Abstract
Background
Effective non-surgical long-term weight loss solutions remain elusive.
Objective
To compare a behavioral weight loss program (BWL) with a stress management-based program (Emotional Brain Training, EBT) on weight loss, blood pressure, depression, perceived stress, diet and physical activity.
Participants/setting
Individuals with a body mass index (BMI) of ≥ 28 and ≤ 45 kg/m2 were recruited in Lexington, Kentucky in January 2014 and randomized to a behavioral weight loss program (BWL) or a stress management program (EBT) for a 20 week intervention. Of those recruited, 49 participants were randomized to EBT or BWL. Randomization and allocation to group were performed using SAS software (v 9.3, 2011, SAS Institute Inc.). Weight, blood pressure, depression, perceived stress, dietary intake and physical activity were measured at baseline, 10, and 20 weeks.
Main outcome measures
Weight and Body Mass Index (BMI)
Statistical analyses
Linear models for change over time were fit to calculate 95% confidence intervals of intervention effects.
Results
BWL produced greater changes in BMI than EBT at both 10(p=0.02) and 20 weeks (p=0.03). At 10 weeks, both EBT and BWL improved BMI, systolic blood pressure, depression and perceived stress (p<0.05). BWL also improved diastolic blood pressure (p=0.005). At 20 weeks, EBT maintained improvements in BMI, systolic blood pressure, depression, and perceived stress while BWL maintained improvements only in BMI and depression (p<0.05).
Conclusions
BWL produced greater weight loss than EBT; however, EBT produced sustained improvements in stress, depression, and systolic blood pressure. A combination of the two approaches should be explored.
Keywords: Obesity, weight loss, stress management, adult, depression
Background
The phrase “obesity epidemic” has become common in the U.S. [1]. This focus on weight has led to a weight loss industry estimated to be a $60 billion per year business [2]. Current efforts at curbing the obesity epidemic are not working, as obesity rates continue to climb [3]. While initial weight loss is possible for many through the use of behavioral weight loss techniques [4], long-term maintenance of weight loss remains difficult for many [5–10]. National data indicate that only 37% of individuals are able to maintain at least a 5% weight loss and only 17% maintain a weight loss of 10% at one year [11]. Data from the National Weight Control Registry indicate that those who are successful with weight maintenance consume a low-calorie diet and exercise regularly [12]. However, for many weight maintenance does not occur, likely because these behaviors are not maintained.
A National Institutes of Health (NIH) working group to improve weight loss maintenance published a report in early 2015 [13]. The working group concluded that, “no approach has worked to change the overall pattern on weight loss and regain.” Current obesity treatment, behavioral weight loss, involves a dietary and exercise prescription with encouragement to self-monitor behaviors. The NIH working group proposed that these behavioral programs fail at producing weight loss maintenance because participants have decreasing adherence to the prescribed treatment of decreased calories and increased exercise; however, they concede that attempts to remedy this problem with adherence to treatment have not been effective [13, 14]. The group then proposed that habitual eating behaviors that led to the state of obesity, possibly “involving dopamine signaling in the brain”, may be responsible for return to old behaviors and difficulty in maintaining weight loss [13].
Chronic and acute stress have been linked to consumption of foods high in sugar and/or fat, often referred to as “emotional eating” [15–17]. Stress is one activator of the dopaminergic (DA) system and has an impact on food intake through the hypothalamic-pituitary-adrenal (HPA) axis. Specifically, recent evidence in humans suggests a direct link from the hypothalamus into the dorsal and ventral striatum, brain regions associated with reward and satiety [18, 19]. Highly palatable foods, such as those high in sugar and fat, increase extracellular dopamine, thus increasing the perception of reward [20, 21]. Furthermore, recent evidence suggests that appetite-regulating hormones, such as ghrelin and glucagon-like peptide-1 (GLP-1) activate reward neurocircuitry, enhancing motivation for rewarding stimuli [22]. Interestingly, studies have shown that individuals who chronically consume high-fat, high-sugar diets show similar adaptations in limbic dopamine function as individuals who chronically abuse drugs [23, 24]. While the link between stress regulation of the HPA and limbic reward centers is still not clearly understood, it suggests a possible mechanism by which stress can govern food intake, resulting in obesity. Furthermore, it provides grounds to suggest that stress management techniques may help to “reset” the neurochemical regulation of food intake, resulting in more maintainable weight-loss interventions [19, 20].
Despite the connection between stress and eating behaviors, there has been little research on the use of stress management to improve weight loss. Christaki et al. evaluated the efficacy of an 8-week stress management program for weight loss in a small sample of adults and found that participants in the stress management group lost significantly more weight than the control group but did not experience greater improvements in perceived stress [25]. In another 12-week weight loss program augmented with stress management techniques, the authors found it to produce a trend in greater weight loss and lower cortisol levels than the control group [26]. Research by Mellin et al. found improvements in weight, blood pressure, and depression when adolescents and adults were treated with a stress reduction program [27, 28]. The skills taught in the early version of this stress reduction program were further developed by modifying the stress-management tools to be consistent with the neurophysiology of self-regulation. The program is now known as the Emotional Brain Training (EBT) program. EBT encourages mindfulness and improvements in lifestyle behaviors, but focuses on cognitive and emotional strategies for managing stress. The strategies used in the program have been developed based on brain physiology. See Table 1. EBT has been shown in two small previous studies to produce changes in weight, blood pressure, perceived stress, and depression [29, 30].
Table 1.
The Components of Emotional Brain Training (EBT)1
| Component | Technique | Description |
|---|---|---|
| Stress Tools Based on Brain State and Perception | ||
| Homeostatic States | ||
| Brain State 1. Feeling great | Sanctuary Tool | Self- and other-compassion statements |
| Brain State 2. Feeling good | Feelings Check Tool | Identification of feelings and needs |
| Brain State 3. A little stressed | Emotional Housekeeping Tool | Expression of negative and positive feelings |
| Non-homeostatic States | ||
| Brain State 4. Definitely stressed | Cycle Tool | Emotional expression; revision of expectations |
| Brain State 5. Stressed out | Damage Control Tool | Stress-reducing statements2 |
| Stress-related Drives for Non-homeostatic Eating | ||
| Survival Circuit Reconsolidation | Emotional revision of stress-related expectations | |
| Cravings Zapper | Emotional expression and distraction | |
| Stress-related Homeostatic Lifestyle Program | ||
| Sleep | 7 – 9 hours/night | |
| Exercise | 30 – 60 minutes/day | |
| Unprocessed Foods | 60% to 90% of intake | |
Health professionals complete 24 hours of distance learning seminars and experiential training to be provisionally certified and an additional 16 hours of supervised program delivery to become fully-certified.
Repetition of statements that promote homeostasis: Do not judge. Minimize harm. Know it will pass.
The objective of the current study was to compare the EBT program to a behavioral weight loss (BWL) program on weight and secondary outcomes, over the course of 20 weeks.
Methods
This was a parallel, randomized controlled clinical trial. Participants were randomized to: (1) a behavioral weight loss program (BWL) (n=25, 51%) or (2) a stress management program, (EBT) (n=24, 49%). The University of Kentucky Medical Institutional Review Board approved the study protocol and all participants provided written informed consent.
Participants
Recruitment occurred in January 2014 in Lexington, KY. Adults aged 25 to 55 years with a body mass index (BMI) of ≥ 28 and ≤ 45 kg/m2 and at least one additional risk factor for metabolic syndrome were recruited through social media, flyers and listservs. Exclusion criteria included: a medical diagnosis of orthopedic or joint problems that may prohibit regular exercise; reported heart problems, chest pain, faintness or dizzy spells; hospitalization for a psychiatric disorder within the last year; a history of anorexia or bulimia nervosa; a medical diagnosis of cancer (except skin cancer) or HIV; pregnant, nursing or planning to become pregnant within the study period; less than 9 months post-partum; weight loss of ≥ 10 pounds in the last six months; on more than two medications for hypertension control; taking insulin, Coumadin, or Lasix, greater than stage 3 kidney disease.
All participants were screened via telephone and eligible subjects were invited to a study information session. At this session, blood pressure and waist circumference were measured to determine eligibility. Interested and qualified participants were asked to obtain approval from their physician, sign the study consent form, and fill out questionnaires. Participants then scheduled an appointment at the clinic for baseline assessments.
Intervention arms
The project coordinator enrolled participants and the principal investigator randomized participants. Randomization and allocation to trial group were performed using SPSS (v 19.0, 2010). Each study group was divided into smaller groups of eight or nine participants. Groups met once per week, in 90-minute sessions, for ten weeks with a trained interventionist. The EBT intervention was based on the introductory material for the EBT program. Participants were instructed in the five stress management skills using the Wired for Freedom workbook, from the EBT program. The lifestyle changes included getting adequate sleep, exercising daily, and consuming a whole foods diet. See Table. The BWL intervention was based on materials from the Diabetes Prevention Program (DPP). This intervention emphasized self-monitoring of caloric intake and physical activity. Participants were encouraged to reduce calories by 500–1000 kcal per day and to increase exercise to a daily total of 60 minutes through moderate intensity physical activity. The weekly lessons were on topics such as eating out, increasing physical activity, changing environmental cues, motivation, and goal setting. In both the EBT and BWL programs, 60 minutes of exercise per day and consumption of a healthy diet was encouraged.
During weeks 11–20, participants did not meet weekly with the interventionists. They were told to continue using the skills and information they had learned during the first 10 weeks of the intervention. Each group member also received a weekly email from the study coordinator during this time. After the 20-week follow-up assessments, the study concluded in July 2014.
Outcome measures and follow-up
Height, weight, and blood pressure were measured in the clinic by a nurse blinded to group assignment. Weight measurements were taken with a Tanita digital scale, model # BWB-800 (Arlington Heights, IL). Height was assessed with a free standing stadiometer. Participants wore a hospital gown with no shoes for weight and height measurements. Body mass index (BMI) was calculated as kilograms of body weight divided by height in meters squared (kg/m2). Blood pressure was measured once in the seated position with a GE Healthcare Dash 2500 monitor.
At baseline, demographic data were collected. At baseline, 10, and 20 weeks, participants completed the Perceived Stress Scale (PSS) [14,31] and the Center for Epidemiologic Studies Depression Scale (CES-D) [32]. The PSS was designed to determine how unpredictable, uncontrollable, and overloaded subjects find their lives [14] and has been validated for use in an adult population [31]. PSS scores range from 0 to 40 and norms for various subgroups range from 11.9 to 14.7 [31]. The CES-D scale is designed for use by the general population and is a self-reported questionnaire, which has demonstrated adequate reliability and validity [32]. The CES-D is scored on a scale from 1 to 60 and a score of 16 or above is considered depressed [32].
Dietary and physical activity data were also collected at baseline, 10, and 20 weeks. Participants completed the Block 2005 food frequency questionnaire (FFQ). The Block FFQ is composed of 110 food items. The validity (r=0.59) and reliability (r=0.75) of the FFQ has been established [33, 34]. Participants wore a medical grade piezoelectric activity monitor (NL–1000; New Lifestyles Inc, Lees Summit, MO) for 7 consecutive days thus allowing total steps taken and time spent (mins) in moderate-to-vigorous physical activity (MVPA) to be assessed.
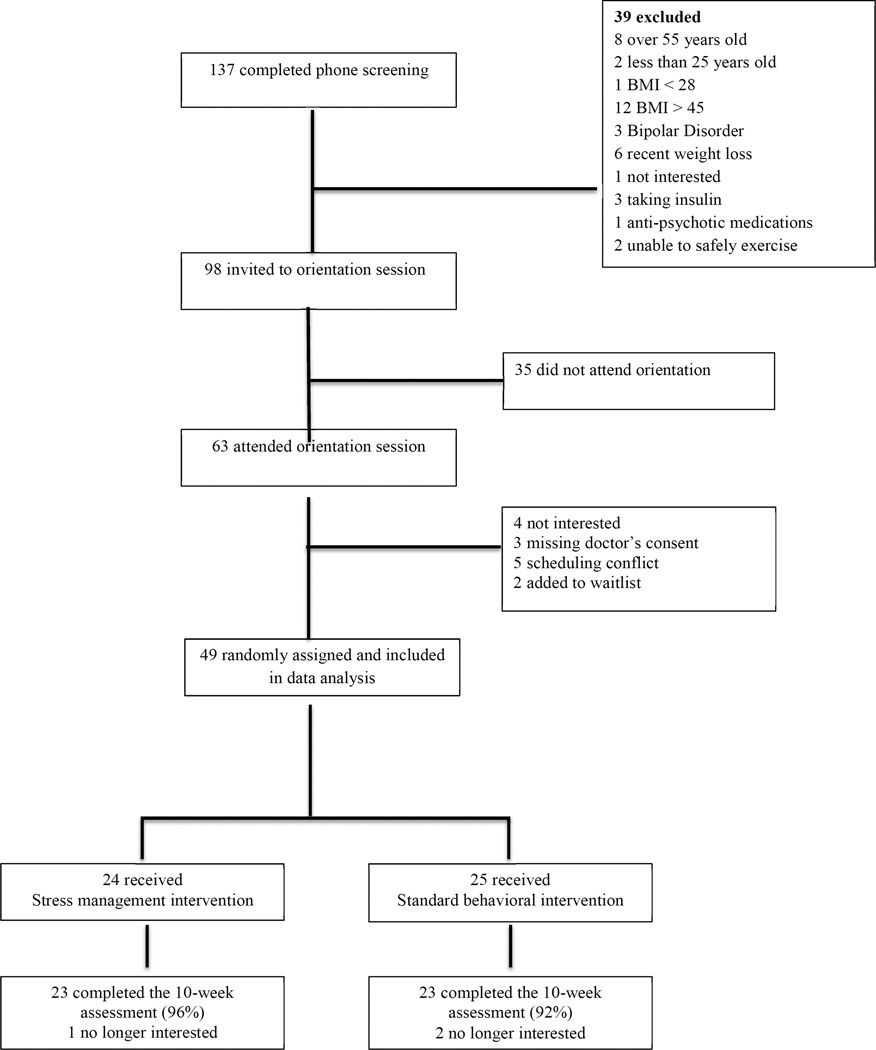
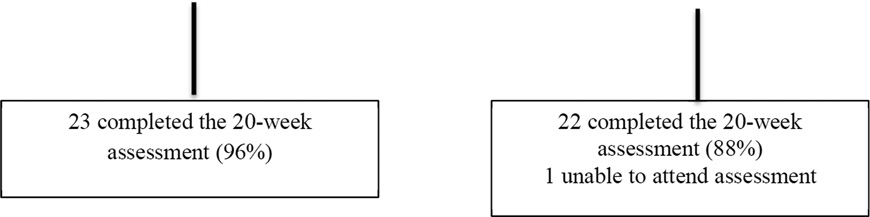
Participants received $50 for completing the 10-week and $75 for completing the 20- week assessments. See Figure 1.
Figure 1.
Participant Flow Diagram From Initial Screening to Follow-Up Assessments
Abbreviation: BMI= body mass index
Statistical analysis
This was an exploratory analysis of a new strategy, EBT; therefore a sample size calculation was not possible. The analysis presented is for completers only, n=45. All analyses were performed using SAS software (version 9.3, 2010, SAS Institute Inc.). Descriptive statistics were used to characterize participants. Continuous variables were compared using two-sample t-tests if the normality assumption held or Wilcoxon rank-sum test otherwise. The normality of the variables was checked using the Kolmogorov-Smirnov test. Categorical variables were compared using Chi-square tests. Generalized estimating equations (GEEs) with unstructured correlation between changes over time (baseline to 10 and 20 weeks) were used to calculate 95% confidence intervals of intervention effects. A p-value of less than 0.05 was considered significant. Changes from baseline were calculated and modeled as outcomes. To estimate adjusted means, models included gender, ethnicity and the corresponding baseline measure as covariates. Cohen’s d was calculated to estimate the effect size.
Results
Sample
A total of 49 participants signed consent forms, attended the baseline assessment and at least one group session. The completion rate was 94% at 10 weeks and 92% at 20 weeks. See Figure 1. The participants were 82.2% female and 82.2% Caucasian. The average age was 45.0 ± 7.9 years and the average BMI was 36.5 ± 4.6 kg/m2, which is in the obese range. Blood pressure was slightly elevated at baseline for both groups. The average CES-D score was 10.4 ± 9.8 for the EBT group and 7.5 ± 6.4 for the BWL group. Neither average was above the 16 point cut-off for depression; however individual scores ranged from 0 to 42. The average PSS score was 18.3 ± 6.6 in the EBT group and 16.1 ± 7.7 in the BWL group. Both of these averages were above the norms for the PSS. The majority of participants (55.6%) had at least a bachelor’s degree. See Table 2.
Table 2.
Baseline Participant Characteristics (n=49)
| EBT group (n=24) | BWL group (n=25) | p-value | |
|---|---|---|---|
| Age | 45.5 ± 8.0 | 44.0 ± 8.8 | 0.536 |
| Female | 20 (83.3) | 21 (84.0) | 1 |
| Minoritya | 4 (16.7) | 5 (20.0) | 0.459 |
| Higher educationb | 12 (50.0) | 15 (60.0) | 0.482 |
| Weight (kg) | 101.9 ± 10.8 | 99.0 ± 16.7 | 0.470 |
| BMI | 37.0 ± 4.9 | 36.0 ± 4.3 | 0.447 |
| Systolic Blood Pressure (mmHg) | 133 ± 14 | 127 ± 14 | 0.166 |
| Diastolic Blood Pressure (mmHg) | 74 ± 8 | 76 ± 11 | 0.451 |
| Perceived Stress | 18.3 ± 6.6 | 16.1 ± 7.7 | 0.305 |
| Depression | 10.4 ± 9.8 | 7.5 ± 6.4 | 0.279 |
| Total Dietary Calories/Day | 2025 ± 451 | 1796 ± 605 | 0.141 |
| Total MVPA Minutes/Day | 80.2 ± 65.5 | 71.0 ± 58.7 | 0.622 |
Two-sample t-tests were used if the normality assumption held or Wilcoxon rank-sum test if not.
Abbreviations: EBT = emotional brain training; BWL = behavioral weight loss; BMI=body mass index;
Asian, African American, or Hispanic/Latino
Bachelor's or Graduate/Professional
Main outcomes
The adjusted outcomes for all of the variables are presented in Table 3. At 10 weeks, 13% of the EBT group and 26% of the BWL group had lost 5% or greater of their initial body weight. At 20 weeks, 13% of EBT and 41% of BWL participants had a 5% or greater weight loss. The BWL group had greater improvements than the EBT group in BMI at 10 weeks (p=0.02). At 20 weeks, BWL maintained a greater improvement in BMI than EBT (p=0.03). BWL lost an average of 3.9 ± 3.3 kg and EBT lost an average of 1.6 ± 3.8 kg at 20 weeks. The between group differences in weight loss were significant at 10 (p= 0.019) and 20 weeks (p=0.022). The estimated effect size (d) was 0.30 at 20 weeks.
Table 3.
Changes in Body Mass Index, Blood Pressure, Perceived Stress, Depression, Dietary Intake and Physical Activity (n=49)
| Adjusted change from baseline | ||||
|---|---|---|---|---|
| To 10-week | p-value | To 20-week | p-value | |
| Body mass index, kg/m2 | ||||
| EBT | −0.5 | 0.012 | −0.6 | 0.032 |
| (−0.8 to −0.1) | (−1.1 to −0.0) | |||
| BWL | −1.1 | <0.001 | −1.3 | <0.001 |
| (−1.5 to −0.7) | (−1.8 to −0.9) | |||
| Difference | 0.6 | 0.021 | 0.8 | 0.030 |
| (0.1 to 1.1) | (0.1 to 1.4) | |||
| Systolic blood pressure, mmHg | ||||
| EBT | −4.2 | 0.035 | −6.6 | <0.001 |
| (−8.0 to −0.3) | (−10.2 to −3.0) | |||
| BWL | −4.6 | 0.038 | −4.9 | 0.063 |
| (−8.9 to −0.3) | (−10.0 to 0.3) | |||
| Difference | 0.4 | 0.892 | −1.7 | 0.625 |
| (−5.8 to 6.7) | (−8.5 to 5.1) | |||
| Diastolic blood pressure, mmHg | ||||
| EBT | −2.1 | 0.213 | 0 | 0.997 |
| (−5.4 to 1.2) | (−4.0 to 4.0) | |||
| BWL | −2.5 | 0.005 | −2.6 | 0.092 |
| (−4.3 to −0.7) | (−5.6 to 0.4) | |||
| Difference | 0.4 | 0.825 | 2.6 | 0.309 |
| (−3.3 to 4.2) | (−2.4 to 7.6) | |||
| Perceived stress | ||||
| EBT | −5.2 | <0.001 | −4.5 | <0.001 |
| (−7.0 to −3.4) | (−6.8 to −2.2) | |||
| BWL | −2.3 | 0.044 | −1.8 | 0.195 |
| (−4.5 to −0.1) | (−4.5 to 0.9) | |||
| Difference | −2.9 | 0.043 | −2.7 | 0.136 |
| (−5.7 to 0.1) | (−6.2 to 0.8) | |||
| Depression | ||||
| EBT | −2.6 | 0.009 | −3.1 | 0.006 |
| (−4.6 to −0.7) | (−5.3 to −0.9) | |||
| BWL | −2.5 | 0.036 | −2.9 | 0.012 |
| (−4.9 to −0.2) | (−5.2 to −0.6) | |||
| Difference | −0.1 | 0.948 | −0.2 | 0.922 |
| (−3.1 to 2.9) | (−3.3 to 3.0) | |||
| Total kilocalorie intake/ day | ||||
| EBT | −303.1 | 0.002 | −449.9 | <0.001 |
| (−492.1 to −114.0) | (−661.3 to −238.5) | |||
| BWL | −469.9 | <0.001 | −437.4 | <0.001 |
| (−654.0 to −285.7) | (−678.2 to −196.7) | |||
| Difference | 166.8 | 0.226 | −12.5 | 0.938 |
| (−103.1 to 436.7) | (−326.3 to 301.4) | |||
| Total MVPA minutes/ day | ||||
| EBT | 20.0 | 0.057 | −6.0 | 0.518 |
| (−0.6 to 40.5) | (−24.2 to 12.2) | |||
| BWL | 25.5 | 0.142 | 0.0 | 0.999 |
| (−8.6 to 59.7) | (−29.1 to 29.1) | |||
| Difference | −5.6 | 0.788 | −6.0 | 0.738 |
| (−46.0 to 34.9) | (−41.2 to 29.2) | |||
Generalized estimating equations (GEEs) were used to calculate 95% confidence intervals.
Abbreviations: CI = confidence interval; EBT = emotional brain training; BWL = behavioral weight loss; MVPA= moderate to vigorous physical activity.
The follow up scores and change scores from baseline were modeled as outcomes with covariates including the corresponding baseline measure, gender, and ethnicity.
Secondary outcomes
The EBT group had a greater improvement in PSS than the BWL group at 10 weeks (p=0.04). The difference in perceived stress between the groups no longer existed at 20 weeks (p=0.14). No other differences between the two groups existed at 10 or 20 weeks. See Table 3.
Process Data
EBT participants attended 8.7 ± 2.0 and BWL participants attended 7.7 ± 2.3 of the 10 weekly sessions (p=0.12). The EBT group had a completion rate of 96% and the BWL group had a completion rate of 88%.
Discussion
This pilot study sought to compare a stress management program and a behavioral weight loss program. Both groups lost a statistically significant amount of weight; however BWL produced greater weight loss than EBT at 10 and 20 weeks. A greater percentage of BWL participants also achieved a clinically significant, 5% or greater, weight loss than EBT participants [35]. However, the effect size for the difference between groups was small (d=0.3).
Weight loss in the BWL group was comparable to that seen in similar length studies, while weight loss in the EBT group was less than what is typically seen [36–41]. It is probable that an emphasis on behavioral weight loss techniques is needed to produce clinically significant weight loss and the EBT group did not receive this type of education. Christaki et al. found greater weight loss (4.4kg), when implementing a stress management based program for women, likely because they also placed study participants on a low-calorie diet to produce an energy deficit each day [25]. Cox et al. also found greater weight loss (2.7kg), than was found with the EBT program, when adding a stress management program to an adaptation of the DPP, a behavioral weight management program [26]. A combination of the two, behavioral weight loss and stress management, appears to be better than stress management alone at producing weight loss in the short-term.
The completion rates for both intervention groups were close to what is normally seen in clinical weight loss trials [36, 42, 43]. The number of sessions attended was 77% and 87%, which is higher than seen in another stress management based study [26]. Both of these facts are strengths of the study and indicate the feasibility of implementing these types of interventions in similar populations.
The BWL group had non-significant decreases in systolic and diastolic blood pressure at 20 weeks of 4.9 and 2.6 mm Hg respectively. According to the NHLBI systematic review on evidence on managing obesity, a 2–3 mmHg mean reduction in both systolic and diastolic blood pressure can be expected with a 5% weight loss [44]. Considering that only 41% of BWL participants had a 5% or greater weight loss at 20 weeks, this is within the range of what is expected. The EBT group also saw a significant decrease in systolic blood pressure of 6.6 mm Hg at 20 weeks. This was similar to what was found by Mellin et al. [29] and Webber et al. [30] when using a version of the EBT program. It appears that this type of stress management treatment may have an impact on blood pressure independent of weight loss.
The EBT group maintained a significant decrease in stress at 20 weeks. This is different than what was found in two previous stress management studies. Christaki et al. did not find any statistical difference between the stress management group and the control group in change on the PSS in that 8-week trial [25]. Cox et al. found a decrease in stress levels over the course of the 12-week intervention for both the stress management intervention and control group, however the change in stress levels, as measured by the PSS, did not differ between the two groups [26]. However, in two previous studies which used a version of the EBT program, there were significant decreases in perceived stress [29, 30].
The differences between these two groups were weight and stress. It has been demonstrated previously that behavioral programs produce weight loss in the short-term, however generally fail in regards to weight loss maintenance. Since stress is linked to eating habits and obesity, a further exploration of a combination of these two approaches may be warranted. Stress is a significant contributor to non-homeostatic caloric intake and food has been indicated as a preferred self-medication method in response to chronic stress [19,45]. Thus, addressing stress levels and increasing stress resilience in the context of the obesity treatment may have much more profound as well as sustained effects than behavioral intervention alone. The current study points to the possibility that EBT may address the issue of increasing stress resiliency.
More research is needed to fully understand the HPA-reward link. Imaging studies as well as animal studies may provide insight into the underlying neural mechanisms that control emotional eating. Recent evidence in humans however suggests a direct link from the hypothalamus into the dorsal and ventral striatum, brain regions typically associated with reward and satiety [19, 46]. Furthermore, studies have shown that VTA neurons contain melanocortin (MC4) receptors, which are known to be key regulators of food intake and weight gain [47]. The link between MC4 receptors and reward (dopamine release) is still poorly understood, however the presence of these receptors on dopaminergic neurons suggests a clear link between stress regulation of the HPA and limbic reward centers, which likely ultimately impacts food intake.
Limitations
Blood pressure was measured only once. The small sample size of primarily white females does not allow for generalization of the results. The study was of short duration, however, this was a pilot study done to provide proof of concept and preliminary data for a larger, longer study.
Conclusions
In the short-term, BWL is superior at producing weight loss to the EBT program. However, in this short study, the EBT program had a small but significant impact on weight and a significant sustained impact on blood pressure, perceived stress, and depression. Because of the difficulty in maintaining weight loss through traditional methods and the connection of stress and obesity, further exploration of the EBT program in a larger more diverse sample over a longer period of time is needed. A combination of the behavioral weight loss and the stress management programs might be beneficial.
Highlights.
The stress management program produces sustained changes in stress level.
Because stress-induced caloric intake may be a risk factor for weight regain, a combination of the behavioral weight loss and stress management approach is suggested.
Acknowledgments
Funding NHLBI grant #1R56HL116517-01A1 and support provided by National Center for Advancing Translational Sciences (UL1TR000117)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s contributions
Kelly Webber secured the grant funding, conducted the study, and drafted and refined the manuscript. Lindsey Mayes administered the behavioral intervention, collected data, and contributed to writing the background section. Erin Casey contributed to the introduction and background sections of the manuscript and completed the references for the manuscript. Yuriko Katsumata conducted the statistical analysis and prepared Tables 1 and 2. Laurel Mellin created Table 1.
Trial Registration at www.clinicaltrials.gov #NCT02023515.
Competing Interests
Dr. Laurel Mellin is the founder of Emotional Brain Training.
Contributor Information
Kelly H. Webber, Kelly.webber@uky.edu, 206E Funkhouser Bldg., University of Kentucky, Lexington, KY 40506-0054, Phone: 859-257-4351, Fax: 859-257-3707.
Erin M. Casey, 206B Funkhouser Bldg., University of Kentucky, Lexington, KY 40506-0054.
Lindsey Mayes, French-East 363C, 3202 Eden Ave, University of Cincinnati, Cincinnati OH 45267-0394.
Yuriko Katsumata, University of Kentucky, 111 Washington Ave., Lexington, KY 40536-003.
Laurel Mellin, University of California San Francisco, 138 Woodland Avenue, San Anselmo, CA 94960.
References
- 1.Boero N. All the News that's Fat to Print: The American "Obesity Epidemic" and Media. Quantitative Sociology. 2007;30:41–60. [Google Scholar]
- 2.LaRosa J. U.S. Weigh Loss Market Worth $60.9 Billion. 2011, Marketdata Enterprises. PRWeb [Google Scholar]
- 3.Centers for Disease Control and Prevention. Health, United States, 2013. Centers for Disease Control and Prevention. [cited 2015 August 6]; Available from: http://www.cdc.gov/nchs/fastats/obesity-overweight.htm.
- 4.Dunkley AJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37(4):922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 5.Wadden TA, et al. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(Suppl):151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 6.Ohsiek S, Williams M. Psychological factors influencing weight loss maintenance: an integrative literature review. J Am Acad Nurse Pract. 2011;23(11):592–601. doi: 10.1111/j.1745-7599.2011.00647.x. [DOI] [PubMed] [Google Scholar]
- 7.Purcell K, et al. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2(12):954–962. doi: 10.1016/S2213-8587(14)70200-1. [DOI] [PubMed] [Google Scholar]
- 8.Skender ML, et al. Comparison of 2-year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions. J Am Diet Assoc. 1996;96(4):342–346. doi: 10.1016/S0002-8223(96)00096-X. [DOI] [PubMed] [Google Scholar]
- 9.Jehn ML, et al. One year follow-up of overweight and obese hypertensive adults following intensive lifestyle therapy. J Hum Nutr Diet. 2006;19(5):349–354. doi: 10.1111/j.1365-277X.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- 10.Booth HP, et al. Effectiveness of behavioural weight loss interventions delivered in a primary care setting: a systematic review and meta-analysis. Fam Pract. 2014;31(6):643–653. doi: 10.1093/fampra/cmu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraschnewski JL, et al. Long-term weight loss maintenance in the United States. Int J Obes (Lond) 2010;34(11):1644–1654. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wing RR, Phelan S. Long-term weight loss maintenance. American Journal of Clinical Nutrition. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 13.MacLean PS, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015;23(1):7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen S, et al. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 15.Imperato A, et al. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577(2):194–199. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- 16.Wedding D. Behavior and Medicine. 5th. Cambridge, MA: Hogrefe Publishing; 2010. [Google Scholar]
- 17.Lovallo W. Stress and Health: Biological and Psychological Interactions. SAGE Publications; 1997. [Google Scholar]
- 18.Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 2013;73(9):827–835. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao A, et al. Food cravings mediate the relationship between chronic stress and body mass index. J Health Psychol. 2015;20(6):721–729. doi: 10.1177/1359105315573448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martel P, Fatino M. Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol Biochem Behav. 1996;53:221–226. doi: 10.1016/0091-3057(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 21.Small DM, et al. Feeding-induced dopamine release in dorsal striatum correlates with meal plesantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 22.Engel JA, Jerlhag E. Role of appetite-regulating pepetidesin the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs. 2014;28:875–886. doi: 10.1007/s40263-014-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayanaswami V, et al. Diet-induced obesity: dopamine, dopamine transporter function, impulsivity and motivation. International Journal of Obesity. 2012:1–9. doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings DE, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 25.Christaki E, et al. Stress management can facilitate weight loss in Greek overweight and obese women: a pilot study. J Hum Nutr Diet. 2013;26(Suppl 1):132–139. doi: 10.1111/jhn.12086. [DOI] [PubMed] [Google Scholar]
- 26.Cox TL, et al. Stress management-augmented behavioral weight loss intervention for African American women: a pilot, randomized controlled trial. Health Educ Behav. 2013;40(1):78–87. doi: 10.1177/1090198112439411. [DOI] [PubMed] [Google Scholar]
- 27.Mellin L, et al. The Solution Method: 2-year trends in weight, blood pressure, exercise, depression, and functioning of adults trained in development skills. J Am Diet Assoc. 1997;97(10):1133–1138. doi: 10.1016/S0002-8223(97)00274-5. [DOI] [PubMed] [Google Scholar]
- 28.Mellin LM, et al. Adolescent obesity intervention: validation of the SHAPEDOWN program. J Am Diet Assoc. 1987;87(3):333–338. [PubMed] [Google Scholar]
- 29.Mellin L. Emotional plasticity theory: Preliminary evaluation of changes in stress-related variables in obese adults (dissertation) Prescott Valley, AZ: Northcentral University. Proquest; 2013. p. 3570245. [Google Scholar]
- 30.Webber K, et al. A stress management-based approach to weight loss produces changes in weight, blood pressure and perceived stress; 13th annual Meeting of International Society of Behavioral Nutrition and Physical Activity; San Diego, CA. 2014. [Google Scholar]
- 31.Cohen S, et al. A global measure of perceived stress. J Health Soc Behav. 1988;21:385–396. [PubMed] [Google Scholar]
- 32.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 33.Block G, et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 34.Boucher B, et al. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9:84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 35.Williamson DA, et al. Is 5% Weight Loss a Satisfactory Criterion to Define Clinically Significant Weight Loss? Obesity. 2015;23(12):2319–2320. doi: 10.1002/oby.21358. [DOI] [PubMed] [Google Scholar]
- 36.Webber K, Rose S. A Pilot Internet-Based Behavioral Weight Loss Intervention with or without Commercially Available Portion-Controlled Foods. Obesity. 2013;21(9):E354–E359. doi: 10.1002/oby.20331. [DOI] [PubMed] [Google Scholar]
- 37.Smith DE, et al. Motivational interviewing to improve adherence to a behavioral weight-control program for older obese women with NIDDM. Diabetes Care. 1997;20:52–54. doi: 10.2337/diacare.20.1.52. [DOI] [PubMed] [Google Scholar]
- 38.Palmeira AL, et al. Predicting short-term weight loss using four leading health behavior change theories. Int J Behav Nutr Phys Act. 2007;4:14. doi: 10.1186/1479-5868-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teixeira PJ, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes Relat Metab Disord. 2004;28:1124–1133. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- 40.Teixeira PJ, et al. Exercise motivation, eating, and body image variables as predictors of weight control. Med Sci Sports Exerc. 2006;38:179–188. doi: 10.1249/01.mss.0000180906.10445.8d. [DOI] [PubMed] [Google Scholar]
- 41.Womble L, et al. A randomized controlled trial of a commercial Internet weight loss program. Obes Res. 2004;12:1011–1018. doi: 10.1038/oby.2004.124. [DOI] [PubMed] [Google Scholar]
- 42.Webber KH, et al. The effect of a motivational intervention on weight loss is moderated by level of baseline controlled motivation. Int J Behav Nutr Phys Act. 2010;7:4. doi: 10.1186/1479-5868-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webber KH, et al. A Randomized Comparison of Two Motivationally Enhanced Internet Weight Loss Programs. Behav Res Ther. 2008;46:1090–1095. doi: 10.1016/j.brat.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Managing Overweight and Obesity in Adults: Systematic Evidence Review from the Obesity Expert Panel. [Accessed November 30, 2015]; http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/obesity-evidence-review.
- 45.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21(3):159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poller WC, et al. A glutamatergic projection from the lateral hypothalamus targets VTA-projecting neurons in the lateral habenula of the rat. Brain Res. 2013;1507:45–60. doi: 10.1016/j.brainres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 47.Leriche M, et al. Presence of pro-opiomelanocortin mRNA in the rat medial prefrontal cortex, nucleus accumbens and ventral tegmental area: studies by RT-PCR and in situ hybridization techniques. Neuropeptides. 2007;41(6):421–431. doi: 10.1016/j.npep.2007.08.004. [DOI] [PubMed] [Google Scholar]