Abstract
A significant proportion of MSM acquire HIV from their primary relationship partners. Rectal microbicides are currently being studied to determine their efficacy for HIV prevention, yet willingness to use rectal microbicides among male couples is largely unknown. Dyadic data from 333 HIV-negative and HIV-discordant male couples, representing 631 HIV-negative men, were used to assess anal douching practices and willingness to use a rectal microbicide for HIV prevention. 17% of men douched 100% of the time before having anal sex within their primary partner. Among those who had sex outside of their relationship, 36% had douched 100% of the time before having anal sex with a casual MSM partner. Most men (63%) indicated a willingness to use a theoretically effective rectal microbicide prior to anal sex for HIV prevention. If found effective, rectal microbicides delivered as an anal douche may be an acceptable format for HIV prevention to some MSM who already engage in anal douching. Understanding current douching practices will be important in successfully promoting the uptake of rectal microbicides.
Keywords: male couples, enema, douche, microbicide willingness, HIV prevention
INTRODUCTION
Gay, bisexual and other men who have sex with men (MSM) are disproportionately burdened by HIV/AIDS in the US, particularly ethnic and racial minority MSM [1]. Estimates also indicate between one- and two-thirds of MSM acquire HIV from their primary male relationship partners (i.e., male couples) [2, 3]. High rates of HIV transmission between primary partners are influenced by three synergistic behaviors: a higher number of sexual acts with primary partners, a higher likelihood of receptive anal intercourse with primary partners, and lower levels of condom use for anal intercourse with primary partners [3]. Condomless anal sex (CAS) is regularly practiced within male couples’ relationships [4–6], and some of these partnered men engage in concurrent CAS within and outside of their relationship (i.e., with both their primary partner and casual partners) [4, 5, 7–10]. Additionally, engagement in receptive CAS (i.e., as the receptive partner) is associated with higher prevalence of HIV/STI infection among MSM [11, 12].
Newer biomedical-focused HIV prevention methods are being developed to help reduce the risk for acquisition and/or transmission of HIV among MSM. For example, rectal microbicides could become an important preventive method, especially for MSM who engage in receptive CAS. Rectal microbicides are products applied inside the rectum that are intended to protect against HIV through anal sex [13, 14]. To disrupt the pathway of sexual HIV-transmission, microbicides can be incorporated into various topical solutions and applied inside the rectum as an enema or douche before engaging in receptive CAS [14]. Current rectal microbicide clinical trials suggest potential efficacy for reducing HIV transmission if used correctly and consistently [13, 15–17]. If rectal microbicides are found efficacious to reduce HIV incidence, their uptake will depend on a better understanding of MSM’s willingness to use a rectal microbicide for HIV prevention and MSM’s behavior practices that precede or are concurrent with anal sex, among them anal douching practices—a behavior similar to that needed for rectal application of a microbicide douche.
Understanding douching practices is especially important for several reasons. First, douching has been associated with HIV infection in epidemiologic studies [18–22] and one histologic study suggested that douching could alter the rectal mucosa [23], together raising the concern of increased susceptibility to HIV from douching. Thus, it will be important to evaluate the effectiveness of any rectal microbicide in the context of douching prior to microbicide use, or at least to educate individuals who regularly douche that the effect of douching on subsequent microbicide use is unclear. Second, if douching prior to microbicide use were to be found safe (i.e., if it did not reduce microbicide effectiveness or increase susceptibility to HIV), the coupling of microbicide application following douching should be used as a method of promoting microbicide use among those who regularly douche prior to sex. And finally, were a gel-based microbicide product to be found efficacious in preventing HIV, the development and study of a similar compound within a douche formulation may be warranted if a substantial number of MSM regularly douche prior to CAS.
Few studies have examined anal douching practices among MSM. Carballo-Dieguez and colleagues (2008, 2010) reported that anal douching is a commonly practiced behavior among MSM who engage in receptive CAS [24, 25]. They noted that men’s frequency of douching before and after their engagement in receptive CAS differed according to their HIV status such that a higher proportion of HIV-positive MSM douched before having anal sex compared to HIV-negative MSM (96% vs. 53%); men’s two most common motivators to douche were for hygienic purposes or a request by their sex partner [24, 25]. A recent study by Noor and Rosser (2014) also found that among 4,992 MSM from 16 US cities, 52% of men reported douching at least once, 35% douched within the prior three months, and 88% douched before having receptive anal sex [26]. Of the men who did douche (at least once), 65% had used water as their douche product [26].
The acceptability of rectal microbicides and MSM’s willingness to use them have also been assessed among samples of MSM in Thailand, South America and the US [27–32]. Among a sample of Peruvian MSM, having a history of anal douching was positively associated with willingness to use a rectal microbicide [29]. Other studies noted that the type of product used (e.g., liquid, gel, or suppository), level of protection offered from HIV, cost and accessibility, and interpersonal dynamics between sex partners (e.g., trust, power) all influence the acceptability of using a rectal microbicide for HIV prevention [27, 30, 32]. While these studies have assessed anal douching practices and willingness to use a rectal microbicide among MSM, this body of literature is limited. We are not aware of any studies that have assessed anal douching practices specifically among male couples and their willingness to use a rectal enema microbicide for HIV prevention.
Given the high rates of HIV infection that occur within male couples’ relationships [2, 3] and the potential that rectal microbicides may have for averting new HIV infections, new research is needed with this particular population. To address this knowledge gap, dyadic data from a US Internet study comprised of 275 HIV-negative and 58 HIV-discordant male couples was used to 1) describe HIV-negative partnered men’s use of douching for anal sex—by type of douche product used and frequency of douching—both within and outside of their relationship; 2) describe men’s willingness to use a rectal enema for HIV prevention; 3) assess which individual- and couple-level factors are associated with men’s willingness to use a rectal enema for HIV prevention. Findings from this study may help inform interventions to promote the uptake of rectal microbicides for HIV prevention among male couples with a HIV-negative partner if and when this intervention is found to be efficacious.
METHODS
The Medical College of Wisconsin Institutional Review Board approved the study protocol; methods have been previously described (blinded refs). Recruitment for this study sample was conducted through Facebook banner advertising in 2011. Advertisements targeted partnered men who reported in their Facebook profile being ≥ 18 years of age, living in the U.S., interested in men, and being in a relationship, engaged, or married. Banner advertisements briefly described the purpose of the study and included a picture of a male couple. Men were eligible to participate if they: were ≥ 18 years of age; lived in the U.S.; were in a sexual relationship with another male and had oral and/or anal sex with this partner within the previous three months. A partner referral system was embedded in the online survey to enable data collection from both men in the couple. Post-hoc analyses of response consistency in several variables and email addresses were used to verify couples’ relationships. Every fifth couple that completed the survey was modestly compensated (e.g., $20 USD e-gift card for each partner).
Of a total of 7,994 Facebook users who clicked on an advertisement, 4,056 (51%) answered eligibility questions; 722 (18%), representing both men of 361 MSM couples provided consent and completed the original study questionnaire. A total of 606 HIV-negative and 25 unknown serostatus MSM, representing 275 concordantly negative and 58 HIV-discordant male couples (N = 333 dyads), are included in this analysis; 28 concordant HIV-positive male couples were excluded from the present analysis.
Measures
We assessed the frequency and type of douche that participants used before having anal sex with their primary partner with two items. First, participants were asked “Which kind of product did you typically use to ‘douche’ before having anal sex with your partner?” with response options of ‘saline-based rectal enema like Fleet’, ‘water’, ‘suppository laxative’, ‘other’, ‘a combination of these products’, and ‘Does not apply. I did not have anal sex (i.e., bottom with my partner)’. To assess frequency of douching, participants were asked “What percentage of the time did you douche before having anal sex with your partner?” with a possible response range of 0 to 100%. Items of similar format were used to assess douche type and douching frequency before anal sex with casual sex partners.
Participants’ willingness to use a rectal enema for HIV prevention was assessed by 1 item—“How likely would you use a rectal enema that contained an HIV prevention medication to help lower your chances of contracting HIV?”—with a 5-point Likert-type scale that had response options ranging from 0 (Not at all likely) to 4 (Extremely likely).
Several demographic (e.g., age, race) and relationship characteristics (e.g., relationship length) were assessed as well as self and primary partner’s HIV status; engagement in CAS within the relationship; and whether sex had occurred with any casual sex partners within the previous three months, including CAS with casual sex partners. Other characteristics about this sample have been reported, including their use of risk-reduction strategies, HIV and STI testing behaviors, aspects about their sexual agreements, and attitudes toward using pre-exposure prophylaxis and couples-based HIV testing and counseling [8, 9, 43, 44, 45].
Validated scales were also used to assess dynamics within male couples’ relationships, including their levels of trust [33], relationship commitment [34], and communication patterns [35]. These same validated scales have been detailed in-depth elsewhere, including their subscales, response options, and reliability coefficients [6–8].
Analysis
Dyadic data from 333 dyads with 631 HIV-negative partnered men were analyzed using Stata v12 (StataCorp, College Station, TX) following recommended guidelines [36, 37]. Descriptive statistics were calculated. Responses from both partners were used to create couple-level dummy variables to describe and assess demographic and behavioral factors at the couple level. Independent individual- and couple-level variables that were significantly (P < 0.05) associated with the outcome (i.e., willingness to use a rectal enema for HIV prevention) in the bivariate random-effects regression models were included in a multivariate random-effects multilevel regression model with maximum likelihood estimation. Relationship HIV-status was included as potential confounder for the model. The coefficients, standard errors, and statistical significance for the factors in the bivariate and multivariate models are reported.
RESULTS
Table 1 describes characteristics of the 333 male couples. Couples’ average relationship length was approximately 5 years. About a third of couples were nonwhite or mixed race; another third had both partners who earned at least a Bachelor’s degree. The majority of couples lived in an urban or suburban setting in the US with fairly equal distribution across the four regions in the US (30% West, 25% Midwest, 28% South, 17% Northeast). Most participants reported being employed, having a primary care provider, being in a concordantly HIV-negative relationship, and cohabitating. The median age of the sample and the average age difference between partners were 29 and 4.9 years, respectively.
Table 1.
Characteristics of sample
| Couples demographic characteristics | % (N = 333 dyads) |
|---|---|
| HIV status of relationship | |
| In HIV discordant relationship | 17% (58) |
| In concordantly HIV negative relationship | 83% (275) |
| Race | |
| White | 66% (220) |
| Mixed or nonwhite race | 34% (113) |
| Ethnicity | |
| Hispanic: One or both men reported yes | 14% (48) |
| Non-Hispanic | 86% (285) |
| Education level: Both men had at least a Bachelor’s degree | 34% (112) |
| Employment status: Both men employed | 66% (220) |
| Had primary care provider: One or both men reported yes | 61% (203) |
| Establishment of sexual agreement: Both men concurred yes | 51% (171) |
| Geographical location | |
| Urban/suburban | 88% (279) |
| Rural | 12% (54) |
| US region a | |
| West | 30% (201) |
| Midwest | 25% (163) |
| South | 28% (186) |
| Northeast | 17% (116) |
|
| |
| Mean (SD) or years of age | |
|
| |
| Age difference between partners | 4.9 (5.7) |
| Individual age range | 18 – 68 |
| 25th quartile | 24 |
| 50th quartile (median) | 29 |
| 75th quartile | 38 |
| Relationship length [range: 0.25 – 35 years] | 4.8 (5.4) |
| Cohabitation length [range: 0.08 – 31.7 years] b | 5.0 (5.7) |
|
| |
| Couples sexual behavior | % (N = 333 dyads) |
|
| |
| CAS practiced within relationship | 83% (278) |
| Sex outside of relationship | 30% (101) |
| CAS outside of relationship a | 63% (64) |
| CAS within & outside of relationship a | 53% (54) |
|
| |
| Couples averaged relationship dynamics c | Mean (SD) |
|
| |
| Investment model for relationship commitment [range: 0 – 6] | |
| Commitment level | 5.40 (0.67) |
| Relationship satisfaction | 4.92 (0.88) |
| Investment size d | 4.72 (0.81) |
| Quality of alternatives d | 3.72 (1.07) |
| Communication patterns scale [range: 0 – 9] | |
| Constructive communication | 7.27 (1.26) |
| Mutual avoidance and withdrawal | 3.45 (1.44) |
| Trust scale [range: 1 – 7] | |
| Dependability | 5.61 (0.83) |
| Faith | 6.03 (0.80) |
| Predictability | 5.33 (0.96) |
Note
Regional data represent the individual men because not all couples reported living together.
Data represents participants who reported living with their main partner for at least one month or longer.
Between couple-level relationship characteristics represent the average of both partners responses to a factor.
Investment size refers to the existence of concrete or tangible resources in the relationship that would be lost or greatly reduced if the relationship ends. Quality of alternatives is the perception that being single or an attractive alternative partner existed outside of the main relationship, and that this alternative would provide superior outcomes when compared with the current relationship.
Most couples practiced CAS within their relationship. In 30% of couples, one or both partners had sex outside of the relationship; in 63% of these couples, one or both partners had CAS with a casual MSM partner and in 53%, one or both partners had CAS within and outside of their relationship.
With respect to relationship dynamics, on average, men reported being committed to their relationship, trusting of their primary male partner, and communicating constructively.
Types of douches used for anal sex
The top three types of anal douches that HIV-negative partnered men reported using before having anal sex with their primary partner (n=367) were: water (64%); ‘other’ (21%); a saline-based rectal enema (12%). Among those who had sex outside of their relationship, the top three types of anal douches that men (n=78) reported using before having anal sex with a casual MSM partner were: water (72%); ‘other’ (15%); saline-based rectal enema (9%).
Frequency of douching for anal sex
Figure 1 illustrates the frequency that partnered men douched before having anal sex by partner type (primary relationship partner vs. casual MSM partner). The frequency that participants douched before having anal sex with their primary partner ranged from 0 to 100% of time; 17% of participants indicated they douched 100% of the time (herein referred to as ‘always’), 47% douched 1% to 99% of the time (herein referred to as ‘sometimes’), and 36% never douched (0%). The frequency that partnered men douched before having anal sex with a casual MSM partner ranged from 0 to 100% with 36% indicating they always douched, 33% sometimes douched, and 31% reporting that they never douched (e.g., 0%). The frequency and types of anal douches that partnered men used before having anal sex – regardless of partner type – did not statistically differ by the couples’ HIV status.
Figure 1.
Percentage of time that HIV-negative partnered men douched before having anal sex, by partner type
Note. Three HIV-negative study participants did not respond to these two survey items, respectively.
Willingness to use a rectal microbicide enema for HIV prevention
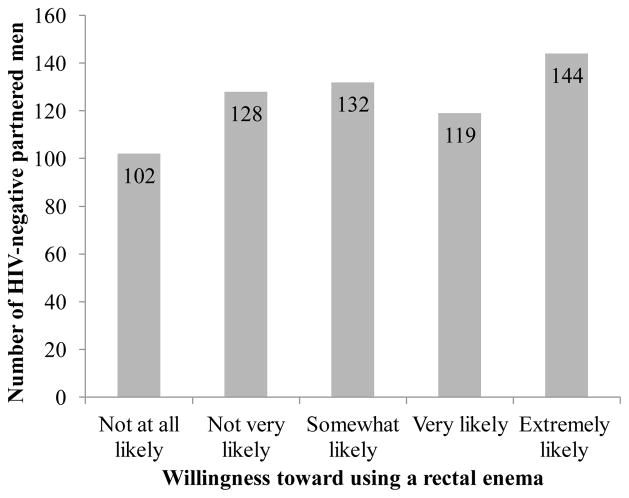
Figure 2 depicts that approximately 42% of the HIV-negative partnered men reported being very-to-extremely likely to use a rectal enema for HIV prevention with another 21% being somewhat likely to use it; the modal response to willingness was extremely likely (23%). Men’s willingness to use a rectal enema for HIV prevention did not significantly differ by relationship HIV status.
Figure 2.
HIV-negative partnered men’s willingness to use a rectal enema for HIV prevention
Findings from the bivariate and final multivariate random-effects multilevel regression models are provided in Table 2. The final random-effects multilevel regression model revealed that several factors were associated with HIV-negative partnered men’s willingness to use a rectal enema for HIV prevention. After controlling for relationship HIV status couples’ practice of CAS within their relationship, participants’ willingness to use a rectal enema was positively associated with being Hispanic (β=0.44, SE=0.22, p < 0.05), living in a rural environment (β=0.36, SE=0.18, p < 0.05), and/or being in a mixed race or nonwhite relationship (β=0.30, SE=0.14, p < 0.05). Willingness to use a rectal microbicide enema was also positively associated with taking the receptive role during CAS with the primary partner (β=0.34, SE=0.15, p < 0.05), using a douche before having anal sex with the primary partner (β=0.42, SE=0.13, p < 0.01), and/or being in a relationship where at least one of the partners engaged in concurrent CAS within and outside of the relationship (β=0.70, SE=0.17, p < 0.001). Willingness to use a rectal microbicide enema was negatively associated with couples in which both partners had earned at least a Bachelor’s degree (β= −0.27, SE=0.13, p < 0.05), and in couples who perceived greater levels of relationship alternatives (e.g., being single or having a different partner was more appealing than being with their current primary partner) (β= −0.11, SE=0.06, p < 0.05). No other factors were significantly associated with willingness to use a rectal enema for HIV prevention.
Table 2.
Factors significantly associated with willingness to use a rectal enema for HIV prevention among 631 HIV-negative partnered MSM in 275 HIV-negative and 58 HIV-discordant male couples: Results from bivariate and final multivariate random-effects multilevel regression models
| Bivariate models | Final multivariate model | |
|---|---|---|
|
| ||
| Couples’ demographic factors | β (SE) | β (SE) |
| Geographical location | ||
| Rural (ref) | 0.44 (0.18)* | 0.36 (0.18)* |
| Urban/suburban | ||
| HIV status of relationship | ||
| Negative concordant (ref) | 0.12 (0.09) | 0.07 (0.09) |
| Discordant | ||
| Ethnicity | ||
| Hispanic (ref) | 0.68 (0.20)** | 0.44 (0.22)* |
| Non-Hispanic | ||
| Race | ||
| Mixed or nonwhite (ref) | 0.33 (0.13)** | 0.30 (0.14)* |
| White | ||
| Education level | ||
| Both men had Bachelor’s or higher degree (ref) | −0.29 (0.13)* | −0.27 (0.13)* |
| One or neither partner had a Bachelor’s degree | ||
|
| ||
| Sexual behaviors and douching | β (SE) | β (SE) |
|
| ||
| Individual’s engaged as receptive role for CAS with main partner | 0.42 (0.12)*** | 0.34 (0.15)* |
| Couple practiced CAS in relationship prior 3 months | 0.33 (0.16) | −0.23 (0.20) |
| Individual’s douche use before anal sex with main partner (vs. not) | 0.58 (0.12)*** | 0.42 (0.13)** |
| Individual’s douche use before anal sex with a casual MSM partner (vs. not) | 0.52 (0.21)* | -- |
| CAS within and outside of relationship prior 3 months | ||
| One or both men reported yes (ref) | 0.66 (0.16)*** | 0.70 (0.17)*** |
| Both partners reported no | ||
|
| ||
| Couples’ averaged relationship dynamics | β (SE) | β (SE) |
|
| ||
| Investment model for relationship commitment | ||
| Commitment level | −0.23 (0.09)* | -- |
| Quality of alternatives | −0.20 (0.06)*** | −0.11 (0.06)* |
| Communication patterns | ||
| Mutual avoidance and withdrawal | 0.09 (0.04)* | -- |
| Trust scale | ||
| Predictability | −0.13 (0.06)* | -- |
| Dependability | −0.16 (0.07)* | -- |
Notes.
Results from final random-effects multilevel regression model controlled for couples’ HIV serostatus and practice of CAS within their relationship. All data represent 537 obs., 301 dyads, χ2 (10) = 77.84, P < 0.001, Log likelihood = −900.33
P < 0.05,
P < 0.01,
P < 0.001
DISCUSSION
Clinical trials are currently testing the efficacy of rectal microbicides for averting new HIV infections among MSM [13]. If rectal microbicides are found to be efficacious, additional data will be needed to understand how to encourage uptake of this new HIV prevention method. Findings from the present study help address this critical knowledge gap by providing insight into HIV-negative partnered men’s douching practices and their willingness to use a rectal enema for HIV prevention.
While microbicides, delivered through gels and lubricants, may be found to be effective in the prevention of HIV transmission from ongoing studies [13–17, 27], our data suggests that adoption of a rectal microbicide delivered through a rectal enema when douching may be beneficial for some partnered MSM. Over a third of partnered men in this sample reported douching sometime prior to having anal sex with a primary partner, and 17% indicated they always douched prior to having anal sex with their primary partner. In this sample a higher proportion of partnered men always douched before having anal sex with a casual MSM partner than with their primary male partner (33% vs. 17%). These findings are relevant. Current research has opted to use the enema as a dosing strategy for rectal microbicides because the use of an enema requires little behavior change by men who already douche before having receptive anal sex [17]. Moreover, the medium that is used (e.g., water, saline-based, etc.) will be another important factor to consider for the uptake of rectal microbicides for HIV prevention among partnered men. In this sample, water was the most commonly used medium amongst the partnered men who douched before having anal sex. As such, current and future research will need to encourage these men to adapt to use other potential mediums, such as a gel lubricant, for delivery of a rectal microbicide [17].
Not all partnered men douched before having anal sex. Approximately a third of the men in this sample did not douche before having anal sex with their primary partner and/or a casual MSM partner. Willingness to use a rectal microbicide enema for HIV prevention may require significant behavior change amongst these partnered men. Additional research is needed to determine whether these men would adopt this behavior or whether they would consider using other HIV preventive options that are already available (e.g., pre-exposure prophylaxis or nonoccupational post-exposure prophylaxis).
When presented with the theoretical concept of a rectal enema that could prevent HIV transmission, the majority of HIV-negative partnered men in this nation-wide sample reported being likely to use this form of prevention if it were effective. Furthermore, partnered men who lived in a rural area, compared to those living in an urban or suburban area, were more willing to use a rectal enema for HIV prevention. This finding is important as many male couples who live in rural areas may not have easy access to other HIV prevention services and because the prevalence of HIV in rural areas continues to increase [38]. As rectal microbicides become a realistic possibility for HIV prevention in the near future, efforts must be made to help make these prevention options accessible and available to those living in rural areas so that couples’ geographical locations will not exacerbate disparities in HIV incidence.
Although there was no significant difference in men’s willingness to use rectal enema by their relationship HIV-status, ethnicity and race may also play a role in their willingness to use this potential new prevention method. Hispanic partnered MSM and partnered men in mixed or non-white same-sex relationships were more likely to use a rectal enema for HIV prevention. Uptake of a rectal enema for HIV prevention among minority male couples may prove to be very beneficial and effective since racial and ethnic minority MSM remain disproportionately affected by and at greater risk for HIV acquisition compared to their white counterparts [1, 39–41]. Once rectal microbicides become a viable method for HIV prevention, additional research will be needed to assess how best to educate racial and ethnic minority male couples about this option, as well as their uptake of it.
Additionally, well-educated couples (i.e., with Bachelor’s degrees or higher) were less willing to use a rectal enema as an HIV prevention method compared to those who were less educated. Prior studies that assessed men’s perceptions of rectal microbicides through focus groups found that many MSM had limited knowledge about this form of prevention [32, 42]. It is possible that men who are more educated may question the utility and/or effectiveness of whether a rectal enema with HIV medication can be used to help prevent HIV, or they may have a higher prevalence of other HIV prevention options, such as pre-exposure prophylaxis, that may seem more appealing. Thus, the use of a rectal enema as an HIV prevention method among certain groups of male couples may require further research to assess how best to inform male couples about this preventive option in context with other available prevention methods.
Furthermore, partnered men who engaged in receptive CAS with their primary partner, concurrent CAS within and outside of their relationship, and/or douched before having anal sex with their primary partner were more willing to use a rectal enema as a HIV prevention method compared to those who did not engage in these sexual behaviors. Prior research has reported similar results regarding rectal douching being a common practice among MSM who engage in receptive CAS [24, 25]. For this subgroup of partnered men, their desire to engage in CAS and/or take on the receptive role [for anal sex] may influence their desire to use a rectal enema for HIV prevention, particularly if condom use is not a realistic and/or desirable option for them. This finding justifies the need for development of additional HIV prevention methods beyond condoms, such as rectal microbicides as well as the promotion of existing, newer methods (e.g., pre-exposure prophylaxis, nonoccupational post-exposure prophylaxis).
With respect to relationship dynamics, few of these factors were associated with willingness to use a rectal enema for HIV prevention. Future research should investigate how other dynamics within couples’ relationships, such as intimate partner violence and power, may affect men’s willingness to use a rectal microbicide. Partnered men’s willingness to use a rectal enema for HIV prevention was negatively associated with being in a relationship that perceived to have higher levels of quality of alternatives (i.e., perception of being single or with someone else). This finding may suggest that partnered men who perceive themselves to be in a more ‘stable’ relationship may be more likely to use this type of preventive method than those who may be uncertain about the future of their current relationship; results obtained from the bivariate multilevel regression models help confirm this possible explanation. However, further research that uses a mixed methods approach should assess how this particular dynamic, as well as other potential dynamics, may influence couples’ willingness and uptake of using a rectal enema for HIV prevention.
Limitations
The use of a cross-sectional study design with a convenience sample inhibits casual inference and the ability to generalize these results to all Internet-using male couples or those who do not use Facebook. Although identifying information was not collected, biases of participation, social desirability, and recall may have influenced participants to inaccurately report information. Further, other factors that were not measured could affect partnered men and male couples’ willingness to use a rectal enema, including their mental health (e.g., depression or anxiety), other relationship dynamics (e.g., intimacy, power), presence or history of intimate partner violence, and perceived risk for acquiring HIV. Further, willingness to use a rectal enema for HIV prevention was assessed using an unbalanced item, which may have impacted participants to select a more positive attitude compared to them potentially having a neutral stance about their willingness. Future studies may benefit by addressing these limitations with improved methods and inclusion of additional measures to further assess male couples’ willingness to use a rectal enema for HIV prevention via different modes of delivery including the use of a gel and an anal douche.
Conclusion
Despite these limitations, our findings present implications for the future use of a rectal microbicide enema for HIV prevention among partnered, HIV-negative MSM. HIV-negative men in HIV-concordant and HIV-discordant relationships are willing to use rectal enemas as an HIV prevention tool, especially among those who engage in CAS, douche before having CAS, and engage in CAS within and outside of their relationships. As new types of HIV prevention methods become available, our findings may help identify perceived barriers for their future use. We encourage additional research to better understand how dynamics within and outside of male couples’ relationships may encourage or inhibit willingness to use a rectal microbicide enema for HIV prevention, including their social (e.g., peers) and healthcare environments. Our findings illustrate that some partnered, HIV-negative MSM are willing to use a rectal microbicide enema for HIV prevention.
Of course, uptake of an effective new rectal microbicide as an HIV-prevention method is a step beyond an individual’s willingness to use a product that could theoretically prevent HIV. When an effective topical rectal microbicide becomes available, achieving high levels of uptake among MSM who could benefit from its use will be essential if this prevention strategy is to have a sizeable impact on HIV incidence. Effectively promoting uptake of a rectal microbicide will require an understanding of this population’s willingness to use a microbicide and the factors associated with such willingness. By understanding the current levels of willingness to use a microbicide and differences between those who are more and less willing to use one, promotional messaging can be tailored to different groups accordingly. Thus, data provided by this study offer value in informing population-level strategies to increase uptake of a rectal microbicide.
Acknowledgments
Data collection for this manuscript was supported by the center (P30-MH52776; PI Kelly J) and NRSA (T32-MH19985; PI Pinkerton S) grants from the National Institute of Mental Health. The authors graciously thank the participants for their time and input.
Contributor Information
Jason W. Mitchell, Email: jwm35@med.miami.edu.
Amber S. Sophus, Email: axs840@miami.edu.
Ji-Young Lee, Email: jxl636@miami.edu.
Andrew E. Petroll, Email: apetroll@mcw.edu.
References
- 1.Centers for Disease Control and Prevention. [Accessed June 17, 2015];Fact Sheet: HIV among gay and bisexual men. http://www.cdc.gov/hiv/risk/gender/msm/facts/index.html.
- 2.Goodreau S, Carnegie N, Vittinghoff E, et al. What drives the US and Peruvian HIV epidemics in men who have sex with men (MSM) PLoS. 2012;7(11):e50522. doi: 10.1371/journal.pone.0050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan P, Salazar L, Buchbinder S, Sanchez T. Estimating the proportion of HIV transmissions from main sex partners among men who have sex with men in five US cities. AIDS. 2009;23:1153–62. doi: 10.1097/QAD.0b013e32832baa34. [DOI] [PubMed] [Google Scholar]
- 4.Hoff C, Chakravarty D, Beougher S, Neilands T, Darbes L. Relationship characteristics associated with sexual risk behavior among MSM in committed relationships. AIDS Patient Care STDs. 2012;26:738–45. doi: 10.1089/apc.2012.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell JW. Characteristics and allowed behaviors of gay male couples’ sexual agreements. J Sex Res. 2014;51:316–28. doi: 10.1080/00224499.2012.727915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell JW, Harvey SM, Champeau D, Seal DW. Relationship factors associated with HIV risk among a sample of gay male couples. AIDS Behav. 2012;16:404–11. doi: 10.1007/s10461-011-9976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez AM, Beougher SC, Chakravarty D, et al. Relationship dynamics as predictors of broken sexual agreements about outside sexual partners: Implications for HIV prevention among gay couples. AIDS Behav. 2012;16:1584–85. doi: 10.1007/s10461-011-0074-0. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell JW, Harvey SM, Champeau D, Moskowitz DA, Seal DW. Relationship factors associated with gay male couples’ concordance on aspects of their sexual agreements: Establishment, type, and adherence. AIDS Behav. 2012;16:1560–9. doi: 10.1007/s10461-011-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell JW, Petroll AE. Patterns of HIV and sexually transmitted infection testing among men who have sex with men couples in the United States. Sex Transm Dis. 2012;39:871–6. doi: 10.1097/OLQ.0b013e3182649135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell JW, Petroll AE. Factors associated with men in HIV-negative gay couples who practiced UAI within and outside of their relationship. AIDS Behav. 2013;17:1329–37. doi: 10.1007/s10461-012-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. The Lancet. 2012;380(9839):367–77. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150:306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 13.Microbicide trials network. [Accessed June 17, 2015];Microbicides: A Promising Strategy. Retrieved from http://www.mtnstopshiv.org/node/82.
- 14.McGowan I. Rectal microbicides: can we make them and will people use them? AIDS Behav. 2011;15:66–71. doi: 10.1007/s10461-011-9899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGowan I. Microbicides for HIV prevention: reality or hope? Curr Opin Infect Dis. 2010;23(1):26–31. doi: 10.1097/QCO.0b013e328334fe70. [DOI] [PubMed] [Google Scholar]
- 16.Kelly CG, Shattock RJ. Specific microbicides in the prevention of HIV infection. J Internal Med. 2011;270:509–19. doi: 10.1111/j.1365-2796.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- 17.NIH RePORTER. [Accessed September 30, 2015];Development of rectal enema as microbicide (DREAM) (PI: Hendrix, CW) https://projectreporter.nih.gov/project_info_description.cfm?aid=8883371&icde=26605907&ddparam=&ddvalue=&ddsub=&cr=5&csb=default&cs=ASC.
- 18.Moss AR, Osmond D, Bacchetti P, et al. Risk factors for AIDS and HIV seropositivity in homosexual men. Am J Epidemiol. 1987;125:1035–47. doi: 10.1093/oxfordjournals.aje.a114619. [DOI] [PubMed] [Google Scholar]
- 19.Chmiel JS, Detels R, Kaslow RA, et al. Factors associated with prevalent human immunodeficiency virus infection in the multicenter AIDS cohort study. Am J Epidemiol. 1987;126:568–75. doi: 10.1093/oxfordjournals.aje.a114696. [DOI] [PubMed] [Google Scholar]
- 20.Koziol DE, Saah AJ, Odaka N, et al. A comparison of risk factors for human immunodeficiency virus and hepatitus B virus infections in homosexual men. Ann Epidemiol. 1993;3:434–41. doi: 10.1016/1047-2797(93)90073-d. [DOI] [PubMed] [Google Scholar]
- 21.Deininger S, Muller R, Guggenmoos-Holzmann I, et al. Behavioral characteristics and laboratory parameters on homo- and bisexual men in West Berlin: An evaluation of five years of testing and counseling on AIDS. Klin Wochenschr. 1990;68:906–13. doi: 10.1007/BF01649037. [DOI] [PubMed] [Google Scholar]
- 22.Beinzle U, Guggenmoos-Holzmann I, Zwingenberger K, et al. Lymphadenopathy and anitbodies to HTLV-III in homosexual men. Klin Wochenschr. 1985:597–602. doi: 10.1007/BF01733012. [DOI] [PubMed] [Google Scholar]
- 23.Schmelzer M, Schiller LE, Meyer R, Rugari SM, Case P. Safety and effectiveness of large-volume enema solutions. Appl Nurs Res. 2004;17:265–74. [PubMed] [Google Scholar]
- 24.Carballo-Dieguez A, Bauermeister JA, Ventuneac A, Dolezal C, Balan I, Remien RH. The use of rectal douches among HIV-uninfected and infected men who have unprotected receptive anal intercourse: Implications for rectal microbicides. AIDS Behav. 2008;12(6):860–6. doi: 10.1007/s10461-007-9301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carballo-Dieguez A, Bauermeister JA, Ventuneac A, Dolezal C, Mayer K. Why rectal douches may be acceptable rectal-microbicide delivery vehicles for men who have sex with men. Sex Transm Dis. 2010;37(4):228–33. doi: 10.1097/OLQ.0b013e3181bf9b2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noor SW, Rosser BR. Enema use among men who have sex with men: A behavioral epidemiologic study with implications for HIV/STI prevention. Arch Sex Behav. 2014;43(4):755–69. doi: 10.1007/s10508-013-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carballo-Dieguez A, Dolezal C, Bauermeister JA, O’Brien W, Ventuneac A, Mayer K. Prevalence for gel over suppository as delivery vehicle for a rectal microbicide: results of a randomized, crossover acceptability trial among men who have sex with men. Sex Transm Infect. 2008;84(6):483–7. doi: 10.1136/sti.2008.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinsler JJ, Cunningham WE, Nurena CR, et al. Using conjoint analysis to measure the acceptability of rectal microbicides among men who have sex with men in four South American cities. AIDS Behav. 2012;16(6):1436–47. doi: 10.1007/s10461-011-0045-5. [DOI] [PubMed] [Google Scholar]
- 29.Kinsler J, Galea J, Lama J, et al. Rectal douching among Peruvian men who have sex with men, and acceptability of a douche-formulated rectal microbicide to prevent HIV infection. Sex Transm Infect. 2013;89:62. doi: 10.1136/sextrans-2012-050630. [DOI] [PubMed] [Google Scholar]
- 30.Newman PA, Roungprakhon S, Tepjan S. A social ecology of rectal microbicide acceptability among young men who have sex with men and transgender women in Thailand. J Intl AIDS Soc. 2013;1(16):18476. doi: 10.7448/IAS.16.1.18476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thienkra W, Todd C, Chaikummao S, et al. Prevalence and correlates of willingness to participate in a rectal microbicide trial among men who have sex with men in Bangkok. AIDS Care. 2014;26(11):1359–69. doi: 10.1080/09540121.2014.913763. [DOI] [PubMed] [Google Scholar]
- 32.Galea JT, Kinsler JJ, Imrie J, Nurena CR, Sanchez J, Cunningham WE. Rectal douching and implications for rectal microbicides among populations vulnerable to HIV in South America: A qualitative study. Sex Transm Infect. 2014;90(1):33–5. doi: 10.1136/sextrans-2013-051154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rempel JK, Holmes JG, Zanna MP. Trust in close relationships. J Pers Soc Psychol. 1985;49:95–112. [PubMed] [Google Scholar]
- 34.Rusbult CE, Martz JM, Agnew CA. The investment model scale: Measuring commitment level, satisfaction level, quality of alternatives, and investment size. Persl Relation. 1985;5:357–91. 1998. [Google Scholar]
- 35.Christensen A, Shenk JL. Communication, conflict, and psychological distance in nondistressed, clinic, and divorcing couples. J Consult Clin Psychol. 1991;59:458–63. doi: 10.1037//0022-006x.59.3.458. [DOI] [PubMed] [Google Scholar]
- 36.Kenny D, Kashy D, Cook W. Dyadic data analysis. New York: Guilford Press; 2006. [Google Scholar]
- 37.Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. College Station: Stata Press; 2008. [Google Scholar]
- 38.National Rural Health Association [NRHA] Policy Brief: HIV/AIDS in rural America: Disproportionate impact on minority and multicultural populations 2014 [Google Scholar]
- 39.Calabrese SK, Rosenberger JG, Schick VR, Novak DS, Reece M. An event-level comparison of risk-related sexual practices between black and other-race men who have sex with men: condoms, semen, lubricant, and rectal douching. AIDS Patient Care STDS. 2013;27(2):77–84. doi: 10.1089/apc.2012.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. [Accessed June 17, 2015];HIV Among Latinos. http://www.cdc.gov/hiv/risk/racialEthnic/hispanicLatinos/facts/index.html.
- 41.Centers for Disease Control and Prevention. [Accessed June 17, 2015];HIV Among African American Gay and Bisexual Men. http://www.cdc.gov/hiv/risk/racialethnic/bmsm/facts/index.html.
- 42.Kubicek K, Arauz-Cuadra C, Kipke M. Attitudes and perceptions of biomedical HIV prevention methods: Voices from young men who have sex with men. Arch Sex Behav. 2015;44(2):487–97. doi: 10.1007/s10508-014-0398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell JW, Stephenson R. HIV-negative partnered men’s willingness to use pre-exposure prophylaxis and associated factors among an Internet sample of US HIV-negative and HIV-discordant male couples. LGBT Health. 2015;2(1):35–40. doi: 10.1089/lgbt.2014.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell JW. Gay male couples’ attitudes toward using couples-based voluntary HIV counseling and testing. Arch Sex Behav. 2014;43(1):161–171. doi: 10.1007/s10508-013-0211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell JW. HIV-negative and HIV-discordant gay male couples’ use of HIV risk-reduction strategies: Differences by partner type and couples’ HIV status. AIDS Behav. 2013;17(4):1557–1569. doi: 10.1007/s10461-012-0388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]