Abstract
Anxiety disorders are among the most prevalent psychological disorders, have significant negative impacts on quality of life and the healthcare system, and yet effective treatments remain elusive. Manipulating the endocannabinoid system has demonstrated potential for treating anxiety, although the side effects of direct manipulations of cannabinoid receptors keeps them from widespread clinical use. Disrupting the degradation enzyme fatty acid amide hydrolase (FAAH) enhances endogenous signaling and may produce similar efficacy without the side effects. The current experiments examine the effects of low (5.6 mg/kg) or moderate (10.0 mg/kg) doses of OL-135, a FAAH inhibitor, on the acquisition and consolidation of classical fear conditioning, a common model of trauma-induced anxiety. The acquisition of contextual, but not auditory, fear conditioning was disrupted by both doses of OL-135. Shock reactivity was not affected. Due to the additional neural circuitry required for contextual, but not auditory, fear conditioning, these data suggest that endocannabinoid signaling outside the amygdala may be critical for a subset of fearful memories.
Anxiety disorders affect more than 25% of Americans over their lifetime, making it among the most common psychological disorders. In the past few decades incredible progress has been made in elucidating the neural circuitry underlying fear and anxiety, such that there is now a solid understanding of the relevant neural circuits, including the role of broad neuroendocrine and neuromodulatory systems [1, 2]. However, despite this progress in basic science, few, if any, novel treatments have reached clinical practice.
In recent years, the role of the endocannabinoid system in anxiety has gained a great deal of attention [3]. Cannabinoids have long been used for their psychoactive properties. There are two well-studied endogenous ligands, anandamide and 2-Arachidonoylglycerol (2-AG), which both appear to have a role in modulating levels of anxiety [4]. In addition, there are two major receptors, CB1 and CB2. CB1 (and perhaps CB2) is found throughout the brain and appears to be involved in anxiety [4]. Interestingly, the relationship between cannabinoid levels and anxiety is not straightforward. Although low doses of CB1 and CB 2 agonists can be anxiolytic, higher doses of CB1 agonists can be anxiogenic [3]. These different properties are likely due to CB1 receptors on different neural populations, but complicate the pharmacological application of CB1 agonists clinically. In addition, significant side effects prevent broad use of direct agonists and antagonists in the clinic [e.g. 5].
Based, in part, on the strategy adopted by anti-depressants to modulate the serotonin system using selective serotonin reuptake inhibitors (SSRI), one approach that is currently being adopted to reduce side effects is to target the endocannabinoid degradation enzyme Fatty Acid Amide Hydrolase (FAAH) [6]. FAAH appears to preferentially target anandamide [7], although under some conditions it also appears to affect 2-AG. By targeting degradation enzymes, non-specific saturation of cannabinoid receptors can be avoided and selective enhancement of existing endogenous signaling can be achieved [8]. This reduces side effects and makes FAAH an attractive target for anxiolytic effects [7].
Although FAAH inhibition appears to be promising for reducing unconditioned anxiety [9, 10], there is currently little information on the effects of FAAH inhibition on the acquisition of conditioned fear. This is a critical absence. Classical fear conditioning, in which previously neutral stimuli take on aversive attributes due to associations with traumatic stimuli, is a well-studied adaptive phenomenon in which the neural circuitry is exquisitely understood. In addition, excessive or abnormal fear conditioning is likely one of the mechanisms by which posttraumatic stress disorder (PTSD) and similar types of anxiety are acquired. Interestingly, a recent review demonstrates that the effects of direct endocannabinoid manipulation on conditioned fear appears to be different from that on unconditioned, innate anxiety [11], making it critical to study independently. Although CB1 agonists, at least at moderate doses, are anxiolytic on unconditioned anxiety, they sometimes increase the expression of contextual conditioned fear [12]. Moreover, unlike unconditioned anxiety, CB1 antagonism or genetic knockout sometimes is found to reduce the acquisition of conditioned fear [12]. However, it is important to note that both sets of findings are controversial, in that the opposite pattern can also be observed [13].
The effects of FAAH inhibition on conditioned fear are difficult to predict. Although FAAH inhibition appears to be anxiolytic and thus may reduce conditioned fear, there are several rodent studies that suggest FAAH inhibition can enhance memory formation and storage for aversively motivated tasks [14, 15]. Given the important role that acquired fears play in the formation and maintenance of anxiety disorders, a general memory enhancing effect of FAAH inhibition would be potentially problematic for therapeutic use. Therefore, the current experiments examine the effects of OL-135, a well characterized FAAH inhibitor, on the acquisition and consolidation of classical fear conditioning.
To test this, 60 adult male Sprague-Dawley rats (Charles River), weighing approximately 200–300g were divided into two experiments. Experiment 1 examined the effects of Pre-Training FAAH disruption. Experiment 2 examined the effects of Post-Training FAAH disruption. Rats were handled for five days before the study began and were randomly assigned to treatment groups. All experiments were conducted with the approval of The University of New England’s Institutional Animal Care and Use Committee (IACUC) and according to PHS and NIH guidelines.
The OL-135 [16] was stored at −80°C in powder form, until approximately 60 min before behavioral testing. Three doses were prepared (0, 5.6, 10.0 mg/kg) with a vehicle consisting of 1:1:18 ethanol, cremophor, and saline. Drug administration occurred 30 minutes prior to training (Experiment 1) or immediately (within 5 min) after training (Experiment 2) via intraperitoneal (IP) injection.
Conditioning was conducted in four Startfear chambers using their proprietary “Freezing” software using previously published methods [17], with the sensitivity adjusted for use in adult subjects. Briefly, on the first day (training) subjects were given five minutes of habitation followed by 10 pairings of a 10-s 67-db 4-kHz tone and a 2-s 0.3-mA shock. On the second day (context testing) subjects were transferred into the same conditioning chamber as the day before for five minutes during which freezing was assessed. On the third day (auditory testing) subjects were transported into a new conditioning chamber with the opposite context. Freezing was assessed during a five-minute habituation period followed by 10 presentations of the auditory cue during which freezing was also assessed. In all cases, freezing was defined as a lack of relative cage movement below a fixed threshold for 0.5s. The threshold was determined in pilot studies by an investigator trained to recognize the freezing response. Percent freezing scores were calculated by comparing the time spent below the movement threshold with the total time for each event, including averaging over all 10 tones.
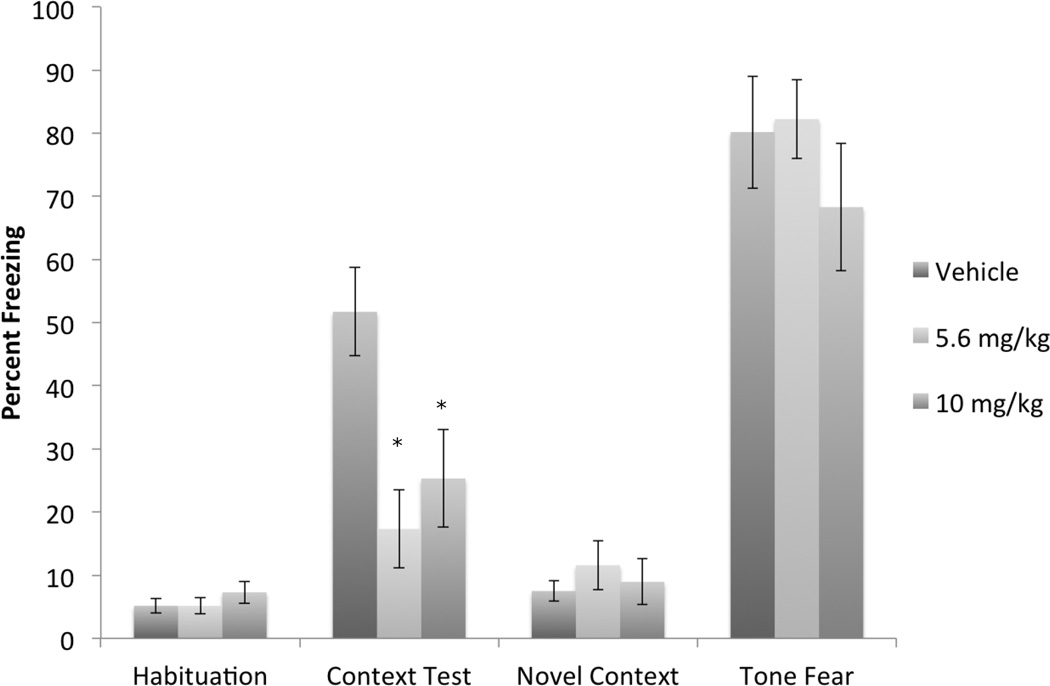
Experiment 1 was designed to examine the effects of FAAH inhibition on the acquisition of conditioned fear. It was analyzed as a 3 (drug dose) × 4 (test session: habituation, context freezing, novel context, auditory freezing) mixed model repeated measures ANOVA using conditioning freezing as the dependent variable (Figure 1). Due to a significant departure from sphericity, the Greenhouse-Geisser Epsilon correction was used. There was no main effect of drug dose. As expected, there was a main effect of test session, F(2.11, 72.07) = 106.39, p<.0001. There was also a significant interaction between drug dose and test session F(4.2394,72.07) = 2.47, p<.05. A one-way ANOVA examining drug dose on contextual freezing demonstrated a significant effect of drug F(2,34) =4.36, p<.05. The Dunnett’s post-hoc test found significant differences between the control group and both drug doses (ps<.05). A one-way ANOVA on the effects of drug dose on tone fear showed no significant differences, demonstrating that only contextual freezing was affected by FAAH inhibition.
Figure 1.
Effects of pre-training OL-135 on acquisition of fear conditioning. Subjects were injected with vehicle (n=12) or 5.6 (n=13) or 10.0 (n=12) mg/kg OL-135 prior to fear conditioning. Both doses of OL-135 significantly impaired the subsequent expression of contextual fear conditioning, but not the simultaneously acquired auditory fear conditioning. * = significant from Vehicle, p<.05.
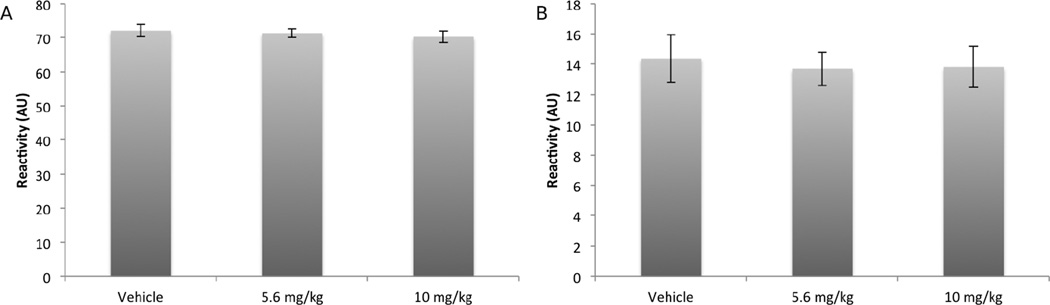
To ensure the effects of the drug were on fear conditioning and not antinociceptive effects, we also assessed reactivity to the aversive stimulus, both as a maximal value and as an average value (Figure 2). Between subjects one-way ANOVAs of drug dose on maximum and average shock reactivity found no significant differences
Figure 2.
A) Effects of pre-training OL-135 on maximum shock reactivity during the acquisition of fear conditioning. Maximum reactivity generally occurred during the first shock presentation. .B): Effects of pre-training OL-135 on shock reactivity averaged across all shocks during the acquisition of fear conditioning. There was no effect of drug dose on movement during the shock.
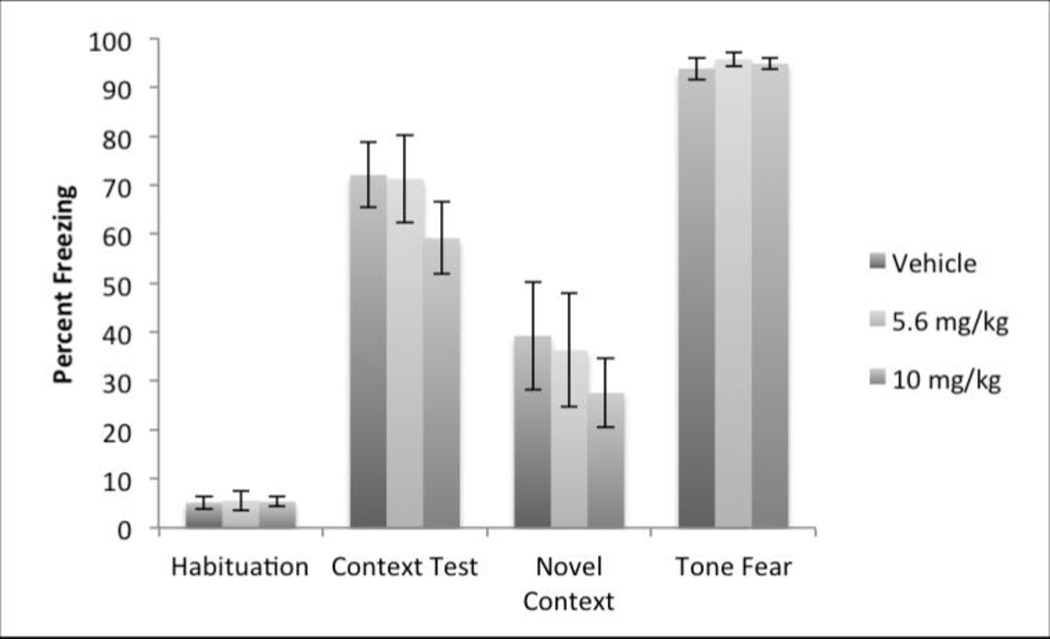
Experiment 2 was designed and analyzed identically (Figure 3). Again, due to a significant departure from sphericity, the Greenhouse-Geisser Epsilon correction was used. Here there was a significant effect of test, F(1.9, 38.33) = 142.7315, p<.0001, as expected. However, there was no main effect of drug dose, or a test × drug dose interaction.
Figure 3.
There were no differences between post-training vehicle (n=8), 5.6 (n=7) or 10.0 (n=8) mg/kg OL-135 injections on the subsequent expression of fear conditioning.
The current experiments demonstrate a specific impairment of the acquisition or early consolidation of contextual fear conditioning due to inhibition of FAAH, consistent with a role for the endogenous cannabinoid system in this form of learning. Post-training injections did not impair conditioning, suggesting that the role of the endocannabinoid does not extend to the later consolidation phases of contextual fear conditioning. Furthermore, the impairment of contextual fear acquisition is not caused by alterations in sensory function as shock reactivity and auditory fear conditioning remained intact. Finally, as post-training injections did not disrupt learning, it is unlikely that the impairment is due to residual effects of the drug during the test session.
Although to the best of our knowledge these experiments are the first to look at the effects of FAAH inhibition on the acquisition of fear conditioning, there are a number of studies examining FAAH inhibition on both appetitive and aversive memory as well as innate anxiety-based tasks. For example, FAAH inhibition is anxiolytic in the light/dark test [9], the elevated plus maze [10] and other tests, although this may depend upon a number of environmental factors. FAAH inhibition also appears to enhance extinction of fear conditioning, which may be related to the drugs anxiolytic effects [11].
However, despite these anxiety-reducing properties, FAAH inhibition appears to enhance memory, especially those based on aversive motivation. For example, URB597 enhances the acquisition of passive avoidance, an effect found to be dependent on the PPAR-alpha receptor [14]. This suggests an anxiety-producing effect. Aversively-motivated memory tasks that do not directly involve anxiety also appear to be enhanced by FAAH inhibition. For example, both acquisition and extinction of the Morris-Water maze are enhanced by OL-135 or genetic knockout of FAAH [15]. However, appetitively-motivated tasks may be relatively unaffected by FAAH inhibition [18].
The effects of FAAH inhibition appear to generally mimic the effects of direct CB1 antagonism or knockout in disrupting the acquisition, but not consolidation, of contextual, but not auditory, fear [11]. This is of interest because FAAH inhibition, by disrupting endocannabinoid degradation, should generally increase cannabinoid levels. However, it is important to note that there is not full agreement on the effects of CB1 manipulation on fear conditioning, as there are reports in the literature of CB1 antagonists disrupting various aspects of conditioning [e.g. 11, 13], although these differences may be due to different timing of injections or the specific brain structures targeted. An alternative hypothesis is that the enhanced anandamide levels caused by the FAAH disruption are acting on a variety of receptors, such as both CB1 and TRPV1, which may have opposing effects [19]. This may lead to different behavioral changes. Nevertheless, that the effects of FAAH inhibition more closely mimic those reported by CB1 antagonism confirm that there is a clear difference between broad and non-specific activation caused by direct CB1 agonists/antagonists and the enhancement of endogenously active signaling caused by FAAH inhibition [8].
Fear conditioning is a powerful tool to examine the anxiolytic properties of pharmaceuticals. The neural circuitry is very well understood and it has great face validity for certain forms of anxiety, such as specific phobias and post-traumatic stress disorder [1, 2]. Moreover, fear conditioning is enhanced in patients with anxiety disorders [20] and fear conditioning may be one of the mechanisms that contribute to the formation of anxiety early in life. Importantly, different forms of fear conditioning require different neural circuitry. Conditioning to a unimodal, discrete, simple, cue such as the auditory fear conditioning in the current experiments, appears to depend upon a small circuit centered on the lateral, basolateral, and central nuclei of the amygdala and including subcortical efferent and afferent structures. In contrast, contextual fear conditioning often recruits additional neural circuitry, including the hippocampus and various cortical regions [21, 22]. Interestingly, treatments such as propranolol, a beta-adrenergic antagonist, in humans disrupt only contextual and not auditory conditioning [23], similar to the results of the current studies. It is worth noting that this pattern precludes a simple interpretation of the effects of FAAH inhibition (or beta-receptor antagonism). Although these drugs are generally considered anxiolytic, that simple fear conditioning remains intact suggests these drugs have a more subtle effect on a subset of fear and anxiety circuitry.
Indeed, the data demonstrating that auditory fear conditioning is spared by FAAH inhibition suggests that the critical site of action for FAAH inhibition’s effect on contextual fear conditioning is likely not in the essential fear circuit, including parts of the amygdala. This is somewhat surprising given the number of CB1 receptors in the amygdala, the known role of the amygdala in fear and anxiety and the established effects of FAAH inhibition on the amygdala [7, 24]. However, as other regions such as the hippocampus, entorhinal cortex and prefrontal cortex are also required for contextual fear conditioning and contain large numbers of CB1 receptors and robust FAAH expression [24], it appears likely that these regions may be critical for its anxiolytic effects on fear conditioning. However, it is important to note that some amygdalar sub-nuclei, such as the basolateral nucleus, may be preferentially involved in contextual fear conditioning and thus the target of OL-135’s effects in the current experiments. Further work is necessary to clarify this issue.
A role for endocannabinoid in fear and anxiety is well established [e.g. 11], although relatively few studies have examined this using FAAH inhibition. Despite the apparently similar effects on conditioned fear that were observed in the current studies, FAAH inhibitors may be preferable over CB1 antagonists for the treatment of anxiety disorders. Due to significant side effects, CB1 antagonists have been withdrawn from the market and clinical research has slowed on this class of compounds [5]. In contrast, FAAH inhibitors appear to be much more well tolerated and avoid many of the pitfalls of direct endocannabinoid receptor modulation [8]. Indeed, clinical trials of PF-04457845 with PTSD patients are currently underway.
The doses of OL-135 used in the current study were chosen to be at or below the threshold dose for antinociception [25]. Indeed, we observed no evidence for reduced shock responsiveness or a general impairment of conditioning. There are several reasons why this could be. The current studies used a relatively robust shock, which may be sufficient to overcome any anti-nociceptive effects of the doses we used. In addition, most of the work on anti-nociceptive prosperities of OL-135 have used tactile or thermal sensitivity. Electric shock may activate different or additional pathways that are not affected by OL-135. However, it is also important to note that the current experiments did not find evidence for a linear dose-response relationship, which may have been apparent with a lower dose, nor can we rule out that larger doses would impair shock sensitivity or fear conditioning more generally. Additional studies with a broader range of doses would be necessary to answer those questions.
In summary, FAAH inhibition disrupted the acquisition of contextual fear conditioning at a dose that did not show any evidence of antinociceptive properties. These effects are consistent with those reported from direct CB1 antagonism. Nevertheless, these findings are of interest because FAAH inhibition may show greater promise in the clinic than direct CB1 manipulation.
Research Highlights.
FAAH Inhibitor OL-135 disrupts the acquisition, but not consolidation, of contextual fear conditioning
Auditory fear conditioning and shock reactivity remain unaffected.
Together, these data suggest a specific role for endocannabinoids in a subset of fear conditioning paradigms.
Acknowledgments
Funding for this project was provided though National Institutes of Health grants DA015648 to D.L.B. and MH093950 to M.A.B provided additional support. Support for student fellowships and internships was provided by UNE’s Center for Excellence in the Neurosciences, Office of Research and Scholarship, and College of Arts and Sciences. Funding sources had no involvement in the design, conduct or interpretation of this research. Thanks to many members of the Burman and Bilsky laboratories who helped with various technical tasks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 2.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. Journal of neurophysiology. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 3.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annual review of psychology. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 4.Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biological psychiatry. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology. 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 7.Gaetani S, Cuomo V, Piomelli D. Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends in molecular medicine. 2003;9:474–478. doi: 10.1016/j.molmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Otrubova K, Ezzili C, Boger DL. The discovery and development of inhibitors of fatty acid amide hydrolase (FAAH) Bioorganic & medicinal chemistry letters. 2011;21:4674–4685. doi: 10.1016/j.bmcl.2011.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, et al. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, et al. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology. 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chhatwal JP, Ressler KJ. Modulation of fear and anxiety by the endogenous cannabinoid system. CNS spectrums. 2007;12:211–220. doi: 10.1017/s1092852900020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikics E, Dombi T, Barsvari B, Varga B, Ledent C, Freund TF, et al. The effects of cannabinoids on contextual conditioned fear in CB1 knockout and CD1 mice. Behavioural pharmacology. 2006;17:223–230. doi: 10.1097/00008877-200605000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Sink KS, Segovia KN, Collins LE, Markus EJ, Vemuri VK, Makriyannis A, et al. The CB1 inverse agonist AM251, but not the CB1 antagonist AM4113, enhances retention of contextual fear conditioning in rats. Pharmacology, biochemistry, and behavior. 2010;95:479–484. doi: 10.1016/j.pbb.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, et al. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learning & memory. 2009;16:332–337. doi: 10.1101/lm.1145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- 16.Boger DL, Miyauchi H, Du W, Hardouin C, Fecik RA, Cheng H, et al. Discovery of a potent, selective, and efficacious class of reversible alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase effective as analgesics. Journal of medicinal chemistry. 2005;48:1849–1856. doi: 10.1021/jm049614v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burman MA, Erickson KJ, Deal AL, Jacobson RE. Contextual and auditory fear conditioning continue to emerge during the periweaning period in rats. PloS one. 2014;9:e100807. doi: 10.1371/journal.pone.0100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manwell LA, Satvat E, Lang ST, Allen CP, Leri F, Parker LA. FAAH inhibitor, URB-597, promotes extinction and CB(1) antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats. Pharmacology, biochemistry, and behavior. 2009;94:154–162. doi: 10.1016/j.pbb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Aguiar DC, Moreira FA, Terzian AL, Fogaca MV, Lisboa SF, Wotjak CT, et al. Modulation of defensive behavior by Transient Receptor Potential Vanilloid Type-1 (TRPV1) channels. Neuroscience and biobehavioral reviews. 2014;46(Pt 3):418–428. doi: 10.1016/j.neubiorev.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour research and therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 22.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current opinion in neurobiology. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grillon C, Cordova J, Morgan CA, Charney DS, Davis M. Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology. 2004;175:342–352. doi: 10.1007/s00213-004-1819-5. [DOI] [PubMed] [Google Scholar]
- 24.Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends in pharmacological sciences. 2013;34:637–644. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, et al. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. British journal of pharmacology. 2006;148:102–113. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]