Abstract
Objective
To describe the incidence of age-related macular degeneration (AMD) and associated risk factors in four racial/ethnic groups (white, black, Hispanic and Chinese) residing in the United States (U.S.).
Design
Prospective cohort study.
Participants
3811 participants, aged 46–86 years old, from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, with retinal data collected twice, on average, eight years apart.
Methods
Fundus images, taken using a digital camera through dark-adapted pupils using a standard protocol and the same equipment at both study visits, were graded centrally for early and late AMD based on drusen size, type and area, increased retinal pigment, retinal pigment epithelial depigmentation, neovascular lesions, and geographic atrophy using the modified Wisconsin Age-Related Maculopathy Grading System. Demographic, clinical, and laboratory measures were included in multivariable regression models to determine their impact on the variation in AMD incidence among racial/ethnic groups.
Main outcome measures
Incident early and late AMD.
Results
The overall 8-year age- and sex-standardized incidence of early and late AMD was 4.1% and 2.3%, respectively, with incidence of early and late AMD highest in whites (5.3% and 4.1%), intermediate in Chinese (4.5% and 2.2%) and Hispanics (3.3% and 0.8%), and lowest in blacks (1.6% and 0.4%). Adjusting for age and sex, blacks had 70% lower risk of developing early AMD than whites and this decreased only slightly to 67% lower risk after multivariable adjustment. Adjusting for age, sex and race/ethnicity, hyperopia was associated with early AMD [Odds Ratio (OR)=1.51; 95% Confidence Interval (CI): 1.04–2.20], as was astigmatism [OR=1.47; 95% CI: 1.00–2.16], but not myopia (p=0.29). Age, race/ethnicity, current smoking, hyperopia, and AMD-susceptibility genotypes CFH RS1061170 and ARMS2 RS3793917 were independently associated with incident early AMD in multivariable models for the combined sample. However, the only statistically significant factor consistently associated with incident early AMD across the four racial/ethnic groups was increasing age. Risk factors for late AMD were not assessed due to its low incidence, particularly across racial/ethnic groups.
Conclusion
Variation in the incidence of early AMD exists among racial/ethnic groups in the U.S. and is not explained by the clinical, genetic, and environmental factors included in this study.
Keywords: Age-related macular degeneration, incidence, race/ethnicity, risk factors
Age-related macular degeneration (AMD) is an important cause of vision loss in older adults.1 Previous population-based epidemiological studies, mostly involving a single race or ethnicity, have provided estimates of the prevalence and incidence of AMD in whites, blacks and other racial/ethnic groups,2–13 with significant variability reported and confirmed in a meta-analysis.1 Few studies have directly compared either the prevalence or incidence of AMD among racial/ethnic groups. An earlier analysis2 from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort showed that prevalent early AMD was highest in whites (5.4%) and lowest in blacks (2.4%), while controlling for age and sex. The highest prevalence of any AMD occurred in individuals aged 75 to 84 years old, varying from 7.4% in blacks to 15.8% in whites and Chinese Americans. Similar variation in prevalence of AMD for whites, blacks, and Mexican Americans was reported in a National Health and Nutrition Examination Survey (NHANES) study.3 Yet, most intra-study racial/ethnic comparisons of AMD have been based on cross-sectional prevalence data,2–7 and few had longitudinal data to report on incident AMD.11, 14–16
The reasons for racial/ethnic variations are unclear. Differences in the prevalence of AMD among the racial/ethnic groups have been attributed to differences in the frequency and impact of genetic markers (e.g. AMD candidate genes17–18 such as Age Related Maculopathy Susceptibility 2 [ARMS2 RS10490924] and Complement Factor H [CFH Y402H]) and clinical/environmental factors (e.g. smoking status).19–20 However, in cross-sectional analyses in the MESA cohort, the distribution of these AMD candidate genes explained only a small proportion of the variability and age, sex, pupil size, body mass index, smoking history, alcohol use, diabetes, hypertension, and measures of inflammation did not explain the differences in prevalence among the four racial/ethnic groups.2,21 This suggests the need to better understand putative risk factors underlying racial/ethnic differences, which might be more readily discerned in an analysis of AMD incidence.
The MESA cohort is a well-characterized sample of adults from four racial/ethnic groups living in the U.S.22 with longitudinal data providing an opportunity to investigate incident AMD and its associated risk factors. The current study examines the incidence of AMD lesions and the associations between relevant risk factors and AMD outcomes, overall and within the different racial/ethnic groups in the cohort.
Methods
Subjects and Study Design
MESA is a prospective cohort study of adults, aged 45 to 84 years sampled from six communities in the U.S., who were free of clinical cardiovascular disease at enrollment between July 2000 and July 2002. Briefly, the MESA cohort was composed of 6814 men and women who were recruited at six field centers in Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota using locally available resources, such as lists of residences, dwellings, and telephone exchanges. Supplemental sources were utilized to ensure adequate samples of minorities and elders during the final recruitment period. The details of the MESA study design and methodology have been described elsewhere.22
Fundus photography was included in the second (2002–2004) and fifth (2010–2012) examinations of the study. Of the 6814 participants examined at baseline, 6176 participants returned for a second examination when retinal images were first acquired, of whom 5867 (95.0%) had sufficient retinal imaging data to determine baseline AMD status. Among the 5867 with AMD status at baseline, 3811 individuals participated in the fifth study visit (first follow-up retinal examination) about 8 years later, were not aphakic, and had gradable retinal photographs at both baseline and follow-up visits to determine incident AMD status.
The Tenets of the Declaration of Helsinki were adhered to and all necessary institutional review board approvals were granted. Written informed consent was obtained from all participants.
Fundus Photography and Image Grading
A standardized study protocol was followed at baseline and follow up examinations. Fundus photography has been described in detail previously.23 In brief, participants were seated in a darkened room where a 45-degree, 6.3-megapixel digital nonmydriatic camera (Canon, Lake Success, NY) was used to capture two photographic fields of each eye, the first centered on the optic disc and the second centered on the fovea. Images were sent to the University of Wisconsin-Madison Ocular Epidemiology Reading Center. The images were evaluated twice (preliminary and detailed) using EyeQ Lite software (an image-processing database for storage, retrieval, and manipulation of digital images)23 by trained graders who followed a modification of the Wisconsin Age-Related Maculopathy Grading scheme24–25 and were masked to the health status of the participant.
Participants were evaluated for early AMD lesions, including soft distinct drusen (defined by size between 63 and 300μm in diameter with sharp margins and a round nodular appearance with a uniform density from center to periphery), soft indistinct drusen (defined by same size as soft distinct drusen but having indistinct margins and a softer, less solid appearance), increased retinal pigment (deposition of granules or clumps of gray or black pigment in or beneath the retina), and retinal pigment epithelium (RPE) depigmentation (faint grayish-yellow or pinkish-yellow areas of varying density and configuration without sharply defined borders), and advanced features of maculopathy, including pure geographic atrophy and signs of exudative macular degeneration (e.g. subretinal hemorrhage, subretinal fibrous scar, retinal pigment epithelial detachment, and/or serous detachment of the sensory retina or laser or photodynamic treatment for neovascular AMD). Early AMD was defined by the presence of any soft drusen and pigmentary abnormalities (increased or decreased retinal pigment) or the presence of large soft drusen ≥125μm in diameter with a large drusen area >500μm in diameter or large ≥125μm indistinct soft drusen in the absence of signs of late AMD. Late AMD was defined by the presence of either geographic atrophy (GA) or exudative macular degeneration or both.
Covariates
Detailed questionnaires and clinical examinations were administered to all participants. Demographic and lifestyle characteristics in this analysis included age at baseline retinal examination, sex, race/ethnicity (white, black, Hispanic, Chinese), birth place (U.S. or foreign-born), education (less than high school, completed high school/graduate record examination equivalent, completed some college, college graduate), marital status (single, married, divorced, widowed), occupation (employed, unemployed, not seeking employment), annual family income (< $20,000, $20,000–39,999, $40,000–74,999, $75,000+), cigarette smoking status (never, past, current smoker), pack-years of cigarette smoking, exposure to second-hand smoke (hours per week), alcohol use (current use and drinks per week), and physical activity measured in metabolic equivalent-minutes per week.
Individual health risk factors, including seated systolic and diastolic blood pressure (mmHg), hip and waist circumference (centimeters), hypertension, body mass index (BMI), body surface area, weight at age 20 years, weight at age 40 years, diabetes, microalbuminuria, periodontitis or gum disease, refractive error status, self-reported health conditions, and medication use (e.g. aspirin use, anti-hypertensives, lipid-lowering agents, etc.) were assessed. BMI was calculated as measured weight (kilograms) divided by height (meters) squared. Hypertension (yes or no) was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or self-reported hypertension with use of anti-hypertensive medication. Diabetes status (yes/no) was defined as fasting glucose ≥ 7.0 mmol/L (≥ 126 mg/dl) and/or use of insulin or oral hypoglycemic medicine. Microalbuminuria was defined as a urine albumin: creatinine ratio (UACR) ≥ 30 mg/g. Refractive error status was determined based on the spherical equivalent in the eye with the largest absolute value where myopia was defined as a spherical equivalent of −1.0 diopter or less, hyperopia as a spherical equivalent of 1.0 diopter or more, or emmetropia (no myopia or hyperopia), as previously reported.26 Subsequently, myopia was dichotomized as yes (myopia) or no (emmetropia or hyperopia) and hyperopia was dichotomized as yes (hyperopia) or no (emmetropia or myopia). Astigmatism, defined as more than 1.0 diopter of cylinder value in the worse eye, was also assessed. Cardiovascular disease measures and history, including ankle-brachial index, calcium volumes, major Q-wave abnormalities, pulse wave large and small vessel compliance, ultrasound measure of carotid distensibility, increased intimal-medial thickness, carotid stenosis, and history of myocardial infarction (MI) and stroke, were also collected. Total cholesterol (mg/dl), high-density lipoprotein (HDL) cholesterol (mg/dl), low-density lipoprotein (LDL) cholesterol (mg/dl), triglycerides (mg/dl), fasting glucose (mg/dl), oxidized LDL cholesterol (mg/dl), homocysteine (μmol/l), von Willebrand factor (%), soluble intercellular adhesion molecule-1 (sICAM-1) (ng/ml), plasminogen activator inhibitor type 1 (PAI-1) (ng/ml), factor VIIIc (%), C-reactive protein (mg/l), fibrinogen antigen (mg/dl), and interleukin-6 (pg/ml) were measured centrally using standard methods. Data were available for Age-Related Maculopathy Susceptibility 2 (ARMS2 RS3793917) from CardioMetaboChip genotyping and Complement Factor H (CFH RS1061170) from candidate gene genotyping as previously described.27–28
Statistical Methods
Participant characteristics at baseline were compared using analysis of covariance and logistic regression models, controlling for age and sex, between individuals who completed both retinal examinations (visits 2 and 5) and individuals who did not return for the follow up examination. Individuals without any AMD at baseline were considered at risk for incident early and late AMD; individuals at risk of incident late AMD could have had either no AMD or early AMD at baseline. Incidence of AMD (early and late) in the worse eye were calculated overall, and for each racial/ethnic group, in all participants and in men and women separately. Incidence of specific AMD lesions were also determined overall and for each racial/ethnic group. Incidence estimates for all participants were age- and sex-standardized and those by sex were age-standardized to the 2010 U.S. Census population.
Multivariable logistic regression of incident early AMD, adjusting for age, sex, and race/ethnicity, was completed for each baseline characteristic separately in order to determine which variables should be included in subsequent models; a p-value < 0.10 was necessary for inclusion in final models. Final multivariable logistic regressions models included the variables: age, sex, race/ethnicity (for analyses using all participants), cigarette smoking, diastolic blood pressure, body mass index, weight at age 40 years, medication use (diuretics, lipid-lowering agents), total serum cholesterol, triglycerides, internal carotid intimal-medial thickness, Z score maximum IMT, hyperopia, astigmatism, and a SNP in each of ARMS2 and CFH genes in order to assess the impact of different risk factors on incident early AMD overall. The joint impact of ARMS2 and CFH genotype on AMD incidence in each ethnic group was estimated and depicted graphically, with p-values calculated using Cochran-Armitage tests for trend across genotypes within each racial/ethnic group. Multivariable regression analyses were not completed for incident late AMD due to its low incidence in the cohort. Odds ratios (OR) and 95% confidence intervals (CI) were reported. Analyses were two-sided with a 5% significance level and conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Of the 5867 individuals whose initial AMD status at baseline and race/ethnicity were collected, 3811 (65.0%) participated in the follow-up retinal examination. Supplemental Table 1 describes a detailed list of baseline characteristics for these 3811 MESA participants alongside those for the 585 individuals who returned for MESA exam five but did not contribute AMD data, the 868 individuals who refused participation in exam five, and the 603 individuals who died prior to exam five, with bolded values indicating significant differences between the respective group and the 3811 participants included in subsequent analyses. Participants who returned for exam 5 and had AMD data collected were younger; less likely to smoke cigarettes; less likely to have astigmatism; and had greater large artery elasticity indices. To summarize, returning participants appeared to be younger and healthier than their counterparts.
Among those at risk for incident AMD, Table 1 shows, for the total group and by race/ethnicity, the baseline characteristics included as covariates in subsequent multivariable analyses. After adjusting for age and sex, virtually all characteristics differed significantly across racial/ethnic groups, including the genetic profiles of ARMS2 (RS3793917) and CFH Y402H (RS1061170). The prevalence of smoking, the most consistent risk factor for AMD besides increasing age, was highest in White and lowest in Chinese individuals.
Table 1.
Selected Baseline Characteristics from Individuals who Participated in Exams 2 and 5 and did not have Late Age-Related Macular Degeneration at Baseline, Overall and by Racial/Ethnic Group, in the Multi-Ethnic Study of Atherosclerosis
| Characteristic* | All Participants (n=3802) [Mean (SD) or % (n)] | Whites Participants (n=1549) [Mean (SD) or % (n)] | Blacks Participants (n=949) [Mean (SD) or %] | Hispanic Participants (n=841) [Mean (SD) or %] | Chinese Participants (n=463) [Mean (SD) or % (n)] | p-value** |
|---|---|---|---|---|---|---|
|
|
|
|||||
| Age (yrs) | 60.8 (9.1) | 61.1 (9.2) | 60.6 (8.7) | 60.3 (9.2) | 60.5 (9.1) | 0.001 |
| Sex (% male) | 46.9% (1783) | 47.6% (737) | 44.3% (420) | 47.0% (395) | 49.9% (231) | 0.291 |
| Race/Ethnicity | ||||||
| White | 40.7% (1549) | -- | -- | -- | -- | -- |
| Black | 25.0% (949) | -- | -- | -- | -- | -- |
| Hispanic | 22.1% (841) | -- | -- | -- | -- | -- |
| Chinese | 12.2% (463) | -- | -- | -- | -- | -- |
| Cigarette Smoking | ||||||
| Past | 41.1% (1552) | 47.4% (729) | 41.7% (392) | 39.5% (330) | 21.9% (101) | < 0.001 |
| Current | 11.1% (419) | 10.2% (157) | 15.7% (148) | 10.8% (90) | 5.2% (24) | < 0.001 |
| Diastolic Blood Pressure, Seated (mmHg) | 70.6 (10.0) | 69.0 (9.6) | 73.7 (9.9) | 70.5 (10.0) | 69.9 (9.7) | < 0.001 |
| Body Mass Index (kg/m2) | 28.4 (5.4) | 27.9 (5.2) | 30.2 (5.5) | 29.5 (5.4) | 24.1 (3.2) | < 0.001 |
| Weight at Age 40 years (lbs) | 156.1 (32.7) | 158.5 (33.3) | 165.0 (33.4) | 154.9 (29.0) | 131.8 (21.4) | < 0.001 |
| Medication Use | ||||||
| Diuretics | 13.3% (483) | 13.2% (191) | 21.4% (196) | 8.8% (71) | 5.5% (25) | < 0.001 |
| Lipid-Lowering Agents | 21.4% (776) | 25.5% (371) | 19.5% (179) | 17.7% (143) | 18.2% (83) | < 0.001 |
| Total Cholesterol (mg/dl) | 192.2 (35.0) | 193.6 (34.5) | 188.9 (36.0) | 194.1 (36.3) | 191.1 (31.5) | < 0.001 |
| Triglycerides (mg/dl) | 131.4 (81.9) | 131.4 (77.1) | 103.2 (57.4) | 152.3 (96.2) | 150.5 (94.6) | < 0.001 |
| Large Artery Elasticity Index (ml/mmHg × 10) | 14.2 (5.5) | 14.5 (5.7) | 14.3 (5.7) | 13.7 (5.1) | 13.9 (5.3) | < 0.001 |
| Internal Carotid Intimal-Medial Thickness (mm) | 1.0 (0.5) | 1.0 (0.5) | 1.0 (0.6) | 1.0 (0.5) | 0.8 (0.3) | < 0.001 |
| Z Score Maximum Intimal-Medial Thickness | −0.2 (0.9) | −0.2 (0.9) | −0.1 (0.9) | −0.2 (0.8) | −0.5 (0.7) | < 0.001 |
| Hyperopia | 36.8% (1355) | 34.9% (526) | 36.2% (328) | 47.4% (389) | 24.9% (112) | < 0.001 |
| Astigmatism | 43.4% (1598) | 44.6% (672) | 42.1% (381) | 38.7% (317) | 50.8% (228) | < 0.001 |
| Genotypes | ||||||
| ARMS2 RS3793917 | < 0.001 | |||||
| CC | 6.6% (234) | 3.8% (56) | 5.7% (47) | 5.6% (45) | 19.6% (86) | |
| CG | 36.2% (1285) | 34.0% (503) | 34.0% (279) | 34.5% (278) | 51.3% (225) | |
| GG | 57.2% (2027) | 62.3% (923) | 60.2% (494) | 59.9% (482) | 29.2% (128) | |
| CFH Y402H RS1061170 | < 0.001 | |||||
| AA | 58.3% (950) | 39.6% (170) | 44.8% (168) | 57.9% (235) | 89.6% (377) | |
| AG | 33.8% (551) | 47.6% (204) | 44.5% (167) | 33.7% (137) | 10.2% (43) | |
| GG | 8.0% (130) | 12.8% (55) | 10.7% (40) | 8.4% (34) | 0.2% (1) | |
-- = cannot be calculated; SD=standard deviation.
Measured at baseline examination (collected prior to visit 5 when follow-up eye examination occurred). Characteristics were selected for inclusion in this Table if the characteristic had a p<0.10 in preliminary analyses of incident AMD in which each variable was considered separately, controlling for age, sex, and race/ethnicity. See Supplemental Table 1 for other characteristics considered. CFH Y402H RS1061170 was available only for a subset of participants (n=2451).
P-values represent the results of testing for equality among racial/ethnic groups, controlling for age and sex. General linear models were utilized for continuous variables and logistic regression models were utilized for categorical variables.
The overall incidence of early AMD was 4.1% (N=129) in the 3685 individuals without any AMD at baseline and late AMD was 2.3% (N=34) in the 3802 individuals without late AMD at baseline (Table 2). Among the 34 participants with incident late AMD, 5 exhibited no signs of AMD and 29 exhibited signs of early AMD at the baseline examination. Overall, white participants had the highest percentage of incident AMD (early or late) followed by Chinese, Hispanic, and black participants, although Chinese men were more likely (but not significantly) to have incident AMD compared to white men. Women were slightly (but not significantly) more likely to have early but not late AMD compared to men.
Table 2.
Incidence of Early and Late Age-Related Macular Degeneration in the Worse Eye by Sex and Race/Ethnicity in the Multi-Ethnic Study of Atherosclerosis (2010–2012)
| Early AMD*
|
Late AMD**
|
|||
|---|---|---|---|---|
| No. at Risk | % (N) | No. at Risk | % (N) | |
|
|
||||
| Overall | 3685 | 4.1% (129) | 3802 | 2.3% (34)† |
| White | 1486 | 5.3% (72) | 1549 | 4.1% (23) |
| Black | 938 | 1.6% (15) | 949 | 0.4% (1) |
| Hispanic | 808 | 3.3% (24) | 841 | 0.8% (4) |
| Chinese | 453 | 4.5% (18) | 463 | 2.2% (6) |
| Men | 1727 | 3.7% (56) | 1783 | 2.8% (18) |
| White | 706 | 3.7% (27) | 737 | 1.6% (12) |
| Black | 417 | 1.6% (6) | 420 | 1.1% (1) |
| Hispanic | 379 | 3.9% (13) | 395 | 0.2% (1) |
| Chinese | 225 | 4.7% (10) | 231 | 3.7% (4) |
| Women | 1958 | 4.3% (73) | 2019 | 2.1% (16) |
| White | 780 | 6.4% (45) | 812 | 4.0% (11) |
| Black | 521 | 1.6% (9) | 529 | 0.0% (0) |
| Hispanic | 429 | 2.6% (11) | 446 | 1.1% (3) |
| Chinese | 228 | 4.3% (8) | 232 | 1.1% (2) |
Incidence estimates overall (all participants and by race/ethnicity) were age- and sex-standardized to the 2010 U.S. Census population; incidence estimates stratified by sex (all men or women and by race/ethnicity) were age-standardized to the 2010 U.S. Census population.
Early AMD was defined as no AMD at baseline examination (visit 2) but the presence of early AMD at follow-up examination (visit 5).
Late AMD was defined as either no AMD or early AMD at baseline examination (visit 2) but the presence of late AMD at follow-up examination (visit 5).
Among participants with late AMD at follow-up examination (visit 5) (n=34), five participants exhibited no AMD and 29 participants presented with early AMD at baseline examination (visit 2).
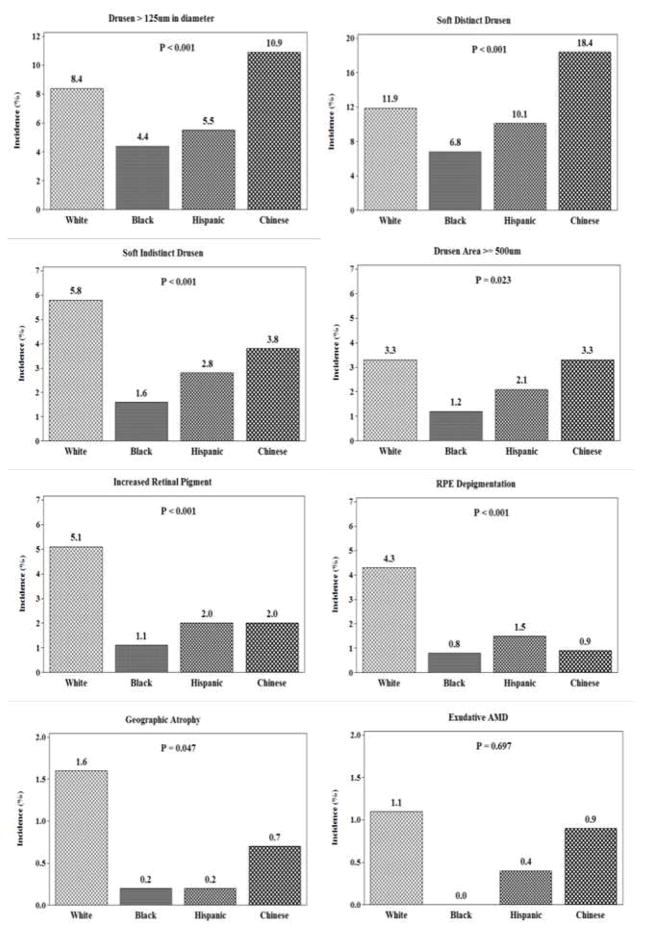
The overall incidence of large drusen was 7.4% (n=240), any soft drusen was 11.6% (n=360), large area of drusen was 2.8% (n=90), pigmentary abnormalities was 3.3% (n=105), exudative AMD was 0.7% (n=21) and geographic atrophy was 0.9% (n=25, 12 of whom also had exudative AMD). There were significant differences in incidence of early AMD lesions and geographic atrophy by racial/ethnic group, after age and sex adjustment (Figure 1). The number of participants with exudative macular degeneration was too small to detect significant differences among racial/ethnic groups. White or Chinese participants exhibited the highest incidences for all of the specific AMD lesions.
Figure 1.
Incidence of age-related macular degeneration (AMD) lesions by racial/ethnic group in the Multi-Ethnic Study of Atherosclerosis (2010–2012). Incidence estimates were age- and sex-standardized to the 2010 U.S. Census population. P-values represent the results of logistic regression testing for equality among racial/ethnic groups, controlling for age and sex. As a caution, the ranges for incidence (along the vertical axis) were not consistent across individual lesion plots due to some lesions having significantly fewer incident cases than other lesions.
Factors determined to be significant for incident early AMD, after adjustment for age, sex, and race/ethnicity, and deemed eligible for subsequent multivariable analyses (p < 0.10) included smoking [OR (current vs. never smoked): 1.99 (95% CI: 1.06, 3.73); p=0.03], greater BMI [OR (per 1.0 kg/m2): 1.21 (95% CI: 1.00, 1.21); p=0.05], greater weight at age 40 years [OR (per 1.0 lb): 1.22 (95% CI: 0.97, 1.54); p=0.09], history of diuretics use [OR (vs. not using diuretics): 1.56 (95% CI: 0.98, 2.48); p=0.06], history of lipid lowering agents use [OR (vs. not using lipid lowering agent): 0.65 (95% CI: 0.41,1.03); p=0.07], serum total cholesterol [OR (per SD): 0.85 (95% CI: 0.70,1.03); p=0.10], serum triglycerides [OR (per SD): 0.79 (95% CI: 0.61, 1.00); p=0.05], internal carotid intimal medial thickness (IMT) [OR (per SD): 1.14 (95% CI: 0.98, 1.32); p=0.09], Z-score maximum IMT [OR (per SD): 1.18 (95% CI: 1.00, 1.41); p=0.05], large artery elasticity index [OR (per SD): 0.79 (95% CI: 0.62, 1.01); p=0.06], hyperopia [OR (vs. no hyperopia): 1.51 (95% CI: 1.04, 2.20); p=0.03], and astigmatism [OR (vs. no astigmatism): 1.47 (95% CI: 1.00, 2.16); p=0.05]. Additionally, participants homozygous or heterozygous for the variant risk allele were at increased risk of incident early AMD compared to participants homozygous for the wild type allele for the ARMS2 RS3793917 gene [OR: 2.48 (95% CI: 1.25, 4.92); p=0.01 and OR: 1.83 (95% CI: 1.23, 2.73); p<0.01, respectively], and this was also true for the CFH RS106170 gene [OR: 5.68 (95% CI: 2.15, 15.01) and OR: 2.83 (95% CI: 1.38, 5.80); p=0.01; p<0.01, respectively].
Since differences in refractive errors among the racial/ethnic groups were known to exist, additional analyses examining the association between specific refractive errors and incident AMD were completed in the entire cohort and by racial/ethnic group (Table 3). After adjustment for age, sex, and race/ethnicity, no significant associations were found between myopia and incident early or late AMD when myopia was compared against emmetropia or compared against no myopia (emmetropia or hyperopia). There was, after adjustment for age, sex, and race/ethnicity, a statistically significant association between hyperopia and early (but not late) AMD when compared to those with emmetropia and it remained significantly associated with early (but not late) AMD when comparing hyperopia versus no hyperopia (emmetropia or myopia). Interactions between age and refractive error (either myopia or hyperopia) were not statistically significant. In analyses controlling for age and sex and stratified by race/ethnicity, hyperopia was significantly associated with incident early AMD only in Chinese, although the data in white and Hispanic participants were consistent with a non-statististically significant increase in AMD among those with hyperopia. Astigmatism narrowly missed reaching statistical significance (p=0.05) in the overall group. In sex-specific analyses of data from all racial ethnic groups combined, hyperopia was significantly associated with early AMD in men (p<0.05) and was elevated, but not statistically significantly higher, in women (data not shown). In contrast, astigmatism was significantly associated with early AMD in women (p=0.03) but not in men (data not shown).
Table 3.
Incidence of Early and Late Age-Related Macular Degeneration in the Worse Eye by Refractive Error, Overall and for Each Race/Ethnicity, in the Multi-Ethnic Study of Atherosclerosis
| Myopia | Status | N | Age-and Sex-Standardized Incidencesa
|
Odds Ratio (95% CI)b
|
||
|---|---|---|---|---|---|---|
| Early AMD | Late AMD | Early AMD | Late AMDc | |||
| Overall | No | 2431 | 4.1% (96) | 2.2% (22) | 1 [reference] | 1 [reference] |
| Yes | 1140 | 4.0% (31) | 2.4% (10) | 0.80 (0.52, 1.22); p=0.289 | 1.32 (0.60, 2.88); p=0.462 | |
| White | No | 917 | 5.5% (52) | 3.7% (14) | 1 [reference] | |
| Yes | 531 | 5.3% (20) | 4.5% (7) | 0.89 (0.52, 1.54); p=0.678 | ||
| Black | No | 637 | 1.5% (11) | 0.6% (1) | 1 [reference] | |
| Yes | 259 | 1.8% (4) | 0.0% (0) | 1.02 (0.32, 3.28); p=0.974 | ||
| Hispanic | No | 634 | 3.0% (19) | 0.8% (3) | 1 [reference] | |
| Yes | 154 | 2.4% (3) | 0.9% (1) | 0.76 (0.22, 2.64); p=0.669 | ||
| Chinese | No | 243 | 5.5% (14) | 2.9% (4) | 1 [reference] | |
| Yes | 196 | 3.9% (4) | 1.7% (2) | 0.41 (0.13, 1.32); p=0.137 | ||
|
| ||||||
| Hyperopia | Status | N | Early AMD | Late AMD | Early AMD | Late AMDc |
|
| ||||||
| Overall | No | 2267 | 3.5% (59) | 2.6% (19) | 1 [reference] | 1 [reference] |
| Yes | 1304 | 5.2% (68) | 2.0% (13) | 1.51 (1.04, 2.20); p=0.025 | 0.81 (0.39, 1.68); p=0.528 | |
| White | No | 949 | 4.8% (36) | 3.8% (11) | 1 [reference] | |
| Yes | 499 | 6.7% (36) | 3.6% (10) | 1.40 (0.86, 2.29); p=0.179 | ||
| Black | No | 574 | 1.7% (8) | 0.9% (1) | 1 [reference] | |
| Yes | 322 | 1.4% (7) | 0.0% (0) | 0.95 (0.33, 2.71); p=0.922 | ||
| Hispanic | No | 415 | 1.9% (8) | 0.6% (2) | 1 [reference] | |
| Yes | 373 | 4.2% (14) | 0.9% (2) | 1.52 (0.61, 3.76); p=0.366 | ||
| Chinese | No | 329 | 3.5% (7) | 2.6% (5) | 1 [reference] | |
| Yes | 110 | 7.4% (11) | 1.5% (1) | 3.29 (1.18, 9.17); p=0.023 | ||
|
| ||||||
| Astigmatism | Status | N | Early AMD | Late AMD | Early AMD | Late AMDc |
|
| ||||||
| Overall | No | 2012 | 3.2% (47) | 2.3% (12) | 1 [reference] | 1 [reference] |
| Yes | 1562 | 4.5% (80) | 2.3% (20) | 1.47 (1.00, 2.16); p=0.053 | 0.92 (0.43, 1.97); p=0.834 | |
| White | No | 802 | 4.5% (28) | 5.1% (9) | 1 [reference] | |
| Yes | 646 | 5.7% (44) | 3.5% (12) | 1.41 (0.84, 2.35); p=0.192 | ||
| Black | No | 514 | 2.0% (4) | 0.0% (0) | 1 [reference] | |
| Yes | 383 | 1.9% (11) | 0.5% (1) | 2.30 (0.69, 7.64); p=0.176 | ||
| Hispanic | No | 480 | 1.8% (9) | 0.6% (2) | 1 [reference] | |
| Yes | 309 | 3.2% (13) | 0.8% (2) | 1.56 (0.62, 3.95); p=0.344 | ||
| Chinese | No | 216 | 2.7% (6) | 0.7% (1) | 1 [reference] | |
| Yes | 224 | 5.3% (12) | 3.0% (5) | 1.23 (0.43, 3.51); p=0.693 | ||
Myopia is defined as a spherical equivalent of −1.0 diopter or less in the worse eye.
Hyperopia is defined as a spherical equivalent of 1.0 diopter or more in the worse eye.
Astigmatism is defined as more than 1.0 diopter of cylinder value in the worse eye.
Incidence estimates were age- and sex-standardized to the 2010 U.S. Census population.
Analyses compared incident AMD between participants with and without the specific refractive error, overall and by racial/ethnic group, adjusted for age, sex, and race/ethnicity for the overall cohort and for age and sex in analyses stratified by race/ethnicity.
Analyses stratified by race/ethnicity were not completed for late AMD due to small numbers.
Results from the subsequent multivariable models indicate that increasing age, racial/ethnic group, current cigarette smoking, hyperopia, and AMD susceptibility genotype were independently associated with incident early AMD in the cohort (Table 4). These risk factors, with the exception of current cigarette smoking and hyperopia, were also significantly associated with incident large drusen (data not shown). In addition, lower diastolic blood pressure and not using lipid lowering agents were also associated with incidence of large drusen in multivariable analyses (data not shown). Black individuals were at significantly lower odds of incident early AMD and incident large drusen, in particular (data not shown), compared to white individuals. After considering CFH genotype, blacks continued to have significantly lower odds of early AMD, but the association attenuated from an OR of 0.60 to 0.65 for large drusen (data not shown). There was no significant difference in early AMD incidence between Chinese and White individuals, but Chinese participants were significantly more likely to have incident large drusen than whites even after adjustment in multivariable models (data not shown). The ARMS2 genotype was significantly associated with incident early AMD when CFH genotype was not included in the models, but, while odds of incident AMD remained higher for those homozygous for ARMS2 it was not significant when CFH genotype was simultaneously included in the model.
Table 4.
Multivariable Associations between Putative Risk Factors and Incident Early Age-Related Macular Degeneration in the Multi-Ethnic Study of Atherosclerosis
| Characteristic* | Incident Early Age-Related Macular Degeneration
|
|||
|---|---|---|---|---|
| Model 1 (N=2916)
|
Model 2 (N=1331)
|
|||
| Odds Ratio (95% CI) | p-value† | Odds Ratio (95% CI) | p-value† | |
| Age, per year | 1.07 (1.04, 1.10) | < 0.001 | 1.09 (1.03, 1.14) | < 0.001 |
| Sex, Women vs. Men | 0.76 (0.41, 1.40) | 0.376 | 0.85 (0.31, 2.35) | 0.756 |
| Race/Ethnicity | ||||
| Black vs. White | 0.27 (0.13, 0.56) | < 0.001 | 0.32 (0.10, 0.99) | 0.049 |
| Hispanic vs. White | 0.54 (0.30, 0.97) | 0.038 | 0.68 (0.25, 1.85) | 0.452 |
| Chinese vs. White | 0.95 (0.48, 1.89) | 0.883 | 2.05 (0.67, 6.30) | 0.210 |
| Cigarette Smoking | ||||
| Past vs. Never | 1.22 (0.76, 1.96) | 0.402 | 0.77 (0.33, 1.78) | 0.536 |
| Current vs. Never | 1.83 (0.89, 3.80) | 0.103 | 3.35 (1.21, 9.26) | 0.020 |
| Diastolic Blood Pressure, Seated (mmHg), per SD | 0.79 (0.63, 1.00) | 0.054 | 0.70 (0.48, 1.02) | 0.062 |
| Body Mass Index (kg/m2), per SD | 1.23 (0.93, 1.64) | 0.151 | 1.29 (0.82, 2.01) | 0.272 |
| Weight at Age 40 years (lbs), per SD | 1.01 (0.72, 1.41) | 0.976 | 1.02 (0.58, 1.82) | 0.937 |
| Medication Use | ||||
| Diuretics, Yes vs. No | 1.25 (0.71, 2.23) | 0.439 | 2.34 (0.95, 5.78) | 0.066 |
| Lipid-Lowering Agents, Yes vs. No | 0.57 (0.33, 0.99) | 0.045 | 0.65 (0.27, 1.59) | 0.347 |
| Total Cholesterol (mg/dl), per SD | 0.92 (0.72, 1.17) | 0.478 | 0.87 (0.59, 1.29) | 0.479 |
| Triglycerides (mg/dl), per SD | 0.75 (0.56, 1.03) | 0.073 | 0.55 (0.31, 1.00) | 0.050 |
| Large Artery Elasticity Index (ml/mmHg × 10), per SD | 0.76 (0.57, 1.01) | 0.056 | 0.80 (0.48, 1.31) | 0.370 |
| Internal Carotid Intimal-Medial Thickness (mm), per SD | 1.06 (0.77, 1.47) | 0.708 | 0.96 (0.56, 1.65) | 0.874 |
| Z Score Maximum Intimal-Medial Thickness, per SD | 1.05 (0.72, 1.52) | 0.809 | 1.01 (0.57, 1.81) | 0.969 |
| Hyperopia, Yes vs. No | 1.73 (1.12, 2.66) | 0.013 | 2.39 (1.18, 4.85) | 0.015 |
| Astigmatism, Yes vs. No | 1.28 (0.82, 1.99) | 0.285 | 1.39 (0.67, 2.88) | 0.378 |
| Genotypes | ||||
| ARMS2 RS3793917 | ||||
| CC vs. GG | 2.91 (1.39, 6.09) | 0.005 | 2.04 (0.62, 6.69) | 0.238 |
| CG vs. GG | 2.02 (1.29, 3.16) | 0.002 | 1.38 (0.66, 2.89) | 0.392 |
| CFH Y402H RS1061170 | ||||
| AG vs. AA | -- | -- | 2.55 (1.13, 5.74) | 0.024 |
| GG vs. AA | -- | -- | 4.14 (1.25, 13.76) | 0.020 |
-- = not calculated; CI=confidence interval.
Measured at baseline examination (collected prior to visit 5 when follow-up eye examination occurred).
Multivariable models adjusted for characteristics simultaneously. Model 1 includes age, sex, race/ethnicity, cigarette smoking, diastolic blood pressure, body mass index, weight at age 40 years, medication use (diuretics, lipid-lowering agents), total serum cholesterol, triglycerides, internal carotid intimal-medial thickness, Z score maximum IMT, hyperopia, astigmatism, and ARMS2 RS3793917, and model 2 includes all variables in model 1 plus CFH Y402H RS1061170. The analytic sample for model 1 is N=2999 (99 cases of incident early AMD) and for model 2 is N=1375 (39 cases of incident early AMD). Variables were selected for inclusion in multivariable models if they had a p<0.10 in preliminary analyses in which each variable was considered separately, controlling for age, sex, and race/ethnicity.
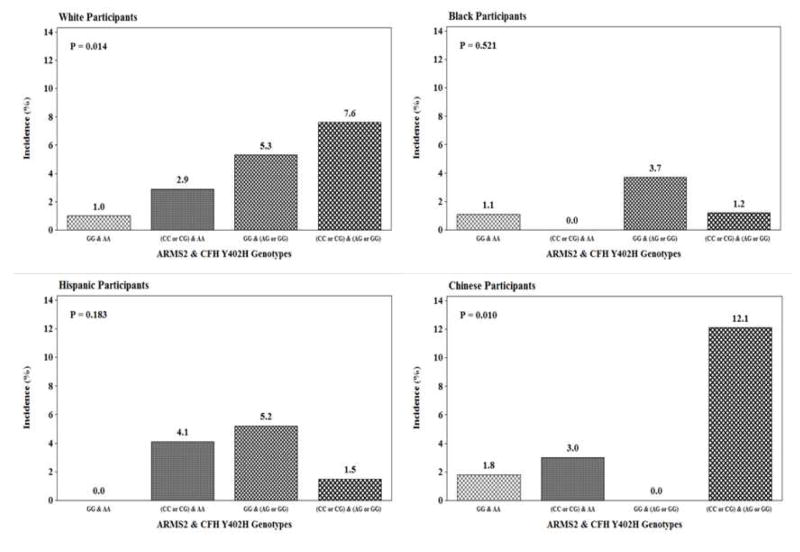
To investigate further the joint impact of both ARMS2 and CFH genotypes, the incidence of AMD was depicted graphically (Figure 2) for each racial/ethnic group separately, with the lowest genetic risk conferred by two homozygous genes represented in the first bar, the risk associated with putative ARMS2 allele and low risk CFH allele in the second bar, the putative CFH allele and low risk ARMS2 allele in the third bar, and high risk alleles for both ARMS2 and CFH in the final bar. The joint presence of the high risk alleles was strongly associated with AMD incidence in whites and Chinese but not in Hispanics.
Figure 2.
Incidence of early age-related macular degeneration (AMD) by ARMS2 RS3793917 and CFH Y402H RS1061170 genotypes for each racial/ethnic group in the Multi-Ethnic Study of Atherosclerosis (2010–2012). P-values represent the results of Cochran-Armitage tests for trend across genotypes for each racial/ethnic group.
Discussion
MESA provided a unique opportunity to examine the 8-year overall incidence of AMD in four major racial/ethnic groups (white, black, Hispanic and Chinese) living in six U.S. communities. Furthermore, it allowed us to examine clinical, genetic and environmental factors related to incident AMD and to assess the potential impact of these factors on racial/ethnic variation in AMD incidence. The overall incidence of early and late AMD was 4.1% and 2.3%, respectively, and after adjustment for age and sex, was highest in whites, intermediate in Chinese and Hispanics, and lowest in blacks. Age, current smoking status, hyperopia, and AMD-susceptibility genotypes CFH RS1061170 and ARMS2 RS3793917 were associated with incident early AMD when considering the whole cohort. However, none of these risk factors, besides age, were consistently associated with incident early AMD among all four racial/ethnic groups. In other words, the clinical, genetic and environmental factors measured in MESA and considered for this analysis did not explain the observed racial/ethnic variation in AMD incidence.
The incidence of early and late AMD in this study is lower than reports from other studies.8–9, 11–13, 15–16, 29–31 Some of the discrepancy may be due to different definitions of AMD used across studies, as well as the lack of age and sex standardization in most previous reports. It is important to note that MESA participants were enrolled into a cardiovascular cohort study, in which they were assessed to be free from clinical cardiovascular disease at baseline. MESA incorporated an eye component, as opposed to being a dedicated eye study (e.g., Beaver Dam Eye Study), and issues of selection bias may also play a role. Nevertheless, the lower incidence observed in MESA is consistent with the lower prevalence observed at the baseline MESA retinal examination2 and in the 2005–2008 National Health and Nutrition Examination Survey.3 Finally, since most previous reports of incidence were based on studies carried out a decade or longer before MESA was initiated, this lends credence to the concept of an age-period-cohort effect observed in the Beaver Dam Eye Study, which reported a lower incidence of AMD in later birth cohorts.32
In MESA, while adjusting for age, sex, smoking status, hyperopia and CFH RS1061170, the odds of developing incident early AMD at the 8 year follow-up was 67% lower in blacks compared to whites. The 8-year overall incidences of exudative AMD and geographic atrophy were also lower in blacks compared to whites (0.0% vs. 1.1% and 0.2% vs. 1.6%, respectively). These findings are consistent with lower prevalences of early AMD (2.4% vs 5.4%), exudative AMD (0.19% vs. 0.22%) and geographic atrophy (0.1% vs. 0.4%) in blacks compared to whites seen in MESA and in most population-based cross-sectional studies in which whites and blacks have been compared.2–3, 33–37
There are few population-based studies in which the incidence of AMD in blacks and whites have been compared.14–16 In the Salisbury Eye Evaluation (SEE) project, while adjusting for age and sex, the two-year overall incidences of large retinal drusen, increased retinal pigment, and geographic atrophy were found to be lower in blacks compared to whites although these differences were not statistically significant, in part, due to the lack of power. The Barbados Eye study also examined the incidence of AMD in blacks at 4 and 9 year follow-up; the 9-year overall incidence of early AMD was 12.6% and for late AMD was 0.7% (geographic atrophy and exudative AMD were each 0.5%). The estimate of 9-year incident late AMD in blacks in the Barbados Eye Study was considerably less than that found in whites in the Beaver Dam Eye Study over its 10 year duration of follow-up.
The lower incidence of AMD in blacks compared to whites has been attributed to racial variations in melanin pigmentation in the choroid and retinal pigment epithelium.38 Melanin is thought to decrease the risk of developing AMD by protecting the retina against the damaging effects of free radicals associated with photo-oxidation and light absorption.39 The lower incidence of early AMD in blacks compared to whites has also been attributed to differences in the distributions of protective genes such as CFH-related 1/3 deletions, C2/BF haplotype and deleterious CFH and other gene SNPs between the races.18, 40–42 Known risk factors such as cigarette smoking, history of alcohol drinking, body mass index, hypertension status, diabetes status, measures of subclinical CVD, markers of inflammation or use of anti-inflammatory agents did not explain the differences in the incidence of AMD between blacks and whites in MESA. This was also consistent with the lack of an effect of these variables on white and black differences in the prevalence of any AMD in the baseline MESA examination.40, 43
In MESA, while adjusting for other factors, the odds of developing early AMD were not significantly different between whites and Hispanics. In the population-based Los Angeles Latino Eye Study (LALES),11 the 4-year incidence of early AMD was 7.5% and late AMD was 0.2%, lower than that found in non-Hispanic whites in the Beaver Dam Eye Study or the Blue Mountains Eye Study.44–45 In the LALES, the lower prevalence and incidence of late AMD was attributed to a lower frequency of persons homozygous for the CFH Tyr402His polymorphism variant, which was present in only 3% of the Mexican-American population compared to 9% to 21% of whites in the Beaver Dam Eye Study. In MESA, the distributions of CFH RS1061170 were similar in Hispanics and whites.
Despite the infrequency of CFH RS1061170 in Chinese Americans in MESA, the incidence of early AMD was similar to that found in whites. Similar incidence of AMD in Chinese Americans and whites was also reported in another study using data from an administrative database,46 and a meta-analysis using pooled data suggested that whites and Asians have similar prevalence of early and late AMD.47 Furthermore, studies have shown differences in the frequency of major genes and the impact of known AMD-susceptibility genes on Asians and whites.48 Similary, in MESA, the prevalence and incidence of early AMD in Chinese Americans may be partly explained by differences between Chinese Americans and whites in the distributions of genes associated with early AMD. In earlier analyses,21 we identified six SNPs of five genes, ARMS2 RS10490924, PLEKHA1 RS4311997 and RS2421018, MRPL10 RS3209, ABCA4 RS548122, and SOD3 RS2284659 to be associated with the prevalence of early AMD. In all six of these SNPs, the risk allele was more frequent in Chinese Americans than in whites. The higher frequency of these risk alleles in Chinese Americans may have explained a similar incidence of early AMD as whites despite having a low frequency of CFH RS1061170.
In MESA, we examined factors associated with pathways such as atherosclerosis, lipids, inflammation, oxidative stress that were hypothesized to play a role in the pathogenesis of AMD. While adjusting for age, sex, and race/ethnicity, we found few associations that were statistically significant. These included relationships between current smoking and hyperopia, both of which were previously reported as important factors associated with AMD.19, 49 The non-statistically significant but elevated odds ratio associated with astigmatism in the multivariable model deserves further consideration in subsequent studies, particularly since participants in this study were significantly less likely to have astigmatism compared to those at baseline who did not contribute follow-up data. There were also a number of borderline associations including a 35% lowering of the odds of incident early AMD in those with a positive history of using lipid lowering agents compared to those not taking these medications and a 56% increase in the odds of those with a history of taking diuretics. Greater IMT thickness of the internal carotid artery was weakly and directly and large artery elasticity was weakly and inversely associated with incident early AMD. These results were consistent with findings from the Beaver Dam Eye Study, where internal carotid IMT and pulse pressure were directly related to the incidence of AMD.50 Elastin loss associated with atherosclerotic peripheral vascular disease has been reported to be associated with the presence of AMD.51 Our study was underpowered to examine associations of many factors with the incidence of early AMD within the racial/ethnic groups because of their infrequency and/or because of the low incidence of early AMD in the study.
Although our study has many strengths (objective grading of AMD and a large multiethnic cohort), caution must be exercised when interpreting the findings for several reasons. Chance, bias and unadjusted confounding may have affected the interpretation of our findings. Due to the lower sensitivity of color digital images used in MESA for detecting subretinal vessels or pseudodrusen in the macular area compared to more recent studies using spectral domain optical coherence tomography (SD-OCT), their presence would likely be underestimated in MESA. The power to detect some associations may have been limited by the low incidence of AMD and its specific lesions and also some of the risk factors under study, particularly in analyses stratified by race/ethnicity. Not all factors were measured in the entire sample, further reducing the study power. Finally, biases that affected the relationships may have been caused by nonparticipation or selective mortality as those who participated in the follow-up were younger and generally healthier.
In summary, we report 8-year incidence of AMD in four racial/ethnic groups in the U.S. Results from this study on incident AMD are consistent with the prevalence data showing unexplained lower prevalence and incidence in blacks compared to whites. Age, race/ethnicity, smoking status, hyperopia, and AMD-susceptibility genotypes CFH RS1061170 and ARMS2 RS3793917 were associated with the incidence of early AMD when examining the whole cohort. However, no risk factors besides age were consistently associated with incident early AMD among the four racial/ethnic groups. The genetic, clinical and environmental measures assessed in the study did not explain racial/ethnic differences in AMD incidence.
Supplementary Material
Acknowledgments
Financial Support
MESA and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-001079, UL1-TR-000040, and DK063491. Funding support for the eye datasets were provided by grant HL069979 and by an award from the NIH Intramural Research Program ZIAEY000403. Cardiometabochip genotyping data was supported in part by grants and contracts R01HL98077, N02-HL-64278, HL071205, UL1TR000124, DK063491, RD831697, and P50 ES015915. The funders had no Incidence of AMD in Four Racial/Ethnic Groups in the United States role in data collection, management, analysis and interpretation of the data, preparation, writing and approval of the manuscript, or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
The authors have no proprietary or commercial interest in any materials discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BEK, Knudtson MD, et al. Prevalence of age-related macular degeneration in four racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Ophthalmology. 2006;113:373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Chou CF, Klein BE, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 4.Cheung CM, Tai ES, Kawasaki R, et al. Prevalence and risk factors for age-related macular degeneration in a multiethnic Asian cohort. Arch Ophthalmol. 2012;130:480–486. doi: 10.1001/archophthalmol.2011.376. [DOI] [PubMed] [Google Scholar]
- 5.Munoz B, Klein R, Rodriguez J, et al. Prevalence of age-related macular degeneration in a population-based sample of Hispanic people in Arizona: Proyecto VER. Arch Ophthalmol. 2005;123:1575–1580. doi: 10.1001/archopht.123.11.1575. [DOI] [PubMed] [Google Scholar]
- 6.Cheung CM, Li X, Cheng CY, et al. Prevalence, racial variations, and risk factors of age-related macular degeneration in Singaporean Chinese, Indians, and Malays. Ophthalmology. 2014;121:1598–1603. doi: 10.1016/j.ophtha.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Joachim N, Mitchell P, Younan C, et al. Ethnic variation in early age-related macular degeneration lesions between white Australians and Singaporean Asians. Invest Ophthalmol Vis Sci. 2014;55:4421–4429. doi: 10.1167/iovs.14-14476. [DOI] [PubMed] [Google Scholar]
- 8.Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001;42:2237–41. [PubMed] [Google Scholar]
- 9.Wang JJ, Rochtchina E, Lee AJ, et al. Ten-year incidence and progression of age-related maculopathy: the blue Mountains Eye Study. Ophthalmology. 2007;114:92–8. doi: 10.1016/j.ophtha.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BEK, Knudtson MD, et al. Fifteen-Year Cumulative Incidence of Age-Related Macular Degeneration: The Beaver Dam Eye Study. Ophthalmology. 2007;114:253–62. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Varma R, Foong AWP, Lai M-Y, et al. Four-year incidence and progression of age-related macular degeneration: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149:741–51. doi: 10.1016/j.ajo.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Leeumen R, Klaver CC, Vingerling JR, et al. The risk and natural course of age-related maculopathy: follow-up at 6 ½ years in the Rotterdam study. Arch Ophthalmol. 2003;121:519–526. doi: 10.1001/archopht.121.4.519. [DOI] [PubMed] [Google Scholar]
- 13.Buch H, Nielsen NV, Vinding T, et al. 14-year incidence, progression, and visual morbidity of age-related maculopathy: the Copenhagen City Eye Study. Ophthalmology. 2005;112:787–798. doi: 10.1016/j.ophtha.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 14.Chang MA, Bressler SB, Munoz B, West SK. Racial differences and other risk factors for incidence and progression of age-related macular degeneration: Salisbury Eye Evaluation (SEE) project. Invest Ophthalmol Vis Sci. 2008;49:2395–2402. doi: 10.1167/iovs.07-1584. [DOI] [PubMed] [Google Scholar]
- 15.Leske MC, Wu SY, Hyman L, et al. Four-year incidence of macular changes in the Barbados Eye Studies. Ophthalmology. 2004;111:706–711. doi: 10.1016/j.ophtha.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Leske MC, Wu SY, Hennis A, et al. Nine-year incidence of age-related macular degeneration in the Barbados Eye Studies. Ophthalmology. 2006;113:29–35. doi: 10.1016/j.ophtha.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Myers CE, Meuer SM, et al. Risk alleles in CFH and ARMS2 and the Long-term Natural History of Age-Related Macular Degeneration, The Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131:383–92. doi: 10.1001/jamaophthalmol.2013.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holliday EG, Smith AV, Cornes BK, et al. Insights into the Genetic Architecture of Early Stage Age-Related Macular Degeneration: A Genome-Wide Association Study Meta-Analysis. PLoS ONE. 2013;8:e53830. doi: 10.1371/journal.pone.0053830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein R, Knudtson MD, Cruickshanks KJ, Klein BEK. Further observations on the association between smoking and the long-term incidence and progression of age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:115–21. doi: 10.1001/archopht.126.1.115. [DOI] [PubMed] [Google Scholar]
- 20.Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003;121:785–92. doi: 10.1001/archopht.121.6.785. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Li X, Kuo JZ, et al. Associations of candidate genes to age-related macular degeneration among racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Am J Ophthalmol. 2013;156:1010–1020. doi: 10.1016/j.ajo.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Meuer SM, Moss SE, et al. Detection of age-related macular degeneration using a nonmydriatic digital camera and a standard film fundus camera. Arch Ophthalmol. 2004;122:1642–6. doi: 10.1001/archopht.122.11.1642. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Davis MD, Magli YL, et al. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 26.Pan CW, Klein BE, Cotch MF, et al. Racial variations in the prevalence of refractive errors in the United States: the multi-ethnic study of atherosclerosis. Am J Ophthalmol. 2013;155:1129–1138. doi: 10.1016/j.ajo.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voight BF, Kang HM, Ding J, et al. The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet. 2012;8:e1002793. doi: 10.1371/journal.pgen.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson NB, Berardi C, Decker PA, et al. Trans-ethnic meta-analysis identifies common and rare variants associated with hepatocyte growth factor levels in the Multi-Ethnic Study of Atherosclerosis (MESA) Ann Hum Genet. 2015;79:264–274. doi: 10.1111/ahg.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein R, Klein BE, Tomany SC, et al. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam eye study. Ophthalmology. 2002;109:1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 30.Jonasson F, Fisher DE, Eiriksdottir G, et al. Five-year incidence, progression, and risk factors for age-related macular degeneration: the age, gene/environment susceptibility study. Ophthalmology. 2014;121:1766–1772. doi: 10.1016/j.ophtha.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudnicka AR, Kapetanakis W, Jarrar Z, et al. Incidence of late-stage age-related macular degeneration in American whites: systematic review and meta-analysis. Am J Ophthalmol. 2015;160:85–93. doi: 10.1016/j.ajo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Knudtson MD, Lee KE, et al. Age-period-cohort effect on the incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2008;115:1460–1467. doi: 10.1016/j.ophtha.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein R, Rowland ML, Harris MI. Racial/ethnic differences in age-related maculopathy. Third National Health and Nutrition Examination Survey. Ophthalmology. 1995;102:371–81. doi: 10.1016/s0161-6420(95)31012-3. [DOI] [PubMed] [Google Scholar]
- 34.Klein R, Klein BE, Jensen SC, et al. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–1065. doi: 10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 35.Klein R, Clegg L, Cooper LS, et al. Prevalence of age-related maculopathy in the Atherosclerosis Risk in Communities Study. Arch Ophthalmol. 1999;117:1203–1210. doi: 10.1001/archopht.117.9.1203. [DOI] [PubMed] [Google Scholar]
- 36.Klein R, Klein BE, Marino EK, et al. Early age-related maculopathy in the cardiovascular health study. Ophthalmology. 2003;110:25–33. doi: 10.1016/s0161-6420(02)01565-8. [DOI] [PubMed] [Google Scholar]
- 37.Bressler SB, Munoz B, Solomon SD, West SK. Racial differences in the prevalence of age-related macular degeneration: the Salisbury Eye Evaluation (SEE) project. Ophthalmology. 2008;126:241–245. doi: 10.1001/archophthalmol.2007.53. [DOI] [PubMed] [Google Scholar]
- 38.Weiter JJ, Delori FC, Wing GL, Fitch KA. Relationship of senile macular degeneration to ocular pigmentation. Am J Ophthalmol. 1985;99:185–187. doi: 10.1016/0002-9394(85)90230-2. [DOI] [PubMed] [Google Scholar]
- 39.Jampol LM, Tielsch J. Race, macular degeneration, and the Macular Photocoagulation Study. Arch Ophthalmol. 1992;110:1699–1700. doi: 10.1001/archopht.1992.01080240039024. [DOI] [PubMed] [Google Scholar]
- 40.Klein R, Knudtson MD, Klein BE, et al. Inflammation, complement factor h, and age-related macular degeneration: the Multi-ethnic Study of Atherosclerosis. Ophthalmology. 2008;115:1742–1749. doi: 10.1016/j.ophtha.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hageman GS, Hancox LS, Taiber AJ, et al. Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med. 2006;38:592–604. [PMC free article] [PubMed] [Google Scholar]
- 42.Grassi MA, Fingert JH, Scheetz TE, et al. Ethnic variation in AMD-associated complement factor H polymorphism p.Tyr402His. Hum Mutat. 2006;27:921–925. doi: 10.1002/humu.20359. [DOI] [PubMed] [Google Scholar]
- 43.Klein R, Klein BE, Knudtson MD, et al. Subclinical atherosclerosis cardiovascular disease and early age-related macular degeneration in a multiracial cohort: the Multiethnic Study of Atherosclerosis. Arch Ophthalmol. 2007;125:534–543. doi: 10.1001/archopht.125.4.534. [DOI] [PubMed] [Google Scholar]
- 44.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109:1092–1097. doi: 10.1016/s0161-6420(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 46.Stein JD, Vanderbeek BL, Talwar N, et al. Rates of nonexudative and exudative age-related macular degeneration among Asian American ethnic groups. Invest Ophthalmol Vis Sci. 2011;52:6842–6848. doi: 10.1167/iovs.11-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117:921–927. doi: 10.1016/j.ophtha.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Cheng CY, Yamashiro K, Chen LJ, et al. New loci and coding variants confer risk for age-related macular degeneration in East Asians. Nat Commun. 2015;6:6063. doi: 10.1038/ncomms7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan CW, Ikram MK, Cheung CY, et al. Refractive errors and age-related macular degeneration: a systematic review and meta-analysis. Ophthalmology. 2013;120:2058–2065. doi: 10.1016/j.ophtha.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 50.Klein R, Klein BE, Jensen SC. The relation of cardiovascular disease and its risk factors to the 5-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:1804–1812. doi: 10.1016/s0161-6420(97)30023-2. [DOI] [PubMed] [Google Scholar]
- 51.Chen K, Weiland JD. Discovery of retinal elastin and its possible role in age-related macular degeneration. Ann Biomed Eng. 2014;42:678–684. doi: 10.1007/s10439-013-0936-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.