Abstract
Objective
Extended-duration thromboprophylaxis for 4 weeks after discharge has been demonstrated to reduce venous thromboembolic events (VTE) in cancer patients undergoing abdominopelvic surgery and is recommended in national guidelines. We examined the utilization and effectiveness of extended-duration low molecular weight heparin prophylaxis in high-risk cancer patients.
Methods
We analyzed patients with colon, ovarian, and uterine cancer who underwent surgery from 2009–2013 and who were recorded in the MarketScan database. Multivariable models and propensity score analysis with inverse probability of treatment weight were developed to examine uptake and predictors of use of post-discharge low molecular weight heparin (LMWH), as well as associated adverse events (transfusion, and hemorrhage).
Results
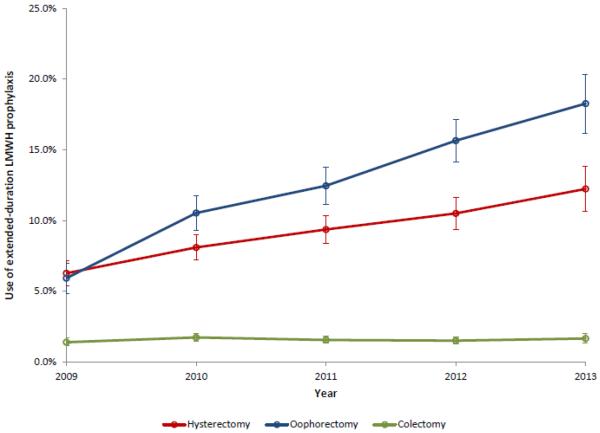
A total of 63,280 patients were identified. Use of extended-duration prophylaxis increased from 2009 to 2013 from 1.4% to 1.7% (P=0.67) for colectomy, 5.9% to 18.3% for ovarian cancer surgery (P<0.001), and 6.3% to 12.2% (P<0.001) for hysterectomy for endometrial cancer. There was no association between use of extended-duration prophylaxis and reductions in VTE for any of the procedures: colectomy (2.4% with extended-duration prophylaxis vs. 2.9% without prophylaxis, OR=0.84; 95% CI, 0.54-1.31), ovarian cancer-directed surgery (3.7% vs. 3.6%, OR=1.01; 95% CI, 0.76-1.33), hysterectomy (2.1% vs. 2.1%; OR=0.96; 95% CI, 0.67-1.38). Extended-duration prophylaxis was associated with an increased risk of adverse postoperative events: 2.20 (95% CI, 1.51-3.19) after colectomy, 1.24 (95% CI, 0.92-1.68) following ovarian cancer-directed surgery and 0.99 (95% CI, 0.66-1.48) for hysterectomy for endometrial cancer.
Conclusion
Use of extended-duration thromboprophylaxis is low among high-risk cancer patients undergoing surgery.
Introduction
Venous thromboembolism is a major cause of morbidity and mortality for surgical patients.1 Among surgical patients not receiving prophylaxis, 15–20% will develop an asymptomatic deep venous thrombosis (DVT) while up to 0.9% will develop a fatal pulmonary embolism (PE).1–4 Certain sub-groups of patients, such as those undergoing orthopedic or oncologic surgery, are at particularly high-risk.1,5,6 The risk of venous thromboembolic events (VTE) among patients undergoing cancer-directed surgery is two to three-fold higher than in non-cancer patients.6 Given the high-risk of VTE, strategies using pharmacologic prophylaxis have been tested in numerous prospective trials and recommended in national guidelines for more than two decades.1,5,6
Even after hospital discharge, the risk of venous thromboembolic disease remains elevated for several weeks to months following surgery.7–10 A study of nearly one million women from the United Kingdom found that, compared to patients who had not undergone surgery, the relative risk for VTE 7–12 weeks postoperatively was 19.6, while patients 4–6 months after surgery were 9-fold more likely to develop a VTE.7 To reduce the risk of VTE during the postoperative, post-discharge period, several randomized trials have investigated extended-duration VTE prophylaxis with low molecular weight heparin (LMWH) in high-risk patients who underwent abdominal or pelvic surgery.11–13 These studies have demonstrated that extended-duration prophylaxis, typically for 4 weeks, reduces the risk of DVT by at least 50%.8,14–17 Importantly, extended prophylaxis was not associated with an increased risk of bleeding complications in these studies.8,14–17
Based on the efficacy of extended prophylaxis, national consensus guidelines have recommended extended-duration prophylaxis with LMWH for 4 weeks after hospital discharge in cancer patients who undergo abdominal or pelvic surgery.6,8,18,19 Despite these recommendations, little is known about the patterns of extended-duration prophylaxis use in actual clinical practice. We examined the utilization and effectiveness of extended-duration low molecular weight heparin VTE prophylaxis in high-risk cancer patients who underwent abdominal and pelvic surgery.
Methods
Data Source
The Truven Health MarketScan database was used for analysis.20 The database contains a sample of patients enrolled in commercial health plans sponsored by approximately 100 payers. The database captures claims on over 50 million covered lives, includes all inpatient and outpatient medical claims and prescription drug data.20 The database collects detailed information on monthly enrollment and allows longitudinal data capture on patients. Data was de-identified and deemed exempt by the Columbia University Institutional Review Board.
Patients and Procedures
We selected patients with high-risk abdominopelvic malignancies, including gynecologic cancers and colorectal tumors, in which extended-duration VTE prophylaxis has previously been evaluated. Specifically, our cohort consisted of patients with colorectal (ICD9 153.x, 154.x), ovarian (ICD9 183.x), or uterine (ICD9 182.x) cancer who underwent colectomy, oophorectomy and/or hysterectomy or cytoreduction, or hysterectomy, respectively, from 2009 through 2013 (Supplemental Table 1). Patients who underwent colectomy were further classified as having undergone either a minimally invasive (laparoscopic or robotic-assisted) procedure or an open colectomy. Women in the ovarian cancer cohort were stratified based on whether concurrent cytoreduction was performed. Hysterectomy for uterine cancer was classified as open, vaginal, or minimally invasive (laparoscopic or robotic-assisted).
Patients with incomplete coverage for 3 months prior or 3 months after the primary procedure and those without pharmacy benefits were excluded. Similarly, patients with a preoperative diagnosis of either a deep vein thrombosis or pulmonary embolism and those receiving anticoagulation (oral or injectable) prior to the admission for the surgical procedure were excluded from the analysis. We recorded the diagnosis of venous thromboembolism (both DVT and PE) both during and after discharge from the hospital.
Outcomes and Covariates
The primary outcome was the provision of extended-duration thromboprophylaxis. We chose a permissive definition of extended-duration prophylaxis, defined as prescription and receipt of low molecular weight heparin (enoxaparin, dalteparin, tinzaparin, parnaparin, certoparin, reviparin, nadroparin, bemiparin) within one week of discharge from the admission for the surgical procedure of interest. Patients who developed a venous thromboembolic event during the index hospitalization were excluded from this portion of the analysis as they would have received therapeutic anticoagulation. Similarly, those patients who received a therapeutic dose of LMWH or who had a diagnosis of a DVT or PE after discharge but prior to receipt of LMWH were categorized as not having received extended-duration prophylaxis. Patients who received prophylaxis but subsequently developed a VTE were retained in the analysis.
Bleeding complications including transfusion and hemorrhage were examined. These events were measured both during the index hospitalization as well as within 3 months after discharge. A composite measure for adverse events was developed and included the occurrence of either of these events. Patients who had a code for a complication both during the hospitalization and after discharge were only coded as having had a complication during hospitalization since it is impossible to determine if the postoperative code represented a separate occurrence of the event.
Clinical and demographic characteristics analyzed included age (≤34, 35–44, 45–54, 55–64 and ≥65 years), gender (male or female), year of surgery (2009–2013), and region (northeast, north central, south, west, unknown). Comorbidity was measured using the Charlson comorbidity score and classified as 0, 1, or ≥2.21 The Charlson index is a weighted measures of comorbid medical conditions that has been validated and used extensively in health services research.21
Statistical Analysis
Utilization of extended-duration prophylaxis as well as rates of VTE, both in-hospital and after discharge, are reported descriptively. Frequency distributions between categorical variables were compared using χ2 tests. Multivariable logistic regression models were developed to determine the association between clinical and demographic characteristics and receipt of extended-duration prophylaxis. Results are reported as odds ratios with 95% confidence intervals.
To account for imbalances in the cohort, a propensity score (PS) analysis was utilized to analyze the association between receipt of extended-duration prophylaxis and the occurrence of VTE and the adverse events. A propensity score is the predicted probability of receipt of a treatment, extended-duration prophylaxis in this analysis.22–24 To estimate the PS, a logistic regression model was fit to determine predictors of use of extended-duration prophylaxis. The model included age, sex, year, region, comorbidity, hysterectomy, oophorectomy, colectomy, and hospital complications (any instance of transfusion, or hemorrhage) and all possible two-way interaction terms. The inverse probability of treatment weight (IPTW) was then calculated from the PS. To reduce the bias introduced by influential weights, the variance of the IPTW was decreased by stabilization and trimming at cutoffs of 0.1 and 10.25,26
To verify the robustness of our findings, a number of sensitivity analyses were performed. We altered the definition of extended-duration prophylaxis and defined prophylaxis as use of LMWH within 6 weeks after surgery. Further, sub-group analyses after exclusion of patients who underwent minimally invasive surgery (colectomy or hysterectomy) or ovarian cancer surgery that did not require cytoreduction were performed. All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). All statistical tests were two-sided. A P-value of <0.05 was considered statistically significant.
Results
A total of 63,280 procedures patients were identified, including 40,068 who underwent colectomy, 10,260 who underwent ovarian cancer-directed surgery, and 14,518 who underwent hysterectomy for endometrial cancer. The rate of extended prophylaxis increased for each procedure from 2009 to 2013: 1.4% to 1.7% (P=0.67) for colectomy, 5.9% to 18.3% for ovarian cancer surgery (P<0.001), and 6.3% to 12.2% (P<0.001) for hysterectomy for endometrial cancer (Figure 1). The clinical characteristics of the cohort are displayed in Table 1.
Figure 1.
Use of extended-duration low molecular weight heparin prophylaxis stratified by type of primary surgery performed.
Table 1.
Clinical and demographic characteristics of the cohort stratified by extended-duration low molecular weight heparin.
| Colectomy1,4 | Oophorectomy2,4 | Hysterectomy3,4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No LMWH | Extended-Duration LMWH | P-value | No LMWH | Extended-Duration LMWH | P-value | No LMWH | Extended-Duration LMWH | P-value | |||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | ||||
| 39,437 | (98.4) | 631 | (1.6) | 9,001 | (87.7) | 1,259 | (12.3) | 13,213 | (91.0) | 1,305 | (9.0) | ||||
| Age | <0.001 | 0.002 | 0.36 | ||||||||||||
| ≤34 | 710 | (1.8) | 22 | (3.5) | 532 | (5.9) | 47 | (3.7) | 198 | (1.5) | 25 | (1.9) | |||
| 35–44 | 2,643 | (6.7) | 50 | (7.9) | 985 | (10.9) | 114 | (9.1) | 892 | (6.8) | 79 | (6.1) | |||
| 45–54 | 9,063 | (23.0) | 199 | (31.5) | 2,446 | (27.2) | 352 | (28.0) | 2,958 | (22.4) | 290 | (22.2) | |||
| 55–64 | 13,037 | (33.1) | 223 | (35.3) | 3,250 | (36.1) | 500 | (39.7) | 6,092 | (46.1) | 627 | (48.1) | |||
| ≥65 | 13,984 | (35.5) | 137 | (21.7) | 1,788 | (19.9) | 246 | (19.5) | 3,073 | (23.3) | 284 | (21.8) | |||
| Sex | 0.04 | — | — | ||||||||||||
| Female | 19,031 | (48.3) | 330 | (52.3) | — | — | — | — | — | — | — | — | |||
| Male | 20,406 | (51.7) | 301 | (47.7) | — | — | — | — | — | — | — | — | |||
| Year | 0.52 | <0.001 | <0.001 | ||||||||||||
| 2009 | 7,024 | (17.8) | 100 | (15.9) | 1,731 | (19.2) | 109 | (8.7) | 2,618 | (19.8) | 175 | (13.4) | |||
| 2010 | 8,968 | (22.7) | 158 | (25.0) | 2,166 | (24.1) | 255 | (20.3) | 3,353 | (25.4) | 295 | (22.6) | |||
| 2011 | 9,573 | (24.3) | 152 | (24.1) | 2,144 | (23.8) | 305 | (24.2) | 3,236 | (24.5) | 334 | (25.6) | |||
| 2012 | 8,533 | (21.6) | 131 | (20.8) | 1,872 | (20.8) | 347 | (27.6) | 2,600 | (19.7) | 305 | (23.4) | |||
| 2013 | 5,339 | (13.5) | 90 | (14.3) | 1,088 | (12.1) | 243 | (19.3) | 1,406 | (10.6) | 196 | (15.0) | |||
| Region | <0.001 | <0.001 | <0.001 | ||||||||||||
| Northeast | 7,444 | (18.9) | 92 | (14.6) | 2,026 | (22.5) | 220 | (17.5) | 3,313 | (25.1) | 275 | (21.1) | |||
| North central | 10,441 | (26.5) | 206 | (32.7) | 2,063 | (22.9) | 447 | (35.5) | 3,678 | (27.8) | 560 | (42.9) | |||
| South | 14,331 | (36.3) | 233 | (36.9) | 2,928 | (32.5) | 331 | (26.3) | 3,444 | (26.1) | 251 | (19.2) | |||
| West | 6,738 | (17.1) | 88 | (14.0) | 1,870 | (20.8) | 240 | (19.1) | 2,666 | (20.2) | 197 | (15.1) | |||
| Unknown | 483 | (1.2) | 12 | (1.9) | 114 | (1.3) | 21 | (1.7) | 112 | (0.9) | 22 | (1.7) | |||
| Comorbidity 5 | <0.001 | 0.35 | 0.56 | ||||||||||||
| 0 | 26,787 | (67.9) | 476 | (75.4) | 7,219 | (80.2) | 1,030 | (81.8) | 9,340 | (70.7) | 935 | (71.7) | |||
| 1 | 8,469 | (21.5) | 123 | (19.5) | 1,341 | (14.9) | 176 | (14.0) | 2,920 | (22.1) | 286 | (21.9) | |||
| ≥2 | 4,181 | (10.6) | 32 | (5.1) | 441 | (4.9) | 53 | (4.2) | 953 | (7.2) | 84 | (6.4) | |||
| Colectomy type | 0.01 | — | — | ||||||||||||
| Open | 28,726 | (72.8) | 489 | (77.5) | — | — | — | — | — | — | — | — | |||
| Laparoscopic | 10,711 | (27.2) | 142 | (22.5) | — | — | — | — | — | — | — | — | |||
| Ooophorectomy type | — | <0.001 | — | ||||||||||||
| Cytoreduction | — | — | — | — | 5,706 | (63.4) | 998 | (79.3) | — | — | — | — | |||
| No cytoreduction | — | — | — | — | 3,295 | (36.6) | 261 | (20.7) | — | — | — | — | |||
| Hysterectomy type | — | — | <0.001 | ||||||||||||
| Abdominal | — | — | — | — | — | — | — | — | 7,630 | (57.8) | 886 | (67.9) | |||
| Laparoscopic | — | — | — | — | — | — | — | — | 3,508 | (26.6) | 262 | (20.1) | |||
| Robotic | — | — | — | — | — | — | — | — | 1,847 | (14.0) | 155 | (11.9) | |||
| Vaginal | — | — | — | — | — | — | — | — | 228 | (1.7) | 2 | (0.2) | |||
| Hospital complications | |||||||||||||||
| Transfusion | 2,231 | (5.7) | 27 | (4.3) | 0.14 | 443 | (4.9) | 59 | (4.7) | 0.72 | 301 | (2.3) | 39 | (3.0) | 0.11 |
| Hemorrhage | 535 | (1.4) | 8 | (1.3) | 0.85 | 163 | (1.8) | 15 | (1.2) | 0.11 | 198 | (1.5) | 7 | (0.5) | 0.005 |
| Composite | 2,658 | (6.7) | 34 | (5.4) | 0.18 | 587 | (6.5) | 73 | (5.8) | 0.33 | 469 | (3.6) | 44 | (3.4) | 0.74 |
511 patients who had VTE during the hospitalization were excluded (N=40,068).
196 patients who had VTE during the hospitalization were excluded (N=10,260).
122 patients who had VTE during the hospitalization were excluded (N=14,518).
Postoperative LMWH is defined as daily dose of enoxaparin ≤ 40 mg and/or dalteparin ≤ 5000 IU within 1 week after hospitalization (starting from date of discharge), and no VTE diagnosis before LMWH claims. 171, 147, and 166 patients in the colectomy, oophorectomy and hysterectomy cohort who received therapeutic LMWH (Lovenox > 40 mg and/or Fragmin > 5000 IU) and/or who had diagnosis of VTE before LMWH were grouped as no for postoperative LMWH.
Comorbidity defined as number of comorbid medical conditions as defined by the Charlson index.
Among patients who underwent colectomy, those >54 years of age and patients with ≥2 comorbidities were less likely to receive extended-duration prophylaxis while patients operated on in 2010 and patients in the North Central U.S. more frequently received prophylaxis (Table 2). Compared to open colectomy, the odds ratio for receipt of extended-duration prophylaxis was 0.77 (95% CI, 0.63–0.93) after laparoscopic colectomy. For both hysterectomy and oophorectomy, performance of the procedure in more recent years was associated with receipt of prophylaxis. For oophorectomy, patients residing outside of the Northeast and those undergoing cytoreduction more commonly received prophylaxis. Women who underwent abdominal hysterectomy were more likely to receive prophylaxis than women who underwent either vaginal or minimally invasive hysterectomy.
Table 2.
Multivariable logistic regression models of predictors of use of extended-duration prophylaxis low molecular weight heparin.
| Colectomy | Oophorectomy | Hysterectomy | |
|---|---|---|---|
| Age | |||
| ≤34 | Referent | Referent | Referent |
| 35–44 | 0.61 (0.37–1.01) | 1.21 (0.84–1.73) | 0.69 (0.42–1.11) |
| 45–54 | 0.73 (0.46–1.14) | 1.43 (1.03–1.97)* | 0.76 (0.49–1.17) |
| 55–64 | 0.58 (0.37–0.91)* | 1.47 (1.07–2.03)* | 0.77 (0.50–1.18) |
| ≥65 | 0.34 (0.22–0.54)* | 1.29 (0.92–1.80) | 0.68 (0.44–1.06) |
| Sex | |||
| Female | Referent | — | — |
| Male | 0.85 (0.73–1.00)* | — | — |
| Date | |||
| 2009 | Referent | Referent | Referent |
| 2010 | 1.34 (1.04–1.73)* | 1.92 (1.52–2.43)* | 1.39 (1.15–1.70)* |
| 2011 | 1.24 (0.96–1.61) | 2.37 (1.88–2.98)* | 1.72 (1.42–2.08)* |
| 2012 | 1.19 (0.91–1.56) | 3.07 (2.45–3.86)* | 1.96 (1.61–2.39)* |
| 2013 | 1.33 (0.99–1.78) | 3.72 (2.92–4.73)* | 2.39 (1.92–2.97)* |
| Region | |||
| Northeast | Referent | Referent | Referent |
| North central | 1.65 (1.28–2.11)* | 2.18 (1.83–2.60)* | 1.84 (1.58–2.15)* |
| South | 1.23 (0.96–1.57) | 1.09 (0.91–1.31) | 0.84 (0.70–1.00) |
| West | 1.07 (0.79–1.43) | 1.26 (1.03–1.53)* | 0.92 (0.76–1.12) |
| Unknown | 1.82 (0.99–3.35) | 1.72 (1.04–2.82)* | 2.38 (1.47–3.85)* |
| Comorbidity | |||
| 0 | Referent | Referent | Referent |
| 1 | 0.91 (0.74–1.12) | 0.89 (0.75–1.06) | 0.93 (0.80–1.07) |
| ≥2 | 0.53 (0.37–0.76)* | 0.83 (0.61–1.12) | 0.84 (0.66–1.06) |
| Colectomy type | |||
| Open | Referent | — | — |
| Laparoscopic | 0.77 (0.63–0.93)* | — | — |
| Oophorectomy type | |||
| No cytoreduction | — | Referent | — |
| Cytoreduction | — | 2.14 (1.85–2.48)* | — |
| Hysterectomy type | |||
| Abdominal | — | — | Referent |
| Laparoscopic | — | — | 0.66 (0.57–0.76)* |
| Robotic | — | — | 0.64 (0.53–0.76)* |
| Vaginal | — | — | 0.08 (0.02–0.31)* |
| Hospital complications (composite) | |||
| No | Referent | Referent | Referent |
| Yes | 0.86 (0.60–1.21) | 0.82 (0.63–1.06) | 0.84 (0.61–1.15) |
P<0.05
The VTE rate associated with colectomy was 1.3% during hospitalization and 2.9% during the 3-month postoperative period (Table 3). Similar trends were noted for ovarian cancer; the in-hospital VTE rate was 1.9% while 3.7% of women were diagnosed with VTE postoperatively. The corresponding VTE rates after hysterectomy for endometrial cancer were 0.8% during hospitalization and 2.1% postoperatively. VTE rates were lower for patients who underwent minimally invasive compared to open procedures.
Table 3.
Occurrence of venous thromboembolic disease during and after hospitalization and use of extended-duration low molecular weight heparin prophylaxis.
| In-hospital analysis | Post-discharge analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| VTE in-hospital | VTE post-hospital* | LMWH post hospital*ǂ | ||||||
| N | N | (%) | N* | N | (%) | N | (%) | |
| Colectomy | ||||||||
| Colectomy (all) | 40,579 | 511 | (1.3) | 40,068 | 1,160 | (2.9) | 631 | (1.6) |
| Open | 29,647 | 432 | (1.5) | 29,215 | 922 | (3.2) | 489 | (1.7) |
| Laparoscopic | 10,932 | 79 | (0.8) | 10,853 | 238 | (2.2) | 142 | (1.3) |
| Oophorectomy | ||||||||
| Oophorectomy (all) | 10,456 | 196 | (1.9) | 10,260 | 376 | (3.7) | 1,259 | (12.3) |
| No cytoreduction | 6,836 | 132 | (1.9) | 6,704 | 267 | (4.0) | 998 | (14.9) |
| Cytoreduction | 3,620 | 64 | (1.8) | 3,556 | 109 | (3.1) | 261 | (7.3) |
| Hysterectomy | ||||||||
| Hysterectomy (all) | 14,640 | 122 | (0.8) | 14,518 | 307 | (2.1) | 1,305 | (9.0) |
| Abdominal hysterectomy | 8,623 | 107 | (1.2) | 8,516 | 227 | (2.7) | 886 | (10.4) |
| Laparoscopic hysterectomy | 3,779 | 9 | (0.2) | 3,770 | 54 | (1.4) | 262 | (7.0) |
| Robotic-assisted hysterectomy | 2,006 | 4 | (0.2) | 2,002 | 22 | (1.1) | 155 | (7.7) |
| Vaginal hysterectomy | 232 | 2 | (0.9) | 230 | 4 | (1.7) | 2 | (0.9) |
511, 196, 122 patients who had VTE during the hospitalization were excluded from the colectomy (N=40,068), oophorectomy (N=10,260), and hysterectomy (N=14,518) cohorts, respectively.
LMWH post-discharge hospital is defined as daily dose of enoxaparin ≤ 40 mg and/or dalteparin ≤ 5000 IU within 1 week after hospitalization (starting from date of discharge), and no VTE diagnosis before LMWH. 171, 147, and 166 patients who received therapeutic LMWH (enoxaparin > 40 mg and/or dalteparin > 5000 IU) and/or who had diagnosis of VTE before LMWH were grouped as no for LMWH post hospital in colectomy, oophorectomy and hysterectomy cohort respectively.
After propensity score balancing, there was not a statistically significant association between use of extended-duration LMWH prophylaxis and reduction in the rate of VTE for any of the 3 procedures: colectomy (2.4% extended-duration prophylaxis vs. 2.9% without prophylaxis, OR=0.84; 95% CI, 0.54-1.31), ovarian cancer-directed surgery (3.7% vs. 3.6%, OR=1.01; 95% CI, 0.76-1.33), or hysterectomy (2.1% vs. 2.1%; OR=0.96; 95% CI, 0.67-1.38) (Table 4, Supplemental Table 2). Similar findings were noted when the analysis was limited to patients who underwent laparotomy after exclusion of minimally invasive procedures (colectomy and hysterectomy) or in those who underwent cytoreduction (Supplemental Table 3).
Table 4.
Perioperative outcomes stratified by receipt of extended-duration low molecular weight heparin prophylaxis.
| Colectomy | Oophorectomy | Hysterectomy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Events5 | (%) | Odds Ratio (95% CI) | Events5 | (%) | Odds Ratio (95% CI) | Events5 | (%) | Odds Ratio (95% CI) | |
| VTE 1 | |||||||||
| Extended-Duration LMWH | |||||||||
| No | 1,146 | (2.9) | Referent | 329 | (3.6) | Referent | 283 | (2.1) | Referent |
| Yes | 20 | (2.4) | 0.84 (0.54–1.31) | 62 | (3.7) | 1.01 (0.76–1.33) | 33 | (2.1) | 0.96 (0.67–1.38) |
| Transfusion 2 | |||||||||
| Extended-Duration LMWH | |||||||||
| No | 379 | (1.0) | Referent | 166 | (2.0) | Referent | 98 | (0.8) | Referent |
| Yes | 17 | (2.2) | 2.18 (1.34–3.56)* | 31 | (2.0) | 1.00 (0.68–1.47) | 11 | (0.7) | 0.90 (0.48–1.70) |
| Hemorrhage 3 | |||||||||
| Extended-Duration LMWH | |||||||||
| No | 367 | (1.0) | Referent | 87 | (1.0) | Referent | 149 | (1.2) | Referent |
| Yes | 13 | (1.6) | 1.70 (0.97–2.97) | 26 | (1.6) | 1.59 (1.02–2.46)* | 19 | (1.2) | 1.05 (0.65–1.69) |
| Composite (transfusion, hemorrhage) 4 | |||||||||
| Extended-Duration LMWH | |||||||||
| No | 664 | (1.8) | Referent | 233 | (2.8) | Referent | 224 | (1.8) | Referent |
| Yes | 30 | (3.9) | 2.20 (1.51–3.19)* | 55 | (3.4) | 1.24 (0.92–1.68) | 27 | (1.8) | 0.99 (0.66–1.48) |
p-value < 0.05. LMWH post hospital is defined as daily dose of enoxaparin ≤ 40 mg and/or dalteparin ≤ 5000 IU within 1 week after hospitalization (starting from date of discharge), and no VTE diagnosis before LMWH. 171, 147, and 166 patients who received therapeutic LMWH (Lovenox > 40 mg and/or Fragmin > 5000 IU) and/or who had diagnosis of VTE before LMWH were grouped as no for LMWH post hospital in the colectomy, oophorectomy, and hysterectomy cohort and included in deriving the propensity score. Age, sex, year, region, comorbidity, hysterectomy, oophorectomy, colectomy, and hospital complications (any of transfusion or hemorrhage) and all possible two-way interactions were included in the models for propensity score.
Number of patients in the pseudocohort after applying IPTW is 40,258, 10,765, and 14,848 for colon, ovarian, and uterine cohort.
171, 147, and 166 patients who received therapeutic LMWH (enoxaparin > 40 mg and/or dalteparin > 5000 IU) and/or who had diagnosis of VTE before LMWH, and 2,245, 496, and 334 patients who had transfusion in hospital were excluded from the colectomy, oophorectomy, and hysterectomy cohort. Number of patients after applying IPTW is 37,838, 10,083, and 14,334 for the colectomy, oophorectomy, and hysterectomy cohortrespectively.
171, 147, and 166 patients who received therapeutic LMWH (enoxaparin > 40 mg and/or dalteparin > 5000 IU) and/or who had diagnosis of VTE before LMWH, and 539, 175, and 200 patients who had hemorrhage in hospital were excluded from the colectomy, oophorectomy, and hysterectomy cohort. Number of patients after applying IPTW is 39,549, 10,435, and 14,479 for the colectomy, oophorectomy, and hysterectomy cohortrespectively.
171, 147, and 166 patients who received therapeutic LMWH (enoxaparin > 40 mg and/or dalteparin > 5000 IU) and/or who had diagnosis of VTE before LMWH, and 2,677, 651, and 503 patients who had any complications in hospital were excluded from the colectomy, oophorectomy, and hysterectomy cohort. Number of patients after applying IPTW is 37,407, 9,926, and 14,165 for the colectomy, oophorectomy, and hysterectomy cohortrespectively.
Number of events was weighted by the inverse probability of treatment weighting and rounded to integer.
Extended-duration prophylaxis was associated with an increased risk of adverse postoperative events for colectomy (Table 4). The odds ratio for the composite endpoint (transfusion, or hemorrhage) associated with extended-duration prophylaxis was 2.20 (95% CI, 1.51-3.19) after colectomy, 1.24 (95% CI, 0.92-1.68) after ovarian cancer-directed surgery and 0.99 (95% CI, 0.66-1.48) after hysterectomy.
Discussion
Our findings suggest that the use of extended-duration thromboprophylaxis is low among cancer patients undergoing surgery. The use of extended-duration prophylaxis has increased slightly over time. In contrast to randomized controlled trials, we found no association between use of extended-duration prophylaxis and reduction in the risk of VTE but noted a small increased risk of adverse events.
A large number of randomized studies have examined the efficacy of VTE prophylaxis during the immediate postoperative period.1,8 These studies have demonstrated the efficacy of prophylaxis in reducing thromboembolic events and recommendations for perioperative VTE prophylaxis have long been in place to help guide clinicians.1,8 Despite the strength of these recommendations, compliance with in-hospital prophylaxis guidelines is highly variable.27–30 A worldwide analysis of over 18,000 surgical patients found that only 62% received some form of prophylaxis.28 Similarly, even among high-risk surgical oncology patients, prophylaxis is often omitted.29
Additionally, randomized trials as well as systematic reviews have suggested that extended-duration LMWH is efficacious in reducing the risk of nonfatal VTE after surgery.11–17 One analysis predicted that use of extended-duration prophylaxis among high-risk patients would result in 13 fewer events per 1000 patients without an increased risk of hemorrhagic complications.8 However, these studies have not demonstrated a statistically significant reduction in mortality and furthermore, the benefits of extended-duration prophylaxis are smaller in lower risk patients.8 Based on the abundance of data, extended-duration prophylaxis has been recommended in the American College of Chest Physicians (ACCP) guidelines for VTE prophylaxis since 2004.8,18,19 Despite these guidelines, we noted a surprisingly low rate of extended-duration prophylaxis for patients with colon, ovarian, and uterine cancer undergoing cancer-directed surgery. Over the course of the study the rates of extended-duration prophylaxis rose and then declined by 2013.
A number of patient, physician, and hospital-related factors influence utilization of VTE prophylaxis.28,30–33 The type of procedure performed appears to be one of the most important predictors of use of VTE prophylaxis.28,33 The rates of in-hospital VTE prophylaxis are highest for colorectal and gastrointestinal procedures and lower for gynecologic and urologic procedures.28,33 To date, there has been little data specifically evaluating use and predictors of extended-duration VTE prophylaxis. While we noted an overall low rate of use of extended-duration prophylaxis, use was highest for women undergoing surgery for ovarian cancer and appeared to be increasing over time.
A multitude of factors likely contribute to the low use of extended-duration prophylaxis. Administration of extended-duration prophylaxis is complex and requires a subcutaneous injection that must be taught to patients prior to discharge. LMWH is expensive, and for most patients, requires insurance coverage to offset out-of-pocket expense. Lastly, physician factors including lack of awareness about the potential value of extended-duration prophylaxis may also contribute to the low use of the intervention.
Our data raise important questions about the comparative effectiveness of extended-duration LMWH after cancer-directed surgery. Not only was the use of extended-duration prophylaxis not associated with a lower rate of VTE, treatment was also associated with an increased risk of adverse events in some sub-groups. For a number of interventions, efficacy in highly selected patients enrolled in clinical trials has not translated into effectiveness when applied to the general population.34,35 There are a number of possible explanations for our findings. Given the low overall rate of use of extended-duration prophylaxis in our cohort, those at higher risk may have preferentially received extended-duration prophylaxis. Alternatively, patients in the general population may be at lower risk than those enrolled in clinical trials, particularly as the use of lower risk minimally invasive surgery has increased, and as such, the risk-benefit ratio of extended prophylaxis is skewed. Lastly, utilization of LMWH in those prescribed the drug may have been suboptimal. Regardless of the etiology, these findings clearly warrant further investigation.
While our study benefits from the inclusion of a large cohort of patients treated across the U.S., we recognize a number of important limitations. First, use of prophylactic low molecular weight heparin may have been under captured in a small number of patients. To mitigate this bias, we performed a series of sensitivity analyses attempting to capture not only LMWH, but also other anticoagulants, similarly, we used a variety of windows of time and our findings were largely unchanged. Additionally, given the cost associated with the drug, it is unlikely that many patients received LMWH without a claim. Second, we are unable to perform complete risk adjustment as data is lacking for several factors such as surgical complexity, tumor characteristics, preoperative laboratory values, and estimated blood loss that may have influenced the decision to prescribe extended-duration prophylaxis. As such, both measured and unmeasured confounding factors may have biased our estimates of the efficacy of extended-duration LMWH. Third, our data is limited to patients who are commercially insured and may not be generalizable to other surgical populations within the U.S. A further intrinsic limitation of the MarketScan dataset is the limited demographic data available for the cohort. Fourth, we relied on administrative data and thus could only capture billed services and outcomes. We cannot exclude the possibility of undercapture of some outcomes and we are unable to capture asymptomatic DVTs. Lastly, our analysis focused on prescription of LMWH, however, we cannot confirm the patients who filled the prescription were compliant with use of the drug.
Going forward, further prospective, comparative effectiveness studies of the safety and efficacy of extended-duration LMWH in real world populations would be of great value. Given the potential benefits of extended-duration prophylaxis, efforts to raise awareness may increase compliance. Further, pragmatic interventions aimed at promoting utilization of extended-duration prophylaxis may be of benefit to cancer patients undergoing surgery.
Supplementary Material
Highlights.
-
-
Use of extended-duration thromboprophylaxis is low among high-risk cancer patients undergoing surgery.
-
-
The use of extended-duration prophylaxis has increased slightly over time.
Acknowledgements
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants) from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest or disclosures.
References
- 1.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 2.Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227–40. doi: 10.1097/00000658-198808000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mismetti P, Laporte S, Darmon JY, Buchmuller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–30. doi: 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaides A, Irving D, Pretzell M, et al. The risk of deep-vein thrombosis in surgical patients. Br J Surg. 1973;60:312. [PubMed] [Google Scholar]
- 5.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S–77S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 7.Sweetland S, Green J, Liu B, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583. doi: 10.1136/bmj.b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S–77S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber O, Bounameaux H, Borst F, Rohner A. Postoperative pulmonary embolism after hospital discharge. An underestimated risk. Arch Surg. 1992;127:310–3. doi: 10.1001/archsurg.1992.01420030076014. [DOI] [PubMed] [Google Scholar]
- 10.Spyropoulos AC, Hussein M, Lin J, Battleman D. Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102:951–7. doi: 10.1160/TH09-02-0073. [DOI] [PubMed] [Google Scholar]
- 11.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–80. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 12.Lausen I, Jensen R, Jorgensen LN, et al. Incidence and prevention of deep venous thrombosis occurring late after general surgery: randomised controlled study of prolonged thromboprophylaxis. Eur J Surg. 1998;164:657–63. doi: 10.1080/110241598750005534. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost. 2006;4:2384–90. doi: 10.1111/j.1538-7836.2006.02153.x. [DOI] [PubMed] [Google Scholar]
- 14.Akl EA, Terrenato I, Barba M, Sperati F, Muti P, Schunemann HJ. Extended perioperative thromboprophylaxis in patients with cancer. A systematic review. Thromb Haemost. 2008;100:1176–80. [PubMed] [Google Scholar]
- 15.Bottaro FJ, Elizondo MC, Doti C, et al. Efficacy of extended thromboprophylaxis in major abdominal surgery: what does the evidence show? A meta-analysis. Thromb Haemost. 2008;99:1104–11. doi: 10.1160/TH07-12-0759. [DOI] [PubMed] [Google Scholar]
- 16.Kakkar VV, Balibrea JL, Martinez-Gonzalez J, Prandoni P, Group CS. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost. 2010;8:1223–9. doi: 10.1111/j.1538-7836.2010.03892.x. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009:CD004318. doi: 10.1002/14651858.CD004318.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 19.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed June 21, 2015];Truven Health Analytics. MarketScan. at http://truvenhealth.com/your-healthcare-focus/life-sciences/marketscan-databases-and-online-tools.
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102:1780–93. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmila MR, Birkmeyer NJ, Arbabi S, Osborne NH, Wahl WL, Dimick JB. Introduction to propensity scores: A case study on the comparative effectiveness of laparoscopic vs open appendectomy. Arch Surg. 2010;145:939–45. doi: 10.1001/archsurg.2010.193. [DOI] [PubMed] [Google Scholar]
- 24.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. Jama. 2007;297:278–85. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15:234–49. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371:387–94. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 28.Kakkar AK, Cohen AT, Tapson VF, et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330–8. doi: 10.1097/SLA.0b013e3181c0e58f. [DOI] [PubMed] [Google Scholar]
- 29.Wright JD, Lewin SN, Shah M, et al. Quality of Venous Thromboembolism Prophylaxis in Patients Undergoing Oncologic Surgery. Ann Surg. 2011 doi: 10.1097/SLA.0b013e31821287ac. [DOI] [PubMed] [Google Scholar]
- 30.Wright JD, Hershman DL, Shah M, et al. Quality of perioperative venous thromboembolism prophylaxis in gynecologic surgery. Obstet Gynecol. 2011;118:978–86. doi: 10.1097/AOG.0b013e31822c952a. [DOI] [PubMed] [Google Scholar]
- 31.Ritch JM, Kim JH, Lewin SN, et al. Venous thromboembolism and use of prophylaxis among women undergoing laparoscopic hysterectomy. Obstet Gynecol. 2011;117:1367–74. doi: 10.1097/AOG.0b013e31821bdd16. [DOI] [PubMed] [Google Scholar]
- 32.Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician's recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4:E15–21. doi: 10.1002/jhm.526. [DOI] [PubMed] [Google Scholar]
- 33.Wright JD, Lewin SN, Shah M, et al. Quality of venous thromboembolism prophylaxis in patients undergoing oncologic surgery. Ann Surg. 2011;253:1140–6. doi: 10.1097/SLA.0b013e31821287ac. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J, Sharma DB, Gray SW, Chen AB, Weeks JC, Schrag D. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. Jama. 2012;307:1593–601. doi: 10.1001/jama.2012.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. Jama. 2010;303:2479–85. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.