Abstract
Introduction
Serine/threonine kinase 11 (STK11), better known as LKB1, is a tumor-suppressor commonly mutated in lung adenocarcinoma (LUAD). Previous work has shown that mutational inactivation of the STK11 pathway may serve as a predictive biomarker for cancer treatments including phenformin and COX-2 inhibition. Although immunohistochemistry and diagnostic sequencing are employed to measure STK11 pathway disruption, there are serious limitations to these methods emphasizing the importance to validate a clinically useful assay.
Methods
An initial STK11 mutation mRNA signature was generated using cell line data and refined using three large, independent patient databases. The signature was validated as a classifier using The Cancer Genome Anatomy Project (TCGA) LUAD cohort as well as a 442-patient LUAD cohort developed at Moffitt. Finally, the signature was adapted into a NanoString -based format and validated using RNA samples isolated from FFPE tissue blocks corresponding to a cohort of 150 LUAD patients. For comparison, STK11 immunochemistry was also performed.
Results
The STK11 signature was found to correlate with null mutations identified by exon sequencing in multiple cohorts using both microarray and NanoString formats. While there was a statistically significant correlation between reduced STK11 protein expression by IHC and mutation status, the NanoString-based assay showed superior overall performance with a −0.1588 improvement in area under the curve in receiver-operator characteristic curve analysis (p<0.012).
Conclusion
The described NanoString-based STK11 assay is a sensitive biomarker to study emerging therapeutic modalities in clinical trials.
Keywords: STK11, LKB1, NanoString, biomarker, COX-2 inhibition
INTRODUCTION
STK11 is a serine/threonine kinase, also known as liver kinase β1 (LKB1), which was discovered as the gene responsible for familial Peutz-Jeghers syndrome1. While somatic STK11 mutations are uncommon in many cancers, including lung squamous cell carcinoma2, the incidence of STK11 mutations in lung adenocarcinoma (LUAD) is exceeded only by KRAS and TP533–10. The STK11 protein is catalytically active as a heterotrimeric complex with the STE20-related adaptor protein α (STRADα) and mouse protein-25 (MO25). Many mutations in STK11 have been found to interfere with its ability to bind these partners11 rendering the expressed protein inactive. STK11 has multiple targets that likely mediate its tumor-suppressor activity. Foremost, STK11 is responsible for phosphorylating the AMP-activated kinase (AMPK) at amino acid residue T172 under conditions of energy stress12. Our published work links STK11 to the regulation of cyclooxygenase 2 (COX-2) and many other genes via indirect regulation of the CRTC-1 transcription factor13–15. Additionally, we find that STK11 mutations can be linked to immunosuppression via potential influence on the NF-κB signaling pathway16.
While there are currently no drugs in routine clinical use that specifically target STK11 there is a growing number of approaches that may differentially benefit patients with disrupted STK1117–25. Therapies that reactivate AMPK, such as metformin, phenformin20, and AICAR22, and an AMP mimetic (2-deoxyglucose)21 have been shown to render STK11-mutant tumors more susceptible to chemotherapies, possibly by promoting a transition from adenocarcinoma to squamous cell carcinoma17. Recent work suggests that STK11 mutation enhances the sensitivity to energetic stress that is induced by erlotinib treatment25. Additionally, drugs that target the downstream proteins that are up-regulated by loss of STK11 such as mTOR inhibitors (e.g. rapamycin24 and everolimus23), cyclooxygenase 2 (COX-2) inhibitors (e.g. NS-398 and Niflumic acid13), reactive oxygen species-inducing agents17, and lysyl oxidase inhibitors (e.g. BAPN26) have shown efficacy in STK11-mutant cells as compared to their WT counterparts. In summary, despite extensive preclinical data, the lack of progress to develop clinical treatments for patients with STK11-null cancers is partly due to the lack of a validated method to reliably score STK11 status using available formalin fixed archival (FFPE) samples.
Immunohistochemistry (IHC)27 and diagnostic sequencing are both potential means to measure STK11 pathway disruption. However, many of the variants identified in STK11 lead to single amino acid changes that may not alter STK11 function16, 28. Furthermore, STK11 function can be lost by epigenetic inactivation29 or by homozygous and intragenic deletions7, 30 which are difficult to detect by genomic sequencing. In fact, recent work suggests that as many as one-half of instances of STK11 functional loss are undetected by sequencing31. Given the potential of assessing STK11 status as a biomarker in LUAD, a robust STK11 mutation signature was adapted into a NanoString™ Elements-based format, which is amenable to use with formalin-fixed, paraffin-embedded (FFPE) samples. This assay was then validated using RNA isolated from tissue blocks from a cohort 150 LUAD surgical specimens. For comparison, STK11 immunochemistry was performed. Results demonstrate that the NanoString-based assay yields superior overall performance to STK11 IHC suggesting that NanoString may be a useful tool in a multi-analyte approach to identify LUAD patients with disruption of the STK11 pathway.
MATERIALS AND METHODS
Expression Vectors, Cell Culture and Molecular Assays
Detailed methods are provided in the Supplementary Materials. Briefly, the pcDNA3-FLAG-STK11 vector was purchased from Addgene (Plasmid #8590;Cambridge, MA, USA). pNTAPb and the pNTAPb-Mef2a affinity-tagged negative control were purchased from Agilent Technologies (Kit #240103, Santa Clara, CA, USA) as component of the InterPlay N-terminal Mammalian TAP System. Cells were cultured in RMPI supplemented with 10% fetal bovine and transfected with vector (pNTAPb), Mef2a control, or one of three STK11 variants, D194Y, P281fs*6, or F354L with Lipofectamine-2000 in serum-free media. Proteins were visualized using horseradish peroxidase conjugated secondary antibodies and enhanced chemiluminescence (ECL; Amersham Biosciences, GE Life Sciences, Pittsburgh, PA, USA. NanoString assays were performed with 150-ng aliquots of RNA using the NanoString nCounter Analysis system. TMA (tissue microarray) slides were cut into 4 µM sections and stained with anti-STK11 at a 1:100 dilution.
Clinical and Molecular Data Sets
Five public datasets were used, including; 1) the Molecular Classification of Lung Adenocarcinoma (MCLA) microarray study32, 2) the Cancer Genome Anatomy Project (TCGA) LUAD RNAseq study28 and 3–5) three microarray studies from the Gene Expression Omnibus33 (GSE3021934, GSE3774535 and GSE1481436). The MCLA32 and GSE1481436 sets were combined to form the MCLA + cohort. Data from a 442-LUAD patient cohort (herein referred to as MLOS, Moffitt LUAD, Overall Survival cohort) that consented to the Moffitt Cancer Center's Total Cancer Care Protocol (TCC™, see Table S1) was also used. Surgical tissue blocks from 150 patients (see Table S2) that were treated at Moffitt were used to construct a TMA as described in the Supplementary Methods and as the source for RNA for NanoString analysis. Herein, these 150 patients are referred to as the MLCom cohort (Moffitt LUAD, Complete) since it was possible to acquire medical charts and tissue blocks for these patients. Most MLCom’ members are also in the MLOS cohort. All patient-related work was approved by the University of South Florida Institutional Review Board.
Statistical Analysis
Statistical analyses were performed using R Project for Statistical Computing version 3.2.2 (http://www.r-project.org/) and SAS 9.4 (SAS institute; Cary, NC). Chi-squared test exact method with Monte Carlo estimation was used to test for differences in the distributions of mutational status by study population characteristics. Principle component analysis (PCA) was used to summarize STK11 signature scores in a single number. Briefly, PCA is used to find orthogonal vectors that capture the largest sources of variance within a dataset37, 38 The scores from the first principle component (PC1) of a gene signature are commonly used to score samples within a dataset. The gene loadings, p[1], corresponding to the first principle component, reflect the relative weights of each gene with respect to their contribution to the sample scores. The second principle component in our analyses did not correlate with STK11 mutation status; however, it was used as a means to plot the first principle component in two-dimensions.
Measures of test performance and agreement of the STK11 gene signature to predict STK11 mutation status, including true positives, true negatives, false positives, false negatives, sensitivity, specificity, negative predictive value, false positive rate, and false discovery rate were calculated. For the TCGA samples, the first principle component (t[1]) of the STK11 signature was used to assign WT and STK11 phenotypes (WT: t[1] ≤ 0, STK11: t[1] > 0) phenotype to the samples. The MLOS cohort sample phenotypes were assigned in a similar fashion (WT: t[1] ≤ 2.1, STK11: t[1] > 2.1). Splice site mutations were treated as mutant for two-state analysis purposes. Receiver operator characteristic (ROC) curves were used to compare overall assay sensitivity and specificity based on the area under the curve (AUC) calculations.
RESULTS
Expression of stable, but catalytically inactive, STK11 variants may obscure STK11 pathway mutations
Previous work has identified three alternatively spliced isoforms of STK11; 1) the full-length 50-kDa isoform, 2) a 48-kDa isoform with an alternative C-terminus that is expressed in the testis39 and 3) an oncogenic, but kinase inactive, 42-kDa isoform that is mainly expressed in normal heart and skeletal muscle40 that is reported to be expressed (along with other shortened STK11 isoforms) at high levels in tumors with mutations in STK11 codons 1 and 241. Such isoforms could potentially obscure standard IHC assessment of STK11 pathway status. Thus, we sought to examine the expression of STK11 isoforms in established cells lines and tumor. Forty-two cell lines and fifty-six tumor samples with known STK11 status were examined by STK11 western blotting (Figure 1). Figure 1A reveals that the full length 50-kDa STK11 band was present in STK11 wildtype (WT) cell lines, as expected, with H2170 cells being the exception. The 48-kDa isoform was observed in only two cell lines (H292 and HCC4006, both STK11 WT) and was co-expressed with the 50-kDa band. In H2170, H520, H1395, Calu-6 and H1581 cells intense faster migrating bands were observed in the 42-kDa size range which were generally co-expressed with the 50-kDa isoform. Full-length STK11 protein expression was absent in all STK11 mutant cell lines, as expected.
Figure 1. STK11 protein expression in cell lines and tumor samples.
(A) Cell lines and (B) patient tumors of previously determined STK11 mutational status16 were subjected to western blotting for STK11. Samples specifically highlighted in the text are boxed. Repeated cell lines were obtained from different laboratory sources. The three STK11 isoforms are indicated (C) Empty pNTAPb vector (Vector), a vector expressing a negative control affinity-tagged Mef2a protein (Mef2a control), WT STK11, and each of the three indicated STK11 variants were transfected into H1299 cells, immunoprecipitated using streptavidin beads, and blotted for binding to MO25, and STRAD. (D) A549 cells were transfected with the indicated combination of plasmids, extracted and blotted for AMPK phosphorylated at T172, total AMPK and β-actin. The first lane (−) represents empty vectors (pcDNA3 and pNTAPb); the second lane (+) represents cells transfected with pcDNA3 WT STK11 and empty pNTAPb; lanes labeled D194Y received pNTAPb-D194Y alone (−), or pNTAPb-D194Yplus WT in 1:1 (+), 1:5 (++), and 1:10 (+++) ratios. Lanes labeled F354L received pNTAPb-F354L alone (−), or pNTAPb-F354L plus WT in 1:1 (+), 1:5 (++), and 1:10 (+++) ratios.
In patient tumor samples, STK11 western blotting revealed a wide range of protein isoform expression for both STK11 WT and variant tumors (Figure 1B). Expression of both the 42- and 48-kDa isoforms were much more common in tumors than cell lines and in many tumors the shorter isoforms were expressed at higher levels than the full length protein. We do not observe expression of the 42-kDa isoform to be correlated with STK11 mutations as previously reported41. Considering only the 50-kDa full-length protein, many STK11 WT tumors expressed less STK11 protein than STK11 variant tumors. Figure 1B highlights three examples of common/recurring STK11 variants, D194Y, F354L and P281fs*6, which were further explored. STK11 variants D194Y and F354L expressed high protein levels in four patients, whereas a patient with the P281fs*6 variant (and other similar frame shift variants) expressed very little STK11 protein. The F354L variant, which has previously been found in Asian populations at approximately 10% frequency42, is likely a polymorphism. It was explored further; however, because it has also been reported to affect cell polarity through an AMPK-dependent mechanism43. First, it was determined if these three variants could bind the other members of the catalytically active STK11 trimeric complex, MO25 and STRAD. A negative control vector, WT STK11 or each of the three variants were then transfected into H1299 cells and extracts subjected to co-immunoprecipation (co-IP) with STK11 antibody/streptavidin beads. The exogenous WT STK11, as well as the D194Y and F354L variants, were stably expressed and efficiently co-immunoprecipitated (IPed) MO25 and STRAD (Figure 1C) relative to the control vector. However, the P281fs*6 mutant was unstable (as expected) and did not co-IP either MO25 or STRAD. Next, the two stable variants were tested for dominant negative activity by transfecting the variant alone, and the variant plus WT STK11 in 1:1, 1:5, and 1:10 ratios, into A549 cells which lack endogenous STK11. Catalytic activity was measured by examining downstream phosphorylation of AMPK via a phospho-specific antibody. The D194Y mutant was able to suppress activation of AMPK by the WT STK11 at all ratios examined (Figure 1D). Conversely, the F354L variant phosphorylated AMPK in the presence, or absence, of WT protein and is therefore likely a common polymorphism.
Development and validation of a 122-gene STK11 mutation signature
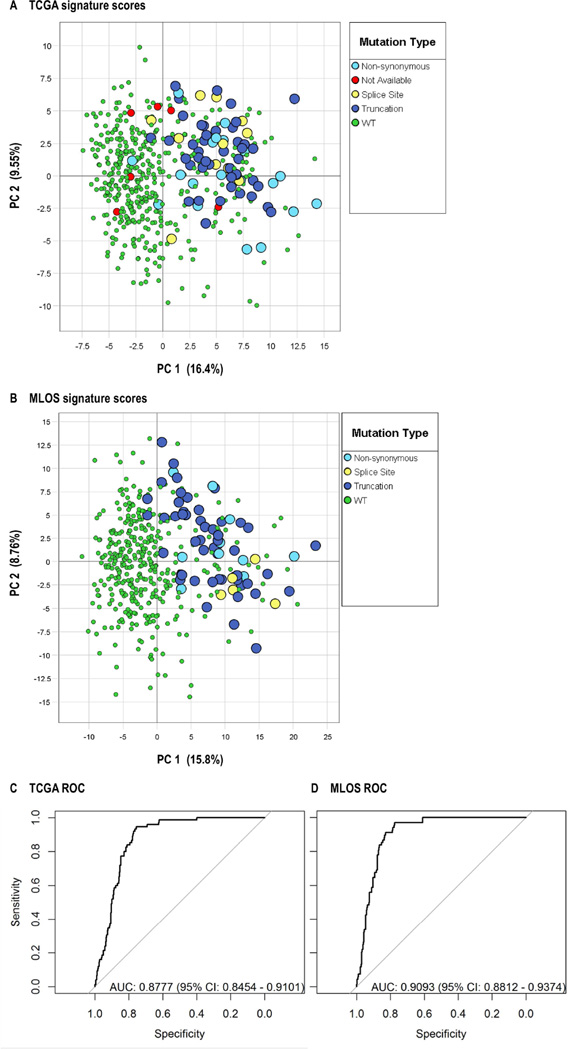
Cell line data and three published Affymetrix-based gene expression databases32, 34, 35 representing nearly 1000 non-small cell lung cancer (NSCLC) patients were used to develop and refine a 122-gene STK11 mutation signature. Development of this signature is described in the Supplementary Materials and the included genes along with their relative contributions (loading coefficients) to the PCA-based signature are listed in Table S7. The ability of this STK11 signature to classify LUAD tumors from two large datasets with available expression and mutation/copy number data was tested using the TCGA LUAD RNAseq dataset28 including 488 patient samples and a second recently described16 microarray-based expression dataset from 442 LUAD patients (herein the MLOS cohort, described in Table S1). Figure 2A and 2B show the results of PCA analysis of the two large patient datasets in which PC1 (the STK11 score, x-axis) and PC2 (unrelated to STK11 mutation, y-axis) for each patient (color coded as either STK11 WT or one of three types of mutations) is plotted. Various measures of agreement with sequenced STK11 mutation status were then calculated (Table 1) and demonstrate that the sensitivity of the signature relative to determined mutations is good (0.95–0.97), but specificity (0.73–0.74) is poor based on sequencing as the standard. These are the expected results since there may be many tumors with WT STK11 DNA that down regulate the STK11 protein or pathway via other mechanisms27, 31.
Figure 2. Validation of the STK11 mutation signature in multiple large patient-derived datasets.
(A–C) PCA using 182 Affymetrix probesets derived from cell data was performed on published patient-derived datasets and the loading coefficients from the first (p[1]) and second (p[2]) principle components from human tumors are plotted. The signal variation covered by each principle component is indicated as a percentage of total variation. Blue symbols represent probesets with a negative loading coefficient in cell lines and red symbols represent those with positive values in cell lines. (A) GSE3021934 (B) MCLA+, represents the Director's Challenge Consortium for the MCLA32 combined with GSE1481436 and (C) GSE3774535 (D, E) PCA was performed on the indicated patient-derived datasets using the refined STK11 signature. (F, G) Summary signature scores (for first PC 1) and second (PC 2) principle components for each individual patient are plotted. WT patients are plotted with green symbols, splice-site variants are plotted in blue, exon mutations are plotted with yellow symbols and TCGA samples of unknown mutation status are plotted in red. (C, D) ROC curves for the TCGA and LUAD 422 (MLOS) cohorts.
Table 1.
Measures of test performance between the STK11 signature and detected DNA variation in TCGA and the MLOS cohorts
| Detected DNA Variation |
Signature Prediction |
N | Class | Metric | Value | 95% CI | |
|---|---|---|---|---|---|---|---|
| TCGA | |||||||
| Mutant | STK11+ | 71 | TP (True Positives) | Sensitivity | 0.947 | (0.869, 0.985) | |
| Mutant | WT | 4 | FN (False Negatives) | Specificity | 0.732 | (0.686, 0.77) | |
| WT | STK11+ | 109 | FP (False Positives) | Precision | 0.394 | (0.322, 0.469) | |
| WT | WT | 298 | TN (True Negatives) | NPV (Negative Predictive Value) | 0.987 | (0.966, 0.996) | |
| False Positive Rate | 0.268 | (0.22, 0.313) | |||||
| False Discovery Rate | 0.606 | (0.53, 0.677) | |||||
| MLOS | |||||||
| Mutant | STK11+ | 66 | TP (True Positives) | Sensitivity | 0.971 | (0.897, 0.996) | |
| Mutant | WT | 2 | FN (False Negatives) | Specificity | 0.741 | (0.693, 0.784) | |
| WT | STK11+ | 97 | FP (False Positives) | Precision | 0.405 | (0.328, 0.484) | |
| WT | WT | 277 | TN (True Negatives) | NPV (Negative Predictive Value) | 0.993 | (0.974, 0.999) | |
| False Positive Rate | 0.259 | (0.215, 0.306) | |||||
| False Discovery Rate | 0.595 | (0.515, 0.671) |
Adaptation of the STK11 signature to clinical specimens using NanoString
Given the potential of assessing STK11 status as a clinical biomarker the STK11 mutation signature was adapted into a NanoString™ Elements-based format (see Tables S3–5), which is amenable to use with formalin-fixed, paraffin-embedded (FFPE) samples. NanoString assays utilize fluorescently barcoded probes that hybridize to targeted RNA molecules fixed to a slide. Advantages of the technology include; 1) no enzymatic steps that can be inhibited by contaminants, 2) the assay works well on the partially-degraded RNA that is generally retrieved from fixed tissues and 3) since RNA molecules are detected/counted individually the data analysis is simple. This NanoString panel was used to examine RNA samples isolated from paraffin blocks from a cohort 150-patient LUAD patients (Table S2). Ten of the 150 blocks did not yield adequate NanoString counts using 150 ng of RNA. Figure 3 highlights the results of PCA. Figure 3A demonstrates that the loading coefficients of most genes in the signature have the same sign as in cell lines despite the great difference in source material (fresh frozen tissue versus fixed tissue) and in spite of the difference in methodology (microarray versus NanoString). Figure 3B demonstrates that the first principle component significantly segregates STK11 WT and mutant tumors. Figure 3C–F shows waterfall plots of the first principle component score of each patient highlighting patients that are mutant in STK11, KRAS, TP53 or EGFR in red. Clearly, the signature correlates with STK11 mutations (Wilcoxon Rank Sum Test, p-value < 0.0001), whereas mutation in KRAS and TP53 do not show a statistically significant tendency (p-values 0.9965 and 0.2548, respectively) in spite of the fact that STK11 and KRAS mutations are known to favor co-occurrence (p-value = 0.003)44. In contrast, EGFR mutants have a significantly lower STK11 score (Wilcoxon Rank Sum Test, p-value < 0.0132) which would be expected since STK11 and EGFR mutations are mutually exclusive in LUADs44.
Figure 3. A NanoString-based STK11 assay applied to clinical samples.
(A) PCA using 122 NanoString codesets was performed on 140 RNAs isolated from FFPE blocks and the loading coefficients from the first (p[1]) and second (p[2]) principle components from human tumors are plotted. The signal variation covered by each principle component is indicated as a percentage of total variation. Blue symbols represent probesets with a negative loading coefficient in cell lines and red symbols represent those with positive values in cell lines. (B) Summary signature scores (for first (PC 1) and second (PC 2) principle components for each individual patient are plotted. See Methods for details of analysis. WT patients are plotted with green symbols, splice-site variants are plotted in blue, exon mutations are plotted with yellow symbols. (C–F) Waterfall plotsof the NanoString-based STK11 signature score. The number of WT and mutant samples are indicated for each gene. P values indicating correlation by the Wilcoxon Rank Sum Test demonstrated significant correlation with STK11 and EGFR (mutually exclusive) mutation status. (C) STK11, (D) KRAS, (E) TP53 and (F) EGFR.
Direct comparison between STK11 IHC and NanoString
Previous work has demonstrated STK11 IHC as a means to assess STK11 mutation status27. Having established in Figure 1B that direct measurement of STK11 protein in patient tumors might have complications, STK11 IHC and the STK11 NanoString assay were directly compared. To do this, a tissue microarray representing cell lines of known STK11 status (Figure 1A), 54 normal lung tissue cores and 140 LUAD tumors (135 of which were also assayed by STK11 NanoString) was stained with STK11 antibody, as previously described27. Comparison of the IHC staining of H1299 cells (Figure 4Ai) which expresses the WT 50-kDa STK11 protein (Figure 1A) and A549 cells (Figure 4Aii) which express no STK11 (Figure 1A) demonstrates the specificity of the STK11 IHC. Figure 4Aiii and 4Aiv demonstrate that STK11 staining in normal lung epithelial tissue (alveolar or bronchial) was generally light, cytoplasmic and diffuse and was set to a maximum value of +4 value. IHC staining of tumor tissue ranged from +4 to 0 (see Figure S2 for examples of each) and the IHC scores for the various cores are listed in Table 2. Figure 4Av and Avi highlight two IHC +4 carcinomas that are STK11 WT and mutant, respectively. Figure 4Avii and Aviii highlight two STK11 IHC negative carcinomas that are STK11 WT and mutant, respectively. These examples demonstrate the potential limitations of STK11 IHC. Figure 4B compares STK11 IHC with STK11 NanoString assay using receiver-operator characteristics modeling. For the NanoString assay, the AUC (or C-statistic) was 0.8377, whereas IHC staining achieved only a 0.6789 AUC. Thus, the NanoString-based assay shows superior overall performance with a 0.1588 improvement in AUC relative to IHC (p<0.0119, see Table 2).
Figure 4. Direct comparison between STK11 IHC and NanoString.
STK11 IHC was performed on a TMA representing cell lines, normal tissues and LUAD tumors (MLCom cohort). Scale bar represents 200 µM. (A) STK11 IHC in representative cores; i) Core 231, H1299 cells with WT STK11 and expresses 52-kDa isoform (see Figure 1A), ii) Core 207, A549 cells with Mut STK11 and lacking expression of any STK11 isoform (see Figure 1A), iii) Core 141, Normal Alveolar Tissue, +4 staining, iv) Core 78, Normal Bronchial Tissue, +4 staining, v) Core 1, Carcinoma with WT STK11, +4 staining, vi) Core 139, Carcinoma with Mutant STK11, +4 staining, vii) Core 29, Carcinoma with WT STK11, 0 staining, negative, and viii) Core 66, Carcinoma with Mutant STK11, 0 staining, negative. (B) ROC curves comparing STK11 NanoString with IHC (AUC was 0.8377 for NanoString versus 0.6789 for IHC). Table S6 identifies cores by STK11 mutation status.
Table 2.
STK11 IHC results and test performance comparison with the STK11 NanoString Assay
| Number of Patient’s with indicated IHC scores | ||||||
| IHC Pathology Score | 0 | +1 | +2 | +3 | +4 | |
| Normal Cores | 0 | 0 | 0 | 3 | 51 | |
| Carcinomas | ||||||
| STK11 Mutant | 7 | 6 | 2 | 5 | 7 | |
| STK11 WT | 15 | 6 | 6 | 18 | 68 | |
| Receiver-operator characteristics modeling | ||||||
| Model | AUC | 95% | Difference in AUC and 95% CI | p-value | ||
| NanoString | 0.8377 | (0.76, 0.9154) | 0.1588 (0.035, 0.2825) | 0.0119 | ||
| IHC Pathology Score | 0.6789 | (0.566, 0.7918) | ||||
Discussion
This work describes a NanoString -based assay that can assess deregulation of the STK11 pathway in patient samples derived from FFPE tissues. The genes comprising the assay were validated in multiple datasets representing six different studies representing 1,894 patient samples and the assay itself was further validated using RNA isolated from FFPE tissues. Since anti-STK11 antibodies can cross-react with non-specific species and occasional null mutations do not affect STK11 steady-state protein levels, this assay showed a higher sensitivity and specificity than STK11 IHC using exome sequencing as the standard16. In data not shown, no correlation between STK11 mutation status and cell cycle progression whether measured by Ki-67 staining or by assessment of a previously defined proliferative signature was observed45. Nor was any statistically significant prognostic value associated with STK11 mutations or the STK11 signature whether considered alone or in the context of concurrent KRAS or TP53 mutations. Finally, analysis did not reveal any association between STK11 disruption and metastasis among KRAS mutated patients as has been previously shown27.
We propose that this new NanoString assay can serve as the gold standard to be subsequently tested along with a combination of exon sequencing and IHC as predictive biomarkers for newly emerging immunotherapies16 or other anti-cancer treatments exploiting AMPK activation such as metformin/phenformin or COX-2 inhibition studies13. This NanoString-based STK11 assay will likely emerge as the best of the three methods for several reasons. First, the assay is based on a very comprehensive examination of existing data on STK11 incorporating both extensive cell line data as well as thousands of patient samples. The strong recapitulation of the cell line data into patient samples supports the robust nature of the assay and preservation of at least a subset of the critical biological pathways disrupted downstream of STK11 mutations. The fact that the signature is validated in multiple human tissue datasets, with multiple technical methodologies (including microarray, RNA Seq and NanoString) ensures that the signature it is likely to be broadly applicable going forward. In fact, during the development of this STK11 signature, a 16-gene STK11 signature (classifier) was published31. Ten of the 16 genes in the published signature are also in our signature suggesting significant convergence of the best classifiers using different approaches. In our analysis, nine of these ten genes have individual p-values below 0.05 suggesting that these genes will translate very well. We also note that the NanoString-based assays are emerging as a reliable and highly general and flexible method to assess gene expression in very limited fixed tissues46. A NanoString-based version of the PAM50 panel was recently FDA-approved for prognosis in breast cancer47. We find the NanoString methodology to be highly reproducible and given its general simplicity, we anticipate that it will be readily adapted clinically as an alternative to quantitative PCR and sequencing methods. The straightforward multiplex nature of the NanoString assay will allow us to further optimize the codesets included in the assay as additional data is acquired.
Supplementary Material
Acknowledgments
The many generous gifts of reagents are acknowledged in the Materials and Methods. We would also like to thank A. Kasprzak for microscopy, N. Clark for IHC, F. Kinose for cell line assistance, and the Moffitt Cancer Registry (Director: Karen A. Coyne) for contribution in data curation.
Sources of Funding:
This work was supported by National Institutes of Health/National Cancer Institute grants CA90489 (W.D.C.) and a Specialized Programs of Research Excellence (SPORE) P50 CA119997 (W.D.C.), a USF Presidential Fellowship (B.E.E.), and by the Tissue Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
The authors declare no conflict of interest.
REFERENCES
- 1.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 2.Hammerman PS, Hayes DN, Wilkerson MD, et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Cespedes M, Parrella P, Esteller M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- 4.Shah U, Sharpless NE, Hayes DN. LKB1 and lung cancer: more than the usual suspects. Cancer Research. 2008;68:3562–3565. doi: 10.1158/0008-5472.CAN-07-6620. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Cespedes M. The role of LKB1 in lung cancer. Fam Cancer. 2011;10:447–453. doi: 10.1007/s10689-011-9443-0. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y, Ge G, Ji H. LKB1 in lung cancerigenesis: a serine/threonine kinase as tumor suppressor. Protein Cell. 2011;2:99–107. doi: 10.1007/s13238-011-1021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto S, Iwakawa R, Takahashi K, et al. Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene. 2007;26:5911–5918. doi: 10.1038/sj.onc.1210418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 9.Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudeau J, Scott JW, Resta N, et al. Analysis of the LKB1-STRAD-MO25 complex. J Cell Sci. 2004;117:6365–6375. doi: 10.1242/jcs.01571. [DOI] [PubMed] [Google Scholar]
- 12.Woods A, Johnstone SR, Dickerson K, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Cao C, Gao R, Zhang M, et al. Role of LKB1-CRTC1 on glycosylated COX-2 and response to COX-2 inhibition in lung cancer. J Natl Cancer Inst. 2015;107:358. doi: 10.1093/jnci/dju358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu Y, Lin S, Li JL, et al. Altered LKB1/CREB-regulated transcription co-activator (CRTC) signaling axis promotes esophageal cancer cell migration and invasion. Oncogene. 2012;31:469–479. doi: 10.1038/onc.2011.247. [DOI] [PubMed] [Google Scholar]
- 15.Komiya T, Coxon A, Park Y, et al. Enhanced activity of the CREB co-activator Crtc1 in LKB1 null lung cancer. Oncogene. 2010;29:1672–1680. doi: 10.1038/onc.2009.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schabath MB, Welsh EA, Fulp WJ, et al. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene. 2015 doi: 10.1038/onc.2015.375. 10.1038/onc.2015.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Han X, Li F, et al. LKB1 Inactivation Elicits a Redox Imbalance to Modulate Non-small Cell Lung Cancer Plasticity and Therapeutic Response. Cancer Cell. 2015;27:698–711. doi: 10.1016/j.ccell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Cheng K, Walton Z, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483:613–617. doi: 10.1038/nature10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carretero J, Shimamura T, Rikova K, et al. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell. 2010;17:547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shackelford DB, Abt E, Gerken L, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inge LJ, Coon KD, Smith MA, et al. Expression of LKB1 tumor suppressor in non-small cell lung cancer determines sensitivity to 2-deoxyglucose. The Journal of Thoracic and Cardiovascular Surgery. 2009;137:580–586. doi: 10.1016/j.jtcvs.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Carretero J, Medina PP, Blanco R, et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26:1616–1625. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
- 23.Klumpen HJ, Queiroz KC, Spek CA, et al. mTOR inhibitor treatment of pancreatic cancer in a patient With Peutz-Jeghers syndrome. J Clin Oncol. 2011;29:e150–e153. doi: 10.1200/JCO.2010.32.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahoney CL, Choudhury B, Davies H, et al. LKB1/KRAS mutant lung cancers constitute a genetic subset of NSCLC with increased sensitivity to MAPK and mTOR signalling inhibition. British Journal of Cancer. 2009;100:370–375. doi: 10.1038/sj.bjc.6604886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whang YM, Park SI, Trenary IA, et al. LKB1 deficiency enhances sensitivity to energetic stress induced by erlotinib treatment in non-small-cell lung cancer (NSCLC) cells. Oncogene. 2015 doi: 10.1038/onc.2015.140. 10.1038/onc.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Xiao Q, Ma H, et al. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc Natl Acad Sci U S A. 2010;107:18892–18897. doi: 10.1073/pnas.1004952107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calles A, Sholl LM, Rodig SJ, et al. Immunohistochemical loss of LKB1 is a biomarker for more aggressive biology in KRAS mutant lung adenocarcinoma. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-3112. [DOI] [PubMed] [Google Scholar]
- 28.TCGA. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esteller M, Avizienyte E, Corn PG, et al. Epigenetic inactivation of LKB1 in primary tumors associated with the Peutz-Jeghers syndrome. Oncogene. 2000;19:164–168. doi: 10.1038/sj.onc.1203227. [DOI] [PubMed] [Google Scholar]
- 30.Gill RK, Yang SH, Meerzaman D, et al. Frequent homozygous deletion of the LKB1/STK11 gene in non-small cell lung cancer. Oncogene. 2011;30:3784–3791. doi: 10.1038/onc.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman JM, Amann JM, Park K, et al. LKB1 Loss induces characteristic patterns of gene expression in human tumors associated with NRF2 activation and attenuation of PI3K-AKT. J Thorac Oncol. 2014;9:794–804. doi: 10.1097/JTO.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shedden K, Taylor JM, Enkemann SA, et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med. 2008;14:822–827. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NCBI. Gene Expression Omnibus. [Accessed 17 April 2015]; Available at http://www.ncbi.nlm.nih.gov/geo/
- 34.Rousseaux S, Debernardi A, Jacquiau B, et al. Ectopic activation of germline and placental genes identifies aggressive metastasis-prone lung cancers. Sci Transl Med. 2013;5:186ra166. doi: 10.1126/scitranslmed.3005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botling J, Edlund K, Lohr M, et al. Biomarker discovery in non-small cell lung cancer: integrating gene expression profiling, meta-analysis, and tissue microarray validation. Clin Cancer Res. 2013;19:194–204. doi: 10.1158/1078-0432.CCR-12-1139. [DOI] [PubMed] [Google Scholar]
- 36.Zhu CQ, Ding K, Strumpf D, et al. Prognostic and predictive gene signature for adjuvant chemotherapy in resected non-small-cell lung cancer. J Clin Oncol. 2010;28:4417–4424. doi: 10.1200/JCO.2009.26.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jolliffe I. Principal component analysis and factor analysis. Principal component analysis. 2002;1:150–166. [Google Scholar]
- 38.Ringner M. What is principal component analysis? Nat Biotechnol. 2008;26:303–304. doi: 10.1038/nbt0308-303. [DOI] [PubMed] [Google Scholar]
- 39.Towler MC, Fogarty S, Hawley SA, et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem J. 2008;416:1–14. doi: 10.1042/BJ20081447. [DOI] [PubMed] [Google Scholar]
- 40.Dahmani R, Just PA, Delay A, et al. A novel LKB1 isoform enhances AMPK metabolic activity and displays oncogenic properties. Oncogene. 2015;34:2337–2346. doi: 10.1038/onc.2014.182. [DOI] [PubMed] [Google Scholar]
- 41.Pecuchet N, Laurent-Puig P, Mansuet-Lupo A, et al. Different prognostic ompact of STK11 mutations in non-squamous non-small-cell lung cancer. Oncotarget. 2015 doi: 10.18632/oncotarget.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao B, Sun Y, Zhang J, et al. Spectrum of LKB1, EGFR, and KRAS mutations in chinese lung adenocarcinomas. J Thorac Oncol. 2010;5:1130–1135. doi: 10.1097/JTO.0b013e3181e05016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forcet C, Etienne-Manneville S, Gaude H, et al. Functional analysis of Peutz-Jeghers mutations reveals that the LKB1 C-terminal region exerts a crucial role in regulating both the AMPK pathway and the cell polarity. Hum Mol Genet. 2005;14:1283–1292. doi: 10.1093/hmg/ddi139. [DOI] [PubMed] [Google Scholar]
- 44.Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen DT, Hsu YL, Fulp WJ, et al. Prognostic and predictive value of a malignancy-risk gene signature in early-stage non-small cell lung cancer. J Natl Cancer Inst. 2011;103:1859–1870. doi: 10.1093/jnci/djr420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veldman-Jones MH, Brant R, Rooney C, et al. Evaluating Robustness and Sensitivity of the NanoString Technologies nCounter Platform to Enable Multiplexed Gene Expression Analysis of Clinical Samples. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-0262. [DOI] [PubMed] [Google Scholar]
- 47.Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–345. doi: 10.1093/annonc/mdt494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.