Abstract
The cellular turnover required for skeletal muscle maintenance and repair is mediated by resident stem cells, also termed satellite cells. Satellite cells normally reside in a quiescent state, intermittently entering the cell cycle to fuse with neighboring myofibers and replenish the stem cell pool. However, the mechanisms by which satellite cells maintain the precise balance between self-renewal and differentiation necessary for long-term homeostasis remain unclear. Recent work has supported a previously unappreciated heterogeneity in the satellite cell compartment that may underlie the observed variability in cell fate and function. In this review, we examine the work supporting this notion as well as the potential governing principles, developmental origins, and principal determinants of satellite cell heterogeneity.
Heterogeneity in the Satellite Cell Compartment
Satellite cells were originally identified via electron microscopy in 1961 by Alexander Mauro, located underneath the basal lamina and adjacent to the plasma membrane of the skeletal muscle myofiber [1]. Remarkably, Mauro correctly predicted the origin and function of satellite cells as remnants of embryonic development, prepared to recapitulate this process following muscle injury. Grafting experiments demonstrated that endogenous myogenic cells directly participate in myofiber repair [2], but direct evidence identifying satellite cells as the resident stem cell population remained elusive for several years.
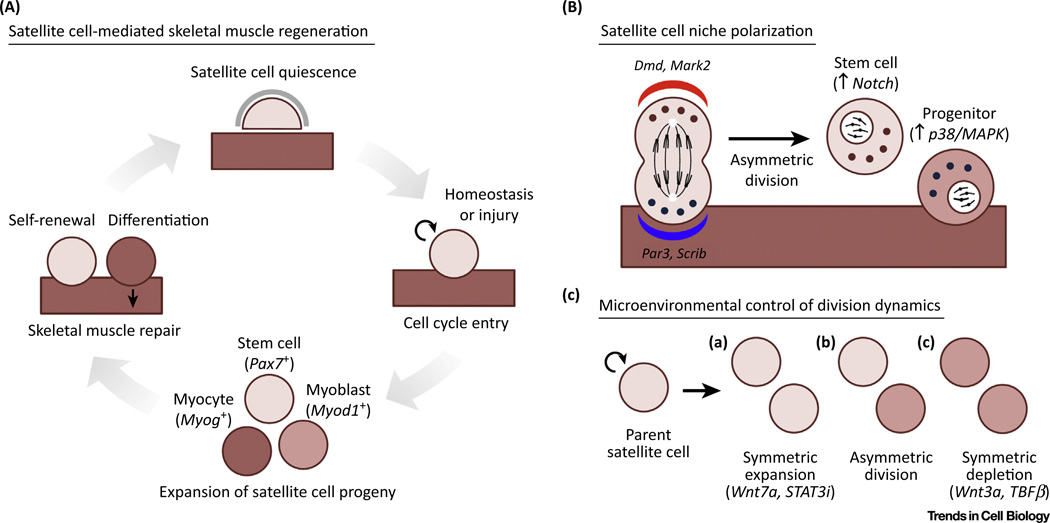
The transcriptional program supporting stem cell function in undifferentiated myogenic cells is dependent upon the paired-box transcription factors Pax3 and Pax7. Pax3 is first expressed in the presomitic mesoderm during development and is required for limb muscle formation, cell survival, and migration [3]. Pax7 was shown to be required for postnatal muscle growth and population of the satellite cell pool [4]. Ablation of both Pax3 and Pax7 allowed satellite cells to adopt alternative cell fates, confirming their crucial role in maintaining myogenic identity [4,5]. The basic helix-loop-helix (bHLH) factors Myod1, Myf5, Myf6 (also known as MRF4) and myogenin, known collectively as the myogenic regulatory factors (MRFs), then act sequentially to advance satellite cells towards myogenic differentiation and fusion to form multinucleated myofibers [6]. The upregulation of Myf5, followed by Myod1, are required for myogenic determination [7,8]. Myogenin works downstream to trigger advancement to the myocyte stage and subsequent terminal differentiation [9]. Reciprocal inhibition exists between Pax7 and the MRFs Myod1 and Myog [10], but neither Pax3 or Pax7 interfere with Myf5 expression [11]. Together, these findings led to the classification of three distinct states as satellite cells differentiate: (i) Pax7+ cells that maintain the stem cell pool, (ii) Myod1+ myogenic progenitors that have entered the myogenic program, and (iii) Myogenin+ myocytes primed for fusion with existing or newly formed myofibers (Figure 1A).
Figure 1. Modes of Satellite Cell Self-Renewal.
(A) Stages of satellite cell-mediated skeletal muscle regeneration. (B) Regulation of daughter cell fate achieved by polarization in the satellite cell niche. (C) Symmetric and asymmetric division events in satellite cells controlled by soluble factors in the microenvironment.
A major hurdle towards assaying the functional potential of satellite cells was overcome by the identification of specific cell surface markers, allowing researchers to employ fluorescence-activated cell sorting (FACS) strategies for their prospective isolation [12]. Intramuscular transplantation of sorted satellite cells revealed their robust capacity for muscle repair and ability to colonize the satellite cell niche. Real-time assessment of satellite cells enabled the dynamic quantification of their expansion and responsiveness to regenerative stimuli [13]. Recombination-based labeling strategies to monitor endogenous satellite cell behavior substantiated these stem cell properties [14–16]. Finally, proper muscle regeneration failed following the genetic ablation of satellite cells [17–19], resolving their identity as a genuine somatic stem cell population indispensable for skeletal muscle repair.
Attempts to more rigorously assess satellite cell behavior uncovered a significant cellular heterogeneity. Clonal analyses revealed variability in gene expression and proliferation dynamics, including time to first division and rate of division [20–22]. These findings were confirmed on myofiber-associated satellite cells, supporting these traits as an inherent property rather than artifact of the isolation procedure [22–24]. Variance in regenerative properties was first evaluated by single myofiber grafting, where donor cell contribution was not proportional to the initial number of associated satellite cells per myofiber [25]. Single cell transplantation experiments later provided conclusive evidence of stem cell behavior at the clonal level, but only in a subset of satellite cells [13]. Functional repopulation assays verified the capacity of satellite cells for long-term self-renewal over serial rounds of regeneration but also observed disparity with regard to repopulation efficiency [26,27]. Altogether, these results support an appreciably complex level of heterogeneity in the satellite cell pool that warrants further investigation.
In this review, we discuss the principles and developmental origins underlying satellite cell heterogeneity. Although several studies have described behavioral diversity on the basis of myofiber type association [28,29] or embryological origin, including those derived from craniofacial and extraocular muscles [30,31], we focus on satellite cells of somitic origin that reside in the limb. A discussion of cellular behavior at the population level summarizes our understanding of the potential basic tenets of satellite cell heterogeneity. Finally, we examine the origin of pool heterogeneity during developmental myogenesis and the implications of key events that occur during this process.
Modes of Self-Renewal in Satellite Cells
To meet the changing needs of skeletal muscle over time, robust mechanisms are required to dynamically modify and later re-establish satellite cell heterogeneity. Division modes, including asymmetric and symmetric self-renewal, contribute to the proportional generation of stem cells and progenitors during tissue repair.
Asymmetric satellite cell self-renewal is advantageous in many circumstances because it produces both stem cells and progenitors after only one round of cell division. When and how the management of daughter cell fate is controlled, however, is still not fully understood. Pioneering studies on myofiber-associated satellite cells in suspension cultures strongly endorse the niche regulation of division asymmetry, wherein the orientation of the mitotic spindle relative to the myofiber axis is associated with daughter cell fate [32]. Many satellite cell divisions were classified as planar, meaning both daughter cells maintained contact with the myofiber. However, asymmetric apical-basal divisions result in only the basal daughter cell advancing through the myogenic program, while the apical daughter cell, which has lost contact with the myofiber, resists MRF upregulation. The Notch signaling pathway integrates juxtacrine, or contact-dependent, information to promote satellite cell self-renewal [33]. Suitably, Notch effector proteins Notch3 and Delta1, a Notch ligand, are partitioned asymmetrically at the surface of either daughter cell following division [32]. While Numb, a contextual Notch antagonist, is also asymmetrically segregated following a subset of satellite cell divisions, it has not consistently been associated with myogenic differentiation [34,35]. Although Numb-deficient satellite cells exhibit myostatin-dependent proliferative defects [36], Numb is also able to prevent p53-dependent senescence during muscle repair [37]. Further studies will be required to clarify the mechanistic role of Numb during division asymmetry.
Continued work demonstrated that factors controlling cell polarity participate in the regulation of asymmetric satellite cell division (Figure 1B). Components of the evolutionarily conserved Par complex, a well-studied set of molecules able to establish cell polarity [38], are required for the asymmetric initiation of myogenic differentiation [39]. This is accomplished by selectively activating the stress-responsive p38α/β MAPK pathway to directly regulate Myod1 transcription [40]. Another member of the cell polarity complex, Scrib, is upregulated during satellite cell activation and also asymmetrically distributed to the progenitor-like daughter cell [41]. Despite its traditional role as a protein essential for myofiber membrane stability, dystrophin also cooperates with Par proteins to regulate cell polarity during asymmetric division [42]. Thus, the absence of dystrophin impairs the generation of progenitors required for muscle repair, adding satellite cell dysfunction to its complex role in muscular dystrophy pathology [43].
Soluble factors are also capable of altering satellite cell fate, augmenting their symmetric expansion in a heterogeneous manner (Figure 1C). Lineage tracing has identified a subset of satellite cells having never expressed Myf5 [32]. This putative stem cell reservoir is capable of improved niche engraftment and preferentially expresses the Wnt7a receptor Fzd7 [44]. Non-canonical Wnt signaling activates the planar cell polarity pathway and promotes symmetric self-renewal, suggesting that this subset is preferentially capable of rapid growth. Pharmacological inhibition of the JAK-STAT signaling pathway stimulated symmetric stem cell divisions and improved satellite cell expansion in both aged and dystrophic muscle [45,46]. Conversely, Wnt3a and transforming growth factor β (TGFβ) signaling oppose self-renewal, coordinating their differentiation and contribution to muscle repair [47,48]. Switching between asymmetric and symmetric modes of division might allow for the efficient generation of satellite stem cells or differentiated progeny during more dynamic periods of tissue growth and repair. Together, these findings help to establish a hierarchical organization within the satellite cell pool, achieving more nuanced levels of control to tightly regulate the regenerative process.
Design Principles Underlying Satellite Cell Heterogeneity
Although studying functional diversity in the satellite cell pool from the standpoint of cell division may be mechanistically informative, it can be difficult to reconcile in terms of whole muscle repair. When studied as a population, these changes in behavioral diversity can be properly contextualized. To appreciate how satellite cell heterogeneity as a whole is maintained, knowledge of the fundamental tenets governing satellite cell fate is needed.
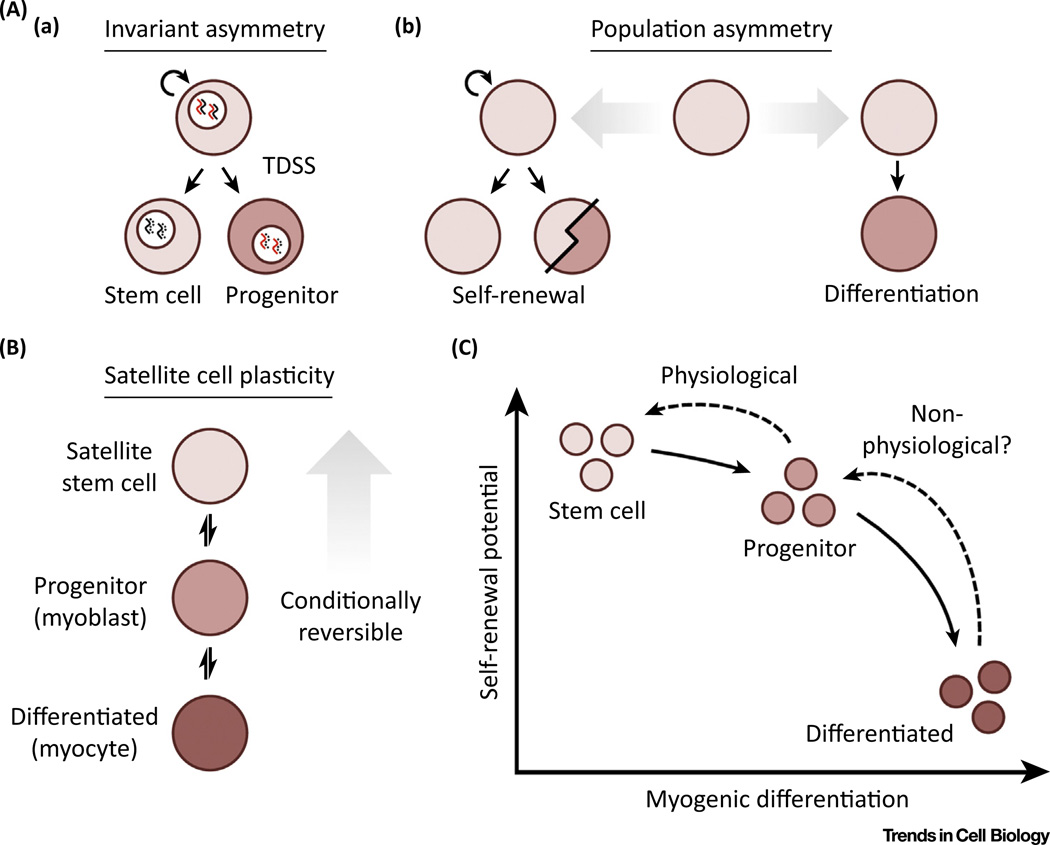
Despite considerable evidence for a hierarchical structure within the satellite cell pool, it is unclear whether the position of a satellite cell within this hierarchy is predetermined or stochastic in nature. An improved understanding for the role of asymmetric divisions in satellite cells has lent support to a model of invariant, or division, asymmetry (Figure 2A). In this scenario, satellite cells undergo prolonged, asymmetric self-renewal to accomplish the enduring maintenance of skeletal muscle as long-lived clones. This aligns with the immortal DNA strand hypothesis, a proposed mechanism by which adult stem cells minimize genomic mutation [49]. Pulse-chase experiments have determined that only satellite cells with relatively high levels of Pax7 are able to reproducibly perform template DNA strand segregation (TDSS) with single chromatid resolution [26]. Pax7HI satellite cells also displayed stem cell markers, a lower metabolic state and longer time to first division, associating phenotypic distinctions with TDSS [26].
Figure 2. Design Principles Underlying Satellite Cell Heterogeneity.
(A) Models of satellite cell behavior: (a) invariant, or division, asymmetry, and (b) population asymmetry. (B) Conditionally reversible plasticity between satellite stem cells and their differentiating progeny. (C) Likelihood of conversion between cellular states as a function of self-renewal potential and myogenic differentiation status.
While Pax7HI satellite cells performing TDSS may represent an enduring stem cell subpopulation, work in the male germline shows that non-random sister chromatid segregation is often interrupted and without a clear proliferative or differentiation phenotype, questioning its ability to truly protect against errors in DNA replication [50]. Relative levels of Pax7 expression, despite being associated with TDSS, are not predictive of improved regenerative capacity or satellite cell niche engraftment [26]. Cell adhesion regulates DNA segregation and satellite cell fate during asymmetric division in situ and in vitro [35,51,52]; thus, monitoring satellite cell division dynamics within their native microenvironment is of critical importance. Intravital imaging experiments have observed apical-basal division events in vivo less frequently than has been reported on isolated single myofibers [22,53]. Reorienting the remaining basal lamina by surgical manipulation increased the rate of apical-basal division but led to disorganized tissue repair, indicating that this 3D architecture favors planar division for proper regeneration [53]. While these findings do not by themselves invalidate this theory, more work is needed to substantiate the long-term sustainability of invariant asymmetry in satellite cells.
If satellite cell fate over time is not predetermined, then how is this order sustained? A model of population asymmetry may offer insight (Figure 2A). Here, the self-renewal of neighboring satellite cells replace those lost to differentiation, balancing population-level heterogeneity. In this model, stemness is more evenly distributed and cell fate determination is apparently stochastic. Efforts to distinguish between these possibilities through in vivo lineage tracing have argued for population asymmetry in several tissues [54–57]. These studies have largely observed neutral drift dynamics, a process of competitive scaling behavior where increased clonality is progressively reached through clone loss and compensatory expansion of neighboring clones. These findings do not rule out intrinsic factors as a contributing variable, especially in pathological circumstances, as genetic perturbations and environmental disruption can lead to non-neutral competition and altered fitness [58,59]. However, they do suggest that intrinsic molecular determinants are not sufficient for long-term self-renewal under homeostatic circumstances. Instead, daughter cell fate is highly subject to spatially ordered, external determinants. This aligns with the causative relationship between disease progression in dystrophic muscle and alterations to the stem cell microenvironment that challenge homeostatic equilibrium [60]. The reductions in satellite cell number and expansion potential with age are also consistent with clone loss over time [61–63]. Typical levels of clonal complexity in the satellite cell pool may succumb to selective external pressures over time, thereby contributing to neutral drift and clonal collapse.
Alternatively, these studies might fail to assess the behavior of stem cells that rarely enter the cell cycle. Satellite cells atop the hierarchy may be maintained as an emergency stem cell reserve while less potent satellite cells are burdened with homeostatic maintenance. Label-retaining cells (LRCs) identified by the histone H2B-GFP/YFP pulse-chase systems may represent these nondividing stem cells. LRCs more efficiently contribute to muscle repair and stem cell pool repopulation following transplantation from healthy, aged, and dystrophic muscle [61,64]. A novel strategy to trace intestinal crypt LRCs using Cre-complementation found that these cells produce differentiated cell types following acute injury but not during homeostasis [65], indicating that these may be two fundamentally different processes controlled by different stem cell subsets. This is supported by the longitudinal assessment of clonal dynamics in the hematopoietic system via transposon-based cellular labeling [66]. Although long-lived stem cells predominantly contribute to the peripheral blood following transplantation, a more complex clonal succession of multipotent progenitors was found to drive steady-state hematopoiesis. Further studies are required to determine whether any of these models of clonal stem cell behavior accurately describes healthy, physiological satellite cell activity.
Plasticity in the Satellite Cell Compartment
One intriguing possibility is that functional diversity might not result from static heterogeneity but from a greater plasticity within the stem cell hierarchy than previously appreciated. Although transdifferentiation to fibrogenic and adipogenic fates has been described in satellite cells [67,68], here we focus on plasticity within the myogenic lineage. Stem cell populations, including satellite cells, are often thought to move linearly and irreversibly towards terminal differentiation. However, this concept is now being challenged (Figure 2B). Combining long-term lineage tracing with in vivo live imaging, several groups have described reversibility between stem cells and their differentiating progeny in different stem cell compartments [69–72]. Oscillation in the expression of key cell fate genes can regulate hematopoietic and neural stem cell multipotency, implicating stochastic processes [73,74]. Laser ablation of specific cell populations in the hair follicle has demonstrated that fate switching can be achieved through spatial repositioning within the niche [75]. In this context, either stem cells or differentiated progeny are dispensable for regeneration so long as the other remains and their interactions with support cell types are preserved. Enterocyte progenitors in the intestinal crypt do not normally exhibit stem cell activity but can revert to a multipotent state following acute injury [76]. Their acquisition of self-renewal potential is position-dependent, as resident stem cell depletion allows enterocytes to populate the stem cell niche at the base of the intestinal crypt. True to form, the progeny of dedifferentiated progenitors again lose their newly acquired stemness once exiting the stem cell niche. Therefore, progenitor cells may only be temporarily predisposed to differentiation and competent for self-renewal in the correct environmental setting. Evidence of the Markov assumption, stating the stochastic nature of cell fate adoption is primarily influenced by the present state, has been found in hematopoietic and cancer stem cells [77,78]. Lineage hierarchies may therefore be best described not by a conventional tree structure but instead by bidirectional cell state transition probabilities (Figure 2C).
Although not yet formally tested in skeletal muscle, satellite cells might also transition between behavioral states during regeneration as part of a dynamic equilibrium, functionally variable at the single cell level but stable as a whole over time. In this scenario, variance in cell division dynamics would not be categorically required to alter stem cell or progenitor pool size. For example, differentiation factors would be predicted to decrease as sufficient myonuclear accretion is reached, driving the remaining undifferentiated cells to the satellite cell pool. This competition-driven plasticity would preserve population diversity in the event of a loss or impairment to any satellite cell subset, mitigating this risk through diversification and increasing the overall robustness of the system. Again, variation in niche signaling in this model becomes the primary means of control over satellite cell heterogeneity. Resident support cell types in skeletal muscle, including fibroadipogenic progenitors (FAPs) and PW1/Peg3+ interstitial cells, play a key role in this process through the production of trophic factors [79–81]. Paracrine secretion of the extracellular matrix (ECM) molecule fibronectin may allow for communication between satellite cell subsets themselves in the context of asymmetric division [82]. These findings place additional emphasis on achieving a better understanding of external cues and cell-cell communication to define the potentially dynamic satellite cell fate status.
The Developmental Origins of Satellite Cell Heterogeneity
Future work examining the fundamental organization and plasticity of satellite cells will require analysis over a sufficient period of stem cell activity. However, skeletal muscle is a slow-turnover tissue. The low number of cell divisions prior to the emergence of aging factors may make it increasingly difficult to separate the physiological and pathological stimuli that drive changes in satellite cell pool composition over time [61]. Instead, the formation and growth of skeletal muscle during development includes a large number of cell fate decisions in a relatively short period of time. Many of these cells ultimately populate the satellite cell pool, as lineage tracing initiated in the dermomyotome has established a somitic origin for satellite cells [83]. Thus, developmental myogenesis may be an appropriate context for the study of satellite cell heterogeneity.
Behavioral heterogeneity emerges quickly in satellite cell precursors, given the differences in proliferative rate observed during embryonic myogenesis [84]. MRF expression patterns vary in prenatal muscle despite their functional redundancy [5,85]. The ablation of Myf5+ cells only temporarily delays myogenesis before full recovery during the late fetal stages [86,87], consistent with the possible satellite stem cell reserve having never expressed Myf5 [32]. The existence of Myod1-independent satellite cell precursors is argued against by lineage tracing, demonstrating that all satellite cells have expressed Myod1 in their developmental history [88]. Ablation of Myod1+ cells abruptly halts myofiber formation during primary myogenesis and eliminates Pax7+ cells by fetal stages [89]. However, the eventual downregulation of Myod1 in cells populating the adult satellite cell niche is consistent with the conditional reversibility of progenitor fate acquisition during development. Future experiments dynamically monitoring Myod1 expression are required to determine its contribution to cellular heterogeneity.
While suitable for studying cellular heterogeneity, developmental studies also offer potential for the investigation of factors involved in the origin of heterogeneity. In particular, the transition to a quiescent state marks a pivotal moment in satellite cell specification. Quiescence is thought to preserve stem cell function, avoiding stress and preserving genomic integrity. However, little is known about the induction of quiescence or the mechanisms by which it is achieved. The functional implications are significant, as cycling activity is inversely correlated to long-term self-renewal potential with age and through telomere shortening [60,90,91]. LRCs, likely the early adopters of quiescence, are afforded enhanced self-renewal potential later in adult life [64]. Although they cannot be firmly distinguished from slowly proliferating cells, concomitant down-regulation of MRF expression and cell cycle inhibitor p27kip1 upregulation supports the notion that early entry into quiescence is responsible.
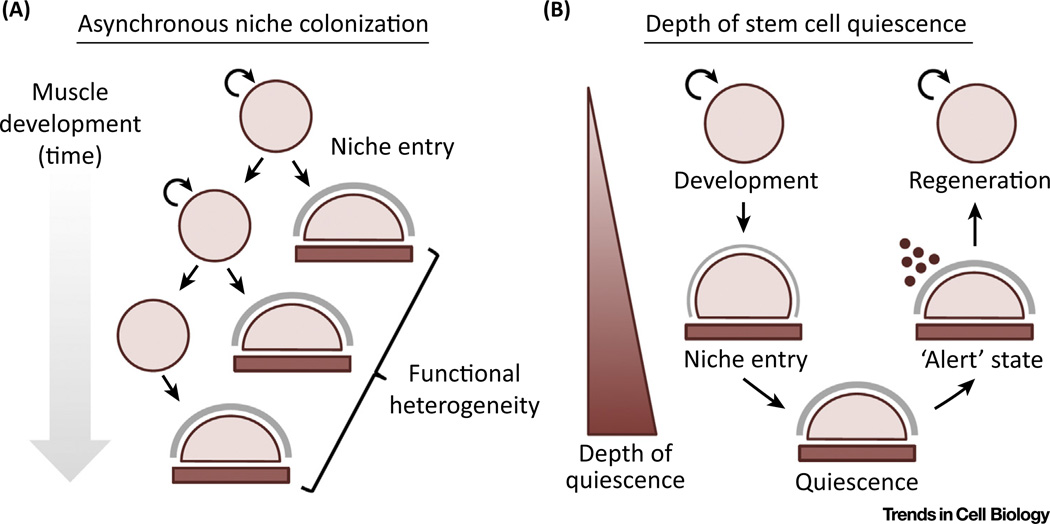
Maturation of the skeletal muscle framework allows for the acquisition of satellite cell fate by anatomical definition only following the late fetal stages [5,85]. Notably, Rbpj-mediated Notch activity in satellite cell precursors themselves is required for niche colonization [92]. The expression of adhesion molecules and assembly of basal lamina components supports the reciprocal nature of microenvironmental interactions and the active role satellite cells play in this process. The Notch ligand Delta is also present on the myofiber surface and positively regulates this transition [93]. Autonomous ECM remodeling underlies distinct stem cell functionalities observed between fetal satellite cell precursors and their adult counterparts, regulating their regenerative potential in a stage-specific manner [94]. Fetal satellite cell precursors were able to more efficiently contribute to muscle repair but less efficient in colonizing the niche, reflective of their primary responsibilities during development or regeneration and potentially adapted over time via selective processes. Thus, the asynchronous entry into the satellite cell niche prior to quiescence may introduce a new level of functional heterogeneity to the satellite cell compartment (Figure 3A).
Figure 3. Developmental Origins of Satellite Cell Heterogeneity.
(A) Contribution of the asynchronous entry into the satellite cell niche to population-level functional heterogeneity. (B) The degree of satellite cell quiescence during development and adult regeneration.
Once quiescence is achieved, key signaling pathways are known to aid its maintenance through inherent mechanisms and protection from environmental stressors, a dynamic that becomes increasingly tenuous with age. Active Notch signaling is required for the maintenance of quiescence [95,96] but is antagonized by excessive levels of TGFβ-dependent pSmad3 activity [48]. Sublaminar niche integrity is also compromised by high levels of the mitogen FGF2 with age, resulting in a loss of quiescence [61]. Sprouty-1 (Spry1), an inhibitor of FGF signaling, can overcome this defect [97]; however, its expression is only retained in the LRC fraction. Whether Spry1 activity is inherently variable or heterogeneously influenced by niche deterioration is uncertain. Calcitonin receptor, a G-protein-coupled receptor (GPCR), suppressed emergence from the niche in a cAMP-dependent manner [98]. Angiopoietin-1/Tie2/TEK signaling and ERK1/2 pathway activation promote satellite cell quiescence [99]. Finally, the selective translational silencing of cell cycle and MRF proteins, achieved by micro RNAs, RNA granule assembly, and the inhibition of translation initiation, preserves the quiescent state and can modify self-renewal potential [100–102].
In addition, heterogeneity has been described with regard to metabolic activity [26]. Basal macroautophagy and proteostasis can become disrupted with age, inducing a switch from reversible quiescence to a p16INK4a-dependent, presenescent state [91,103]. Strategies to augment autophagy prevented senescence and restored their regenerative capacity. SIRT1, a histone deacetylase, links epigenetic regulation to the switch from oxidative to glycolytic metabolism during satellite cell activation [104]. SIRT1 is elevated during quiescence, prevents premature differentiation, and is required for proper regenerative function. Ambiguity regarding the uniformity of quiescence has been raised by the description of a reversible, 'alert' state, characterized by increased mTORC1 signaling, altered transcriptional and metabolic profiles, and enhanced regenerative potential [105]. Key unresolved issues, including whether similar disparities in the depth of quiescence are present normally within unperturbed satellite cells, might be addressed by studying the induction, rather than the maintenance, of satellite cell quiescence (Figure 3B). In any case, the ubiquity and relative stability of quiescence in satellite cells when compared with other high-turnover stem cell compartments provides a unique opportunity for its study as a potential driver of pool heterogeneity.
Concluding Remarks
Skeletal muscle, similar to several other tissues, relies on resident stem cell populations for its life-long homeostatic maintenance and repair. Tight regulation of their composition and activity is key in accomplishing these tasks, as any imbalance between cells intended to self-renew or differentiate may result in progressive tissue failure or tumorigenesis. Therefore, understanding single cell behavior to achieve a population-level understanding of the satellite cell compartment is of critical importance (see Outstanding Questions).
Outstanding Questions.
When and how is the management of daughter cell fate controlled following satellite cell division? To what extent is this process regulated by either intrinsic or extrinsic determinants in the context of skeletal muscle homeostasis?
Is the position of a satellite cell within the hierarchy predetermined or stochastic in nature? Can clonal activity in satellite cells be modeled over time to support either invariant (division) or population asymmetry?
Are the homeostatic maintenance and repair of skeletal muscle distinct processes controlled by different satellite cell subsets? If so, do individual cells maintain their distinct functionality over time? How do these subpopulations interact or communicate with each other to maintain the desired level of population-level heterogeneity?
Can satellite cells reversibly transition between different stages of myogenic differentiation? Can satellite cells repopulate behavioral subgroups if ablated prior to skeletal muscle regeneration? At what point during the differentiation process are satellite cells no longer able to reacquire self-renewal potential?
To what extent is the timing of local niche colonization controlled by satellite cell precursors themselves or environmental factors? When is satellite cell quiescence accomplished once within the satellite cell niche? Once achieved, are there any disparities in the depth of quiescence across the satellite cell pool and is this functionally relevant?
What effect do pathological states, including degenerative disease or aging, have on the governing principles regulating stem cell heterogeneity and cell fate determination?
Determining whether cell fate can be modeled in terms of invariant or population asymmetry would constitute a conceptual advancement in the field. Single cell analysis will assist the identification of cellular subpopulations and cell-to-cell variations. Recombination-based reporter strategies for quantitative lineage tracing would enable clonal composition analyses, longitudinal fate mapping, and the resolution of single cell phylogenies. These studies would also be able to decipher the ability of and conditions required for satellite cell plasticity across the stem cell hierarchy. In parallel, efforts to recapitulate niche characteristics in vitro by bioengineering methods and their cross-validation in vivo would advance our understanding of the microenvironment’s influence during satellite cell fate decisions [21]. Lastly, improved sorting strategies have made the study of cellular heterogeneity increasingly possible in human satellite cells [106,107] and would justify the clinical relevance of further study in this area.
Satellite cells constitute a very promising tool for regenerative medicine approaches. Maintaining their number and function is of particular relevance to skeletal muscle pathologies, including aging or genetic diseases [42,60,61]. Furthering our understanding of the underlying molecular mechanisms and fundamental aspects of stem cell heterogeneity will be relevant to clinical applications exploiting somatic stem cell populations, either through cell replacement or pharmacological manipulation.
Trends.
Satellite cells are a functionally heterogeneous pool of skeletal muscle stem cells, differentially able to generate hierarchically composed pools of stem and progenitor cells during tissue regeneration.
Accumulating evidence supports the dominant role of microenvironmental signaling and stochastic acquisition of satellite cell fate in coordinating satellite cell fate and heterogeneity.
Reversible and competitive plasticity between functional states of self-renewal and early differentiation may underlie overall fitness and the preservation of population diversity during tissue repair.
Developmental myogenesis offers a physiological context to study the evolution of heterogeneity during satellite cell specification as it is critically regulated by local niche entry and the induction of quiescence.
Acknowledgments
A.S. is Associate Professor at Sanford Burnham Prebys Medical Discovery Institute (SBPMDI) and acknowledges support from the National Institutes of Health (NIH) (P30AR06130303, R01AR064873, R03AR063328), the Muscular Dystrophy Association, and the Ellison Medical Foundation (EMF). M.T.T. is a graduate student in the Graduate Program of Biomedical Sciences at SBPMDI and acknowledges support from the NIH (F31AR06592301). We apologize to authors whose work could not be cited owing to space limitations.
References
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge TA, et al. Evidence of fusion between host and donor myoblasts in skeletal muscle grafts. Nature. 1978;273:306–308. doi: 10.1038/273306a0. [DOI] [PubMed] [Google Scholar]
- 3.Tajbakhsh S, et al. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 4.Seale P, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 5.Relaix F, et al. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 6.Weintraub H, et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 7.Rudnicki MA, et al. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- 8.Rudnicki MA, et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 9.Venuti JM, et al. Myogenin is required for late but not early aspects of myogenesis during mouse development. J. Cell Biol. 1995;128:563–576. doi: 10.1083/jcb.128.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olguin HC, et al. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J. Cell Biol. 2007;177:769–779. doi: 10.1083/jcb.200608122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relaix F, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin H, et al. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacco A, et al. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunther S, et al. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell. 2013;13:590–601. doi: 10.1016/j.stem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishijo K, et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009;23:2681–2690. doi: 10.1096/fj.08-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Maltzahn J, et al. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 2013;110:16474–16479. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepper C, et al. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy MM, et al. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambasivan R, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 20.Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel AL, et al. Muscle satellite cell proliferation and association: new insights from myofiber time-lapse imaging. Skelet. Muscle. 2011;1:7. doi: 10.1186/2044-5040-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beauchamp JR, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zammit PS, et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Rocheteau P, et al. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 27.Hall JK, et al. Prevention of muscle aging by myofiber-associated satellite cell transplantation. Sci. Transl. Med. 2010;2:57ra83. doi: 10.1126/scitranslmed.3001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiMario JX, et al. Myoblasts transferred to the limbs of embryos are committed to specific fibre fates. Nature. 1993;362:165–167. doi: 10.1038/362165a0. [DOI] [PubMed] [Google Scholar]
- 29.Hughes SM, Blau HM. Muscle fiber pattern is independent of cell lineage in postnatal rodent development. Cell. 1992;68:659–671. doi: 10.1016/0092-8674(92)90142-y. [DOI] [PubMed] [Google Scholar]
- 30.Sambasivan R, et al. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev. Cell. 2009;16:810–821. doi: 10.1016/j.devcel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Stuelsatz P, et al. Extraocular muscle satellite cells are high performance myo-engines retaining efficient regenerative capacity in dystrophin deficiency. Dev. Biol. 2015;397:31–44. doi: 10.1016/j.ydbio.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuang S, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen Y, et al. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol. 2012;32:2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 35.Shinin V, et al. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat. Cell Biol. 2006;8:677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 36.George RM, et al. Numb-deficient satellite cells have regeneration and proliferation defects. Proc. Natl. Acad. Sci. U.S.A. 2013;110:18549–18554. doi: 10.1073/pnas.1311628110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Roux I, et al. Numb is required to prevent p53-dependent senescence following skeletal muscle injury. Nat. Commun. 2015;6:8528. doi: 10.1038/ncomms9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J. Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 39.Troy A, et al. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38alpha/beta MAPK. Cell Stem Cell. 2012;11:541–553. doi: 10.1016/j.stem.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palacios D, et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–469. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono Y, et al. Muscle stem cell fate is controlled by the cell-polarity protein scrib. Cell Rep. 2015;10:1135–1148. doi: 10.1016/j.celrep.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 42.Dumont NA, et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 2015;21:1455–1463. doi: 10.1038/nm.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 44.Le Grand F, et al. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price FD, et al. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 2014;20:1174–1181. doi: 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tierney MT, et al. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 2014;20:1182–1186. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brack AS, et al. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Carlson ME, et al. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 50.Yadlapalli S, Yamashita YM. Chromosome-specific nonrandom sister chromatid segregation during stem-cell division. Nature. 2013;498:251–254. doi: 10.1038/nature12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conboy MJ, et al. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yennek S, et al. Cell adhesion geometry regulates non-random DNA segregation and asymmetric cell fates in mouse skeletal muscle stem cells. Cell Rep. 2014;7:961–970. doi: 10.1016/j.celrep.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Webster MT, et al. Intravital imaging reveals ghost fibers as architectural units guiding myogenic progenitors during regeneration. Cell Stem Cell. 2016;18:243–252. doi: 10.1016/j.stem.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonaguidi MA, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doupe DP, et al. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 56.Klein AM, et al. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell. 2010;7:214–224. doi: 10.1016/j.stem.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Bondar T, Medzhitov R. p53-mediated hematopoi- etic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolahgar G, et al. Cell competition modifies adult stem cell and tissue population dynamics in a JAK-STAT-dependent manner. Dev. Cell. 2015;34:297–309. doi: 10.1016/j.devcel.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sacco A, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakkalakal JV, et al. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins CA, et al. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 63.Shefer G, et al. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev. Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakkalakal JV, et al. Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state. Development. 2014;141:1649–1659. doi: 10.1242/dev.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buczacki SJ, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 66.Sun J, et al. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 68.Yin H, et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013;17:210–224. doi: 10.1016/j.cmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hara K, et al. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell. 2014;14:658–672. doi: 10.1016/j.stem.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ritsma L, et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rompolas P, et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–499. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeda N, et al. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang HH, et al. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imayoshi I, et al. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science. 2013;342:1203–1208. doi: 10.1126/science.1242366. [DOI] [PubMed] [Google Scholar]
- 75.Rompolas P, et al. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tetteh PW, et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Abkowitz JL, et al. Evidence that hematopoiesis may be a stochastic process in vivo. Nat. Med. 1996;2:190–197. doi: 10.1038/nm0296-190. [DOI] [PubMed] [Google Scholar]
- 78.Gupta PB, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 79.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitchell KJ, et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 81.Uezumi A, et al. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 82.Bentzinger CF, et al. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gros J, et al. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- 84.Picard CA, Marcelle C. Two distinct muscle progenitor populations coexist throughout amniote development. Dev. Biol. 2013;373:141–148. doi: 10.1016/j.ydbio.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 85.Kassar-Duchossoy L, et al. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19:1426–1431. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gensch N, et al. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development. 2008;135:1597–1604. doi: 10.1242/dev.019331. [DOI] [PubMed] [Google Scholar]
- 87.Haldar M, et al. Two cell lineages, myf5 and myf5-independent, participate in mouse skeletal myogenesis. Dev. Cell. 2008;14:437–445. doi: 10.1016/j.devcel.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanisicak O, et al. Progenitors of skeletal muscle satellite cells express the muscle determination gene. MyoD. Dev. Biol. 2009;332:131–141. doi: 10.1016/j.ydbio.2009.05.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wood WM, et al. MyoD-expressing progenitors are essential for skeletal myogenesis and satellite cell development. Dev. Biol. 2013;384:114–127. doi: 10.1016/j.ydbio.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ono Y, et al. Slow-dividing satellite cells retain long-term self-renewal ability in adult muscle. J. Cell Sci. 2012;125:1309–1317. doi: 10.1242/jcs.096198. [DOI] [PubMed] [Google Scholar]
- 91.Sousa-Victor P, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 92.Brohl D, et al. Colonization of the satellite cell niche by skeletal muscle progenitor cells depends on Notch signals. Dev. Cell. 2012;23:469–481. doi: 10.1016/j.devcel.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 93.Schuster-Gossler K, et al. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc. Natl. Acad. Sci. U.S.A. 2007;104:537–542. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tierney MT, et al. Autonomous extracellular matrix remodeling controls a progressive adaptation in muscle stem cell regenerative capacity during development. Cell Rep. 2016 doi: 10.1016/j.celrep.2016.01.072. Published online February 18, 2016. http://dx.doi.org/10.1016/j.celrep.2016.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bjornson CR, et al. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mourikis P, et al. Cell-autonomous Notch activity maintains the temporal specification potential of skeletal muscle stem cells. Development. 2012;139:4536–4548. doi: 10.1242/dev.084756. [DOI] [PubMed] [Google Scholar]
- 97.Shea KL, et al. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell. 2010;6:117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamaguchi M, et al. Calcitonin receptor signaling inhibits muscle stem cells from escaping the quiescent state and the niche. Cell Rep. 2015;13:302–314. doi: 10.1016/j.celrep.2015.08.083. [DOI] [PubMed] [Google Scholar]
- 99.Abou-Khalil R, et al. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 2009;5:298–309. doi: 10.1016/j.stem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheung TH, et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crist CG, et al. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell. 2012;11:118–126. doi: 10.1016/j.stem.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 102.Zismanov V, et al. Phosphorylation of eIF2alpha is a translational control mechanism regulating muscle stem cell quiescence and self-renewal. Cell Stem Cell. 2016;18:79–90. doi: 10.1016/j.stem.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 103.Garcia-Prat L, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 104.Ryall JG, et al. The NAD+-dependentSIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell. 2015;16:171–183. doi: 10.1016/j.stem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodgers JT, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G to G. Nature. 2014;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Charville GW, et al. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Rep. 2015;5:621–632. doi: 10.1016/j.stemcr.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu X, et al. Human satellite cell transplantation and regeneration from diverse skeletal muscles. Stem Cell Rep. 2015;5:419–434. doi: 10.1016/j.stemcr.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]