Abstract
Host-microbe interactions may play a fundamental role in the pathogenesis of atopic dermatitis (AD), a chronic relapsing inflammatory skin disorder characterized by universal colonization with Staphylococcus. To examine the relationship between epidermal barrier function and the cutaneous microbiota in AD, this study employed a spontaneous model of canine AD (cAD). In a cohort of 14 dogs with cAD, the skin microbiota was longitudinally evaluated with parallel assessment of skin barrier function at disease flare, during antimicrobial therapy and posttherapy. Sequencing of the bacterial 16S ribosomal RNA gene revealed decreased bacterial diversity and increased proportions of Staphylococcus (S. pseudintermedius in particular) and Corynebacterium in comparison to a cohort of healthy control dogs (n=16). Treatment restored bacterial diversity with decreased Staphylococcus proportions, concurrent with decreased cAD severity. Skin barrier function, as measured by corneometry, pH, and transepidermal water loss (TEWL) also normalized with treatment. Bacterial diversity correlated with TEWL and pH, but not corneometry. These findings provide insights into the relationship between the cutaneous microbiome and skin barrier function in AD, the impact of antimicrobial therapy on the skin microbiome, and highlight the utility of cAD as a spontaneous non-rodent model of AD.
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that affects approximately 10% of children (Spergel, 2010) and is commonly associated with Staphylococcus aureus colonization (Leyden et al., 1974). Genetic risk conferred by mutations in the gene encoding the epidermal barrier protein filaggrin suggests that barrier dysfunction in part contributes to the disease (O’Regan et al., 2009). Environmental factors, including staphylococcal colonization and infection, may also contribute to disease etiology and/or severity. Recent studies have highlighted the dysbiotic nature of the AD skin microbiome, including a predominance of S. aureus during active flares, suggesting a role for S. aureus and the skin microbiome in atopic inflammation (Kong et al., 2012),
Mouse models have highlighted effects of specific genetic changes in AD, but are limited in their clinical similarity and do not recapitulate the complexity of the human disease (Scharschmidt & Segre, 2008; Marsella & Girolomoni, 2009). Canine atopic dermatitis (cAD) occurs spontaneously and exhibits similar immunological and clinical features of human AD, therefore providing a useful intermediate model (Marsella & Girolomoni, 2009). cAD affects approximately 10% of dogs, and presents with similar lesion distribution, life stage of onset, IgE-specific immune responses, and predisposition to chronic and recurrent superficial bacterial dermatitis and folliculitis (Hillier & Griffin, 2001; Santoro et al., 2015). Epidermal barrier function is impaired in cAD, suggesting a route for epicutaneous sensitization (Santoro et al., 2015). The pathogenesis of cAD is multifactorial and complex interactions between genetics and environment are hypothesized, as in human AD (Bizikova et al., 2015).
Flare states in atopic humans and dogs are associated with colonization and/or superficial infection by Staphylococcus species: S. aureus in humans and S. pseudintermedius or S. schleiferi in dogs (Fazakerley et al., 2009; Furiani et al., 2011; Kong et al., 2012; Leyden et al., 1974; Santoro et al., 2015). Recent studies in an Adam17-deficient mouse model suggest that S. aureus drives lesion formation (Kobayashi et al., 2015). Toxins produced by S. aureus are hypothesized to trigger or exacerbate inflammation in AD (Williams et al., 2015), such as the δ-toxin recently shown to induce mast cell degranulation and promote inflammatory skin disease (Nakamura et al., 2013). Further understanding of host-microbe dynamics during flare, treatment and resolution is critical for improved therapies to manage atopic inflammation.
Herein, an integrated analysis of the canine cutaneous microbiome and the skin barrier in cAD is reported. The skin microbiome was defined using culture-independent sequencing of the 16S ribosomal RNA (rRNA) gene before, during, and after antimicrobial treatment. In parallel, quantitative assessment of the skin barrier was measured using transepidermal water loss (TEWL), epidermal moisture content (corneometry), and pH. The results of this study should be translated to inform future studies of the functional relationship between host cutaneous barrier function and the skin microbiome of humans and dogs.
RESULTS
Summary of study participants and design
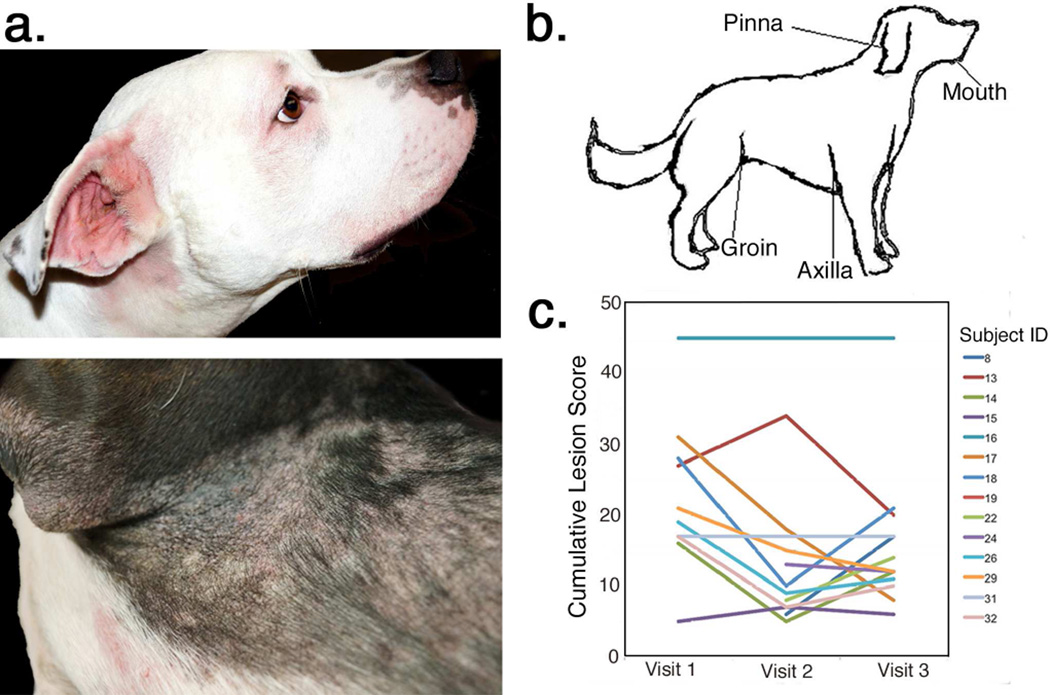
Thirty-two dogs (n=15 affected by cAD and n=17 unaffected) were enrolled from September 3, 2013 to March 7, 2014 at the University of Pennsylvania Matthew J. Ryan Veterinary Hospital (Table 1, Table S1). One dog in each cohort was excluded due to unrelated medical problems. All cAD subjects had active lesions of superficial bacterial dermatitis and folliculitis at enrollment (Figure 1a).
Table 1.
Signalment data of study cohorts.
| cAD | Control | |
|---|---|---|
| N | 14 | 16 |
| Median age, months (range) | 71 (10–120) | 79.5 (8–144) |
| Males:Females | 5:9 | 1:1 |
| Spayed/Neutered | 13 | 15 |
| Average Cumulative Lesion Score | ||
| Visit 1 | 22 | 0 |
| Visit 2 | 14.92 | 0 |
| Visit 3 | 15.93 | 0 |
FIGURE 1. Canine atopic dermatitis.
(a) Typical clinical findings of cAD include alopecia and erythema of the periocular region and muzzle as well as evidence of chronic dermatitis and folliculitis in regions such as the pinna, axilla, and groin. (b) The anatomic sites sampled for microbiomic analysis included the mouth, axilla, groin and concave pinna. (c) Lesion scores (x-axis) measuring cumulative site-specific lesion severity at each study visit (y-axis). Each line represents one subject with cAD (n=14).
The skin was swabbed to sample microbiota at anatomic sites with a predilection for cAD lesions: the pinna, axilla, and groin (Figure 1b). The mouth was also sampled, as licking of the skin is a manifestation of pruritus in dogs. Assessment and sampling occurred at three study visits: visit 1 at initial presentation with a flare of cAD and concurrent bacterial dermatitis; visit 2 at the conclusion of 4–6 weeks of culture- and susceptibility-directed oral antimicrobial therapy; and visit 3 at 4–6 weeks following the conclusion of antimicrobial therapy. Healthy dogs were assessed and sampled contemporaneously.
Site-specific (pinna, axilla, and groin) assessment and semi-quantitative scoring of lesion severity was performed based off of the parameters used for CADESI-03 (Canine Atopic Dermatitis Extent and Severity Index) (Olivry et al., 2007). This scoring was performed to assess the relationship between microbiota, skin barrier, and clinical signs at a given site. Cumulative site-specific lesion scores varied widely in dogs with cAD (range=5–45) (Figure 1c). The components of the site-specific lesion scores, erythema, lichenification, and alopecia, were strongly positively correlated with each other (Table S3). Cumulative lesion scores decreased with antimicrobial treatment from median of 19 (±12.1) at visit 1 to a median of 10 (±13.1) at visit 2, though this decrease was not significant (p=0.56). Subject 16 was identified as a minor outlier, with a cumulative lesion score of 45 and a severe phenotype of chronic cAD in which four weeks of therapy was unlikely to alter the degree of chronic dermatitis and scarring. Upon exclusion of this subject from this particular analysis, the median cumulative lesion score significantly dropped with treatment, from 18 (±7.5) at visit 1 to 9.5 (±8.1) at visit 2 (p=0.035) (Table 1, Table S1, Figure 1c). This minor outlier was not removed from any of the subsequent analyses. Five of 14 subjects had an elevated cumulative lesion score at visit 3 compared to visit 2, corresponding to recrudesce of bacterial dermatitis in the post treatment interval, but this change was not significant across the entire cohort (p=0.26).
The microbiome of cAD skin differs from normal canine skin
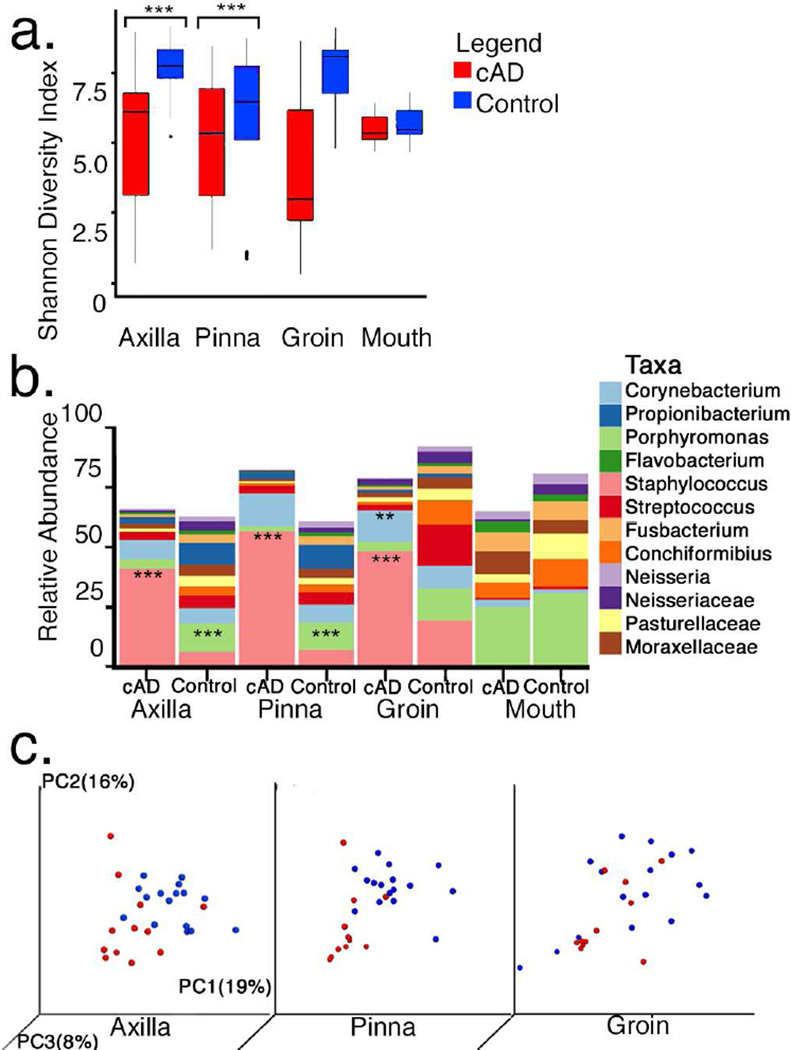
To analyze microbial communities in cAD and control dogs, the V1–V3 region of the 16S rRNA gene was amplified and sequenced from skin swabs. The skin microbiome of cAD and unaffected control dogs differed at visit 1, prior to commencing treatment. Shannon diversity index, an alpha diversity metric that takes into account the number of taxa present as well as their abundance in the community, was significantly lower in the cAD group compared to the control group (p=0.0001; Figure 2a, Figure S1). These differences were significant at the pinna and axilla, but not at the groin or mouth (pinna: p=5.0×10−4; axilla: p=0.006; groin: p=0.075; mouth: p=0.363; Figure 2a) and reiterated with numerous other alpha diversity metrics (Figure S1).
FIGURE 2. The microbiome of cAD during flares and prior to treatment.
(a) Median Shannon diversity index for each anatomic site sampled in cAD dogs (red) compared to unaffected dogs (blue). Line in box represents median, boxes represent interquartile range, whiskers represent lowest and highest values within 1.5-times the interquartile range, and dots represent outliers. (b) Median taxonomic relative abundance (y-axis) of the 12 genera present in greatest abundance. cAD and control dogs are partitioned by anatomical site sampled (x-axis). (c) Principle coordinates analysis of the weighted UniFrac metric comparing cAD (red) and unaffected controls (blue) in the axilla and pinna. Clustering is significant as assessed by the ANOSIM test (axilla: R=0.403, p=0.001; pinna: R=0.639, p= 0.001for axilla and pinna). Percent variability explained by each axis is given.
The predominant bacteria on healthy canine skin were Porphyromonas, Staphylococcus, Streptococcus, Propionibacterium, Corynebacterium and genera belonging to the families Neisseriaceae and Moraxellaceae (Figure 2B, Figure S2). Though the same taxa were present in cAD and control dogs, the relative abundance of taxa varied dramatically between the two groups. Dogs with cAD flares had significantly increased relative abundance of Staphylococcus (cAD median=45%±29%; control median=5%±18%; p<1.0×10−4) across all skin sites. There was a decreased relative abundance of Porphyromonas in the pinna (cAD median=1%±3%; control median=10%±6%; p=1.0×10−4) and axilla (cAD median=2±5%; control median=10%±7%; p=1.0×10−4). In the groin, there was a significant median increase in the relative abundance of Corynebacterium in cAD (cAD median=9%±13%, control median=1%±16%; p=0.003) (Figure 2b, Figure S2). This trend was similar in the axilla and pinna though it did not reach significance. In the oral cavity the most abundant taxa did not differ significantly between control and cAD dogs and consisted of many anaerobes including Porphyromonas, Conchiformibius, Fusobacterium, unclassified Moraxellaceae, Flavobacterium and unclassified Prevotellaceae (Figure 2b, Figure S2). Skin microbial communities of cAD dogs were significantly different from control dogs as determined by the weighted UniFrac metric, a distance metric that takes into account shared phylogeny and is weighted for abundance of observed organisms in the community (R=0.445, p=0.001; ANOSIM test). Similar differences were observed when examining each anatomical site individually and visualizing clustering using principle coordinates analysis (axilla: R=0.403, p=0.001; pinna: R=0.639, p=0.001; groin: R= 0.297, p=0.002; ANOSIM test; Figure 2c). Significant clustering was not associated with patient sex, breed, age, or antimicrobial class used during treatment (Figure S3).
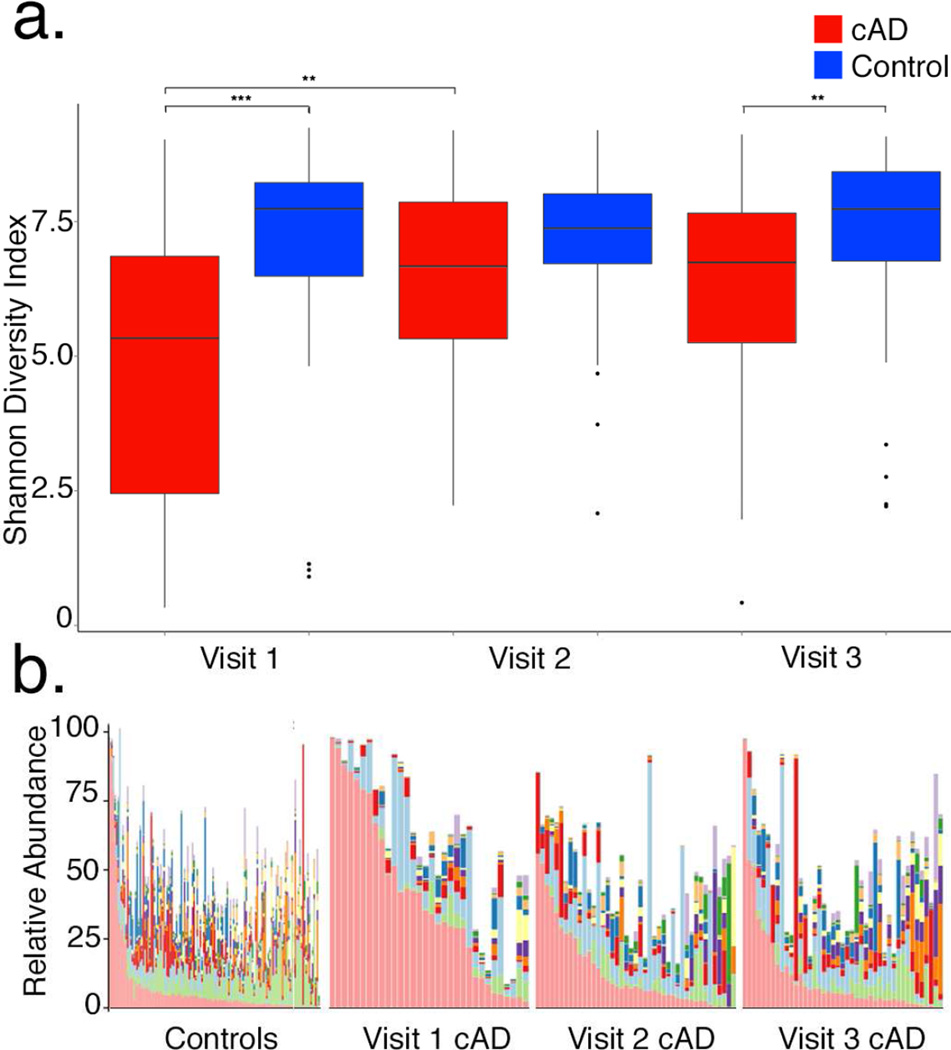
Treatment normalizes the cAD microbiome
The Shannon diversity index increased across skin sites during treatment of dogs with cAD between visit 1 and 2 (p=0.004; Figure 3a), and approached mean Shannon diversity index values observed in the control group at visit 2 (control=7.17±1.44; cAD = 6.53 ± 1.74; Figure 3a). There was no longer a statistically significant difference in Shannon diversity between control and cAD dogs at visit 2 (p=0.090), but significant differences re-emerged at visit 3 during the post-treatment interval (p=0.008; Figure 3a). These findings suggest that antimicrobial therapy restores diversity of the skin microbiome in cAD, but the effects may dissipate once treatment is withdrawn or if bacterial dermatitis recurs.
FIGURE 3. Skin microbiome changes during treatment of cAD.
(a) Change in Shannon Diversity Index longitudinally with treatment. Line in box represents median, boxes represent interquartile range, whiskers represent lowest and highest values within 1.5-times the interquartile range, and dots represent outliers. (b) Median taxonomic relative abundance. See Figure 2 for the taxonomic legend.
To further examine the relationship between treatment, disease severity, and microbial diversity, correlations between microbial diversity and lesion scores were analyzed. The Shannon diversity index was significantly inversely correlated with site-specific erythema (R=−0.467; p<1.0×10−4), alopecia (R=−0.379; p<1.0×10−4), lichenification (R=−0.454; p<1.0×10−4) and total lesion scoring (R=−0.458; p=1.0×10−4) (Table S3), suggesting that decreased microbial diversity is associated with lesion severity at cAD predilection sites.
At the taxonomic level, concurrent with increased diversity, relative abundance of Staphylococcus decreased a median of 45% to 6% ± 16% (p<0.001) across all skin sites of cAD dogs from visits 1 to 2. Corynebacterium spp. increased in cAD dogs (median 8% ± 9%) compared to the control group (4% ± 4%) at visit 3 (p=0.007), relative to control dogs (Figure 2b, Figure S2). Clustering by the weighted UniFrac metric also decreased with treatment when comparing dogs with cAD to control dogs (R=0.0502, p=0.002 at visit 3; R=0.445, p =0.001 at visit 1; ANOSIM test). Together, these data indicate that antimicrobial therapy normalizes the skin microbiome of cAD.
Skin barrier dysfunction correlates with cAD severity and skin microbiome
In parallel with sampling the skin microbiome, barrier function was assessed by TEWL, hydration (corneometry), and pH (Table S2, S3, Figure S4). Site-specific lesion scores were positively correlated with TEWL (R=0.365; p=0.0001) suggesting that more severe cAD is associated with impaired barrier function, and were negatively correlated with pH (R=−0.194; p=0.03). Similarly, Shannon diversity index was negatively correlated with TEWL (R=−0.249; p=0.006), but showed a weakly positive correlation with pH (R=0.18; p=0.05). Additional alpha diversity metrics followed similar trends and were also statistically significant (p<0.05; Table S3). Skin moisture (corneometry) did not correlate with site-specific lesion scores or alpha diversity metrics. These results demonstrate that concurrent with cAD flares, alpha diversity decreases and is correlated with disease severity, TEWL, and pH.
During the treatment of cAD, TEWL and corneometry scores decreased and trended toward the distribution seen in the control group, but were not statistically different between visits 1 and 2 (Table S2, Figure S4). The proportional change in lesion scores in patients with cAD correlated with the change in TEWL from visit 1 to visit 2 (R= 0.682, p=0.02). There was no correlation between changes in lesion scores and TEWL between visits 1 and 3, likely due to recrudescence of bacterial dermatitis in some cAD dogs. The proportional change in corneometry did not correlate with the change in lesion scores from visit 1 to visit 2, but there was a significant correlation between these parameters at visits 1 and 3 (R=0.624, p=0.03). Skin pH did not differ significantly between dogs with cAD and healthy controls across the three visits.
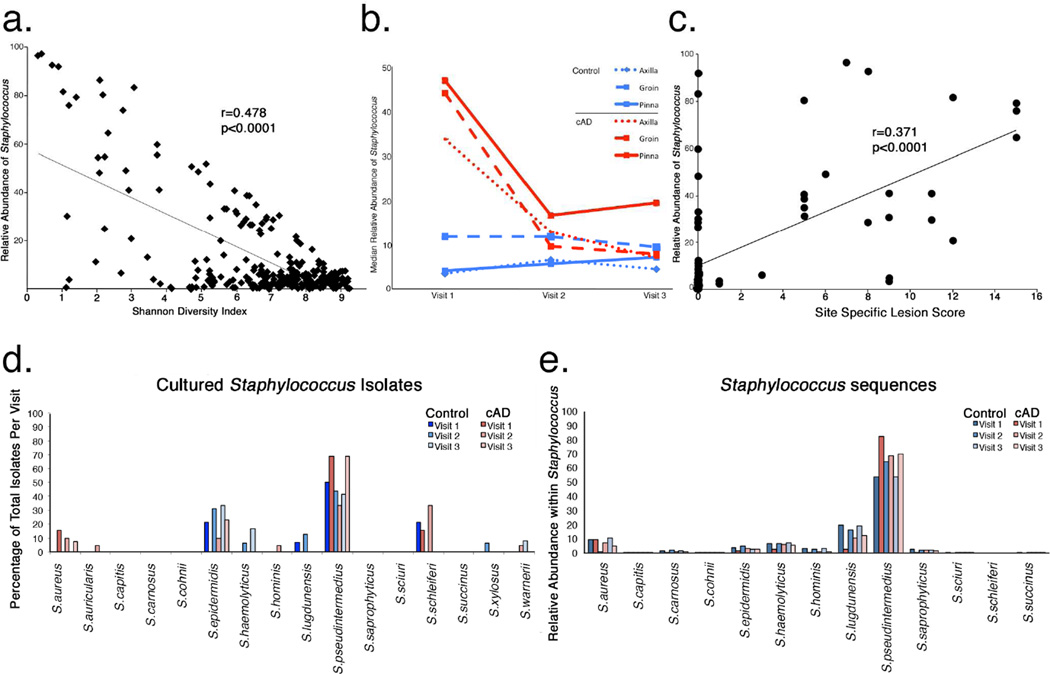
Staphylococcus pseudintermedius predominates in cAD
The relationships between Staphylococcus, cAD severity, and treatment in the canine skin microbiome were correlated. Relative abundance of Staphylococcus significantly inversely correlated with alpha diversity (R=−0.686; p<1.0×10−4; Figure 4a), and decreased in mean relative abundance with treatment (45%, 15%, and 18% at visits 1, 2, and 3, respectively), in contrast to control dogs where mean relative abundance of Staphylococcus remained low across skin sites at each visit (10%; Figure 4b). There was a positive correlation with the relative abundance of Staphylococcus and site-specific lesion scoring (R=0.609; p<1.0×10−4; Figure 4c), suggesting that Staphylococcus increases as disease severity increases.
FIGURE 4. Staphylococcus species and cAD.
(a) Correlation between relative abundance of Staphylococcus (y-axis) and Shannon Diversity Index (x-axis). (b) Change in Staphylococcus relative abundance (y-axis) longitudinally at each skin site in cAD (red lines) and control (blue lines) dogs. (c) Correlation between relative abundance of Staphylococcus (y-axis) and lesion severity (x-axis) as measured by site-specific lesion score. Species-level relative abundance (y-axis) of cultured Staphylococcus isolates (d) and 16S rRNA sequences using phylogenetic placement (e).
Because Staphylococcus species in humans can be commensal (S. epidermidis) or potential pathogens (S. aureus), species identification of Staphylococcus was determined. One microbial culture from a lesional site and axilla in control dogs or when lesions had resolved were performed contemporaneously with microbiome swabs at each visit. The majority of the isolates were identified as S. pseudintermedius in both dogs with cAD and healthy controls, followed by S. epidermidis (a coagulase-negative Staphylococcus), and S. schleiferi (a coagulase-variable Staphylococcus). To speciate and compare 16S rRNA sequence data to cultures, we employed a most recent common ancestor analysis of phylogenetic placement using the pplacer algorithm (Matsen et al., 2010) and a curated database of Staphylococcus spp. genomes (Conlan et al., 2012). The majority of the sequences identified across all samples were attributed to S. pseudintermedius (59.4%) with lesser contribution from S. aureus, S. epidermidis, S. haemolyticus, S. hominis, S. lugdunensis, and S. saprophyticus (Figure 5d). S. schleiferi was not detected, suggesting that the region of the 16S rRNA gene employed in this study may not be able to reliably classify S. schleiferi to the species level.
DISCUSSION
Approximately 70 million dogs live in 40 million households across the United States (American Veterinary Medical Association, 2012), and 10% are afflicted with cAD (Hillier & Griffin, 2001). In this spontaneous large animal model of AD, alterations in skin microbiome parallel those observed in AD patients, including increased relative abundance of Staphylococcus spp. and decreased microbial diversity compared to healthy controls. The longitudinal dynamics of the skin microbiome of cAD during flare and treatment correlate with changes in cutaneous barrier function. This work unveils the dynamic relationship between cutaneous barrier function and the skin microbiome in mammalian health and disease states.
The cutaneous microbiome in cAD has been previously reported in a small cross-sectional study of allergic (n=6) and healthy dogs (n=12), with a similar decrease in microbial diversity, but there were no differences in the relative abundance of Staphylococcus between the two cohorts (Rodrigues Hoffmann et al., 2014). The contrast may be due to different methodologies employed, geography, and differing selection criteria (with dogs in the present study presenting with evidence of bacterial dermatitis and disease flare). Our results are harmonious with longitudinal studies in human AD, where microbial diversity is decreased and Staphylococcus and Corynebacterium predominate during flare states (Kong et al., 2012). Furthermore, the Adam17fl/flSox9-Cre murine model of AD is characterized by high relative abundance of cutaneous Staphylococcus and Corynebacterium that normalized following antimicrobial therapy (Kobayashi et al., 2015).
In cAD there is a predisposition to the development of coagulase positive Staphylococcus (CoPS) colonization and dermatitis as in AD. S. aureus is the primary CoPS of human skin and mucosal sites. S. pseudintermedius is a skin and mucosal commensal in the dog, and as we substantiate independent of culture, the most frequent pathogen isolated from dogs with skin or ear canal infections (Bannoehr & Guardabassi, 2012). Human S. pseudintermedius colonization is rare and primarily restricted to those with regular contact with dogs and cats (Talan et al., 1989; Goodacre et al., 1997; Guardabassi et al., 2004). By the same token, S. aureus is infrequently isolated from infection and carriage sites of dogs in clinical practice and in epidemiological surveys, and is considered a comparatively infrequent canine pathogen (Beck et al., 2012; Morris et al., 2006; 2010). The dog may act as a potential vector of S. aureus, which raises zoonotic and anthropozoonotic concerns for potential transfer of pathogens, drug resistance, and genetic elements (Misic et al., 2015; Song et al.., 2013; Boag et al., 2004; Bramble et al., 2011).
Mechanisms of staphylococcal perturbation of the epidermal barrier are still unclear and may be through direct or indirect (immunostimulatory) means. Mitigation of staphylococcal overgrowth clearly ameliorates disease severity and the epidermal barrier normalizes. However, methicillin and multidrug resistance are now commonplace in both veterinary and human medicine. The findings presented here may inform future efforts to develop alternative (non-antibiotic) approaches to controlling staphylococcal burden in skin disease.
MATERIALS & METHODS
Animal subjects
Dogs with cAD (n=16) and healthy dogs (n=14) were prospectively enrolled in this pilot study at the University of Pennsylvania Matthew J. Ryan Veterinary Hospital, following examination by a board certified veterinary dermatologist (CC, EM, DM). The same dermatologist examined each patient across all time points. Dogs with cAD were included in the study by fulfilling standardized criteria (5 criteria of Favrot, Favrot et al., 2010) and by ruling out dermatoses with similar presentations as depicted by Hensel et al. (Hensel et al., 2015) based on dermatologic examination and clinical histories. Dermatologic examination in all patients involved, but was not limited to, skin and otic cytology, flea combing/direct examination, and skin scraping. Dogs with evidence of other underlying systemic disease, an atopic history that was not clearly documented, or evidence of active ecto-parasitic (including Demodex spp., Sarcoptes spp., and Ctenocephalides felis) infestations were excluded. All subjects were on a strict flea control regimen. Four dogs with cAD had a history of antibiotic exposure within 45 days before enrollment. Therapy was prescribed as indicated by the attending veterinary dermatologist. Systemic antimicrobial therapy was prescribed based on aerobic culture and sensitivity at enrollment. See Table S4a–b for specific therapies prescribed for each patient. Healthy dogs were enrolled in the study during the same time period. A subset of dogs were owned by veterinarians and veterinary technicians. Informed consent was obtained from all owners prior to enrollment. All experiments were carried out according to approved Institutional Animal Care and Use Committee protocols. Site-specific assessment and semi-quantitative scoring of lesion severity was performed and included erythema, lichenification, and self-induced alopecia each made on a 0–5 scale (0-no lesions and 5-most severe). The same dermatologist (CC and DM) assessed/scored each patient at each time point. This scoring system is based on CADESI-03 (Canine Atopic Dermatitis Extent and Severity Index) (Olivry et al., 2007), due to familiarity by the dermatologists, akin to the SCORAD metric used in human dermatology. The lesion score was used to assess for changes at a given site that might correlate with microbial shifts or skin barrier function, and not as a means for documenting changes in disease flare.
Skin microenvironmental assessment
Noninvasive probes (Courage+Khazaka, Cologne, Germany) for measuring subsurface-epidermal water content by the capacitance method (Corneometer ®, CM 825), skin pH (a flat surface Skin-pH Meter ®, PH 905), and transepidermal water loss (TEWL) by diffusion (Tewameter ®, TM300) were used at each visit according to the manufacturer’s instructions. Sampling sites included the axilla, groin, and concave aspect of the pinna. These sites were chosen as they are sparsely haired allowing for corneometry and tewametry measurements and are cAD predilection sites. Dogs were sampled in lateral recumbency with minimal physical restraint. The order of measurements at each site was: corneometry, TEWL, and pH. TEWL measurements were averaged at 1-second intervals for a 30 second period. Five corneometry measurements were performed over a 3cm2 area and averaged. Three consecutive pH readings were averaged. Measurements were performed in the dermatology clinic treatment area. During sampling and assessment, the mean indoor ambient temperature was 22.2°C (18.3°C to 23.9°C) and the mean relative humidity was 25.5% (range: 16% to 68%).
Microbiomic sampling
The oral cavity, axilla, concave pinna, and groin were swabbed (3cm2 region) using Catch-All Sample Collection Swabs (Epicentre Biotechnologies, Madison, WI). Swabs were rubbed vigorously over the skin site or mouth for 10–15 second intervals. Swabs exposed to air in the treatment room and laboratory at extraction were used as negative control samples. Swabs were placed in 300 µL of Yeast Cell Lysis Solution (Epicentre Biotechnologies, Madison, WI), and the tip of the swab was aseptically cut from the handle and stored at −80°C until extraction.
Contemporaneous swabs were taken from a single lesional site of dogs with dermatitis, and submitted for aerobic bacterial culture. Swabs were processed using standard laboratory protocols, and isolates were identified as Staphylococcus spp. by use of a conventional biochemical identification system (MicroScan Walkaway 40 PC20 Gram-positive combo-panel, Dade Behring, Sacramento, California, USA) as described by the manufacturer. The results of the culture and antimicrobial sensitivity testing were used to direct 4–6 weeks of systemic (oral) antimicrobial therapy (Table S4a).
DNA extraction and sequencing
DNA extractions were performed as previously described (Misic et al.., 2015). The V1–V3 hypervariable region of the 16S rRNA gene was amplified using a barcoding strategy as described (Fadrosh et al., 2014) and primers 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 534R (5’-ATTACCGCGGCTGCTGG-3’). Sequencing was performed on the Illumina MiSeq instrument using 300 bp paired-end chemistry at the University of Maryland Institute for Genome Sciences.
16S rRNA gene analysis
Paired-end reads were demultiplexed by Flexbar (Dodt et al., 2012) and assembled using Pear (Zhang et al., 2014), resulting in 17,065,344 sequences. Sequences <465 base pairs and >535 base pairs and sequences with ≥10 homopolymers were removed, resulting in 13,023,611 sequences. QIIME v. 1.8 (Caporaso et al., 2010) was used for further downstream processing and analyses. Sequences were aligned to the Greengenes database, and taxonomy assigned using the RDP classifier (Wang et al., 2007). OTUs were picked using 97% sequence similarity with cd-hit (Li & Godzik, 2006; Fu et al., 2012) and a representative sequence based on the most abundant sequence was used. Each sample was rarified to 4,000 sequences for alpha- and beta-diversity analyses. The pplacer binary (Matsen et al., 2010) was employed as previously described (Gardner et al., 2013) for species level assessment of Staphylococcus using a Staphylococcus reference database (Conlan et al., 2012). Water and processed blank samples were sequenced and processed in parallel and contaminants were identified and removed as previously reported (Misic et al., 2015). Published best practices were used as guidelines (Bokulich et al., 2012). Sequences were deposited in the NCBI Short Read Archive under BioProject Accession No. PRJNA302288.
Statistics
The R Statistical Package (R Core Team, 2015) was used for all computations. Non-parametric Wilcoxon rank-sum tests were used to compare differences between groups. For within subject comparisons, paired Wilcoxon signed-rank tests were used. Pearson’s product-moment correlation coefficients were calculated for correlations and tested using Student’s t test.
Supplementary Material
Acknowledgments
The authors thank Dr. Darcie Kunder, Dr. Fiona Lee, Ms. Colleen Walters, and Mr. Joseph Rogosky for exemplary patient care and assessment, and members of the Grice laboratory for their underlying contributions. Research reported in this publication was supported by a grant from the University of Pennsylvania, School of Veterinary Medicine, Center for Host-Microbial Interactions and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R00-AR060873 and R01-AR066663 to EAG. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AD
Atopic dermatitis
- cAD
Canine atopic dermatitis
- DNA
deoxyribonucleic acid
- rRNA
ribosomal ribonucleic acid
- CADESI
Canine Atopic Dermatitis Extent and Severity Index
- TEWL
transepidermal water loss
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors state no conflict of interest.
REFERENCES
- American Veterinary Medical Association. U.S. Pet Ownership & Demographics Sourcebook. Schaumburg, IL: American Veterinary Medical Association; 2012. [Google Scholar]
- Bannoehr J, Guardabassi L. Staphylococcus pseudintermedius in the dog: taxonomy, diagnostics, ecology, epidemiology and pathogenicity: Staphylococcus pseudintermedius in dogs. Vet Dermatol. 2012 Aug;23(4):253–e52. doi: 10.1111/j.1365-3164.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- Beck KM, Waisglass SE, Dick HLN, Weese JS. Prevalence of meticillin-resistant Staphylococcus pseudintermedius (MRSP) from skin and carriage sites of dogs after treatment of their meticillin-resistant or meticillin-sensitive staphylococcal pyoderma. Vet Dermatol. 2012 Aug;23(4):369–75. e66–e67. doi: 10.1111/j.1365-3164.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- Bizikova P, Pucheu-Haston CM, Eisenschenk MNC, Marsella R, Nuttall T, Santoro D. Review: Role of genetics and the environment in the pathogenesis of canine atopic dermatitis. Vet Dermatol. 2015 Apr;26(2):95–e26. doi: 10.1111/vde.12198. [DOI] [PubMed] [Google Scholar]
- Boag A, Loeffler A, Lloyd DH. Methicillin-resistant Staphylococcus aureus isolates from companion animals. Vet Rec. 2004 Mar 27;154(13):411. [PubMed] [Google Scholar]
- Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2012 Dec 2;10(1):57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramble M, Morris D, Tolomeo P, Lautenbach E. Potential Role of Pet Animals in Household Transmission of Methicillin-Resistant Staphylococcus aureus: A Narrative Review. Vector-Borne Zoonotic Dis. 2011 Jun;11(6):617–620. doi: 10.1089/vbz.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010 May;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan S, Kong HH, Segre JA. Species-level analysis of DNA sequence data from the NIH Human Microbiome Project. PloS One. 2012;7(10):e47075. doi: 10.1371/journal.pone.0047075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt M, Roehr J, Ahmed R, Dieterich C. FLEXBAR—Flexible Barcode and Adapter Processing for Next-Generation Sequencing Platforms. Biology. 2012 Dec 14;1(3):895–905. doi: 10.3390/biology1030895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2(1):6. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrot C, Steffan J, Seewald W, Picco F. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet Dermatol. 2010 Feb;21(1):23–31. doi: 10.1111/j.1365-3164.2009.00758.x. [DOI] [PubMed] [Google Scholar]
- Fazakerley J, Nuttall T, Sales D, Schmidt V, Carter SD, Hart CA, et al. Staphylococcal colonization of mucosal and lesional skin sites in atopic and healthy dogs. Vet Dermatol. 2009 Jun;20(3):179–184. doi: 10.1111/j.1365-3164.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012 Dec 1;28(23):3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furiani N, Scarampella F, Martino PA, Panzini I, Fabbri E, Ordeix L. Evaluation of the bacterial microflora of the conjunctival sac of healthy dogs and dogs with atopic dermatitis. Vet Dermatol. 2011 Dec;22(6):490–496. doi: 10.1111/j.1365-3164.2011.00979.x. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013 Mar;62(3):923–930. doi: 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science. 2009 May 29;324(5931):1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel P, Santoro D, Favrot C, Hill P, Griffin C. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Vet Res. 2015 Dec;11(1) doi: 10.1186/s12917-015-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier A, Griffin CE. The ACVD task force on canine atopic dermatitis (I): incidence and prevalence. Vet Immunol Immunopathol. 2001 Sep 20;81(3–4):147–151. doi: 10.1016/s0165-2427(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity. 2015 Apr;42(4):756–766. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012 May;22(5):850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974 May;90(5):525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinforma Oxf Engl. 2006 Jul 1;22(13):1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Marsella R, Girolomoni G. Canine models of atopic dermatitis: a useful tool with untapped potential. J Invest Dermatol. 2009 Oct;129(10):2351–2357. doi: 10.1038/jid.2009.98. [DOI] [PubMed] [Google Scholar]
- Matousek JL, Campbell KL. A comparative review of cutaneous pH. Vet Dermatol. 2002;13(6):293–300. doi: 10.1046/j.1365-3164.2002.00312.x. [DOI] [PubMed] [Google Scholar]
- Matsen FA, Kodner RB, Armbrust EV. pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics. 2010;11(1):538. doi: 10.1186/1471-2105-11-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misic AM, Davis MF, Tyldsley AS, Hodkinson BP, Tolomeo P, Hu B, et al. The shared microbiota of humans and companion animals as evaluated from Staphylococcus carriage sites. Microbiome. 2015;3:2. doi: 10.1186/s40168-014-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DO, Rook KA, Shofer FS, Rankin SC. Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: a retrospective review of 749 isolates (2003–04) Vet Dermatol. 2006 Oct;17(5):332–337. doi: 10.1111/j.1365-3164.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- Morris DO, Boston RC, O’Shea K, Rankin SC. The prevalence of carriage of meticillin-resistant staphylococci by veterinary dermatology practice staff and their respective pets. Vet Dermatol. 2010 Aug;21(4):400–407. doi: 10.1111/j.1365-3164.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Muñoz-Planillo R, Hasegawa M, et al. Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature. 2013 Oct 30;503(7476):397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivry T, Marsella R, Iwasaki T, Mueller R International Task Force On Canine Atopic Dermatitis. Validation of CADESI-03, a severity scale for clinical trials enrolling dogs with atopic dermatitis. Vet Dermatol. 2007 Apr;18(2):78–86. doi: 10.1111/j.1365-3164.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- O’Regan GM, Sandilands A, McLean WHI, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2009 Sep;124(3 Suppl 2):R2–R6. doi: 10.1016/j.jaci.2009.07.013. [DOI] [PubMed] [Google Scholar]
- R Core Team. R Foundation for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. R: A language and environment for statistical computing. http://www.R-project.org. [Google Scholar]
- Rodrigues Hoffmann A, Patterson AP, Diesel A, Lawhon SD, Ly HJ, Elkins Stephenson C, et al. The skin microbiome in healthy and allergic dogs. PloS One. 2014;9(1):e83197. doi: 10.1371/journal.pone.0083197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro D, Marsella R, Pucheu-Haston CM, Eisenschenk MNC, Nuttall T, Bizikova P. Review: Pathogenesis of canine atopic dermatitis: skin barrier and host-micro-organism interaction. Vet Dermatol. 2015 Apr;26(2):84–e25. doi: 10.1111/vde.12197. [DOI] [PubMed] [Google Scholar]
- Scharschmidt TC, Segre JA. Modeling atopic dermatitis with increasingly complex mouse models. J Invest Dermatol. 2008 May;128(5):1061–1064. doi: 10.1038/sj.jid.5701201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Lauber C, Costello E, et al. Cohabiting family members share microbiota with one another and with their dogs. eLife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2010 Aug;105(2):99–106. doi: 10.1016/j.anai.2009.10.002. quiz 107–9, 117. [DOI] [PubMed] [Google Scholar]
- Vázquez -Baeza Y, Pirrung M, Gonzalez A, Knight R. EMPeror: a tool for visualizing highthroughput microbial community data. GigaScience. 2013;2(1):16. doi: 10.1186/2047-217X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl Environ Microbiol. 2007 Aug 15;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Gallo RL. The Role of the Skin Microbiome in Atopic Dermatitis. Curr Allergy Asthma Rep. 2015;15(11):1–10. doi: 10.1007/s11882-015-0567-4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics. 2014 Mar 1;30(5):614–620. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.