Abstract
Background
Cessation of enteral nutrition prior to an operation/procedure is the most common reason for feeding interruption in critically-ill trauma patients and contributes to substantial calorie deficits. This study reports on a strategy to increase calorie intake by continuing feeds until transfer for operations/procedures.
Methods
Nutrition guidelines were modified in 2006 to allow continuation of feeding in intubated patients up until transfer to the operating room. Prior to 2006, enteral feeding was stopped at least 6 hours prior to surgery. A retrospective cohort design from 2003–2010 compared clinical outcomes in groups of adult trauma subjects before and after guideline changes, and in subjects at other centers without guideline changes.
Results
During the first week, subjects in the pre-implementation cohort (n=245) received a median of 3,787 kcal/person/week, while subjects in the post-implementation cohort (n=368) received a median of 6,662 (p<0.001). There was no change in calorie intake for subjects at other centers (n=1002). The risks for Acute Respiratory Distress Syndrome (ARDS), pneumonia, and mortality were decreased after implementation relative to the pre-implementation cohort (ARDS: RR=0.69, 95% CI [0.59–0.81]; pneumonia: RR=0.82, 95% CI [0.65–1.00]; mortality: RR=0.67, 95% CI [0.46–0.99]). Ventilator-free days increased by 1.4 days (95% CI [0.1–2.7), while ICU and hospital length of stay were unchanged. These outcomes showed similar trends over time at other participating centers.
Conclusion
Allowing intubated trauma patients to continue enteral nutrition until transfer for operations or procedures was associated with increased caloric intake without evidence of increased pulmonary complications. This represents an important strategy to reduce calorie deficits in the trauma ICU.
Level of Evidence
III, Study Type: Therapeutic/Care Management
Keywords: Enteral Nutrition, Nutrition Protocol, nil-per-os, Acute Respiratory Distress Syndrome, Ventilator-Associated Pneumonia
BACKGROUND
Early enteral nutrition (EN) is beneficial in critically-ill trauma patients and improves outcomes relative to parenteral nutrition or starvation.(1–3) However, multiple barriers prevent effective enteral nutrition in the intensive care unit (ICU) and calorie deficits are common.(4, 5) Despite our best efforts, achieving calorie goals in trauma patients is particularly challenging, at least in part because of repeated interruptions in the setting of multiple operations and interventions.(6, 7)
At Harborview Medical Center, we modified our nutrition support guidelines in 2006 with the goal of reducing interruptions in enteral feeding, thereby increasing calorie intake, particularly during the 1st post-injury week. The primary change was to allow continuation of EN up until the time the patient was transferred to the operating room from the intensive care unit, rather than stopping 6 hours prior to surgery (which was our practice prior to 2006 and was the accepted practice in other centers).(8) Continuation of enteral feeds up to the time of surgery was allowed only if the patient: (1) was to remain intubated post-operatively, (2) was not undergoing surgery on the gastrointestinal tract and, (3) was not having any manipulation of the airway, including extubation.
We performed this study in order to determine whether changing our nutritional support guidelines improved caloric intake without increasing pulmonary complications in critically ill trauma victims. As a participating center in the Inflammation and Host Response to Injury Consortium, patients admitted to Harborview Medical Center were enrolled into this prospective cohort from 2003 to 2010. Therefore, data collected as part of that study enabled us to address our aims.
METHODS
The data for this study were collected as part of a prospective, collaborative research program: “Inflammation and the Host Response to Injury” (2U54 GM-062119-07); the details of this have been reported elsewhere.(7) Briefly, subjects were enrolled if they were adult trauma patients who 1) exhibited signs of shock, as evidenced by a base deficit ≥ 6 or hypotension (systolic blood pressure < 90 mmHg) within 60 minutes of arrival to the emergency department, and 2) received a packed red blood cell transfusion after blunt injury. Those with isolated traumatic brain injury, pre-existing organ failure, or immunosuppression were excluded. For the present study, de-identified data were collected from all subjects at participating centers spanning from 2003 to 2010. All delivered enteral or parenteral calories were recorded unless the patient was extubated and eating, as previously reported.(6, 9) Herein, we report caloric intake adjusted for body mass, as kcal/kg/day.
Guidelines for nutritional support as part of the clinical care of the subjects enrolled in the parent study have been previously published.(7) These were evidence-based where possible, and otherwise based upon consensus of the participating consortium members. Many aspects of nutritional care were explicitly addressed and others left to the discretion of the individual institutions involved. For example, the decision for when to interrupt or “hold” feeding prior to operative or other interventional procedures was not specifically addressed. Over the period of time the subjects were enrolled, the general approach across the participating centers was to hold EN support for 6 hours prior to operative and interventional radiological procedures. However, starting in 2006 at Harborview Medical Center, EN support was continued up to the time of operations or procedures if the patient was not undergoing surgery on the GI tract and was to remain intubated postoperatively. In 2006, our center also increased gastric residual volume threshold for holding EN from 150ml to 300ml.
Other elements of enteral nutrition guidelines remained unchanged over this time period. EN was administered in a continuous fashion, via an oro-gastric or naso-gastric tube. If EN support continued beyond 48 hours, a smaller caliber naso-gastric feeding tube was placed. If that patient could not tolerate at least 50% of the goal rate within 48 hours, a fluoroscopically-assisted post-pyloric feeding tube was placed. Surgically placed gastrostomy or jejunostomy tubes had very limited use. If patients required EN support beyond 3–4 weeks, nasal feeding tubes were replaced with percutaneous feeding tubes.
All comparisons were made between subjects enrolled before we adopted our guideline modifications (from 2003–2005; pre-implementation group) and subjects after the modifications (2006 and later; post-implementation group). The kcal/kg/day delivered daily on days 1 through 7 were compared before and after 2006. In order to determine the effects of the guideline change on overall caloric intake, we compared cumulative caloric intake during the first week of admission before and after 2006 using the Mann-Whitney-U test. Multiple linear regression was also performed to assess the association between a change in nutrition protocol (exposure of interest) and calorie intake. Potential confounding factors included in the regression model were categorical variables for age, gender, body mass index (BMI), injury severity score (ISS), APACHE II score, and recent laparotomy.
It is possible that an increase in enteral calorie delivery would increase pulmonary complications. Vomiting and aspiration were not recorded directly in the database. Instead, we examined ventilator associated pneumonia (VAP), acute respiratory distress syndrome (ARDS), duration of mechanical ventilation and ventilator-free days as overall measures of pulmonary morbidity that would reflect the consequences of aspiration. We compared the cohorts before and after 2006 using Poisson regression for categorical outcomes (VAP, ARDS, mortality) or multiple linear regression for continuous outcomes (ventilator-free days and length of stay). Potential confounding factors included in these models were categorical variables for age, gender, BMI, ISS, APACHE II score, and recent laparotomy. Hospital length of stay was included as a potential confounder in the models for VAP and ARDS only. Relative risk ratios and mean estimated differences are presented with the associated 95% confidence intervals. A significance level of 0.05 was used for all tests, and robust standard errors were used for all regression models. All statistical analyses were performed using Stata 12.1 (STATA Corp., College Station, TX).
In more recent years, other participating institutions have modified their feeding guidelines, but these modifications were not in place during the study period. Therefore, subjects enrolled at the other participating centers were also studied to determine whether changes in caloric intake during the first 7 days did or did not occur.
RESULTS
During the study period, 710 subjects were enrolled at Harborview Medical Center. Of these, 23 died on the day of admission and were excluded. Another 74 patients were excluded because they began eating during the first week of admission. This left 245 subjects in the pre-implementation group and 368 in the post-implementation group. Baseline characteristics were similar in the two groups (Table 1). Over the same time period, 1002 subjects enrolled at the other participating centers (Supplemental Digital Content Table 1).
Table 1.
Baseline characteristics for all subjects enrolled at Harborview Medical Center*
| Patient Characteristic | Pre-implementation (n = 245) | Post-implementation (n = 368) |
|---|---|---|
|
| ||
| Male sex | 149 (61) | 244 (66) |
| Age (years) | 40 (24–53) | 42 (23 – 58) |
|
| ||
| Mechanism of injury | ||
| MVC/MCC | 202 (82) | 286 (78) |
| Fall | 22 (9) | 43 (12) |
| Bicycle crash | 10 (4) | 7 (2) |
|
| ||
| APACHE II score | 32 (27 – 35) | 30 (27 – 34) |
| ISS | 33 (25 – 41) | 36 (27 – 48) |
|
| ||
| Severe injury: | ||
| Head | 89 (36) | 122 (33) |
| Chest | 154 (63) | 251 (68) |
| Abdomen | 95 (39) | 176 (48) |
| Spine | 18 (7) | 59 (16) |
|
| ||
| Admission base deficit | −7.5 (−10.3, −5.5) | −7.4 (−10.3, −4.9) |
| # patients transfused | 245 (100) | 368 (100) |
Nominal data are presented as: number (percentage).
Continuous and ordinal data are presented as: median (inter-quartile range). “Severe” injury is defined as abbreviated injury scale ≥3, APACHE II = Acute Physiology and Chronic Health Evaluation II Score; BMI = body mass index; ICU = intensive care unit; ISS = injury-severity-score; MCC = motor cycle crash; MVC=motor vehicle crash.
Guideline modification was associated with increased daily and cumulative caloric intake during the first week in the intensive care unit
Subjects enrolled post-implementation received a higher number of calories on each of the days 1 through 7 (Figure 1a). These daily differences were reflected in cumulative caloric intake. During the first week, subjects in the pre-implementation cohort received a median of 3,787 kcal/person/week (IQR 0–8,599), while subjects in the post-implementation cohort received a median of 6,662 kcal/person/week (IQR 1,980–10,778) (p<0.0001). Mean daily kcal/kg over the first week was also higher post-implementation (12.2 versus 4.4, p <0.001).
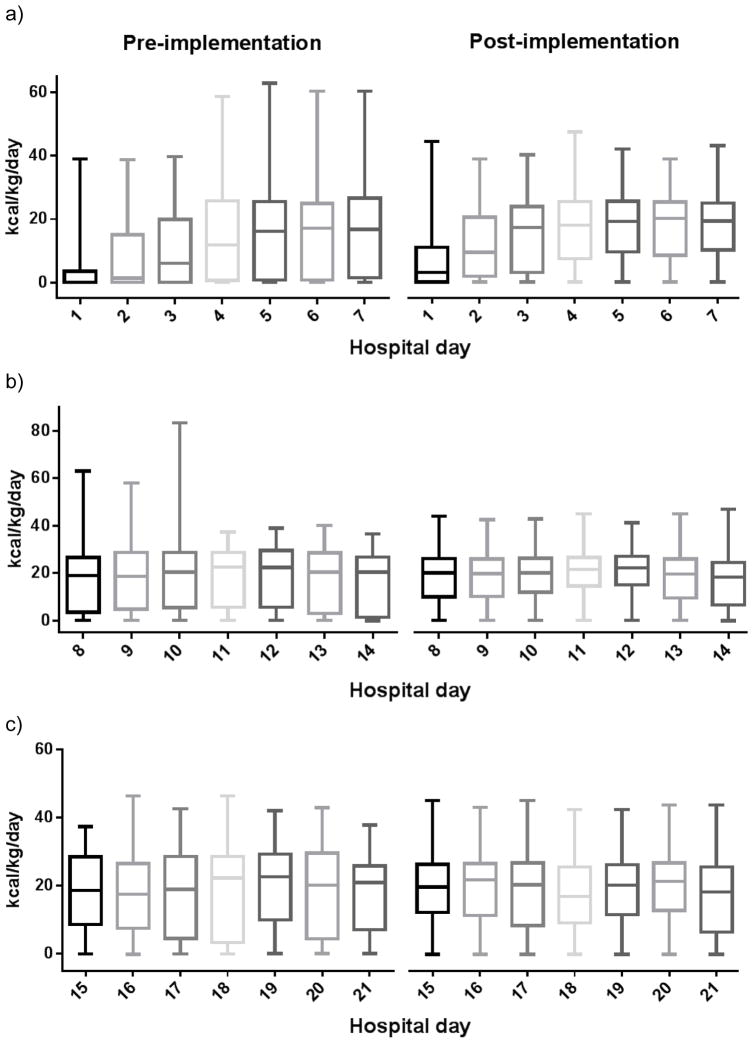
Figure 1.
Distribution of kcal/kg/day for patients at Harborview Medical Center during a) the first, b) the second, and c) the third week of admission. Patients are grouped into cohorts before and after implementation of the modified nutrition guideline in 2006.
We extended our analyses out to the 2nd and 3rd weeks to determine the effect of guideline modifications on subsequent caloric intake. Over the second week, subjects in the pre-implementation cohort received a median of 11,899 kcal/person/week (IQR 8,278–15,315), and those in the post-implementation cohort received a median of 12,126 kcal/person/week (IQR 9,460–14,366) (p=0.53, Figure 1b). Finally, during the third week of ICU admission, subjects before and after implementation had a median of 12,815 kcal/person/week (IQR 9,743–16,094) and 11,700 kcal/person/week (IQR 8,955–14,045) respectively (p=0.20, Figure 1c).
After controlling for potential confounding variables using multiple linear regression, there was still a positive association between increased calorie intake and implementation of modified nutrition guidelines. Post-implementation, subjects received an estimated mean 1965 kcal/person/week more than the pre-implementation cohort, when adjusted for age, gender, BMI, ISS, APACHE II score, and recent laparotomy (95% CI [1237–2694], p<0.001).
Pulmonary complications and duration of mechanical ventilation were not increased despite increased enteral caloric intake during the first post-injury week
The consortium investigators have previously reported that complication and case-fatality rates decreased over the entire study period.(10) However, we have also reported that the incidence of ventilator-associated pneumonia was highest in the subjects who received the most enteral calories during the first week after injury.(6) Therefore, it is important to determine whether complications were increased in our subjects after implementation. Both ventilator-associated pneumonia and ARDS were not increased in the post-implementation period, and there was about one more ventilator-free day (Table 2). Mortality decreased from 15% of the pre-implementation cohort to 8% of the post-implementation cohort (adjusted RR 0.67, 95% CI [0.46–0.99], p=0.04). Pooled results from other participating centers showed no change in the risk of ARDS or VAP, and similar trends for length of stay and ventilator-free days (Supplemental Digital Content Table 2).
Table 2.
Clinical outcomes for patients before and after institution of modified enteral nutrition guidelines at Harborview Medical Center.*
| Outcome | Pre-implementationa | Post-implementation | est. mean difference | 95% CI | p-value | adjustedb difference | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|
| Kilocalorie intake (1st week) | 3,787 (0–8599) | 6,662 (1,980–10,778) | 1951 | 1187, 2716 | <0.001 | 1965 | 1237, 2694 | <0.001 |
| Ventilator-free days | 18 (6,24) | 19 (11, 23) | 1.0 | −0.5, 2.5 | 0.20 | 1.4 | 0.1, 2.7 | 0.04 |
| ICU LOS (days) | 12 (6, 20) | 12 (7, 20) | 0.0 | −1.8, 1.7 | 0.99 | −0.5 | −2.2, 1.2 | 0.54 |
| Hospital LOS (days) | 22 (14, 33) | 24 (15, 34) | 2.8 | −0.6, 6.2 | 0.11 | 2.5 | −1.0, 6.1 | 0.16 |
| Outcome | Pre-implementationa | Post-implementation | RR | 95% CI | p-value | adjustedc RR | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|
| VAP | 85 (35) | 111 (30) | 0.86 | 0.68, 1.08 | 0.19 | 0.82 | 0.65, 1.00 | 0.06 |
| ARDS | 131 (53) | 150 (41) | 0.73 | 0.62, 0.86 | <0.001 | 0.69 | 0.59, 0.81 | <0.001 |
reference group for comparisons
adjusted for age, gender, body mass index, Injury Severity Score, APACHE II score, and recent laparotomy.
adjusted for hospital length of stay, age, gender, body mass index, Injury Severity Score, APACHE II score, and recent laparotomy.
Nominal data are presented as: number (percentage).
Continuous and ordinal data are presented as: median (inter-quartile range). Multiple linear regression was used to analyze continuous data, and Poisson regression was used for categorical data. ARDS = Acute Respiratory Distress Syndrome; CI = confidence interval; est.=estimated; ICU= intensive care unit; LOS = length of stay; RR = relative risk ratio; VAP = Ventilator-Associated Pneumonia.
Changes in caloric intake were not observed at other centers
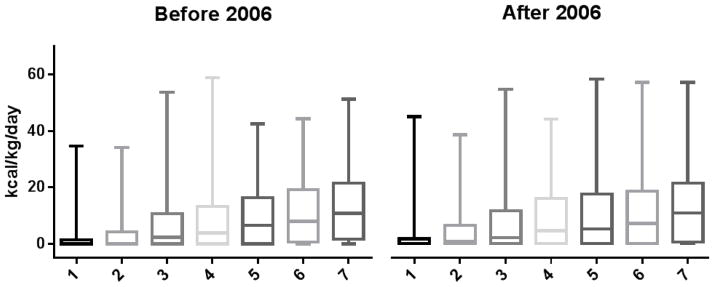
Outcomes for subjects enrolled at the participating centers improved over the 8 years of the study. This, in part, was due to overall changes and improvements in care.(10) It is possible that our observed increase in enteral caloric intake was simply due to an overall trend of increasing enteral intake over time. If this were the case, we would expect to have observed similar increases in enteral caloric intake at the other participating centers. In fact, there were no differences in caloric intake at other hospital centers comparing subjects treated before and after 2006. Daily caloric intake for days 1–7 did not change between the two periods (Figure 2). After the first week of admission, subjects before 2006 received a median of 1480 kcal/person/week (IQR 0–5205) and subjects after 2006 received 1680 kcal/person/week (IQR 0–5720) (p=0.67).
Fig. 2.
Distribution of kcal/kg/day for patients at seven other medical centers during the first week of admission. Patients are grouped into cohorts before and after 2006.
DISCUSSION
Feeding interruptions for operative and other procedures are common in critically-ill trauma victims. Cessation of enteral nutrition 6–12 hours prior to operations and procedures is an ongoing practice in many North American trauma centers, and is an important cause for calorie deficits in critically-ill patients. A recent clinical trial found that 60% of interruptions to enteral feeding in ICU patients were due to fasting for procedures.(11) Another observational study found that 41 of 69 ICU patients underwent procedures and 89% of these patients had a preoperative fasting period with a mean duration of 30 hours or more.(12)
Our study shows that allowing enteral nutrition to continue throughout the perioperative period is associated with a substantial increase in enteral calories received over the first 7 days in the intensive care unit. Our strategy minimizes repeated fasting periods, and reduces the caloric deficits that are prevalent in the critically-ill population. (4, 5, 13) Among the majority of published studies on feeding guidelines, our report is unique in that it specifically addresses the safety of avoiding pre-operative fasting in critically ill patients.(4, 7, 8, 11, 14–16) To our knowledge, only one other recent study has explored this strategy, and also reported no adverse events for the 14 patients included.(17) Of note, our strategy was also associated with less variability in daily calorie intake, particularly by reducing episodes of surplus calories. There were no observed changes in this variability observed at the other centers.
Minimizing calorie deficits in critically-ill trauma patients during the first week of hospitalization is associated with multiple benefits. Attaining consistent caloric intake in the first three days following major trauma has been associated with lower risk for GI intolerance and a lower incidence of pneumonia, relative to trauma patients who were fed later in the hospital course.(18) Randomized controlled trials have also shown that patients with delayed enteral support in the first week have an increased incidence of pneumonia, multiple organ failure, and mortality, relative to patients who are fed more liberally.(19–21) The modified feeding guidelines from our study are associated with higher and more consistent calorie delivery in the first week following trauma. Although the before-after design of our study precludes definitive conclusions about the impact on patient outcomes in this population, the data suggest that the risk for morbidity and mortality in the post-implementation cohort is either similar or reduced, relative to the pre-implementation cohort and relative to patients at other participating centers.
EN has been previously linked to increased gastric-residual volumes, vomiting, aspiration, prolonged mechanical ventilation, and ventilator associated pneumonia (VAP).(22–24) In fact, in a prior study of 1,100 trauma ICU patients, our group observed that those who received the most enteral calories in the first week of admission also experienced the highest incidence of VAP, raising the concern that guidelines encouraging early enteral support may contribute to aspiration and clinically relevant pulmonary morbidity.(6) This observation from our previous study, in part, prompted us to more closely evaluate our feeding practices in our trauma ICU. Our modified nutrition guideline affects an individual patient by increasing total liquid volume in the stomach, both at baseline and at the time of induction for general anesthesia. It is possible that this practice could increase the risk of vomiting, aspiration and subsequent pulmonary morbidity and mortality. However, our data actually show a decrease pulmonary complications and mortality following guideline implementation. Although this study did not collect data on directly observed vomiting or aspiration events, the sequelae of these events (ARDS, pneumonia, prolonged mechanical ventilation, and death) were not increased in patients with a liberal feeding strategy. This is corroborated by a recent randomized trial which found that increasing calorie intake was not associated with any increases in vomiting, aspiration or pneumonia.(11) We therefore conclude that continuing EN in intubated patients up until the time they are transferred from the ICU for an operation/procedure is a safe strategy and that a 6-hour ‘nil per os’ period is not mandatory in this population.
The decreased pulmonary morbidity and all-cause mortality observed after 2006 is unlikely to be completely attributable to modified feeding guidelines, given that other centers without modified feeding guidelines also showed some reductions in these complications. Observed reductions at all centers may also be explained by temporal and national trends in the care of critically-injured trauma patients. The consortium investigators have previously reported a progressive improvement in outcomes over the 10 year study period across all centers.(10) In addition to pulmonary complications such as ARDS and VAP, other organ failures and mortality also decreased over the study period. These reductions, at least in part, were attributed to the application of the various standard operating procedures (SOPs). Given that the overall trend was a reduction in pulmonary complications, it is important to ensure that these trends towards better outcomes did not mask an adverse effect of our center’s change in enteral feeding. In fact, relative to other centers, our center showed similar or greater magnitude reductions for all complications, indicating that modified feeding guidelines are unlikely to have a negative impact on these outcomes.
The safety and feasibility of a generally more liberal approach to enteral feeding is supported by several recent studies. Other published data have shown that increasing enteral caloric intake is not associated with complications, increased length of stay, or mortality. (11, 14) Furthermore, while a liberal feeding strategy does appear to increase gastric residual volumes, evidence linking elevated volumes with aspiration risk is now being called into question.(25) Finally, national guidelines acknowledge that the evidence-basis for perioperative fasting is limited, and reducing the time-interval for preoperative fasting does not appear to confer any additional risk for aspiration. (8, 26)
This report is based upon data collected between 2003 and 2010, and the overall care received must be considered in the context of existing practice and knowledge limitations. For example, the role of supplemental parenteral nutrition is an ongoing debate, (27) and at the time of our study, data regarding harmful effects of parenteral nutrition from the EpANIC trial had not yet been published.(28) We have previously reported our use of parenteral nutrition in this cohort(29) and in keeping with the results of the EpANIC trial, our use was generally limited. However, there have been few other changes in the approach to nutritional support, particularly enteral support, since we enrolled these subjects. As noted above, in 2013, Heyland and colleagues reported that cessation of feeds for procedures was the leading reason for limiting enteral intake.(11)
There are other potential limitations for this study. First, this observational study used a historical control population, making it possible that practice changes and other unmeasured variables biased the study outcomes. Nevertheless, changes in nutrition support guidelines did not occur outside of our center and changes in caloric intake did not occur at the other contributing centers, indicating that our observed association between guideline changes and calorie delivery is real. Second, our measured outcomes (VAP, ARDS, ventilator days) are, at best, indirect measurements for aspiration. Nevertheless, we consider these reasonable surrogate endpoints that were defined and collected in a standard fashion throughout the study. Third, our study population was restricted to severely-injured and intubated patients in an organized surgical ICU with guidelines. In order to generalize our results, other hospitals must have guidelines in place that help minimize aspiration. For example, basic interventions include maintaining the head of bed elevated and avoiding supine positioning. Fourth, one could argue that observed calorie differences were due to our modified gastric residual volume (GRV) threshold (from 150ml to 300ml). However, prior studies have shown that increasing the GRV threshold will result in only nominal changes in calorie delivery.(30) Therefore, although we do not have individual-level data regarding how often feeds were held for elevated GRV, this factor would be unlikely to confound our calorie-based endpoints. All of these limitations would be best addressed by a randomized clinical trial in which patients are monitored directly for complications of enteral feeding and other clinical care is standardized. Our data add important information to the debate regarding the most appropriate way to provide nutritional support to the severely injured.
We conclude that allowing intubated trauma patients to continue enteral nutrition up until they are transferred from the intensive care unit is safe and is associated with increased enteral calorie delivery.
Supplementary Material
Acknowledgments
The Inflammation and the Host Response to Injury “Glue Grant” program is supported by the National Institute of General Medical Sciences. This article was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the National Institute of General Medical Sciences.
Footnotes
There are no additional conflicts of interest declared by the authors.
AUTHOR CONTRIBUTION STATEMENT:
Dr. Brodie Parent, Dr. Grant O’Keefe and Dr. Samuel Mandell contributed to literature search, study design, data collection, data analysis, data interpretation, writing and critical revision of the manuscript. Dr. Ronald Maier, Dr. Joseph Minei, Dr. Jason Sperry, and Dr. Ernest Moore contributed to study design, data interpretation, and critical revision of the manuscript.
Conflicts of interest and disclosures:
The Inflammation and the Host Response to Injury “Glue Grant” program is supported by the National Institute of General Medical Sciences. This article was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the National Institute of General Medical Sciences. There are no additional conflicts of interest declared by the authors.
Contributor Information
Brodie A. Parent, Email: bparent@uw.edu, University of Washington Medical Center, Harborview Department of Surgery, 325 9th Ave, Seattle, WA 98104; c: 202-641-0975, fax: 206-897-5343.
Samuel P. Mandell, Email: mandells@uw.edu, University of Washington Medical Center, Harborview Department of Surgery.
Ronald V. Maier, Email: ronmaier@uw.edu, University of Washington Medical Center, Harborview Department of Surgery.
Joseph Minei, Email: joseph.minei@utsouthwestern.edu, University of Texas Southwestern Medical Center.
Jason Sperry, Email: sperryjl@upmc.edu, University of Pittsburgh Medical Center.
Ernest E. Moore, Email: ernest.moore@dhha.org, University of Colorado Denver Medical Center.
Grant E. O’Keefe, Email: gokeefe@uw.edu, University of Washington Medical Center, Harborview Department of Surgery.
References
- 1.Huynh D, Chapman MJ, Nguyen NQ. Nutrition support in the critically ill. Curr Opin Gastroenterol. 2013 Mar;29(2):208–15. doi: 10.1097/MOG.0b013e32835c9c83. [DOI] [PubMed] [Google Scholar]
- 2.McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009 Jun-Jul;24(3):305–15. doi: 10.1177/0884533609335176. [DOI] [PubMed] [Google Scholar]
- 3.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, Kellum JM, Jr, Welling RE, Moore EE. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992 Aug;216(2):172–83. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binnekade JM, Tepaske R, Bruynzeel P. Daily enteral feeding practice on the ICU: attainment of goals and interfering factors. Crit Care. 2005 Jun;9(3):R218–25. doi: 10.1186/cc3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid C. Frequency of under- and overfeeding in mechanically ventilated ICU patients: causes and possible consequences. J Hum Nutr Diet. 2006 Feb;19(1):13–22. doi: 10.1111/j.1365-277X.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung CK, Whitney R, Thompson CM, Pham TN, Maier RV, O’Keefe GE. Experience with an enteral-based nutritional support regimen in critically ill trauma patients. J Am Coll Surg. 2013 Dec;217(6):1108–17. doi: 10.1016/j.jamcollsurg.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Keefe GE, Shelton M, Cuschieri J, Moore EE, Lowry SF, Harbrecht BG, Maier RV Inflammation the Host Response to Injury Collaborative Research P. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care VIII--Nutritional support of the trauma patient. J Trauma. 2008 Dec;65(6):1520–8. doi: 10.1097/TA.0b013e3181904b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423. doi: 10.1002/14651858.CD004423. [DOI] [PubMed] [Google Scholar]
- 9.Sena MJ, Utter GH, Cuschieri J, Maier RV, Tompkins RG, Harbrecht BG, Moore EE, O’Keefe GE. Early supplemental parenteral nutrition is associated with increased infectious complications in critically ill trauma patients. J Am Coll Surg. 2008 Oct;207(4):459–67. doi: 10.1016/j.jamcollsurg.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuschieri J, Johnson JL, Sperry J, West MA, Moore EE, Minei JP, Bankey PE, Nathens AB, Cuenca AG, Efron PA, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012 May;255(5):993–99. doi: 10.1097/SLA.0b013e31824f1ebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heyland DK, Murch L, Cahill N, McCall M, Muscedere J, Stelfox HT, Bray T, Tanguay T, Jiang X, Day AG. Enhanced protein-energy provision via the enteral route feeding protocol in critically ill patients: results of a cluster randomized trial. Crit Care Med. 2013 Dec;41(12):2743–53. doi: 10.1097/CCM.0b013e31829efef5. [DOI] [PubMed] [Google Scholar]
- 12.Passier RH, Davies AR, Ridley E, McClure J, Murphy D, Scheinkestel CD. Periprocedural cessation of nutrition in the intensive care unit: opportunities for improvement. Intensive Care Med. 2013 Jul;39(7):1221–6. doi: 10.1007/s00134-013-2934-8. [DOI] [PubMed] [Google Scholar]
- 13.Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition. 1996 Jan;12(1):23–9. doi: 10.1016/0899-9007(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 14.Soguel L, Revelly JP, Schaller MD, Longchamp C, Berger MM. Energy deficit and length of hospital stay can be reduced by a two-step quality improvement of nutrition therapy: the intensive care unit dietitian can make the difference. Crit Care Med. 2012 Feb;40(2):412–9. doi: 10.1097/CCM.0b013e31822f0ad7. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie SL, Zygun DA, Whitmore BL, Doig CJ, Hameed SM. Implementation of a nutrition support protocol increases the proportion of mechanically ventilated patients reaching enteral nutrition targets in the adult intensive care unit. JPEN J Parenter Enteral Nutr. 2005 Mar-Apr;29(2):74–80. doi: 10.1177/014860710502900274. [DOI] [PubMed] [Google Scholar]
- 16.Barr J, Hecht M, Flavin KE, Khorana A, Gould MK. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004 Apr;125(4):1446–57. doi: 10.1378/chest.125.4.1446. [DOI] [PubMed] [Google Scholar]
- 17.McElroy LM, Codner PA, Brasel KJ. A pilot study to explore the safety of perioperative postpyloric enteral nutrition. Nutr Clin Pract. 2012 Dec;27(6):777–80. doi: 10.1177/0884533612464656. [DOI] [PubMed] [Google Scholar]
- 18.Kompan L, Vidmar G, Spindler-Vesel A, Pecar J. Is early enteral nutrition a risk factor for gastric intolerance and pneumonia? Clin Nutr. 2004 Aug;23(4):527–32. doi: 10.1016/j.clnu.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Doig GS, Heighes PT, Simpson F, Sweetman EA, Davies AR. Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: a meta-analysis of randomised controlled trials. Intensive Care Med. 2009 Dec;35(12):2018–27. doi: 10.1007/s00134-009-1664-4. [DOI] [PubMed] [Google Scholar]
- 20.Kompan L, Kremzar B, Gadzijev E, Prosek M. Effects of early enteral nutrition on intestinal permeability and the development of multiple organ failure after multiple injury. Intensive Care Med. 1999 Feb;25(2):157–61. doi: 10.1007/s001340050809. [DOI] [PubMed] [Google Scholar]
- 21.Chuntrasakul C, Siltharm S, Chinswangwatanakul V, Pongprasobchai T, Chockvivatanavanit S, Bunnak A. Early nutritional support in severe traumatic patients. J Med Assoc Thai. 1996 Jan;79(1):21–6. [PubMed] [Google Scholar]
- 22.Mizock BA. Risk of aspiration in patients on enteral nutrition: frequency, relevance, relation to pneumonia, risk factors, and strategies for risk reduction. Curr Gastroenterol Rep. 2007 Aug;9(4):338–44. doi: 10.1007/s11894-007-0039-7. [DOI] [PubMed] [Google Scholar]
- 23.Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: a multicenter study. The Nutritional Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit Care Med. 1999 Aug;27(8):1447–53. doi: 10.1097/00003246-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Tejada Artigas A, Bello Dronda S, Chacon Valles E, Munoz Marco J, Villuendas Uson MC, Figueras P, Suarez FJ, Hernandez A. Risk factors for nosocomial pneumonia in critically ill trauma patients. Crit Care Med. 2001 Feb;29(2):304–9. doi: 10.1097/00003246-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, Clavel M, Frat JP, Plantefeve G, Quenot JP, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013 Jan 16;309(3):249–56. doi: 10.1001/jama.2012.196377. [DOI] [PubMed] [Google Scholar]
- 26.Schneider JA, Lee YJ, Grubb WR, Denny J, Hunter C. Institutional practices of withholding enteral feeding from intubated patients. Critical Care Med. 2009 Jul;37(7):2299–302. doi: 10.1097/CCM.0b013e3181a007eb. [DOI] [PubMed] [Google Scholar]
- 27.Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, Thibault R, Pichard C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381(9864):385–93. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 28.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011 Aug 11;365(6):506–17. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 29.Parent B, Shelton M, Nordlund M, Aarabi S, O’Keefe G. Parenteral Nutrition Utilization After Implementation of Multidisciplinary Nutrition Support Team Oversight: A Prospective Cohort Study. JPEN J Parenter Enteral Nutr. 2015 Apr 28; doi: 10.1177/0148607115585354. [DOI] [PubMed] [Google Scholar]
- 30.Elke G, Felbinger TW, Heyland DK. Gastric residual volume in critically ill patients: a dead marker or still alive? Nutr Clin Pract. 2015 Feb;30(1):59–71. doi: 10.1177/0884533614562841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.