Abstract
Hypothesis
Many patients with lung cancers cannot receive platinum-containing regimens due to co-morbid medical conditions. We designed the PPB regimen of paclitaxel, pemetrexed, and bevacizumab to maintain or improve outcomes while averting the unique toxicities of platinum-based chemotherapies.
Methods
We enrolled patients with untreated, advanced lung adenocarcinomas with measurable disease, and no contraindications for bevacizumab. Participants received paclitaxel 90 mg/m2, pemetrexed 500 mg/m2, and bevacizumab 10 mg/kg every 14 days for six months and continued pemetrexed and bevacizumab every 14 days until progression or unacceptable toxicity.
Results
Forty-four patients were treated: 50% women, median age 61 years, and 89% with Karnofsky performance status ≥80%. We genotyped 38 patients: KRAS 16; ALK 3; BRAF V600E 2; HER2/PIK3CA 1; EGFR exon 20 insertion 1; no driver 15. 23 patients achieved a partial response (52%, 95% CI 37 to 68%), including 7/16 with KRAS-mutant tumors. Overall survival at two years was 43% with a median of 17 months (95% CI, 10 to 29). Grade 3/4 treatment-related toxicities included elevated ALT (16%); fatigue (16%); leukopenia (9%); anemia (7%); elevated AST (7%); edema (5%) and pleural effusions (5%). Two patients died of respiratory failure without disease progression.
Conclusions
The PPB regimen of paclitaxel, pemetrexed, and bevacizumab produced a high response rate in patients with lung adenocarcinomas, regardless of mutational status. Survival and toxicities were comparable to phase II reports testing platinum-containing doublets with bevacizumab. These results justify use of the PPB regimen in fit patients where 3 drug regimens including bevacizumab are appropriate.
Keywords: Lung adenocarcinomas, Bevacizumab, Non-platinum chemotherapy, Pemetrexed, Paclitaxel
Introduction
Cisplatin- or carboplatin-containing doublets with or without bevacizumab are standard initial treatments for patients with advanced lung adenocarcinomas.1 However, many patients with lung cancers cannot receive cisplatin or carboplatin because of baseline neuropathy, hearing loss, renal insufficiency, heart failure, or other comorbid medical conditions. Since the majority of patients diagnosed with lung cancers are over age 70,2 these toxicities are more likely and more severe.3 The addition of bevacizumab to a platinum-containing doublet improves response, progression-free survival and overall survival.4, 5
Pemetrexed improves survival in patients with adenocarcinomas when administered both initially with cisplatin 6 and as maintenance.7 As the agent is well tolerated, with predominant side effects being myelosuppression and fatigue, combination therapy and prolonged administration are possible.6-8 Bevacizumab has been widely used with pemetrexed, studied in combination with carboplatin or cisplatin as initial therapy that is then continued until progression.9-12 Progression-free survival significantly improved for patients receiving pemetrexed/carboplatin/bevacizumab followed by pemetrexed/bevacizumab compared to paclitaxel/carboplatin/bevacizumab followed by bevacizumab alone.11 A second trial which randomized patients to either pemetrexed/bevacizumab or bevacizumab alone after induction with pemetrexed/cisplatin/bevacizumab, also demonstrated a significant improvement in progression-free survival in patients continuing both agents after cisplatin therapy was completed.9, 10
Numerous studies have tested regimens without cisplatin or carboplatin, utilizing combinations of gemcitabine, paclitaxel, docetaxel, and vinorelbine.13-19 In one phase I/II trial evaluating the two-drug combination of pemetrexed with paclitaxel, the response rate was 40%, with 1 year survival of 65%, and a grade 3/4 neutropenia rate of 17%.20 A meta-analysis comparing non-platinum to platinum-containing doublets 21 found no difference in overall survival and response between the two types of regimens.
To develop a non-platinum-containing regimen using two active agents plus bevacizumab, we conducted this trial of PPB, the combination of paclitaxel, pemetrexed, and bevacizumab. The regimen was designed empirically to substitute pemetrexed for carboplatin in the carboplatin-paclitaxel-bevacizumab regimen in worldwide use. Pemetrexed is highly active in lung adenocarcinomas and has bested gemcitabine when each was combined with cisplatin in a head-to-head comparison in persons with lung adenocarcinomas. Previous trials have shown that pemetrexed can be combined with either paclitaxel or bevacizumab. This regimen was designed for use in “fit” patients who are candidates to receive a chemotherapy doublet and bevacizumab. Appropriate patients must have a performance status of 70% or greater, normal kidney, liver and bone marrow function, and no contraindications specific to the drugs that are part of the PPB regimen (allergy, hemoptysis, squamous cell histology, recent stroke or heart attack, or peripheral neuropathy greater than 1+).
Materials and Methods
This single arm, open label, single-institution phase II study was reviewed and approved by the Institutional Review Board. All patients provided written informed consent.
Eligibility
All patients had pathologically confirmed lung adenocarcinomas with stage IV disease at diagnosis or metastatic recurrence after definitive local therapy. Inclusion also required Karnofsky Performance Status of ≥70%, and measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST 1.0).22 Patients had leukocytes >4000/mm3; platelets >160,000/mm3; bilirubin <1.2 mg/dL; creatinine clearance ≥40mL/min; alanine aminotransferase and/or aspartate aminotransferase ≤37Units/L (or if one elevated, ≤2.5 times the upper limit of normal); and systolic blood pressure ≤ 150mmHg. Prior neoadjuvant or adjuvant chemotherapy was permitted if it did not contain paclitaxel, pemetrexed or bevacizumab and at least 6 months had elapsed from the date of last administration.
Patients were excluded if they had received systemic therapy for advanced lung cancers or radiation therapy to greater than 25% of the bone marrow within 30 days of starting treatment. Additional exclusion criteria included squamous cell carcinomas, small cell carcinomas, hemoptysis; symptomatic brain metastases with evidence of hemorrhage; history of abdominal fistula, gastrointestinal perforation or intra-abdominal abscess; and myocardial infarction or stroke within 6 months.
Treatment
Patients were initially treated with paclitaxel 90 mg/m2 over 60 minutes on days 1, 8, and 15 in addition to pemetrexed 500 mg/m2 over 10 minutes, and bevacizumab 10 mg/kg over 20 minutes every 14 days. Seven of the first 11 patient who received paclitaxel on days 1, 8, and 15 of each cycle developed AST and ALT elevations. The protocol was amended and the day 8 visits and paclitaxel dose were omitted for all subsequent patients. The trial was amended again to permit patients with dose-limiting paclitaxel-related side effects to continue on study without paclitaxel or paclitaxel could be replaced by albumin-bound paclitaxel if a hypersensitivity reaction to paclitaxel occurred. Each cycle consisted of 28 days, and was repeated up to six times. Subsequently, pemetrexed and bevacizumab were administered every 14 days until progression or unacceptable toxicity. The planned dose intensity for the recommended PPB regimen was 250 mg/m2 per week for pemetrexed, 5 mg/kg per week for bevacizumab, and 45 mg/m2 per week for paclitaxel (60 mg/m2 per week if albumin-bound paclitaxel is used).
Dosing of paclitaxel and pemetrexed was delayed at the start of a cycle for neutrophil count <3,000/μl and/or platelets <100,000/μl and, within a cycle for neutrophil count < 1.0 × 109 cells/L and/or platelets < 75 × 109 cells/L. With the development of grade ≥ 1 to grade ≤ 3 AST and ALT or grade ≥2 pneumonitis/pulmonary infiltrates, paclitaxel was held. Pemetrexed was held if creatinine clearance ≤ 40mL/minute or for grade ≥ 2 mucositis or diarrhea. Upon resuming paclitaxel or pemetrexed, two dose reductions were permitted for paclitaxel (75mg/m2 and 60mg/m2) and pemetrexed could be reduced to 375 mg/m2.
Study Evaluations
Patients were assessed on days 1 and 15 of each 28-day cycle with a history, physical examination, toxicity assessment, complete blood count, and comprehensive metabolic panel. Patients were asked to electronically self-report 13 toxicity- and disease-related symptoms using a Symptom Tracking and Reporting (STAR) system, a validated patient version of the National Cancer Institute's (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.23-26 The symptoms/toxicities collected via the STAR platform included: fatigue, alopecia, epiphora, epistaxis, hoarseness, nausea, mucositis/stomatitis, cough, dyspnea, pain, sensory peripheral neuropathy, anorexia, Karnofsky Performance Status, and myalgias. During the visits, STAR data were provided to clinicians, who then had the option to either accept or modify the grade of these symptoms and toxicities based on their own assessments. The final CTCAE grade and attribution were assigned by the clinician.23
Tumor assessments at baseline included a computed tomography (CT) of the chest, abdomen and pelvis, as well as other relevant sites of disease, and a contrast-enhanced MRI or CT of the brain. Follow-up scans to assess response were obtained after cycles 1, 2, 4 and 6, then every three months. Responses were determined using RECIST 1.0.22 All imaging studies were reviewed by a reference radiologist (MSG).
Statistical Analysis
The primary endpoint was overall response rate (complete response (CR) plus partial response (PR)). Secondary endpoints included progression-free and overall survival and assessment of side-effects. A Simon two-stage design was used to determine the sample size. The null and desired response rates were chosen to be 15% and 35%, respectively. If at least 3 responses were noted among the 19 patients in stage 1, enrollment would be extended to 44. At the end of the trial, if 11 or more of 44 total patients were found to have a complete or partial response, the regimen would be considered effective. This design had a 90% power to detect the difference at a 5% type I error rate. Overall response rates, along with exact two-sided 95% confidence intervals (CI) were calculated. Progression-free and overall survivals were estimated from the date of first treatment using the Kaplan-Meier method.
Additional Objectives
We also assessed the feasibility of obtaining response and side-effect data from this trial in real-time by: a) collecting patient reported outcomes (toxicities and disease-related symptoms) using wireless touch screen laptop computers in the outpatient facilities via the MSK STAR system, with storage of this information in the MSK institutional database (by EMB, MS, MS, LJR), b) using an automated response assessment algorithm to determine uni dimensional (RECIST 1.0) measurements of indicator lesions. Measurements were determined and response data were automatically downloaded into the MSK institutional database (by LHS and MSG), and c) collecting clinician-generated data using a web-based portal, StudyTracker, that automatically downloaded information into the MSK institutional data base (by AL). Each of these objectives was achieved in the context of this trial and reported.23, 27-29
Results
Patients
We enrolled 44 patients between January 2009 and September 2011. Baseline characteristics are listed in Table 1. The majority of the patients were former smokers.
Table 1. Patients.
| Enrolled | 44 |
|
| |
| Women/Men | 22/22 |
|
| |
| Median Age (Range) | 61 (31 – 77) |
|
| |
| Karnofsky Performance Status | |
|
| |
| ≥90% | 19 (43%) |
| 80% | 20 (46%) |
| 70% | 5 (11%) |
|
| |
| Cigarette Smoking History | |
|
| |
| Never | 7 (16%) |
| Former | 30 (68%) |
| Current | 7 (16%) |
| Median Pack-Years Smoked (Range) | 38 (10 – 120) |
|
| |
| Brain Metastases | |
|
| |
| No | 32 (73%) |
| Yes | 12 (27%) |
| Treated | 11 (92%) |
| Untreated | 1 (8%) |
|
| |
| Oncogenic Drivers Identified | |
|
| |
| KRAS‡ | 16 (36%) |
| ALK | 3 (7%) |
| BRAF V600E | 2 (5%) |
| EGFR exon 20 Insertion | 1 (2%) |
| HER2/PIK3CA | 1 (2%) |
| None | 15 (34%) |
| Not Tested | 6 (14%) |
KRAS mutations: G12V (N = 3), G12C (N = 7), G12A (N = 2), G12D (N = 3), G13D (N =1)
Drug Delivery
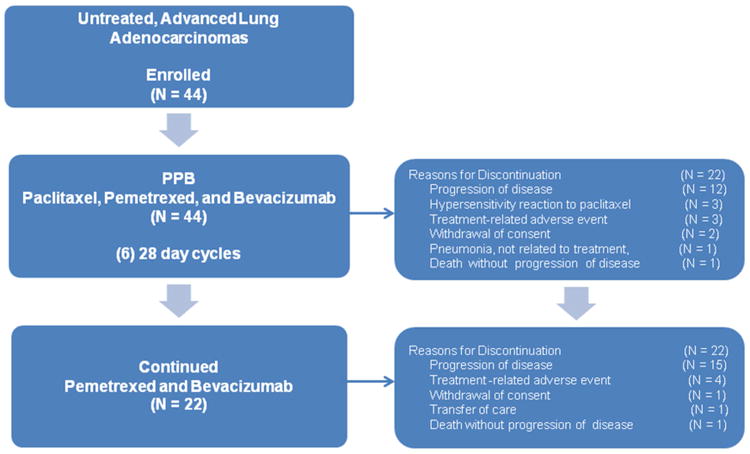
The median number of drug doses and range for each of the agents in the regimen are as follows: pemetrexed 10 doses (range 1 to 36), paclitaxel 6 doses (range 1 to 15) and bevacizumab 11 doses (range 1 to 83). The delivered dose intensity was 220 mg/m2/wk for pemetrexed, 29 mg/m2/wk for paclitaxel, and 4.3 mg/kg per week for bevacizumab. Four patients were treated with albumin-bound paclitaxel after experiencing a hypersensitivity reaction with paclitaxel. They received 1, 2, 5, and 33 doses of albumin-bound paclitaxel without further hypersensitivity symptoms. Fifty percent of the patients (N = 22) completed six cycles of therapy with paclitaxel, pemetrexed, and bevacizumab (median number of cycles in overall cohort, 6; range 1 to 6). Only 2 patients who completed 6 cycles of initial therapy did not continue with maintenance, both due to progression of disease. Twenty patients overall (45%) received at least one dose of pemetrexed and bevacizumab (median number of cycles, 5; range 1-36) after the induction phase (see Figure 1).
Figure 1. Paclitaxel and pemetrexed with bevacizumab (PPB) CONSORT diagram.
Response and Survival
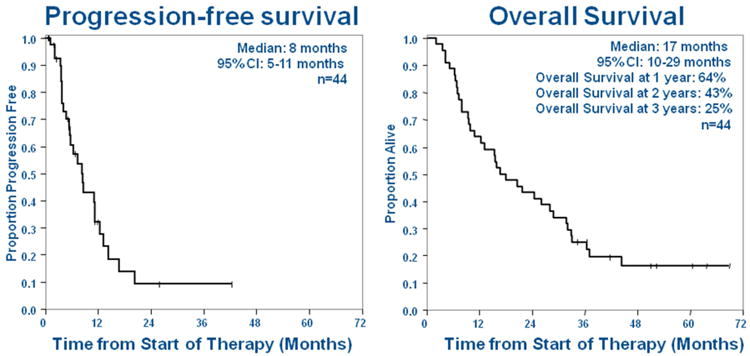
The overall response rate (complete and partial) was 52% (23/44; 95% CI 37 to 68%) (Figure 2). Four additional patients had unconfirmed partial responses. The objective response rate in KRAS-mutant lung cancers, the largest molecularly defined subgroup, was 44% (7/16; 95% CI 23% to 67%). Response could not be determined in 2 patients who did not have follow-up imaging studies, but were included in the denominator for response rate. The median progression free and overall survivals for the entire cohort (n=44) were 8 months (95% CI 5 to 11) and 17 months (95% CI 10 to 29), respectively (Figure 3). The overall survival rates were 64% at 1 year, 43% at 2 years, and 25% at 3 years.
Figure 2. Best change from baseline in measurable lesion size for 42 patients with evaluable indicator lesions: Waterfall Plot.
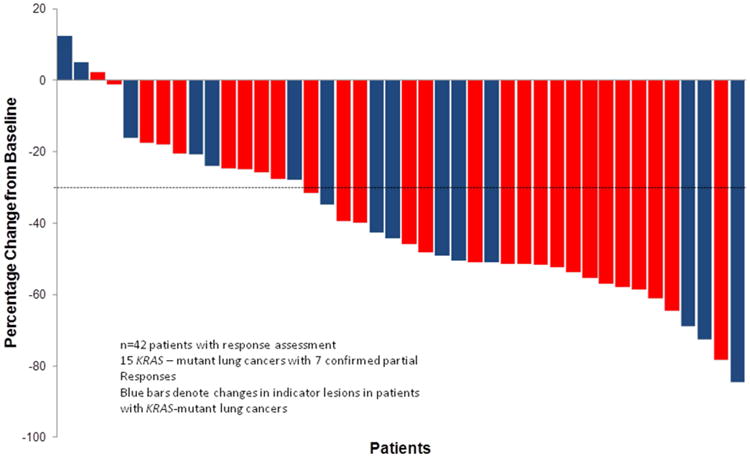
Figure 3. Overall progression-free and overall survival from the date of start of paclitaxel, pemetrexed, and bevacizumab (PPB) treatment (n=44).
Effect of PPB on Symptoms of Lung Cancers Assessed by STAR
Baseline and on-study grading of symptoms of lung cancers are presented in Table 2. The majority of patients with baseline fatigue, pain, dyspnea, cough, and anorexia experienced an improvement of at least 1 CTCAE grade while receiving PPB.
Table 2. Patient reported symptoms of lung cancers collected using the Symptom Tracking and Reporting (STAR) System26-30; baseline CTCAE grades and improvement while on treatment with paclitaxel and pemetrexed with bevacizumab (PPB) by at least one grade in patients with baseline symptoms.
| Symptoms Of Lung Cancers | Baseline Grades of Patient Reported Symptoms (%) | Percent of Patients with Baseline Symptoms with Improvement of at Least One Grade While on Study | ||
|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade ≥2 | ||
| Fatigue | 34 | 50 | 16 | 50% |
| Pain | 36 | 46 | 18 | 82% |
| Dyspnea | 48 | 36 | 16 | 77% |
| Cough | 64 | 23 | 13 | 100% |
| Anorexia | 66 | 25 | 9 | 86% |
Treatment-Related Toxicities
Table 3 summarizes treatment-related toxicities. Paclitaxel was stopped in 7 patients (16%) due to hypersensitivity reactions occurring despite standard premedication. We continued the three drug regimen in 4 of these patients by replacing paclitaxel with albumin bound paclitaxel (120 mg/m2 given over 2 hours without premedication) every 14 days with pemetrexed and bevacizumab30, 31 with no further hypersensitivity symptoms. Grade 3 elevations of ALT occurred in 16% and AST in 7%. There were no grade 4 elevations. Although anemia and leukopenia commonly occurred, only one patient developed febrile neutropenia. Grade 3 lower extremity edema and pleural effusions occurred in 2 patients. One patient developed edema, a pleural effusion and an asymptomatic pericardial effusion, which required drainage. Possible bevacizumab-related side effects included one grade 2 osteonecrosis of the jaw, one grade 2 ischemic event resulting in a partial palsy of cranial nerve IV, one grade 3 wound healing event, and one grade 3 small bowel perforation. One patient developed grade 3 pneumonitis and recovered following treatment with corticosteroids. Two patients (5%) died of respiratory failure without evidence of disease progression. Both individuals presented with dyspnea and bilateral infiltrates and died despite treatment with corticosteroids, antibiotics, and mechanical ventilation.
Table 3. Treatment-related adverse events, all grades (n=44).
| Adverse Event | Any Grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Fatigue | 42 (96%) | 10 (23%) | 25 (57%) | 5 (11%) | 2 (5%) |
| Epistaxis | 39 (89%) | 37 (84%) | 2 (5%) | -- | -- |
| Alopecia | 37 (84%) | 26 (59%) | 11 (25%) | -- | -- |
| Epiphora | 35 (80%) | 21 (48%) | 13 (30%) | 1 (2%) | |
| Hyperglycemia | 35 (80%) | 9 (21%) | 21 (48%) | 5 (11%) | -- |
| ALT Elevation | 33 (75%) | 16 (36%) | 10 (23%) | 7 (16%) | -- |
| AST Elevation | 32 (73%) | 23 (53%) | 6 (14%) | 3 (7%) | -- |
| Hoarseness | 30 (68%) | 25 (57%) | 4 (9%) | 1 (2%) | -- |
| Mucositis/Stomatitis | 28 (64%) | 20 (46%) | 7 (16%) | 1 (2%) | -- |
| Sensory peripheral neuropathy | 28 (64%) | 23 (52%) | 5 (11%) | -- | -- |
| Nausea | 25 (57%) | 18 (41%) | 6 (14%) | 1 (2%) | -- |
| Anorexia | 24 (55%) | 14 (32%) | 9 (21%) | 1 (2%) | -- |
| Anemia | 23 (52%) | 14 (32%) | 6 (14%) | 3 (7%) | -- |
| Leukopenia | 21 (48%) | 6 (14%) | 11 (25%) | 4 (9%) | -- |
| Myalgias | 20 (45%) | 10 (23%) | 8 (18%) | 2 (5%) | -- |
| Edema | 15 (34%) | 9 (21%) | 4 (9%) | 2 (5%) | -- |
| Alkaline phosphatase | 14 (32%) | 14 (32%) | -- | -- | -- |
| Pain | 12 (27%) | 6 (14%) | 4 (9%) | 2 (5%) | -- |
| Hypoalbuminemia | 11 (25%) | 11 (25%) | -- | -- | -- |
| Dyspnea | 10 (22%) | 5 (11%) | 5 (11%) | -- | -- |
| Rhinorrhea | 9 (20%) | 7 (16%) | 2 (5%) | -- | -- |
| Hypertension | 8 (18%) | -- | 7 (16%) | 1 (2%) | -- |
| Neutropenia | 8 (18%) | -- | 6 (14%) | 2 (5%) | -- |
| Creatinine Elevation | 7 (16%) | 7 (16%) | -- | -- | -- |
| Paclitaxel Hypersensitivity | 7 (16%) | 1 (2%) | 4 (9%) | 2 (5%) | -- |
| Rash | 6 (14%) | 5 (11%) | 1 (2%) | -- | -- |
| Cough | 4 (9%) | 3 (7%) | -- | 1 (2%) | -- |
| Pleural effusion | 4 (9%) | -- | 2 (5%) | 2 (5%) | -- |
Molecular Characteristics
Tumor samples for molecular testing were available from 38 patients (86%). (Table 1) We genotyped 38 patients: KRAS 16; ALK 3; BRAF V600E 2; HER2/PIK3CA 1; EGFR exon 20 insertion 1; no driver 15. We documented a partial response in 9 of the 15 individuals with tumors with no oncogenic driver identified. We confirmed partial responses in all 3 patients with ALK-positive lung cancers, the 2 with BRAF-driven tumors and the one individual with an EGFR exon 20 insertion. Following study-treatment discontinuation, 2 of the 3 patients whose tumors harbored ALK-rearrangements were treated with crizotinib and both patients with BRAF V600E-mutant adenocarcinomas received vemurafenib.
Additional Therapies
Overall, 84% of patients (N = 32) received subsequent therapy, with a median of two (range, 1 to 4) additional regimens.
Discussion
This phase II study explored PPB, a non-platinum containing regimen of paclitaxel and pemetrexed with bevacizumab in patients with advanced lung cancers. We found a 52% overall response rate, which met the primary end point of this study and exceeded the 35% response rate we hypothesized. Responses to this regimen were seen across adenocarcinoma genotypes, including tumors with no oncogenic drivers identified and those with KRAS mutations. For patients with EGFR- and ALK-positive lung cancers, we recommend that matched targeted therapies be given before PPB. The huge experience favoring targeted therapies over cisplatin or carboplatin containing chemotherapies in patients with EGFR and ALK-positive lung cancers applies to PPB as well. Toxicities were comparable to the phase II experiences with platinum-containing doublets with bevacizumab. 12, 32, 33
Our study differed from the previous evaluations of non-platinum doublet regimens with third generation chemotherapy agents13-19, 21 as it included bevacizumab, and allowed for pemetrexed and bevacizumab to continue until progression (maintenance therapy), which has become a standard of care in patients with response to initial therapy.1 The design and outcomes of this trial were similar to the 50 patient phase II study of pemetrexed and carboplatin with bevacizumab followed by maintenance pemetrexed and bevacizumab, reported by Patel.12 The overall response rate was 55% and median progression free and overall survivals were 8 and 14 months respectively.12 Three patients died on that study, one without disease progression. In a 34 patient phase II trial of paclitaxel, carboplatin, and bevacizumab (15 mg/m2) without maintenance, the overall response rate was 32% and median time to progression and overall survival were 7 and 18 months respectively.32 Four patients died on this study, all without disease progression. In a recent 67 patient phase II trial of paclitaxel, carboplatin, and bevacizumab with maintenance, the overall response rate was 63% and median progression free and overall survivals were 7 and 16 months respectively. 33 One patient died on study without disease progression. Keeping in mind the limitations of cross trial analyses, the response rates, survival, and number of on study deaths without disease progression seen in our phase II study are comparable to the phase II results with platinum-containing regimens reported by Patel, Johnson, and Besse.12, 32, 33
Fifty percent of patients completed the planned six cycles of pemetrexed, paclitaxel, and bevacizumab and, at progression, 84% of patients went on to receive additional chemotherapeutic agents. There were few grade 3/4 hematologic toxicities and only 1 episode of febrile neutropenia. Paclitaxel was stopped in 7 patients due to hypersensitivity reactions occurring despite standard premedication. We continued the three drug regimen in four by replacing paclitaxel with albumin bound paclitaxel (120 mg/m2 given over 2 hours) every 14 days with the pemetrexed and bevacizumab30, 31 with no further hypersensitivity symptoms. Based on our experience, we would substitute albumin-bound paclitaxel for paclitaxel in this regimen if albumin-bound paclitaxel is available. Initially, we noted elevations of AST and ALT with a day 8 dose of paclitaxel. After enrolling 11 patients, we amended the protocol to eliminate the day 8 paclitaxel dose. After this modification, patients were then able to receive the expected doses of paclitaxel given every 2 weeks with the other agents. Other studies evaluating frequent administrations of paclitaxel have not reported high rates of AST and ALT abnormalities.34, 35 This was also not noted in a prior trial of the combination of paclitaxel and pemetrexed.20 Bevacizumab is not known to cause or accentuate AST and ALT abnormalities alone or in combination.
Grade 3 edema and pleural effusions occurred in 5% of patients, a finding which had not been reported in other trials evaluating the combination of pemetrexed and bevacizumab or pemetrexed alone.8, 9, 12, 36, 37 One patient also developed a pericardial effusion, attributed to pemetrexed. All the effusions occurring without progression at other disease sites were transudates containing no malignant cells. These events have been described. The mechanism is not known.38, 39
Pulmonary toxicity has been described in patients receiving paclitaxel, pemetrexed, and the combination.40-44 While hypersensitivity has been implicated in paclitaxel-induced lung toxicity40, 43 no mechanism has been proposed for pemetrexed. Diffuse alveolar damage was detected in one report.44 Patients on this or any regimen with pemetrexed and paclitaxel should be monitored for clinical and radiographic signs of pulmonary toxicity and corticosteroids should be started promptly whenever treatment-related pulmonary toxicity is a consideration.
The high rate of response seen with the PPB regimen, that increases the dose intensity of pemetrexed and requires visits every two weeks instead of every three, comes at the expense of increased cost. Physicians and patients in each case together need to decide whether the potential benefits in response and symptom improvement with PPB are sufficient to offset the additional financial and time expenditures associated with this regimen. Cost also varies with each country and health care system, necessitating decision making on a case-by-case basis.
PPB, a three drug combination of pemetrexed, paclitaxel, and bevacizumab produced a 52% overall response rate and a one year survival of 64% as an initial therapy in patients with lung adenocarcinomas. This non-platinum-containing chemotherapy regimen including bevacizumab met the study specified definition of effectiveness and improved symptoms of lung cancers. Toxicities were comparable to phase II experiences with platinum doublet regimens plus bevacizumab. This regimen is worthy of further evaluation and use in patients fit to receive three drug regimens including bevacizumab.
Supplementary Material
Acknowledgments
Support: This research was funded in part through the National Institute of Health(NIH)/National Cancer Institute (NCI) Cancer Center Support Grant P30 CA008748 and the Joy Ruane Fund.
Catherine M. Pietanza, MD reports personal fees from Genentech, personal fees from CelGene Corp, personal fees from AbbVie, personal fees from Clovis Oncology, grants and personal fees from Novartis, grants and personal fees from Bristol Myers Squibb, grants from Stemcentrx, Inc, grants from OncoMed Pharmaceuticals, Inc, outside the submitted work;.
Matthew D. Hellmann, MD reports grants and personal fees from BMS, personal fees from Genentech, personal fees from AstraZeneca, personal fees from Inovio Pharmaceuticals, personal fees from Third Rock Ventures, outside the submitted work;.
John J. Fiore, MD has nothing to disclose.
Stephanie Smith-Marrone, MD has nothing to disclose.
Ethan M. Basch, MD has nothing to disclose.
Lawrence H. Schwartz, MD reports other from Novartis, other from Celegene, other from Bioclinica, other from Icon, outside the submitted work; In addition, Dr. Schwartz has a patent Tumor segementation issued.
Michelle S. Ginsberg, MD has nothing to disclose.
Marwan Shouery, MS has nothing to disclose.
Samantha K. Newman, MD has nothing to disclose.
Mary Shaw, BA has nothing to disclose.
Lauren J. Rogak, MA has nothing to disclose.
Alex Lash, MD has nothing to disclose.
Patrick Hilden, MS has nothing to disclose.
Mark G. Kris, MD reports grants from National Institute of Health (NIH)/National Cancer Institute (NCI) Cancer Center Support Grant P30 CA 008748, grants from Memorial Sloan Kettering Cancer Center Joy Ruane Fund, during the conduct of the study; personal fees from AstraZeneca, personal fees from Genentech/Roche, personal fees from ARIAD, personal fees from Daiich Sankyo, personal fees from Array, personal fees from Threshold Pharmaceuticals, outside the submitted work;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NCCN clinical practice guidelines in oncology: National Comprehensive Cancer Network. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 2.Bunn PA, Jr, Lilenbaum R. Chemotherapy for elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2003;95(5):341–3. doi: 10.1093/jnci/95.5.341. [DOI] [PubMed] [Google Scholar]
- 3.Belani CP, Fossella F. Elderly subgroup analysis of a randomized phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for first-line treatment of advanced nonsmall cell lung carcinoma (TAX 326) Cancer. 2005;104(12):2766–74. doi: 10.1002/cncr.21495. [DOI] [PubMed] [Google Scholar]
- 4.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33(19):2197–204. doi: 10.1200/JCO.2014.59.4424. [DOI] [PubMed] [Google Scholar]
- 6.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 7.Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895–902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 8.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 9.Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim JH, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089) J Clin Oncol. 2013;31(24):3004–11. doi: 10.1200/JCO.2012.42.3749. [DOI] [PubMed] [Google Scholar]
- 10.Barlesi F, Scherpereel A, Gorbunova V, Gervais R, Vikstrom A, Chouaid C, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol. 2014;25(5):1044–52. doi: 10.1093/annonc/mdu098. [DOI] [PubMed] [Google Scholar]
- 11.Patel JD, Socinski MA, Garon EB, Reynolds CH, Spigel DR, Olsen MR, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(34):4349–57. doi: 10.1200/JCO.2012.47.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel JD, Hensing TA, Rademaker A, Hart EM, Blum MG, Milton DT, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol. 2009;27(20):3284–9. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- 13.Gridelli C, Gallo C, Shepherd FA, Illiano A, Piantedosi F, Robbiati SF, et al. Gemcitabine plus vinorelbine compared with cisplatin plus vinorelbine or cisplatin plus gemcitabine for advanced non-small-cell lung cancer: a phase III trial of the Italian GEMVIN Investigators and the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21(16):3025–34. doi: 10.1200/JCO.2003.06.099. [DOI] [PubMed] [Google Scholar]
- 14.Smit EF, van Meerbeeck JP, Lianes P, Debruyne C, Legrand C, Schramel F, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group--EORTC 08975. J Clin Oncol. 2003;21(21):3909–17. doi: 10.1200/JCO.2003.03.195. [DOI] [PubMed] [Google Scholar]
- 15.Georgoulias V, Ardavanis A, Tsiafaki X, Agelidou A, Mixalopoulou P, Anagnostopoulou O, et al. Vinorelbine plus cisplatin versus docetaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III randomized trial. J Clin Oncol. 2005;23(13):2937–45. doi: 10.1200/JCO.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Pujol JL, Breton JL, Gervais R, Rebattu P, Depierre A, Morere JF, et al. Gemcitabine-docetaxel versus cisplatin-vinorelbine in advanced or metastatic non-small-cell lung cancer: a phase III study addressing the case for cisplatin. Ann Oncol. 2005;16(4):602–10. doi: 10.1093/annonc/mdi126. [DOI] [PubMed] [Google Scholar]
- 17.Treat JA, Gonin R, Socinski MA, Edelman MJ, Catalano RB, Marinucci DM, et al. A randomized, phase III multicenter trial of gemcitabine in combination with carboplatin or paclitaxel versus paclitaxel plus carboplatin in patients with advanced or metastatic non-small-cell lung cancer. Ann Oncol. 2010;21(3):540–7. doi: 10.1093/annonc/mdp352. [DOI] [PubMed] [Google Scholar]
- 18.Kosmidis P, Mylonakis N, Nicolaides C, Kalophonos C, Samantas E, Boukovinas J, et al. Paclitaxel plus carboplatin versus gemcitabine plus paclitaxel in advanced non-small-cell lung cancer: a phase III randomized trial. J Clin Oncol. 2002;20(17):3578–85. doi: 10.1200/JCO.2002.12.112. [DOI] [PubMed] [Google Scholar]
- 19.Stathopoulos GP, Veslemes M, Georgatou N, Antoniou D, Giamboudakis P, Katis K, et al. Frontline paclitaxel-vinorelbine versus paclitaxel-carboplatin in patients with advanced non-small-cell lung cancer: a randomized phase III trial. Ann Oncol. 2004;15(7):1048–55. doi: 10.1093/annonc/mdh260. [DOI] [PubMed] [Google Scholar]
- 20.Stathopoulos GP, Dimitroulis J, Toubis M, Katis C, Karaindros D, Stathopoulos J, et al. Pemetrexed combined with paclitaxel in patients with advanced or metastatic non-small-cell lung cancer: a phase I-II trial. Lung Cancer. 2007;57(1):66–71. doi: 10.1016/j.lungcan.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Liang X, Zhou X, Huang R, Chu Z, Zhan Q. Non-platinum doublets were as effective as platinum-based doublets for chemotherapy-naive advanced non-small-cell lung cancer in the era of third-generation agents. J Cancer Res Clin Oncol. 2013;139(1):25–38. doi: 10.1007/s00432-012-1294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Pietanza MC, Basch EM, Lash A, Schwartz LH, Ginsberg MS, Zhao B, et al. Harnessing technology to improve clinical trials: study of real-time informatics to collect data, toxicities, image response assessments, and patient-reported outcomes in a phase II clinical trial. J Clin Oncol. 2013;31(16):2004–9. doi: 10.1200/JCO.2012.45.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basch E, Artz D, Dulko D, Scher K, Sabbatini P, Hensley M, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol. 2005;23(15):3552–61. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- 25.Basch E, Artz D, Iasonos A, Speakman J, Shannon K, Lin K, et al. Evaluation of an online platform for cancer patient self-reporting of chemotherapy toxicities. J Am Med Inform Assoc. 2007;14(3):264–8. doi: 10.1197/jamia.M2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basch E, Iasonos A, Barz A, Culkin A, Kris MG, Artz D, et al. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J Clin Oncol. 2007;25(34):5374–80. doi: 10.1200/JCO.2007.11.2243. [DOI] [PubMed] [Google Scholar]
- 27.Basch E, Deal A, Kris M, Scher H, Hudis C, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.63.0830. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basch E, Wood W, Schrag D, Sima C, Shaw M, Rogak L, et al. Feasibility and clinical impact of sharing patient-reported symptom toxicities and performance status with clinical investigators during a phase 2 cancer clinical treatment trial. Clin Trials. 2015 Nov 4; doi: 10.1177/1740774515615540. 2015. E pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes S, Pietanza M, O'Driscoll D, Zheng J, Moskowitz C, Kris M, et al. Comparison of CT volumetric measurements with RESIST response in patients with lung cancer. Eur J Radiol. 2015 doi: 10.1016/j.ejrad.2015.12.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizvi NA, Riely GJ, Azzoli CG, Miller VA, Ng KK, Fiore J, et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol. 2008;26(4):639–43. doi: 10.1200/JCO.2007.10.8605. [DOI] [PubMed] [Google Scholar]
- 31.Paik PK, James LP, Riely GJ, Azzoli CG, Miller VA, Ng KK, et al. A phase 2 study of weekly albumin-bound paclitaxel (Abraxane(R)) given as a two-hour infusion. Cancer Chemother Pharmacol. 2011;68(5):1331–7. doi: 10.1007/s00280-011-1621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Besse B, Le Moulec S, Mazieres J, Senellart H, Barlesi F, Chouaid C, et al. Bevacizumab in Patients with Nonsquamous Non-Small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized, Phase II Study. Clin Cancer Res. 2015;21(8):1896–903. doi: 10.1158/1078-0432.CCR-14-2082. [DOI] [PubMed] [Google Scholar]
- 34.Akerley W, Herndon JE, Egorin MJ, Lyss AP, Kindler HL, Savarese DM, et al. Weekly, high-dose paclitaxel in advanced lung carcinoma: a phase II study with pharmacokinetics by the Cancer and Leukemia Group B. Cancer. 2003;97(10):2480–6. doi: 10.1002/cncr.11375. [DOI] [PubMed] [Google Scholar]
- 35.Belani CP, Ramalingam S, Perry MC, LaRocca RV, Rinaldi D, Gable PS, et al. Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(3):468–73. doi: 10.1200/JCO.2007.13.1912. [DOI] [PubMed] [Google Scholar]
- 36.Zinner RG, Obasaju CK, Spigel DR, Weaver RW, Beck JT, Waterhouse DM, et al. PRONOUNCE: Randomized, Open-Label, Phase III Study of First-Line Pemetrexed + Carboplatin Followed by Maintenance Pemetrexed versus Paclitaxel + Carboplatin + Bevacizumab Followed by Maintenance Bevacizumab in Patients ith Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Thorac Oncol. 2014 doi: 10.1097/JTO.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gridelli C, de Marinis F, Thomas M, Prabhash K, El Kouri C, Blackhall F, et al. Final efficacy and safety results of pemetrexed continuation maintenance therapy in the elderly from the PARAMOUNT phase III study. J Thorac Oncol. 2014;9(7):991–7. doi: 10.1097/JTO.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 38.D'Angelo SP, Kris MG, Pietanza MC, Rizvi NA, Azzoli CG. A case series of dose-limiting peripheral edema observed in patients treated with pemetrexed. J Thorac Oncol. 2011;6(3):624–6. doi: 10.1097/JTO.0b013e318207f788. [DOI] [PubMed] [Google Scholar]
- 39.Bastos BR, Hatoum GF, Walker GR, Tolba K, Takita C, Gomez J, et al. Efficacy and toxicity of chemoradiotherapy with carboplatin and irinotecan followed by consolidation docetaxel for unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2010;5(4):533–9. doi: 10.1097/JTO.0b013e3181ce3e00. [DOI] [PubMed] [Google Scholar]
- 40.Nagata S, Ueda N, Yoshida Y, Matsuda H, Maehara Y. Severe interstitial pneumonitis associated with the administration of taxanes. J Infect Chemother. 2010;16(5):340–4. doi: 10.1007/s10156-010-0058-4. [DOI] [PubMed] [Google Scholar]
- 41.Dhakal B, Singh V, Shrestha A, Rao A, Choong N. Pemetrexed induced pneumonitis. Clin Pract. 2011;1(4):e106. doi: 10.4081/cp.2011.e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breuer S, Nechushtan H. Pemetrexed-induced lung toxicity: a case report. Clin Oncol (R Coll Radiol) 2012;24(1):76–7. doi: 10.1016/j.clon.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg HL, Vannice SB. Pneumonitis related to treatment with paclitaxel. J Clin Oncol. 1995;13(2):534–5. doi: 10.1200/JCO.1995.13.2.534. [DOI] [PubMed] [Google Scholar]
- 44.Kim HO, Lee SY, Shim JJ, Kang KH, Shin BK. A case of pemetrexed-induced acute lung injury in non-small cell lung cancer. J Thorac Oncol. 2010;5(3):401–2. doi: 10.1097/JTO.0b013e3181c5b198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


