Abstract
Background
The optimal management of tricuspid regurgitation (TR) in patients undergoing left ventricular assist device (LVAD) implantation is controversial. This study was undertaken to determine the impact of tricuspid valve repair (TVR) at the time of LVAD implantation on survival.
Methods
The INTERMACS Registry was used to analyze the outcomes of patients undergoing LVAD implantation as destination therapy with or without concomitant TVR.
Results
Among 2,527 patients undergoing implant of a continuous flow LVAD as destination therapy during the study period, 989 (39%) had moderate or severe TR. The management of TR was not uniform among these patients. Patients with moderate and severe TR underwent TVR in 16.7% and 35.3% of cases, respectively. Moderate and severe TR at the time of LVAD implantation were associated with poorer survival over the entire follow up period (p=0.009). Interestingly, TVR at the time of LVAD implantation did not confer improved survival, even among patients with pre-implant moderate or severe TR. A potential explanation for this finding is that patients with pre-implant moderate or severe tricuspid regurgitation who underwent LVAD implant with concomitant TVR commonly developed recurrent, late tricuspid regurgitation (21-27%).
Conclusions
TVR is performed commonly at the time of LVAD implant despite the fact that it does not confer a clear survival benefit. For many patients, LVAD implant alone relieves pre-implant tricuspid regurgitation as effectively as LVAD implant with TVR. Further study is necessary to determine what factors lead to recurrence of late tricuspid regurgitation in LVAD patients both with and without TVR.
Keywords: mechanical support, ventricular assist devices, INTERMACS, destination therapy, tricuspid valve repair
Implantation of left ventricular assist devices (LVADs) is an established treatment for patients with end-stage heart failure. Smaller second and third generation LVADs have been associated with reduced complications including bleeding and infections as well as improved quality of life (1-3). The complication of right ventricular failure, however, continues to occur in a substantial proportion of LVAD recipients and is associated with reduced survival. Right ventricular failure persists as an important obstacle to long term survival after LVAD implant in these patients (4,5).
Functional tricuspid regurgitation (TR) frequently accompanies right ventricular failure in LVAD patients as a result of structural changes that occur in the failing RV (6-9). The treatment of TR with tricuspid valve annuloplasty at the time of LVAD implantation has been identified as a potential means to reduce the frequency and implications of right ventricular failure after LVAD implantation (6,10-12). A number of single-center reports have demonstrated the safety of concomitant tricuspid valve annuloplasty at early follow-up (10-12). The long-term effects of this procedure, however, are unknown and a great deal of practice variation persists among heart failure centers. In this study, we examined the utilization of tricuspid valve repair (TVR) at the time of LVAD implant in the United States and the effect of this concomitant procedure on TR and survival at long-term follow up.
Material and Methods
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) was utilized. INTERMACS is a national registry for patients who have received a durable mechanical circulatory support device that has been approved by the Federal Food and Drug Administration (13).
A total of 127 institutions were enrolled in INTERMACS from June 23, 2006-March 31, 2013 and also submitted data during this time frame. Each hospital had current approval from their institutional review board and each patient signed an informed consent. This study analyzed adult patients who received a primary continuous flow left ventricular assist device with the device strategy of destination therapy at the time of implant.
Pre-implant data were analyzed using basic summary statistics and group comparisons were made using one-way analysis of variance, t-test and chi-square test of association. Time related event data were analyzed using Kaplan-Meier methodology and group comparisons were made with the log-rank test (note that the log-rank test is a univariate Cox proportional hazard test).
The effect of tricuspid valve regurgitation and tricuspid valve intervention on survival was made in both a univariate and multivariate fashion by a parametric hazard regression analysis. The adjusted effect of these variables was assessed after adjustment for significant pre-implant variables. These variables are included in Appendix 1 (risk factors examined).
Results
The INTERMACS was queried to identify subjects who had undergone primary implant of a continuous flow LVAD during the study period. Patients 18 years and younger were excluded. Because the focus of the study was on the long-term outcomes of LVAD patients with TR, only patients receiving an LVAD as destination therapy at the time of surgery were included in the study. Among 8,609 INTERMACS patients undergoing primary implant during the study period, there were 2,527 patients who fit the study criteria described above. Tables 1 and 2 show the categorical and continuous variables of the study population.
Table 1.
Pre-implant characteristics of the study population for categorical variables.
| Pre-implant Characteristics | Total N | Percent |
|---|---|---|
| Male | 2527 | 81.76 |
| White | 2527 | 74.32 |
| Married | 2477 | 71.78 |
| College | 1945 | 50.08 |
| Diabetes | 2519 | 27.15 |
| Inotropes | 2508 | 78.83 |
| Ascites | 2292 | 6.81 |
| COPD | 2476 | 11.35 |
| INTERMACS Patient Profile Level 1: Critical Cardiogenic Shock | 2527 | 10.45 |
| INTERMACS Patient Profile Level 2: Progressive Decline | 2527 | 35.42 |
| INTERMACS Patient Profile Level 3 | 2527 | 31.86 |
| INTERMACS Patient Profile Level 4 | 2527 | 16.38 |
| INTERMACS Patient Profile Level 5 | 2527 | 3.60 |
| INTERMACS Patient Profile Level 6 | 2527 | 1.39 |
| INTERMACS Patient Profile Level 7 | 2527 | 0.91 |
| Destination Therapy | 2527 | 100.00 |
| NYHA = 4 | 2358 | 81.47 |
| Coronary artery disease | 2510 | 7.41 |
| Cerebrovascular accident | 2470 | 5.30 |
| Transient ischemic attack | 2470 | 2.96 |
| Cancer | 2506 | 8.10 |
| Current smoker | 2443 | 7.04 |
| Current drug abuse | 2383 | 1.43 |
| Alcohol abuse | 2446 | 9.65 |
| Blood Type O | 2471 | 44.88 |
| Rheumatologic disease | 1424 | 3.93 |
| Hepatitis B | 1341 | 1.34 |
| Hepatitis C | 1339 | 2.91 |
| Dialysis | 2527 | 1.42 |
| History of coronary artery bypass | 2527 | 34.39 |
| History of Valve Surgery | 2527 | 9.14 |
| Implantable cardioverter defibrillator | 2503 | 84.26 |
| Intra-aortic balloon pump | 2527 | 22.83 |
| Ventilator | 2527 | 4.51 |
| Peripheral vascular disease | 1427 | 11.00 |
| Carotid artery disease | 1377 | 13.80 |
| β-Blockers | 2447 | 79.28 |
| Angiotensin-converting-enzyme inhibitor | 2356 | 47.11 |
| Mitral regurgitation (Moderate/Severe) | 2194 | 59.25 |
| Tricuspid regurgitation (Moderate/Severe) | 2160 | 45.79 |
| Aortic regurgitation (Moderate/Severe) | 2057 | 5.69 |
| Left ventricular ejection fraction (< 20 %) | 2226 | 66.76 |
| Right ventricular ejection fraction (severely reduced) | 1352 | 16.72 |
| Concomitant surgery | 2527 | 40.48 |
| Failure to wean | 2527 | 0.83 |
| Extracorporeal membrane oxygenation | 2527 | 1.35 |
| Patient Profile Modifier TCS | 1964 | 19.60 |
COPD, chronic obstructive pulmonary disease; Failure to wean, inability to wean from cardiopulmonary byass during other cardiac surgical procedure; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; NYHA, New York Heart Association; TCS, temporary circulatory support.
Table 2.
Pre-implant characteristics of the study population for continuous variables.
| NAME | N | Mean | Standard Deviation |
|---|---|---|---|
| Age (years) | 2527 | 63.17 | 11.81 |
| Albumin (g/dL) | 2270 | 3.36 | 0.65 |
| Total bilirubin (mg/dL) | 2272 | 1.36 | 1.71 |
| Body mass index (kg/meter2) | 2492 | 28.40 | 6.86 |
| Brain natriuretic peptide (pg/ml) | 981 | 1206.59 | 1135.56 |
| Body surface area (m2) | 2492 | 2.06 | 0.29 |
| Blood urea nitrogen (mg/dL) | 2489 | 32.51 | 19.25 |
| Cholesterol (mg/dL) | 1181 | 127.22 | 39.70 |
| Cardiac index (L/min/meter2) | 1582 | 2.06 | 0.70 |
| Creatinine (mg/dL) | 2513 | 1.50 | 0.71 |
| C-reactive protein (mg/L) | 474 | 17.02 | 34.93 |
| Diastolic blood pressure (mmHg) | 2461 | 63.33 | 11.61 |
| Hemoglobin (mg/dL) | 2509 | 11.32 | 1.95 |
| Heart Rate | 2488 | 85.73 | 16.89 |
| International normalized ratio (international units) | 2394 | 1.34 | 0.46 |
| LVEDD (cm) | 1717 | 6.74 | 1.05 |
| Left ventricular ejection fraction (%) | 2321 | 9.54 | 65.04 |
| Platelet (K/uL) | 2507 | 189.89 | 77.79 |
| Pre-albumin (mg/dL) | 1288 | 18.72 | 7.32 |
| Protein C (%) | 82 | 84.60 | 34.24 |
| Protein S (%) | 91 | 77.29 | 27.71 |
| Pulmonary diastolic pressure (mmHg) | 1719 | 24.52 | 8.75 |
| Pulmonary systolic pressure (mmHg) | 1732 | 50.42 | 14.51 |
| Pulmonary wedge pressure (mmHg) | 1234 | 23.24 | 8.47 |
| Pulmonary vascular resistance (Woods units) | 1163 | 2.87 | 2.39 |
| Right atrial pressure (mmHg) | 1414 | 12.96 | 8.20 |
| AST (u/L) | 2282 | 52.50 | 176.79 |
| ALT (u/L) | 2295 | 56.90 | 169.20 |
| Sodium (mmol/L) | 2517 | 135.16 | 4.72 |
| Systolic blood pressure (mmHg) | 2468 | 105.17 | 16.53 |
| WBC count (K/uL) | 2511 | 8.22 | 4.71 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; LVEDD, left ventricular end diastolic dimension; WBC, white blood cell.
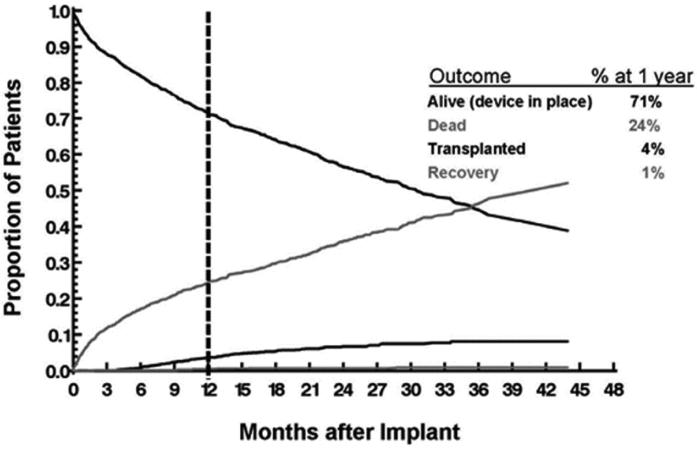
Figure 1 depicts the competing outcomes of survival on LVAD support, death, transplantation, and myocardial recovery. At one year follow up, 71% of study patients were surviving on support, 24% were dead, 4% had been transplanted, and 1% had LVAD explant due to native myocardial recovery. Our intent was to have a limited number of patients transplanted during the follow up period in order to assess the impact of TR on LVAD patients at late follow up. This appears to have been achieved with the study design including only patients with an LVAD implant indication of destination therapy.
Figure 1.
Competing outcomes of survival on LVAD support, death, transplantation, and myocardial recovery among the study population.
LVAD, left ventricular assist device.
TR at the time of LVAD implant was assessed using the most recent echo data available prior to surgery. Significant TR was common among study subjects at the time of LVAD implant (Table 3). Patients with severe (10.5%) and moderate (28.6%) TR accounted for more than a third of overall patients undergoing implantation. Patients with significant TR were not uniformly treated with a tricuspid valve procedure at the time of LVAD implant. Even among patients with severe TR, only 35.3% underwent tricuspid valve repair and 61.7% underwent no procedure. Among patients with moderate TR, 82.0% underwent no concomitant tricuspid valve procedure.
Table 3.
Incidence of tricuspid regurgitation and tricuspid valve treatment strategy at the time of LVAD implantation.
| Tricuspid Valve Procedures | ||||
|---|---|---|---|---|
| TR at time of implant | Repair | Replacement | No Procedure | |
| None | 8.4% (212) | 2.4% (5) | 0% (0) | 97.6% (207) |
| Mild | 38.0% (959) | 6.8% (65) | 0.5% (5) | 92.7% (889) |
| Moderate | 28.6% (723) | 16.7% (121) | 1.2% (9) | 82.0% (593) |
| Severe | 10.5% (266) | 35.3% (94) | 3.0% (8) | 61.7% (164) |
| Unknown | 14.5% (367) | 13.6% (50) | 1.0% (4) | 85.3% (313) |
LVAD, left ventricular assist device; TR, tricuspid regurgitation.
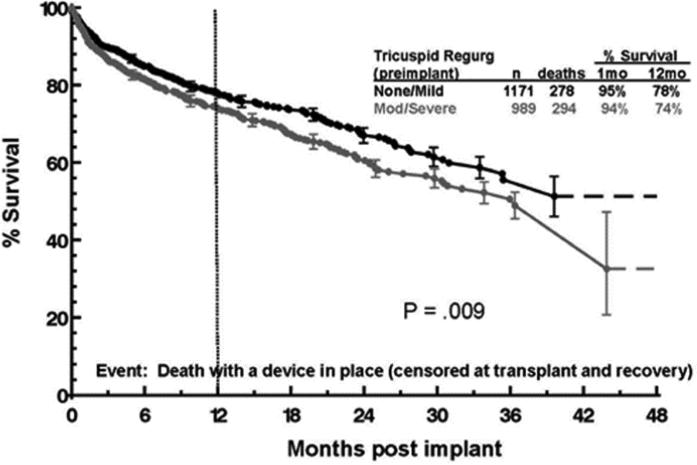
Significant TR at the time of LVAD implant was associated with worsened survival at late follow-up. Figure 2 depicts the Kaplan Meier survival of patients with none or mild TR vs. patients with moderate or severe TR. Patients with moderate and severe TR had significantly poorer survival (p = 0.009) which was apparent over the entire period of follow-up. Both the univariate and the multivariate adjusted effect were significant at the same level (p=0.009). Risk factors for death during follow up are summarized in Table 4.
Figure 2.
Survival of patients with none/mild vs. moderate/severe tricuspid regurgitation at the time of LVAD implant.
LVAD, left ventricular assist device.
Table 4.
Risk factors for death following LVAD implantation.
| Risk Factor for Death | Phases of Hazard | |||
|---|---|---|---|---|
| Early | Constant | |||
| Hazard ratio | p-value | Hazard ratio | p-value | |
| Age (years) | 1.58 | < .0001 | ||
| Body mass index (higher) | 1.49 | .0003 | ||
| Not Married | 1.93 | .0009 | ||
| History of coronary artery bypass | 1.56 | .02 | ||
| Blood urea nitrogen (higher) | 1.10 | .006 | 1.06 | .01 |
| Total bilirubin (higher) | 1.78 | .02 | ||
| Creatinine (higher) | 1.10 | .001 | ||
| Ventilator | 2.18 | .005 | ||
| INTERMACS Level 1 | 2.74 | .0001 | ||
| INTERMACS Level 2 | 1.85 | .004 | ||
| RVAD | 5.05 | < .0001 | ||
| Tricuspid regurgitation | 1.35* | .009 | ||
Hazard ratio represents the increased risk for one level of increase in tricuspid regurgitation, from mild, to moderate, to severe.
INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; RVAD, right ventricular assist device.
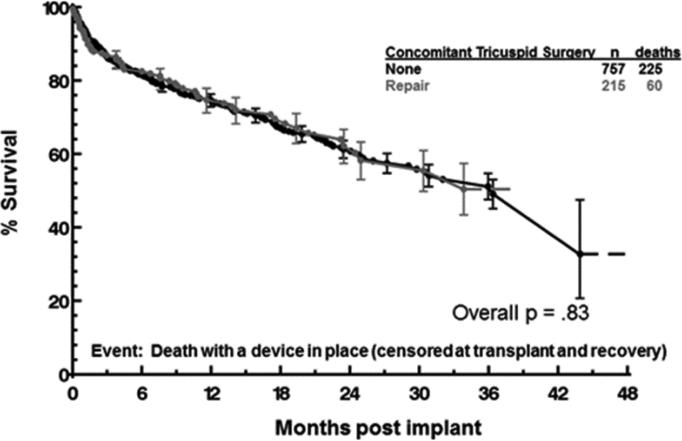
Interestingly, concomitant TVR did not appear to confer a survival benefit among patients with moderate/severe TR at the time of LVAD implant (Figure 3). The Kaplan Meier survival of patients with moderate/severe TR who underwent TVR was not superior to those with moderate/severe TR who underwent no tricuspid valve procedure. The characteristics of patients with moderate/severe TR who underwent TVR vs. no TV procedure are summarized in Tables 5 and 6. The higher frequency of coronary artery disease in the no TV procedure group was the only characteristic with a statistically significant difference between the two groups. Importantly, the frequency of INTERMACS Level 1 patients was the same in both groups (frequency 9.8 percent for both groups, p=1.00). This indicates that the TVR group was not enriched with patients in cardiogenic shock and TVR was performed on patients with a similar risk profile as patients in the no TVR group.
Figure 3.
Survival of patients with moderate/severe tricuspid regurgitation at the time of LVAD implant who underwent concomitant tricuspid valve repair vs. no tricuspid procedure.
LVAD, left ventricular assist device.
Table 5.
Pre-implant characteristics of patients with no tricuspid valve procedure vs. with tricuspid valve repair for categorical variables.
| Pre-implant Characteristics | No TV procedure Percent n=757 | TV repair Percent n=215 | P-value |
|---|---|---|---|
| Male | 78.2 | 83.3 | 0.11 |
| White | 70.8 | 71.6 | 0.81 |
| Married | 67.7 | 67.1 | 0.88 |
| College | 49.1 | 53.6 | 0.30 |
| Diabetes | 23.9 | 24.2 | 0.94 |
| Inotropes | 84.1 | 81.8 | .042 |
| Ascites | 8.2 | 12.4 | 0.07 |
| COPD | 10.7 | 7.6 | .019 |
| INTERMACS Patient Profile Level 1: Critical Cardiogenic Shock | 9.8 | 9.8 | 1.00 |
| INTERMACS Patient Profile Level 2: Progressive Decline | 38.0 | 44.7 | 0.08 |
| INTERMACS Patient Profile Level 3 | 33.7 | 28.8 | 0.18 |
| INTERMACS Patient Profile Level 4 | 12.8 | 13.0 | .094 |
| INTERMACS Patient Profile Level 5 | 3.8 | 2.8 | 0.47 |
| INTERMACS Patient Profile Level 6 | 0.9 | 0.9 | 0.99 |
| INTERMACS Patient Profile Level 7 | 0.9 | 0.0 | 0.16 |
| NYHA = 4 | 82.6 | 84.0 | 0.64 |
| Coronary artery disease | 6.6 | 2.3 | 0.02 |
| Cerebrovascular accident | 4.7 | 5.2 | 0.75 |
| Transient ischemic attack | 3.4 | 1.0 | 0.06 |
| Cancer | 8.4 | 8.6 | 0.92 |
| Current smoker | 6.1 | 6.3 | 0.94 |
| Current drug abuse | 0.8 | 1.9 | 0.18 |
| Alcohol abuse | 9.7 | 7.3 | 0.29 |
| Blood Type O | 44.2 | 38.6 | 0.15 |
| Rheumatologic disease | 3.7 | 5.5 | 0.41 |
| Hepatitis B | 1.2 | 1.0 | 0.82 |
| Hepatitis C | 2.8 | 2.9 | 0.93 |
| Dialysis | 1.3 | 2.3 | 0.29 |
| History of coronary artery bypass | 32.5 | 29.3 | 0.37 |
| History of Valve Surgery | 9.6 | 10.7 | 0.65 |
| Implantable cardioverter defibrillator | 87.8 | 90.6 | 0.27 |
| Intra-aortic balloon pump | 22.2 | 33.0 | 0.11 |
| Ventilator | 3.7 | 1.9 | 0.18 |
| Peripheral vascular disease | 7.9 | 10.0 | 0.47 |
| Carotid artery disease | 11.7 | 13.6 | 0.59 |
| β-Blockers | 78.1 | 74.9 | 0.32 |
| Angiotensin-converting-enzyme inhibitor | 47.2 | 42.6 | 0.26 |
| Mitral regurgitation (Moderate/Severe) | 77.4 | 75.0 | 0.47 |
| Aortic regurgitation (Moderate/Severe) | 6.5 | 7.1 | 0.77 |
| Left ventricular ejection fraction (< 20 %) | 71.2 | 71.0 | 0.97 |
| Right ventricular ejection fraction (severely reduced) | 21.1 | 24.5 | 0.39 |
| Failure to wean | 0.9 | 1.9 | 0.18 |
| Extracorporeal membrane oxygenation | 0.4 | 1.4 | 0.10 |
| Patient Profile Modifier TCS | 17.7 | 20.7 | 0.36 |
COPD, chronic obstructive pulmonary disease; Failure to wean, inability to wean from cardiopulmonary bypass during other cardiac surgical procedure; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; NYHA, New York Heart Association; TCS, temporary circulatory support; TV, tricuspid valve.
Table 6.
Pre-implant characteristics of patients with no tricuspid valve procedure vs. with tricuspid valve procedure for continuous variables.
| NAME | No TV Procedure Percent n=757 | TV Repair Percent n=215 | p-value |
|---|---|---|---|
| Age (years) | 63.19 | 62.18 | 0.29 |
| Albumin (g/dL) | 3.36 | 3.34 | 0.65 |
| Total bilirubin (mg/dL) | 1.53 | 1.56 | 0.78 |
| Body mass index (kg/meter2) | 27.35 | 27.52 | 0.73 |
| Brain natriuretic peptide (pg/ml) | 1385.72 | 1328.57 | 0.71 |
| Body surface area (m2) | 2.01 | 2.04 | 0.15 |
| Blood urea nitrogen (mg/dL) | 32.16 | 36.37 | 0.0039 |
| Cholesterol (mg/dL) | 122.47 | 114.23 | 0.05 |
| Cardiac index (L/min/meter2) | 2.36 | 2.21 | 0.22 |
| Creatinine (mg/dL) | 1.47 | 1.59 | 0.04 |
| C-reactive protein (mg/L) | 14.51 | 16.28 | 0.73 |
| Diastolic blood pressure (mmHg) | 62.61 | 63.12 | 0.58 |
| Hemoglobin (mg/dL) | 11.23 | 11.05 | 0.20 |
| Heart Rate | 87.17 | 87.48 | 0.82 |
| International normalized ratio (international units) | 1.37 | 1.39 | 0.53 |
| LVEDD (cm) | 6.71 | 6.93 | 0.01 |
| Platelet (K/uL) | 188.36 | 184.70 | 0.53 |
| Pre-albumin (mg/dL) | 18.24 | 16.21 | 0.005 |
| Protein C (%) | 73.71 | 81.75 | 0.56 |
| Protein S (%) | 75.00 | 88.25 | 0.29 |
| Pulmonary diastolic pressure (mmHg) | 25.09 | 25.21 | 0.88 |
| Pulmonary systolic pressure (mmHg) | 51.04 | 52.06 | 0.41 |
| Pulmonary wedge pressure (mmHg) | 23.79 | 25.14 | 0.14 |
| Pulmonary vascular resistance (Woods units) | 4.17 | 4.16 | 0.92 |
| Right atrial pressure (mmHg) | 13.80 | 15.35 | 0.05 |
| AST (u/L) | 55.93 | 49.77 | 0.60 |
| ALT (u/L) | 62.77 | 50.58 | 0.27 |
| Sodium (mmol/L) | 134.61 | 133.91 | 0.07 |
| Systolic blood pressure (mmHg) | 103.17 | 102.93 | 0.85 |
| WBC count (K/uL) | 7.95 | 7.59 | 0.15 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; LVEDD, left ventricular end diastolic dimension; WBC, white blood cell; TV, tricuspid valve.
Comment
In this study, we observed a high rate of significant TR among adult patients undergoing primary implant of a continuous flow LVAD as destination therapy in INTERMACS. Treatment of TR with concomitant TVR was not widely performed, and more than half of patients with known severe TR did not undergo a tricuspid valve procedure at the time of LVAD implant. Considerable variation in the use of concomitant TVR was observed among higher volume INTERMACS centers. Centers with at least 20 patients with moderate or severe TR at the time of implant were noted to have TVR rates ranging ranged from 0% to 70% (data not shown).
Significant preoperative TR was associated with a poorer long term survival after LVAD implant and concomitant TVR did not appear to ameliorate this effect. The reason for the failure of concomitant TVR to improve long term survival in these patients is unclear. Standardized echocardiographic follow up is not available through INTERMACS and assessment of tricuspid valve function after surgery is therefore limited. The limited echocardiographic data that is available through INTERMACS suggests a failure rate defined as development of recurrent moderate or severe TR one year after surgery of 21-27% in patients who had TVR at the time of LVAD implantation. This rate of moderate or severe TR one year after LVAD implant with TVR was similar to patients with echocardiographic follow up who had significant TR at the time of surgery and underwent LVAD implant with no concomitant TVR.
The etiology for TVR failure is unclear. Progressive right ventricular failure and changes in right ventricular size and geometry may cause TR recurrence in a subpopulation of LVAD patients. Standard TVR techniques may not be suitable for patients with a cardiomyopathic etiology for valve dysfunction and high risk for ventricular failure.
The limited echocardiographic follow up data suggest that a significant proportion of patients with TR have effective and durable resolution of this with LVAD implant alone. Factors that may contribute to resolution of TR in these patients include etiology of heart failure, the degree of left ventricular unloading by the LVAD, pulmonary vascular resistance, right ventricular size and function, and renal function. How these factors interact to cause progressive right ventricular failure and TR is critical to being able to model or predict which subgroups of patients may or may not benefit from a tricuspid valve procedure at the time of LVAD implant. We did perform an analysis of patients with late recurrence of TR after LVAD implant to attempt to determine pre-LVAD implant risk factors associated with late TR. No variables were significant in this analysis and we must conclude that at this time we are unable to predict which patients will develop late TR after LVAD implantation either with or without concomitant TVR.
This study has a number of important limitations common to retrospective analyses. INTERMACS was designed as a registry and not as a database to specifically address tricuspid valve dysfunction. Assessments of tricuspid valve function were not performed at uniform intervals in the INTERMACS population and our findings may not precisely reflect tricuspid valve function in the entire population. The use of tricuspid valve procedures is not uniform among INTERMACS centers and treatment biases are likely to be present. No detail about the technique used for TVR is available through INTERMACS and there is likely to be variation in how this was performed between centers and surgeons.
Despite its limitations, this study is complementary to the existing literature on the topic because of its use of a multicenter database. Single-center reports have demonstrated the safety of concomitant tricuspid valve repair but are highly prone to selection bias and because of this are ill-suited to comparative studies of patients who have not undergone tricuspid valve repair (10-12). It is interesting to note that in a Society of Thoracic Surgeons (STS) database analysis focusing on short term outcomes, concomitant TVR did not affect surgical mortality but did lead to an increased risk of postoperative renal failure, reoperation, transfusion, and prolonged hospital length of stay (14). The STS analysis and the present study utilize large multicenter databases and therefore mitigate the selection bias inherent in single-center reports. Both studies dispel the notion that concomitant TVR reliably provides favorable short- or long-term effects on this challenging patient population.
Progressive right ventricular failure and associated TR is clearly identified in this study as an unresolved problem limiting the potential for long-term survival among patients supported with LVADs. To a large degree, problems encountered in prior years such as device durability, bleeding, and infectious complications have been at least partially addressed with the second and third generation LVADs currently available (15-18). The related problems of right ventricular failure and TR do not seem ameliorated with current generation devices. Future studies with rigorous follow up of right ventricular and tricuspid valve function are necessary to fully realize the potential benefit of this treatment.
Supplementary Material
Set acknowledgement
This project and the INTERMACS device database are funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201100025C.
Abbreviations and Acronyms
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- LVAD
left ventricular assist device
- TR
tricuspid regurgitation
- TVR
tricuspid valve repair
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Thirty-fourth Annual Meeting of the International Society for Heart and Lung Transplantation, San Diego, CA, April 11, 2014.
Set conflict box: Drs Song, Gelow, Mudd, Chien, and Tibayan discloses a financial relationship with HeartWare and Thoratec; Dr. Naftel with HeartWare.
Set MMC box: The Appendix can be viewed in the online version of this article [INSERT article doi] on http://www.annalsthoracicsurgery.org.
References
- 1.Aaronson KD, Eppinger MJ, Dyke DB, Wright S, Pagani FD. Left ventricular assist device therapy improves utilization of donor hearts. J Am Coll Cardiol. 2002;39:1247–54. doi: 10.1016/s0735-1097(02)01751-5. [DOI] [PubMed] [Google Scholar]
- 2.Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl HB, Hetzer R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation. 2005;112:I37–45. doi: 10.1161/CIRCULATIONAHA.104.525352. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Moskowitz AJ, Packer M, et al. The REMATCH trial: rationale, design, and end points. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure. Ann Thorac Surg. 1999;67:723–30. doi: 10.1016/s0003-4975(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 4.Dang NC, Topkara VK, Mercando M, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006;25:1–6. doi: 10.1016/j.healun.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Miller LW, Nelson KE, Bostic RR, Tong K, Slaughter MS, Long JW. Hospital costs for left ventricular assist devices for destination therapy: lower costs for implantation in the post-REMATCH era. J Heart Lung Transplant. 2006;25:778–84. doi: 10.1016/j.healun.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Kukucka M, Stepanenko A, Potapov E, Krabatsch T, Kuppe H, Habazettl H. Impact of tricuspid valve annulus dilation on mid-term survival after implantation of a left ventricular assist device. J Heart Lung Transplant. 2012;31:967–71. doi: 10.1016/j.healun.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Potapov EV, Stepanenko A, Dandel M, et al. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant. 2008;27:1275–81. doi: 10.1016/j.healun.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Kukucka M, Stepanenko A, Potapov E, et al. Right-to-left ventricular end-diastolic diameter ratio and prediction of right ventricular failure with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2011;30:64–9. doi: 10.1016/j.healun.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Piacentino V, 3rd, Williams ML, Depp T, et al. Impact of tricuspid valve regurgitation in patients treated with implantable left ventricular assist devices. Ann Thorac Surg. 2011;91:1342–6. doi: 10.1016/j.athoracsur.2011.01.053. discussion 1346-7. [DOI] [PubMed] [Google Scholar]
- 10.Maltais S, Topilsky Y, Tchantchaleishvili V, et al. Surgical treatment of tricuspid valve insufficiency promotes early reverse remodeling in patients with axial-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2012;143:1370–6. doi: 10.1016/j.jtcvs.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Piacentino V, 3rd, Ganapathi AM, Stafford-Smith M, et al. Utility of concomitant tricuspid valve procedures for patients undergoing implantation of a continuous-flow left ventricular device. J Thorac Cardiovasc Surg. 2012;144:1217–21. doi: 10.1016/j.jtcvs.2012.07.064. [DOI] [PubMed] [Google Scholar]
- 12.Piacentino V, 3rd, Troupes CD, Ganapathi AM, et al. Clinical impact of concomitant tricuspid valve procedures during left ventricular assist device implantation. Ann Thorac Surg. 2011;92:1414–8. doi: 10.1016/j.athoracsur.2011.05.084. discussion 1418-9. [DOI] [PubMed] [Google Scholar]
- 13.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Robertson JO, Grau-Sepulveda MV, Okada S, et al. Concomitant tricuspid valve surgery during implantation of continuous-flow left ventricular assist devices: a Society of Thoracic Surgeons database analysis. J Heart Lung Transplant. 2014;33:609–17. doi: 10.1016/j.healun.2014.01.861. [DOI] [PubMed] [Google Scholar]
- 15.Frazier OH, Myers TJ, Westaby S, Gregoric ID. Use of the Jarvik 2000 left ventricular assist system as a bridge to heart transplantation or as destination therapy for patients with chronic heart failure. Ann Surg. 2003;237:631–6. doi: 10.1097/01.SLA.0000064359.90219.44. discussion 636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazier OH, Shah NA, Myers TJ, Robertson KD, Gregoric ID, Delgado R. Use of the Flowmaker (Jarvik 2000) left ventricular assist device for destination therapy and bridging to transplantation. Cardiology. 2004;101:111–6. doi: 10.1159/000075991. [DOI] [PubMed] [Google Scholar]
- 17.Hetzer R, Weng Y, Potapov EV, et al. First experiences with a novel magnetically suspended axial flow left ventricular assist device. Eur J Cardiothorac Surg. 2004;25:964–70. doi: 10.1016/j.ejcts.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Slaughter MS, Tsui SS, El-Banayosy A, et al. Results of a multicenter clinical trial with the Thoratec Implantable Ventricular Assist Device. J Thorac Cardiovasc Surg. 2007;133:1573–80. doi: 10.1016/j.jtcvs.2006.11.050. [Erratum appears in J Thorac Cardiovasc Surg. 2007 Sep;134(3):A34].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.