
4.9 (4.7–5.1) |
7.0 (6.9–7.2) |
8.7 (8.6–8.8) |
11.5 (11.3–11.7) |
|
Comparing late and pre-PCV7 years, the largest absolute and relative reductions in pneumonia hospitalizations were at the extremes of age (Table 2). The decline of 551.1 (95% CI 445.1–657.1) pneumonia hospitalizations per 100,000 children aged <2 years or 43.2% (95% CI 34.9%–51.6%) translated to 47,172 fewer hospitalizations in 2009 than expected if rates were unchanged from pre-PCV7 years. The decline of 1300.8 (95% CI 984.0–1617.6) pneumonia hospitalizations per 100,000 adults aged ≥85 years or 22.8% (95% CI 17.3%–28.4%) translated to 73,243 fewer than expected hospitalizations in 2009. Smaller absolute declines of 8.4 (95% CI 0.6–16.2), 85.3 (95% CI 7.0–163.6) and 359.8 (95% CI199.6–520.0) pneumonia hospitalizations per 100,000 adults aged 18–39, 65–74 and 75–84 years resulted in substantial annual pneumonia hospitalization reductions. The overall age-adjusted annual rate difference of 54.8 (95% CI 41.0–68.5) per 100,000 yielded an estimate of 168,182 fewer pneumonia hospitalizations in 2009 than expected based on rates in pre-PCV7 years.
Table 2.
Rate differences per 100,000 in all cause pneumonia hospitalizations per between Pre-PCV7 (1997–1999) and late PCV7 (2007–2009) and estimated absolute reduction in 2009
| Age (years) | US 2009 population (millions) | Rate Difference per 100,000, 1997–1999 to 2007–2009 (95% CI) | % Reduction 1997–1999 to 2007–2009 | Estimated absolute reduction 2009 |
|---|---|---|---|---|
| <2 | 8.6 | 551.1 (445.1 to 657.1) |
43.2 (34.9 to 51.6) |
47,172 (38,099 to 56,245) |
| 2–4 | 12.7 | 51.3 (9.8 to 92.8) |
12.5 (2.4 to 22.6) |
6,536 (1,249 to 11,823) |
| 5–17 | 53.2 | 4.4 (−5.9 to 14.7) |
4.5 (−6.1 to 15.1) |
2,343 (−3,142 to 7,828) |
| 18–39 | 92.5 | 8.4 (0.6 to 16.2) |
7.8 (0.6 to15.1) |
7,771 (555 to 14,988) |
| 40–64 | 100.4 | −33.8 (−57.4 to −10.2) |
−10.1 (−17.1 to −3.0) |
−33,925 (−57.613 to −10,238) |
| 65–74 | 20.8 | 85.3 (7.0 to 163.6) |
6.6 (0.5 to 12.7) |
17,736 (1,455 to 34,016) |
| 75–84 | 13.1 | 359.8 (199.6 to 520.0) |
13.0 (7.2 to18.9) |
47,306 (26,243 to 68,369) |
| 85+ | 5.6 | 1300.8 (984.0 to 1617.6) |
22.8 (17.3 to 28.4) |
73,243 (55,406 to 91,082) |
| Total age adjusted | 307.0 | 54.8 (41.0 to 68.5) |
10.5 (7.9 to 13.1) |
168,182 (125,873 to 210,299) |
DISCUSSION
Early remarkable declines in US pneumonia hospitalizations among young children9 were sustained during the past decade of PCV7 use, decreasing concerns that disease caused by pneumococcal “serotype replacement” would significantly erode the benefits of vaccination.11 Furthermore, pneumonia hospitalizations also declined in other age groups, most notably in older adults, who have a substantial pneumonia disease burden associated with an in-hospital fatality of 7%–12%. Overall, in 2009 there were about 168,000 fewer pneumonia hospitalizations than expected in the US, based on rates from pre-PCV7 years. This estimated annual reduction in pneumonia hospitalizations is five times higher than the annual reduction in IPD.1
Although 20%–60% of community acquired pneumonias are thought to be pneumococcal, only a small proportion of all-cause pneumonias receive a pneumococcal diagnosis (Table 1), and serotype information is usually unavailable.7 Attribution of all-cause pneumonia trends to the PCV7 vaccination program requires understanding expected changes in all-cause pneumonia in young children based on pre-licensure randomized clinical trials and changes in pneumococcal nasopharyngeal carriage that account for indirect vaccination effects.
The 43% decline in annual pneumonia hospitalizations in children aged <2 years is consistent with clinical trial results. In the Kaiser Permanente pre-licensure trial, PCV7 reduced radiographically-defined pneumonia by 30% (95% CI 11%–46%).8 Randomized trials conducted in South Africa, the Philippines and the Gambia reported declines in pneumonia of 17% to 37%.16
PCV7 had a major effect on pneumococcal carriage. Carriage of PCV7 serotypes was markedly reduced by 2009 and non-vaccine serotypes became the dominant colonizers.5,17,18 Indirect protection against IPD in unvaccinated groups was first reported in the Journal in 2003.191
Prior to PCV7 introduction, vaccine serotypes caused 80% of IPD in young children. The proportion of IPD caused by vaccine serotypes was considerably lower in adults, but increased with increasing age reaching 51% in those ≥85 years.20 Seven years after PCV7 introduction, vaccine-serotype IPD was almost eliminated in children aged <5 years, and declined >85% in unvaccinated age groups. Total IPD, which includes disease caused by vaccine serotypes plus non-vaccine serotypes, declined 76% in children aged <5 years, and 43%, 40%, 18%, and 37% in those aged 5–17, 18–49, 50–64, and ≥65 years, respectively.1 These total reductions in IPD encompass the decline in vaccine-serotype disease and the increase in non-vaccine serotype disease.11
The reduction in pneumonia hospitalizations in unvaccinated age groups is perhaps more remarkable than the decline in pneumonia hospitalizations in young children, targeted for vaccination. Indeed, older adults accounted for more than half of the decline in overall pneumonia hospitalizations. Patterns of all-cause pneumonia declines are similar to those observed for total IPD, with the largest relative and absolute reductions at the extremes of age.1,2 For both IPD and pneumonia, the potential for disease reduction was highest at the extremes of age, because vaccine-serotype disease was highest in these age groups before PCV7 introduction.20
Substantial reductions in childhood pneumonia have been reproduced in other countries that introduced PCV7. In Australia, a time-series analysis found 38% and 29% reductions in all-cause pneumonias in children <2 and 2–4 years, two years after vaccine introduction in 2005.21 In the UK, pneumonia hospitalizations in children <15 years, which had been increasing through 2006, when PCV7 was introduced, fell 19% by 2008.22 The association of pneumonia declines with timing of vaccine introduction in multiple countries supports a causal association, and renders other secular trends unlikely contributors.
Attribution of changes in all-cause pneumonia to the PCV7 vaccination program also requires consideration of changes in coding practices, hospital admission thresholds, and other interventions. Despite stable overall hospitalization rates in older adults 2000–2009, there were major declines in hospitalizations coded as pneumonia, congestive heart failure and coronary atherosclerosis.14 Lindenauer et al explored changes in coding of pneumonia over the last decade and found increasing use of sepsis as the first listed diagnosis and pneumonia as a secondary rather than primary (first-listed) diagnosis.23 Our definition of pneumonia included hospitalizations in which sepsis was the primary diagnosis, so our reported rates were not influenced by any known coding changes.
Pneumonia is the only common childhood condition for which hospitalizations declined during the study period,14 and the decline was temporally associated with PCV7 introduction.9 Among children, this decline was associated with no concurrent increases in non-pneumonia respiratory hospitalizations,24 and no increases or actual decreases in outpatient pneumonia visits.25–28 Furthermore, the decline in length of stay and stability of pneumonia case fatality ratios (Table 1) suggest no major increases in admission thresholds.
Influenza vaccination is an unlikely major contributor to these declines in childhood pneumonia since vaccination of young children was uncommon prior to initial (2004) and expanded (2006) recommendations for influenza vaccination of healthy young children, and only reached 30% by 2009.29 Among adults, the major increases in pneumococcal and influenza vaccination pre-date PCV7 introduction. In adults ≥65 years, both pneumococcal polysaccharide and influenza vaccination more than doubled prior to PCV7 introduction from 14% and 30% respectively in 1989 to 50% and 66% in 1999.30 Likewise, adult smoking fell from 40% in the 1970s to 26% in the 1990s.31. During this pre-PCV7 period in which major changes in other immunizations and smoking occurred, pneumonia hospitalizations in adults actually increased.32 Since PCV7 introduction, there have been much more modest changes, pneumococcal and influenza immunizations increased to 60% and 67% in 2008,30 and adult smoking fell to 24%.31
Notwithstanding the substantial reductions in pneumonia observed following PCV7 introduction, some residual burden of pneumococcal disease due to vaccine serotypes remains in unvaccinated age groups.13 In the UK, a novel urinary immunoassay was used to diagnose serotype specific pneumococcal pneumonia in hospitalized adults two years after PCV7 introduction. Investigators estimated that 40% of pneumonias were pneumococcal, that at least 20% of these were due to PCV7 serotypes, and that this proportion increased with increasing age.33 So, two years after vaccine introduction and despite a rapid decline in childhood IPD, a substantial portion of adult pneumonias were still due to vaccine-serotypes. This is consistent with the slower decline in IPD due to PCV7 serotypes in US adults1 and the modest decline in adult pneumonia in the early PCV7 period.
Currently, it is unknown whether direct vaccination of adults with PCV13 vaccine would prevent adult pneumonia and whether such efforts would add substantially to indirect benefits generated by the ongoing infant PCV13 vaccination program which may currently be reducing the transmission of the additional six vaccine serotypes.34,35 PCV13 has recently been recommended for US adults with immunocompromising conditions,36 a group for which efficacy data are already available37 and indirect benefits are likely to be modest.13,38
In summary, the remarkable reduction initially observed in childhood pneumonia was sustained in the decade since PCV7 introduction. More modest relative declines in pneumonia in older adults emerged more slowly and resulted in large absolute reductions in pneumonia hospitalizations.
Acknowledgments
Supported in part by Centers for Disease Control and Prevention Intergovernmental Personnel Agreement 11-IPA1110211 (MRG), 12-IPA1210402 (CGG) and Thrasher Research Fund Award No. 02832-9 (CGG).
References
- 1.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 2.Rosen JB, Thomas AR, Lexau CA, et al. Geographic variation in invasive pneumococcal disease following pneumococcal conjugate vaccine introduction in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53:137–43. doi: 10.1093/cid/cir326. [DOI] [PubMed] [Google Scholar]
- 3.Kayhty H, Auranen K, Nohynek H, Dagan R, Makela H. Nasopharyngeal colonization: a target for pneumococcal vaccination. Expert Rev Vaccines. 2006;5:651–67. doi: 10.1586/14760584.5.5.651. [DOI] [PubMed] [Google Scholar]
- 4.Klugman KP. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect Dis. 2001;1:85–91. doi: 10.1016/S1473-3099(01)00063-9. [DOI] [PubMed] [Google Scholar]
- 5.Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammitt LL, Bruden DL, Butler JC, et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis. 2006;193:1487–94. doi: 10.1086/503805. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;52(Suppl 4):S296–304. doi: 10.1093/cid/cir045. [DOI] [PubMed] [Google Scholar]
- 8.Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006;25:779–81. doi: 10.1097/01.inf.0000232706.35674.2f. [DOI] [PubMed] [Google Scholar]
- 9.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 10.Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio. 2011;2:e00309–10. doi: 10.1128/mBio.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. Jama. 2007;297:1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 13.Muhammad RD, Oza-Frank R, Zell E, et al. Epidemiology of Invasive Pneumococcal Disease Among High-Risk Adults Since the Introduction of Pneumococcal Conjugate Vaccine for Children. Clin Infect Dis. 2012 doi: 10.1093/cid/cis971. [DOI] [PubMed] [Google Scholar]
- 14.HCUP Facts and Figures: Statistics on Hospital-based care in the United States, 2009. Agency for Healthcare Research and Quality; 2009. Accessed 2011, at http://www.hcup-us.ahrq.gov\reports.jsp. [PubMed] [Google Scholar]
- 15.Grijalva CG, Nuorti JP, Zhu Y, Griffin MR. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50:805–13. doi: 10.1086/650573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien KL. Pneumococcal vaccine and the dance of the veils: revealing pneumonia burden in Asia. Pediatr Infect Dis J. 2009;28:463–5. doi: 10.1097/inf.0b013e31819b9085. [DOI] [PubMed] [Google Scholar]
- 17.Pelton SI, Loughlin AM, Marchant CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2004;23:1015–22. doi: 10.1097/01.inf.0000143645.58215.f0. [DOI] [PubMed] [Google Scholar]
- 18.Wroe PC, Lee GM, Finkelstein JA, et al. Pneumococcal carriage and antibiotic resistance in young children before 13-valent conjugate vaccine. Pediatr Infect Dis J. 2012;31:249–54. doi: 10.1097/INF.0b013e31824214ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 20.Feikin DR, Klugman KP, Facklam RR, Zell ER, Schuchat A, Whitney CG. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clin Infect Dis. 2005;41:481–7. doi: 10.1086/432015. [DOI] [PubMed] [Google Scholar]
- 21.Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J. 2010;29:607–12. doi: 10.1097/inf.0b013e3181d7d09c. [DOI] [PubMed] [Google Scholar]
- 22.Koshy E, Murray J, Bottle A, Sharland M, Saxena S. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997–2008. Thorax. 2010;65:770–4. doi: 10.1136/thx.2010.137802. [DOI] [PubMed] [Google Scholar]
- 23.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307:1405–13. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease C. Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine-United States, 1997–2006. MMWR Morb Mortal Wkly Rep. 2009;58:1–4. [PubMed] [Google Scholar]
- 25.Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch Pediatr Adolesc Med. 2007;161:1162–8. doi: 10.1001/archpedi.161.12.1162. [DOI] [PubMed] [Google Scholar]
- 26.Poehling KA, Lafleur BJ, Szilagyi PG, et al. Population-based impact of pneumococcal conjugate vaccine in young children. Pediatrics. 2004;114:755–61. doi: 10.1542/peds.2003-0592-F. [DOI] [PubMed] [Google Scholar]
- 27.Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865–73. doi: 10.1542/peds.2006-0492. [DOI] [PubMed] [Google Scholar]
- 28.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758–66. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Influenza vaccination coverage among children aged 6 months–18 years—eight immunization information system sentinel sites, United States, 2008–09 influenza season. MMWR Morb Mortal Wkly Rep. 2009;58:1059. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Vaccination Trends. available from http://www.cdc.gov/flu/fluvaxview/trends.htm. accessed on March 19, 2013.
- 31.Gallup. U.S. Smoking Rate Still Coming Down. available from http://www.gallup.com/poll/109048/us-smoking-rate-still-coming-down.aspx. accessed March 19, 2013.
- 32.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294:2712–9. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 33.Bewick T, Sheppard C, Greenwood S, et al. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax. 2012 doi: 10.1136/thoraxjnl-2011-201092. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro ED. Prevention of pneumococcal infection with vaccines: an evolving story. JAMA. 2012;307:847–9. doi: 10.1001/jama.2012.194. [DOI] [PubMed] [Google Scholar]
- 35.Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55:265–7. doi: 10.1093/cid/cis364. [DOI] [PubMed] [Google Scholar]
- 36.Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2012;61:816–9. [PubMed] [Google Scholar]
- 37.French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362:812–22. doi: 10.1056/NEJMoa0903029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen AL, Harrison LH, Farley MM, et al. Prevention of invasive pneumococcal disease among HIV-infected adults in the era of childhood pneumococcal immunization. AIDS. 2010;24:2253–62. doi: 10.1097/QAD.0b013e32833d46fd. [DOI] [PubMed] [Google Scholar]


