Abstract
Background
Pegylated interferon α-2b (IFN α-2b) improves disease-free survival in adults with resected stage III melanoma. We conducted a study to determine the feasibility and safety of incorporating pegylated IFN α-2b as adjuvant therapy in the treatment of children and adolescents with high-risk melanoma. Pharmacokinetic studies of IFN α-2b and neuropsychological and quality of life (QOL) assessments were performed.
Patient and Methods
Eligible patients with resected AJCC Stage IIC, IIIA and IIIB cutaneous melanoma received non-pegylated IFN α-2b 20 million units/m2/day intravenously 5 days per week for 4 weeks (induction) followed by pegylated IFN α-2b 1 mcg/kg/dose weekly subcutaneously (SQ) for 48 weeks (maintenance).
Results
Twenty-three patients (15 females, median age 10 years) were enrolled. All patients completed induction therapy; whereas, 5 patients did not complete maintenance therapy either because of recurrent disease (n=2) or toxicity (n=3). The most common grade 3-4 toxicities of pegylated IFN α-2b were neutropenia (35%) and elevated liver transaminases (17%). The median non-pegylated IFN α-2b AUC0-∞ (5,026 pcg*hr/mL) was similar to adults. The median pegylated IFN α-2b exposure (48,480 pcg*hr/mL) was greater than the cumulative weekly exposure for non-pegylated IFN α-2b administered SQ three times per week (TIW). Validated measures demonstrated an improvement in QOL scores and no decline in psychological functioning over the course of therapy.
Conclusions
Pegylated IFN α-2b 1 mcg/kg/dose SQ weekly as maintenance therapy in children and adolescents with high-risk melanoma is feasible with tolerable toxicity and appears to yield higher exposures than non-pegylated IFN α-2b administered SQ TIW.
Keywords: pegylated interferon, melanoma, childhood, pharmacokinetics, high-risk, adjuvant therapy
INTRODUCTION
Compared to adults, the incidence of cutaneous melanoma in the pediatric population is low, with only ~400 new cases diagnosed in the United States per year in patients <20 years of age.[1] Due to the rarity of the disease in this patient population, the treatment generally follows the guidelines outlined for adult patients with melanoma and prospective clinical trials are rare but an area of need as the incidence in the adolescent population is increasing by about 3% per year.[1]
Interferon α-2b (IFN α-2b) has been used as adjuvant therapy in adult patients with surgically resected cutaneous melanoma who are at high risk of recurrence.[2] Various dosages and schedules of IFN α-2b have been evaluated in clinical trials with variable benefit.[3] The studies using high dose IFN α-2b consistently demonstrate an advantage in prolonging time to progression as well as a favorable cost-effectiveness ratio.[4-8] A commonly used IFN α-2b regimen is 20 million units/m2/day intravenously, five times per week for 4 weeks (induction) followed by 10 million units/m2/dose subcutaneously, three times per week (TIW) for 48 weeks (maintenance). The feasibility of administration of this regimen has been reported in a small number of pediatric patients with melanoma and has proven to be tolerable in children.[9-11]
A formulation of IFN α-2b, peginterferon α-2b (PEG Intron®; Merck, Inc), has been developed that contains a single straight-chain molecule of polyethylene glycol (PEG) covalently linked to histadine-34 on IFN α-2b. The PEG moiety decreases clearance, thus increasing the plasma half-life of the IFN α-2b from approximately 4 hours to 40 hours. As a result, the drug can be administered once weekly.[12] Preclinical and clinical studies indicate that the biological activity of IFN α-2b is similar, if not better than conventional interferon.[13, 14] Recently, pegylated IFN α-2b was approved for use in adult patients with high-risk melanoma based on the results of the EORTC 18991 trial in which significant improvement in disease-free survival (DFS) was observed in patients with resected stage III melanoma.[15] The adverse events in adults appear to be similar to non-pegylated IFN α-2b.[16, 17]
Based on the potential benefit observed with pegylated IFN α-2b in adult patients with melanoma and the more convenient once weekly dosing, we conducted a study to determine the feasibility and safety of pegylated IFN α-2b once weekly as a subcutaneous injection following an initial induction phase of high dose IFN α-2b in children and adolescents with resected 2002 American Joint Committee on Cancer (AJCC)[18] Stage IIC, IIIA or IIIB cutaneous melanoma.
PATIENTS AND METHODS
Patients
Eligibility criteria included: age ≤21 years, resected AJCC Stage IIC, IIIA or IIIB cutaneous melanoma, Karnofsky/Lansky performance score ≥50 and no prior therapy for melanoma except surgery. Other organ-specific and prior therapy inclusion/exclusion criteria are provided in Supplementary Materials.
Written informed consent was obtained from patients, parents, or legal guardians, with assent as appropriate. The protocol was approved by the institutional review board of each of the three institutions participating in the study: St. Jude Children’s Research Hospital, Rady Children’s Hospital and MD Anderson Cancer Center.
Treatment Plan
All patients underwent a primary wide local excision (WLE) with a minimum of 1–2 cm margin (if anatomically feasible) surrounding the primary lesion or biopsy scar and a sentinel lymph node biopsy (SLNB). If the sentinel lymph node(s) was positive for disease, then a complete lymph node dissection (CLND) of the involved lymph node basin was performed. Following surgery, patients received induction therapy with recombinant IFN α-2b (Intron A®, Merck, Inc,) 20 million units/m2 per day intravenously on 5 consecutive days per week for 4 weeks. Patients were premedicated with acetaminophen and/or ibuprofen. Following induction therapy, patients received pegylated IFN α-2b 1 mcg/kg/dose weekly subcutaneously for 48 weeks. This dosage of pegylated IFN α-2b was selected based on the results of pharmacokinetic studies in patients with chronic hepatitis C demonstrating a roughly equivalent area under the curve (AUC) to 10 million units TIW of IFN α-2b (dose used in maintenance phase of adult regimen).[14] IFN α-2b and pegylated IFN α-2b were supplied by Schering-Plough/Merck, Inc.
Evaluations, Toxicity Grading and Dose Modifications
Evaluations at baseline, during and after completion of therapy are outlined in Supplementary Materials. Toxicity was graded according to Common Terminology Criteria for Adverse Events (CTCAE) v3.0.
Dose modification was required for any grade 3 or 4 non-hematologic toxicity, with exception of the following toxicities: any drug-related grade fever, chills, pain or fatigue, medically managed grade 3 or 4 nausea or vomiting, grade 3 infection or grade 3 electrolyte abnormalities responsive to oral supplementation. In addition, dose modification was required for any grade 2 cardiopulmonary toxicity, grade 2 elevation in BUN/creatinine, grade 4 neutropenia, or grade 4 thrombocytopenia. Dose modification required holding IFN α-2b or pegylated IFN α-2b therapy until symptoms resolved to < grade 1. A 25% reduction in dosage occurred after the first interruption, 50% reduction after the second interruption and discontinuation if a third dosage modification was required. If > grade 1 toxicity persisted for >2 weeks from when the next dosage of drug was due, therapy was discontinued. IFN α-2b or pegylated IFN α-2b therapy was discontinued for any grade 4 cardiopulmonary toxicity, grade 4 mood alteration or grade 3 elevations in BUN/creatinine.
Missed doses of IFN α-2b were not made up. Dose re-escalation was not permitted. Patients who did not meet the protocol specified off therapy criteria for toxicity could remain on therapy in the absence of recurrent disease.
Quality of Life and Psychologic Assessments
In consenting patients, parent proxy and patient reported QOL outcomes were assessed using the Pediatric Quality of Life Inventory (PedsQL v4.0) and the Pediatric Cancer Quality of Life Inventory (PedsQL v3.0).[19, 20] The Behavioral Assessment System for Children, 2nd Edition (BASC-2)[21] and the Behavioral Rating Inventory of Executive Function (BRIEF)[22] were administered to parents, assessing for any effects on behavior or mood in children undergoing study therapy. The QOL and psychological assessments were obtained at the time points shown in Table III.
TABLE III.
Mean total scores (standard deviation) for each of the quality of life (PedsQL 4.0 and PedsQL3.0) measures and T-scores for each of the psychologic (BASC-2 and BRIEF) measures prior to, during and at the completion of therapy.
| Time point | PedsQL 4.0 Total, Childa, b |
PedsQL 4.0 Total, Parenta, c |
PedsQL 3.0 Cancer Totala, Child |
PedsQL 3.0 Cancer Totala, Parent |
BASC-2 BSId, Parent |
BRIEF GECe, Parent |
|---|---|---|---|---|---|---|
| Pre-Therapy | 75.5 (18.4) | 70.3 (19.1) | -- | -- | 44.9 (8.1) | 47.9 (12.8) |
| Week-2 | 71.6 (18.7) | 71.8 (16.4) | 71.1 (17.2) | 73.2 (13.9) | -- | -- |
| Week-4 | 77.2 (16.3) | 74.4 (17.9) | 76.1 (15.4) | 75.1 (15.2) | 45.9 (8.3) | 50.8 (11.9) |
| Week-8 | 79.3 (17.4) | 79.1 (16.6) | 79.2 (19.2)) | 81.4 (11.6) | -- | -- |
| Week-12 | 77.8 (20.6) | 79.0 (19.0) | 78.5 (14.7) | 78.7 (17.7) | -- | -- |
| Week-24 | 80.6 (15.6) | 82.2 (14.5 | 77.1 (16.0) | 81.6 (17.1) | 44.2 (6.9) | 48.6 (12.4) |
| End of Therapy |
80.4 (16.1) | 87.5 (15.3) | 77.0 (16.5) | 85.6 (13.8) | 47.2 (11.1) | 47.6 (12.6) |
| 6-months Post |
87.5 (12.5) | 86.0 (17.6) | 83.7 (18.0) | 85.0 (11.5) | 42.3 (7.2) | 42.6 (8.1) |
| 12-months Post |
91.0 (7.1) | 87.3 (17.5) | 85.4 (8.9) | 89.1 (11.6) | -- | -- |
Abbreviations: BASC-2, Behavior Assessment System for Children-Second Edition; GEC, Global Executive Composite; BSI, Behavior System Index; BRIEF, Behavior Rating Inventory of Executive Function.
Higher scores reflect better quality of life;
Healthy sample normative means for child report is 83.0 (14.8);
Healthy sample normative means by parent report is 87.6 (12.3);
Higher scores reflect greater behavioral problems;
Higher scores reflect poorer executive functions.
Pharmacokinetics
In consenting patients, serial serum samples (3 mL) for pharmacokinetic studies of IFN α-2b were collected before the first dose week 1, day 1 of induction therapy and 1, 2, 4, 6, 8, 12 and 24 hours post infusion. Serial serum samples (3 mL) for pharmacokinetic studies of pegylated IFN α-2b were collected during maintenance therapy before the first dose and 24, 96 and 168 hours after the dose during weeks 5 and 28. In addition, pre-dose serum samples for pegylated IFN α-2b were collected on weeks 8, 12, 16, 44, and 52. Samples were analyzed for IFN α-2b or pegylated IFN α-2b concentrations by using the VeriKine Human Interferon Alpha ELISA Kit (PBL Assay Science) following the manufacturer’s instructions and concentration-time data were analyzed by nonlinear-mixed effects modeling as implemented in NONMEM 7.3.[23] Details are provided in Supplementary Materials.
Statistical Methods
Event-free survival (EFS) and overall survival (OS) were estimated by the method of Kaplan-Meier.[24] The duration of OS was defined as the interval between time from study enrollment and death from any cause or last follow-up. The duration of EFS was defined as the interval between time from study enrollment and recurrence of disease, development of secondary malignancy, death or last follow-up. Patients who had not met criteria for an event were censored at the time of last follow-up.
Mixed-effects linear models for repeated measures were used to examine the mean change in total QOL, total cancer specific QOL, behavioral, and executive functioning scores over the course of the study. The unstructured correlation structure was selected to account for the correlation among repeated measurements made on the same patient for all models. A two-sided significance level of 0.05 was used for all statistical tests.
RESULTS
Patients
Twenty-three patients with resected AJCC Stage IIC, IIIA or IIIB melanoma were enrolled on study from June 2007 through September 2012. Patient characteristics are summarized in Table I.
TABLE I.
Patient Demographics and Clinicopathologic Characteristics (N = 23)
| Age (years) | No. of Patients (%) | |
|---|---|---|
| Median (range) | 10 (2–20) | |
| Sex | ||
| Female | 15 | |
| Male | 8 | |
| Race | ||
| White | 17 | |
| Black | 2 | |
| Other | 3 | |
| Unknown | 1 | |
| Primary Tumor Site | ||
| Extremity | 10 | |
| Trunk | 8 | |
| Head and Neck | 6 | |
| Histology | ||
| Spitzoida | 10 | |
| Nodular | 5 | |
| Superficial spreading | 5 | |
| Otherb | 3 | |
|
Breslow Thickness (mm)
(n=15) |
||
| Median (range) | 3.4 (1.2-7.8) | |
|
No. of involved lymph
nodes |
||
| Median (range) | 1 (0-3) | |
| Ulceration | ||
| Present | 10 | |
| Absent | 13 | |
| Stagec | ||
| IIC | 2 | |
| IIIA | 11 | |
| IIIB | 10 | |
Spitzoid neoplasms, biologically indeterminate;
Epitheliod (1), spindle cell (1), indeterminate (1);
2002 American Joint Committee on Cancer (AJCC) Staging System
Toxicity
Grade 3 or 4 and grade 1 or 2 toxicities related to induction and maintenance therapy are summarized in Table II and Supplemental Table SI, respectively.
TABLE II.
Grade 3 or 4 Treatment-Related Toxicity during Induction and Maintenance Therapy (N= 23)
| Adverse Eventsa | Induction | Maintenance | |
|---|---|---|---|
| Grade 3 or 4 No. (%) |
Grade 3 or 4 No. (%) |
||
| Hematologic | |||
| Neutropenia | 19 (83) | 8 (35) | |
| Leukopenia | 2 (9) | 2 (9) | |
| Non-hematologic | |||
| Metabolic/Laboratory | |||
| Elevated ALT | 4 (17) | 4 (17) | |
| Elevated AST | 2 (9) | 2 (9) | |
| Hypertriglyceridemia | 1 (4) | - | |
| Elevated amylase | - | 1 (4) | |
| Constitutional | |||
| Fever (absence of neutropenia | 1 (4) | - | |
| Gastrointestinal | |||
| Cholecystitis | - | 1(4) | |
| Musculoskeletal | |||
| Joint effusion | - | 1 (4) | |
| Joint Function | - | 1 (4) | |
| Neurology | |||
| Mood alteration | - | 1 (4) | |
| Pain | |||
| Headache | 1 (4) | 2 (9) | |
Abbreviations: AST, aspartate aminotrasferase; ALT, alanine aminotransferase
Grades according to National Cancer Institute Common Terminology Criteria for Adverse Events (Version 3.0). Each adverse event was counted once (highest grade) for each patient during induction and also once for each patient during maintenance
All patients completed induction with IFN a-2b. The most common grade 3 or 4 toxicities were neutropenia (83%) and elevated liver transaminases (17%). Four patients required dose reductions, three for grade 3 elevation in liver transaminases and one for grade 4 neutropenia. Two patients had a delay in starting maintenance therapy. One had grade 3 hypertriglyceridemia and one had grade 4 neutropenia.
During maintenance, 5 (22%) patients did not complete pegylated IFN a-2b therapy; 3 were removed from protocol therapy because of unacceptable toxicity, including one each with grade 3 joint effusion, grade 3 elevation in aspartate aminotrasferase and alanine aminotransferase, and grade 4 mood alteration (aggressive behavior). Two patients discontinued therapy due to recurrent disease. As with non-pegylated INF a-2b, the most common grade 3 or 4 toxicities were neutropenia (35%) and elevated liver transaminases (17%). Four patients required dose reductions, all due to grade 3 elevation in liver transaminases. Additionally, 4 patients required holding a weekly dose of pegylated interferon, one each for cholecystectomy, viral illness, hematochezia, and behavior issues deemed unrelated to therapy. Two patients did not receive the last dose of pegylated IFN a-2b for non-toxicity related reasons.
Thyroid abnormalities were present in 9 out of 23 participating subjects (39.1%). Autoimmune thyroiditis (i.e., thyroid peroxidase (TPO) and/or thyroglobulin (TGB) antibody titers > ULN) was diagnosed in 8 patients (present at baseline in 1). Only 2 patients experienced resolution (negative antibody titers) during the study time. Among the 8 patients with autoimmune thyroiditis, 6 had abnormal thyroid function: 4 required replacement with levothyroxine, 1 remained asymptomatic and did not require replacement and 1 had a known prior diagnosis of Graves’ disease, developed TPO and TGB antibodies but remained asymptomatic during the study period. One patient developed hypothyroidism during maintenance but had negative TPO and TGB autoantibody titers.
Non-thyroid related autoantibodies [anti-nuclear (ANA), anti-DNA, or anticardiolipin (ACA) antibodies] were detected in 12 patients (present at baseline in 5). One patient who had a low level ACA IgM prior to receiving interferon also developed a positive ANA and anti-DNA during therapy that resolved following the completion of therapy. In the remaining 7 patients, 5 developed positive ANA titers and 2 ACA IgM. All patients were asymptomatic except the one patient who developed a positive ACA IgM and had grade 3 joint pain and effusion. Five of the 12 patients with non-thyroid related autoantibodies also had thyroid related autoantibodies. Excluding patients with autoantibodies prior to interferon therapy, 10 patients developed autoantibodies (thyroid and/or non-thyroid related) while receiving interferon therapy. Of these patients, 3 of 7 with follow-up titers had no detectable autoantibody off treatment.
Outcome
The median follow-up from the time of enrollment was 37.8 months (range, 15.3–64.8 months). Twenty-one patients were alive without evidence of disease recurrence. One 18 year old patient with nodular melanoma of the upper back (ulcerated, Breslow thickness 6.2 mm, mitosis 7/mm2) who had a sentinel node (right axilla) negative for tumor at diagnosis, presented while on maintenance therapy with one isolated cervical lymph node detected by PET/CT. An excisional biopsy of the lymph node revealed malignant melanoma. This patient underwent a CLND followed by radiation to the involved nodal basin and is alive without disease 27 months after the regional recurrence. One patient has died. He was a 14 year old with a superficial spreading melanoma of the shoulder (ulcerated, Breslow Thickness >1.3 mm, mitosis 5/mm2) and a positive sentinel lymph node for tumor at diagnosis with no additional involved nodes found at the time of complete lymph node dissection. He presented with metastatic lung disease while on maintenance therapy and died of disease 18 months from diagnosis. Three-year estimates of OS and EFS are 95.2%±5.8% and 91.3%±7.5%, respectively.
Quality of Life and Psychological Functioning
Mean total scores for QOL and psychological assessments are shown in Table III. Patient reported QOL mean total scores were lowest during week 2 of induction therapy. However, the QOL assessment of patient and parent proxy reported mean total scores significantly improved over time (P ≤ 0.0001). At initiation of interferon therapy, the PedsQL v.4.0 mean total scores were lower, possibly due to the preceding surgical procedures (WLE and SLNB +/− CLND), than the healthy normative means.[25] However, the scores improved to levels equal to or above normative means at 6 and 12 months off therapy. By parent report, few behavioral problems were noted, as scores on the Behavioral Symptoms Index of the BASC-2 were within normative levels at baseline, and did not change over time. Parental report of child executive functions on the BRIEF was within the average range at baseline, and showed significant improvement over time (P <0.05).
Pharmacokinetics
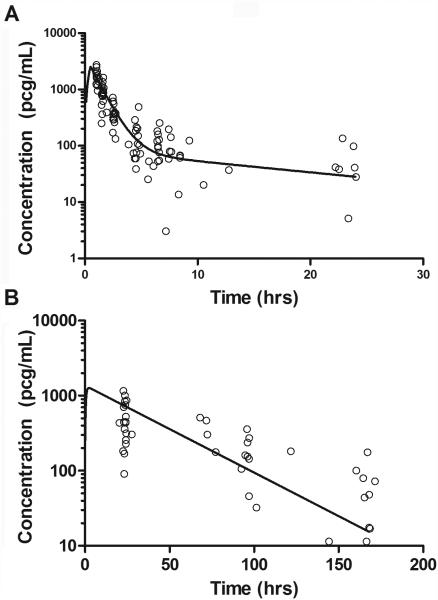
IFN α-2b pharmacokinetic studies were performed in 16 patients (109 samples, ~7 samples/patient). The median (range) systemic clearance (CL), volume of central compartment (Vc), α half-life and β half-life were 15.3 L/hr/m2 (7.5–29.1 L/hr/m2), 25.1 L/m2 (13.9–49.2 L/m2), 0.7 hours (0.4–1.4 hours) and 14.7 hours (12.5–28.2 hours), respectively. The median (range) AUC0-∞ was 5,026 pcg*hr/mL (2,642–10,270 pcg*hr/mL). A representative concentration-time profile for IFN α-2b is depicted in Figure 1A.
Figure 1.
Population concentration-time profile of interferon α-2b (A) and pegylated interferon α-2b (B) superimposed with observed (o) concentrations.
Serial pegylated IFN α-2b pharmacokinetic studies were performed on a total of 9 patients. The median (range) AUC0-∞ was 50,556 pcg*hr/mL (36,166–58,980 pcg*hr/mL) after the first dose (week 5; 7 patients) and 48,480 pcg*hr/mL (34,024–59,857 pcg*hr/mL) at steady state (week 28; 6 patients). The median (range) apparent CL and Vc, and half-life were 19.8 mL/hr/kg (16.7–31.4 mL/hr/kg), 772 mL/kg (594–1410 mL/kg), and 24.8 hours (16.6–40.6 hours), respectively. Based on 63 samples from 17 patients (median of 4 per patient) the median (range) steady-state trough concentrations for pegylated IFN α-2b was 52.8 pcg/ml (13.8–152.4 pcg/ml). A representative pegylated IFN α-2b concentration-time profile is shown in Figure 1B.
DISCUSSION
This is the first prospective study to incorporate pegylated IFN α-2b during maintenance therapy in children and adolescents with malignant melanoma. Our results demonstrate that this therapy is feasible and safe in this patient population.
The use of pegylated IFN α-2b based on the EORTC 18991 in adult patients with resected stage III melanoma that led to the approval of the pegylated IFN α-2b for this indication consisted of an induction phase of 6 mcg/kg/dose SQ weekly for 8 weeks followed by a planned maintenance phase of 3 mcg/kg/dose for 5 years.[16, 17] Although there was an improvement in DFS in patients who were randomized to receive pegylated IFN α-2b, 37% of patients discontinued therapy for toxicity (25% due to fatigue) and the median treatment duration was only 12 months (range, 3.8–33.4). In contrast to this adult study and with the caveat that we employed a lower dose of pegylated INF α-2b, discontinuation of therapy was observed in only 3 (13%) of 23 participants in our study and whereas the most common grade 3 or 4 toxicity was fatigue (16%) and liver function abnormalities (11%) in EORTC 18991,[16] the most common toxicities during pegylated IFN α-2b therapy in our study were neutropenia (35%) and liver function abnormalities (17%). This finding is consistent with our previous study of non-pegylated interferon where grade 3 or 4 neutropenia was also more common in children than constitutional symptoms as observed in adults.[9]
Adult patients undergoing interferon therapy for melanoma not only report an intolerable level of fatigue, but an overall global decrease in quality of life as well as depressive and cognitive difficulties.[26-28] In order to better understand the impact of interferon on QOL and psychological function in pediatric patients with melanoma, we used validated measures to systematically assess these symptoms in our study. Despite a significant percentage of patients coded with grade 1 or 2 fatigue (induction 70%; maintenance 83%) and mood alteration (induction 17%; maintenance 39%) by CTCAE v3.0, our results using the validated measures for QOL and psychological functioning, demonstrated improvement in QOL over the course of therapy and following completion of therapy as reported by the patient and parent proxy. Similarly, we found no decline in psychological functioning, which was within normal limits, and observed an improvement in executive functioning over the course of treatment. While it does not appear that there is an adverse impact of interferon therapy on QOL, depression or executive function, our sample size was small and therefore for each individual patient it is imperative for physicians to assess for mood alterations and symptoms that may impact QOL. This may be challenging in young patients with pre-existing behavioral issues that may be exacerbated by the change in routine associated with the diagnosis and treatment of a malignancy.
Thyroid abnormalities have been reported in up to 50% of cases with interferon therapy[29], but have not been well characterized in the pediatric population. In our study, thyroid dysfunction developed in 35% of patients. The most commonly reported thyroid abnormality, both in the literature and in our study, was autoimmune thyroiditis. It was diagnosed most commonly during the maintenance phase of our study and resolved in only 2 patients during the study period. Autoimmune thyroiditis is caused by damage to the epithelial cells of the thyroid following immune system activation. Non-autoimmune thyroiditis due to direct damage by interferon has also been described and may have occurred in one of our patients with persistent hypothyroidism and negative thyroid autoantibodies.[29] Primary hypothyroidism required treatment in all of our patients who to date continue to require replacement therapy.
The induction of serologic and clinical manifestations of autoimmunity with interferon in patients with melanoma is not restricted to thyroid-related autoantibodies. In a prospective study of adult patients with melanoma who were treated with high dose interferon therapy, 26% developed autoantibodies (anti-thyroid, ANA, anti-DNA and ACA antibodies) or clinical manifestations of autoimmunity and this feature was an independent prognostic factor for improved DFS and OS in that study.[30] In a retrospective study evaluating 3 autoantibodies (ANA, ACA and TGB antibodies) in patients receiving intermediate dose interferon in two clinical trials, seroconversion was not associated with improved outcome.[31] Similar findings were noted in patients receiving pegylated interferon in EORTC 18991.[32] In our study, we performed serologic testing for 5 autoantibodies and found seroconversion in 10 (43%) of 23 patients during therapy. Because of the small number of participants and excellent outcomes in our study, we are unable to comment on whether seroconversion is a prognostic factor for DFS. It is also unclear if there are any clinical implications for those few patients with persistently positive titers.
We evaluated the single-dose disposition of IFN α-2b, and both the single-dose and multiple-dose pharmacokinetics of pegylated IFN α-2b. The median IFN α-2b AUC0-∞ and systemic clearance and pegylated IFN α-2b apparent clearance in our study were in agreement with previous studies in adults after accounting for differences in dosage.[12, 33-35]
Based on prior reports investigating the pharmacokinetics of pegylated IFN α-2b and IFN α-2b in patients with chronic hepatitis C (Merck; data on file), we substituted a subcutaneous dosage of 1 µg/kg/week pegylated IFN α-2b to achieve similar exposures to IFN α-2b 30 million units/week subcutaneously.[14] We found that the median pegylated IFN α-2b exposure was greater than the cumulative weekly exposure reported for IFN α-2b given TIW, suggesting that our patients had a greater exposure than predicted. However, this result should be interpreted with caution because of differences in analytical techniques, bioactive forms of interferon, and patient populations.
The use of adjuvant high dose interferon in adult and pediatric patients with high-risk melanoma is controversial. Those who dispute the use of adjuvant interferon cite modest benefit at the expense of cost and toxicity as arguments against its use. There are additional considerations related to using interferon in the adjuvant setting for the pediatric population. First, in pediatrics, the benefit in OS and DFS versus observation alone is unknown. The low incidence of the disease in this population precludes a large and/or randomized study mimicking those performed in adults to answer this question. The second issue is the diagnostic dilemma of atypical spitzoid tumors (AST) commonly encountered in children. ASTs often have some morphologic features that would suggest that the lesion could be malignant and are frequently associated with a positive sentinel node. However, ASTs rarely metastasize and therefore do not carry the same prognostic significance, particularly in prepubertal children, as conventional melanoma and may not require adjuvant therapy. Nonetheless, because there are small subsets of children with ASTs who develop metastatic disease,[36] we included patients with biologically indeterminate spitzoid neoplasms in our study. Molecular profiling of ASTs will help better define the need for adjuvant therapies in these patients.[37]
Recent approval of immune checkpoint inhibitors (e.g., ipilimumab and nivolumab) and small molecules targeting mutations in BRAF (e.g., vemurafenib) and downstream inhibition of MEK (e.g., trametinib) have led to trials evaluating these agents in the adjuvant setting for high risk patients. The first of the results of these trials was recently published and showed that ipilimumab after complete resection of stage III melanoma improved DFS compared to observation alone.[38] However, ipilimumab-related toxicities were significant. Further, it is important to recognize that the early trials in patients with metastatic disease with both checkpoint inhibitors and those targeting BRAF only benefit a subset of patients. The tumor in ~50% percent of patients with melanoma does not harbor a BRAF mutation.[39-41] Further, the development of resistance to these inhibitors is a common problem.[42] Because of the challenges with these new therapies, adjuvant interferon for high-risk patients deserves consideration. In our study, we show that in children with high-risk melanoma, the convenient weekly SQ pegylated IFN α-2b is feasible as maintenance therapy.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by Cancer Center Support CORE Grant P30 CA 21765 from the National Cancer Institute, the American Lebanese Syrian Associated Charities, and Schering-Plough/Merck, Inc.
The authors would like to thank all of the patients and their families, research nurses, and data managers that participated in this study.
Abbreviations
- ACA
anticardiolipin antibody
- AJCC
American Joint Committee on Cancer
- ANA
anti-nuclear antibody
- AST
atypical spitzoid tumor
- AUC
area under the curve
- BASC-2
Behavioral Assessment System for Children, 2nd Edition
- BRAF
v-raf murine sarcoma viral oncogene homolog B1
- BRIEF
Behavioral Rating Inventory of Executive Function
- BUN
blood urea nitrogen
- CL
systemic clearance
- CLND
complete lymph node dissection
- CTCAE
Common Terminology Criteria for Adverse Events
- DFS
disease-free interval
- DNA
deoxyribonucleic acid
- EFS
event-free survival
- EORTC
European Organisation for Research and Treatment of Cancer
- IFN α-2b
interferon
- MEK
mitogen-activated protein kinase/extracellular signal-regulated kinase
- OS
overall survival
- PedsQL
Pediatric Quality of Life Inventory
- PEG
polyethylene glycol
- QOL
quality of life
- SLNB
sentinel lymph node biopsy
- SQ
subcutaneously
- TGB
thyroglobulin
- TIW
three times per week
- TPO
thyroid peroxidase
- Vc
volume of central compartment
- WLE
wide local excision
Footnotes
Clinical trials registration number: NCT00539591
CONFLICT OF INTEREST STATEMENT
Alberto Pappo is a consultant for Ziopharm Oncology, Inc. All other authors report no conflict of interest.
REFERENCES
- 1.Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: Risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735–41. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U, Eastern Cooperative Oncology G A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–7. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 3.Sabel MS, Sondak VK. Pros and cons of adjuvant interferon in the treatment of melanoma. Oncologist. 2003;8:451–8. doi: 10.1634/theoncologist.8-5-451. [DOI] [PubMed] [Google Scholar]
- 4.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 5.Cormier JN, Xing Y, Ding M, Cantor SB, Salter KJ, Lee JE, Mansfield PF, Gershenwald JE, Ross MI. Cost effectiveness of adjuvant interferon in node-positive melanoma. J Clin Oncol. 2007;25:2442–8. doi: 10.1200/JCO.2007.10.7284. [DOI] [PubMed] [Google Scholar]
- 6.Hillner BE, Kirkwood JM. Economic analyses of benefit from interferon-alpha 2b in high-risk melanoma: Trade-offs between completeness, simplicity and clarity. Eur J Cancer. 1997;33:1345–6. doi: 10.1016/s0959-8049(97)00184-6. [DOI] [PubMed] [Google Scholar]
- 7.Lafuma A, Grob JJ. Cost-effectiveness of interferon-alpha2 as adjuvant therapy in malignant melanoma. Expert Opin Pharmacother. 2003;4:343–9. doi: 10.1517/14656566.4.3.343. [DOI] [PubMed] [Google Scholar]
- 8.Messori A, Becagli P, Trippoli S, Tendi E. A retrospective cost-effectiveness analysis of interferon as adjuvant therapy in high-risk resected cutaneous melanoma. Eur J Cancer. 1997;33:1373–9. doi: 10.1016/s0959-8049(96)00413-3. [DOI] [PubMed] [Google Scholar]
- 9.Navid F, Furman WL, Fleming M, Rao BN, Kovach S, Billups CA, Cain AM, Amonette R, Jenkins JJ, Pappo AS. The feasibility of adjuvant interferon alpha-2b in children with high-risk melanoma. Cancer. 2005;103:780–7. doi: 10.1002/cncr.20860. [DOI] [PubMed] [Google Scholar]
- 10.Chao MM, Schwartz JL, Wechsler DS, Thornburg CD, Griffith KA, Williams JA. High-risk surgically resected pediatric melanoma and adjuvant interferon therapy. Pediatr Blood Cancer. 2005;44:441–8. doi: 10.1002/pbc.20168. [DOI] [PubMed] [Google Scholar]
- 11.Shah NC, Gerstle JT, Stuart M, Winter C, Pappo A. Use of sentinel lymph node biopsy and high-dose interferon in pediatric patients with high-risk melanoma: The hospital for sick children experience. J Pediatr Hematol Oncol. 2006;28:496–500. doi: 10.1097/01.mph.0000212973.28996.e4. [DOI] [PubMed] [Google Scholar]
- 12.Glue P, Fang JW, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S. Pegylated interferon-alpha2b: Pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis c intervention therapy group. Clin Pharmacol Ther. 2000;68:556–67. doi: 10.1067/mcp.2000.110973. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay KL, Trepo C, Heintges T, Shiffman ML, Gordon SC, Hoefs JC, Schiff ER, Goodman ZD, Laughlin M, Yao R, Albrecht JK. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis c. Hepatology. 2001;34:395–403. doi: 10.1053/jhep.2001.26371. [DOI] [PubMed] [Google Scholar]
- 14.Bukowski RM, Tendler C, Cutler D, Rose E, Laughlin MM, Statkevich P. Treating cancer with peg intron: Pharmacokinetic profile and dosing guidelines for an improved interferon-alpha-2b formulation. Cancer. 2002;95:389–96. doi: 10.1002/cncr.10663. [DOI] [PubMed] [Google Scholar]
- 15.Herndon TM, Demko SG, Jiang X, He K, Gootenberg JE, Cohen MH, Keegan P, Pazdur R. U.S. Food and drug administration approval: Peginterferon-alfa-2b for the adjuvant treatment of patients with melanoma. Oncologist. 2012;17:1323–8. doi: 10.1634/theoncologist.2012-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, Punt CJ, Sales F, Gore M, Mackie R, Kusic Z, Dummer R, Hauschild A, Musat E, Spatz A, Keilholz U. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage iii melanoma: Final results of eortc 18991, a randomised phase iii trial. Lancet. 2008;372:117–26. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 17.Eggermont AM, Suciu S, Testori A, Santinami M, Kruit WH, Marsden J, Punt CJ, Sales F, Dummer R, Robert C, Schadendorf D, Patel PM, de Schaetzen G, Spatz A, Keilholz U. Long-term results of the randomized phase iii trial eortc 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage iii melanoma. J Clin Oncol. 2012;30:3810–8. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 18.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr., Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the american joint committee on cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 19.Varni JW, Katz ER, Seid M, Quiggins DJ, Friedman-Bender A. The pediatric cancer quality of life inventory-32 (pcql-32): I. Reliability and validity. Cancer. 1998;82:1184–96. doi: 10.1002/(sici)1097-0142(19980315)82:6<1184::aid-cncr25>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Varni JW, Katz ER, Seid M, Quiggins DJ, Friedman-Bender A, Castro CM. The pediatric cancer quality of life inventory (pcql). I. Instrument development, descriptive statistics, and cross-informant variance. J Behav Med. 1998;21:179–204. doi: 10.1023/a:1018779908502. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds CR, Kamphaus RW. Pearson assessments. Second Bloomington, MN: 2004. Behavior assessment system for children. [Google Scholar]
- 22.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Psychological assessment resources. Lutz, FL: 2000. Behavior rating inventory of executive function. [Google Scholar]
- 23.Nonmem 7.3.0 users guides. ICON Development Solutions; Hanover, MD: 1989 - 2013. B S, S L, B A, and B R. [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 25.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The pedsql in pediatric cancer: Reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer. 2002;94:2090–106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 26.Bottomley A, Coens C, Suciu S, Santinami M, Kruit W, Testori A, Marsden J, Punt C, Sales F, Gore M, Mackie R, Kusic Z, Dummer R, Patel P, Schadendorf D, Spatz A, Keilholz U, Eggermont A. Adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage iii melanoma: A phase iii randomized controlled trial of health-related quality of life and symptoms by the european organisation for research and treatment of cancer melanoma group. J Clin Oncol. 2009;27:2916–23. doi: 10.1200/JCO.2008.20.2069. [DOI] [PubMed] [Google Scholar]
- 27.Trask PC, Paterson AG, Esper P, Pau J, Redman B. Longitudinal course of depression, fatigue, and quality of life in patients with high risk melanoma receiving adjuvant interferon. Psychooncology. 2004;13:526–36. doi: 10.1002/pon.770. [DOI] [PubMed] [Google Scholar]
- 28.Rataj D, Jankowiak B, Krajewska-Kulak E, Van Damme-Ostapowicz K, Nowecki ZI, Rutkowski P, Niczyporuk W. Quality-of-life evaluation in an interferon therapy after radical surgery in cutaneous melanoma patients. Cancer Nurs. 2005;28:172–8. doi: 10.1097/00002820-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Torino F, Barnabei A, Paragliola R, Baldelli R, Appetecchia M, Corsello SM. Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid. 2013;23:1345–66. doi: 10.1089/thy.2013.0241. [DOI] [PubMed] [Google Scholar]
- 30.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos A, Papadopoulos O, Stratigos A, Markopoulos C, Bafaloukos D, Pectasides D, Fountzilas G, Kirkwood JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–18. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 31.Bouwhuis MG, Suciu S, Collette S, Aamdal S, Kruit WH, Bastholt L, Stierner U, Sales F, Patel P, Punt CJ, Hernberg M, Spatz A, ten Hagen TL, Hansson J, Eggermont AM, Group EM, Nordic Melanoma G Autoimmune antibodies and recurrence-free interval in melanoma patients treated with adjuvant interferon. J Natl Cancer Inst. 2009;101:869–77. doi: 10.1093/jnci/djp132. [DOI] [PubMed] [Google Scholar]
- 32.Bouwhuis MG, Suciu S, Testori A, Kruit WH, Sales F, Patel P, Punt CJ, Santinami M, Spatz A, Ten Hagen TL, Eggermont AM. Phase iii trial comparing adjuvant treatment with pegylated interferon alfa-2b versus observation: Prognostic significance of autoantibodies--eortc 18991. J Clin Oncol. 2010;28:2460–6. doi: 10.1200/JCO.2009.24.6264. [DOI] [PubMed] [Google Scholar]
- 33.Daud AI, Xu C, Hwu WJ, Urbas P, Andrews S, Papadopoulos NE, Floren LC, Yver A, Deconti RC, Sondak VK. Pharmacokinetic/pharmacodynamic analysis of adjuvant pegylated interferon alpha-2b in patients with resected high-risk melanoma. Cancer Chemother Pharmacol. 2011;67:657–66. doi: 10.1007/s00280-010-1326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jen JF, Glue P, Ezzet F, Chung C, Gupta SK, Jacobs S, Hajian G. Population pharmacokinetic analysis of pegylated interferon alfa-2b and interferon alfa-2b in patients with chronic hepatitis c. Clin Pharmacol Ther. 2001;69:407–21. doi: 10.1067/mcp.2001.115872. [DOI] [PubMed] [Google Scholar]
- 35.Radwanski E, Perentesis G, Jacobs S, Oden E, Affrime M, Symchowicz S, Zampaglione N. Pharmacokinetics of interferon alpha-2b in healthy volunteers. J Clin Pharmacol. 1987;27:432–5. doi: 10.1002/j.1552-4604.1987.tb03044.x. [DOI] [PubMed] [Google Scholar]
- 36.Barnhill RL. The spitzoid lesion: Rethinking spitz tumors, atypical variants, 'spitzoid melanoma' and risk assessment. Mod Pathol. 2006;19(Suppl 2):S21–33. doi: 10.1038/modpathol.3800519. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Barnhill RL, Dummer R, Dalton J, Wu J, Pappo A, Bahrami A. Tert promoter mutations are predictive of aggressive clinical behavior in patients with spitzoid melanocytic neoplasms. Sci Rep. 2015;5:11200. doi: 10.1038/srep11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebbe C, Ferraresi V, Smylie M, Weber JS, Maio M, Konto C, Hoos A, de Pril V, Gurunath RK, de Schaetzen G, Suciu S, Testori A. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage iii melanoma (eortc 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–30. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 39.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 40.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the braf gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 41.Daniotti M, Ferrari A, Frigerio S, Casieri P, Miselli F, Zucca E, Collini P, Della Torre G, Manoukian S, Peissel B, Bono A, Santinami M, Parmiani G, Rivoltini L, Pilotti S, Rodolfo M. Cutaneous melanoma in childhood and adolescence shows frequent loss of ink4a and gain of kit. J Invest Dermatol. 2009;129:1759–68. doi: 10.1038/jid.2008.422. [DOI] [PubMed] [Google Scholar]
- 42.Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, Chodon T, Guo R, Johnson DB, Dahlman KB, Kelley MC, Kefford RF, Chmielowski B, Glaspy JA, Sosman JA, van Baren N, Long GV, Ribas A, Lo RS. Acquired resistance and clonal evolution in melanoma during braf inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.