Abstract
The fact that the pedunculopontine nucleus (PPN) is part of the reticular activating system places it in a unique position to modulate sensory input and fight-or-flight responses. Arousing stimuli simultaneously activate ascending projections of the PPN to the intralaminar thalamus to trigger cortical high frequency activity and arousal, as well as descending projections to reticulospinal systems to alter posture and locomotion. As such, the PPN has become a target for deep brain stimulation (DBS) for the treatment of Parkinson’s disease (PD), modulating gait, posture, and higher functions. This article describes the latest discoveries on PPN physiology and the role of the PPN in a number of disorders. It has now been determined that high frequency activity during waking and REM sleep is controlled by two different intracellular pathways and two calcium channels in PPN cells. Moreover, there are three different PPN cell types that have one or both calcium channels and may be active during waking only, REM sleep only, or both. Based on the new discoveries, novel mechanisms are proposed for insomnia as a waking disorder. In addition, neuronal calcium sensor protein-1 (NCS-1), which is over expressed in schizophrenia and bipolar disorder, may be responsible for the dysregulation in gamma band activity in at least some patients with these diseases. Recent results suggest that NCS-1 modulates PPN gamma band activity and that lithium acts to reduce the effects of over expressed NCS-1, accounting for its effectiveness in bipolar disorder.
Keywords: Arousal, gamma oscillations, N-type, P/Q-type, sleep/wake
1. Introduction
A recent review thoroughly described the effects of pedunculopontine nucleus (PPN) DBS being used clinically, along with the rationale for using PPN DBS (Garcia-Rill et al. 2014). Of relevance to its clinical use is the discovery of the presence of beta/gamma band activity in every cell in the nucleus (Simon et al. 2010). Basically, beta/gamma oscillations in PPN cells are mediated by high threshold, voltage-dependent N- and P/Q-type calcium channels (Kezunovic et al. 2011). The following review describes the latest findings on PPN physiology that suggest that, a) two different intracellular pathways control gamma band activity during waking compared to REM sleep, b) these two pathways differentially control the two different types of high threshold voltage-dependent calcium channels (N- and P/Q-type), c) there are separate populations of PPN cells bearing one or both channels, d) this organization informs regarding the mechanisms behind the developmental decrease in REM sleep, and e) such findings have implications for the mechanisms behind insomnia, as well as schizophrenia and bipolar disorder. The PPN, as part of the reticular activating system (RAS) and its role in generating gamma band activity suggest its participation in arousal and preconscious awareness (Garcia-Rill et al. 2014; Urbano et al. 2014), therefore, it is not surprising that it is involved in all of these disorders.
2. Two Pathways, Two States
PPN neurons are known to fire at beta/gamma frequencies in vivo during waking and REM sleep, but not during slow wave sleep (Boucetta et al. 2014; Datta and Siwek 2002; Datta et al. 2009; Kayama et al. 1992; Sakai et al. 1990; Steriade et al. 1990). Brainstem transections anterior to the PPN prevented the expression of gamma frequencies in the EEG, while lesions posterior to the PPN allowed the manifestation of cortical gamma activity, and stimulation of the PPN led to gamma band frequencies on the cortical EEG (Lindsley et al. 1949; Moruzzi 1972; Moruzzi and Magoun 1949; Steriade et al. 1969; Steriade et al. 1990; Steriade et al. 1991). That is, PPN activity is reflected in the cortical EEG, although partial lesions of the PPN do not significantly alter sleep and waking architecture (Webster and Jones 1988). This may be because there are other areas modulating sleep and waking homeostasis, including hypothalamic and basal forebrain regions. However, stimulation of these regions must be applied for much longer periods (10–20 sec) (Carter et al. 2012; Han et al. 2014) compared to RAS stimulation (1–2 sec) to induce waking (Carter et al. 2012; Garcia-Rill 2015; Moruzzi and Magoun 1949), In addition, optogenetic studies have found that induction of waking by stimulation of orexin neurons, for example, is blocked by inactivation of the locus coeruleus in the RAS (Carter et al 2012). That is, the RAS may be the final output for the arousal induced by some of these modulatory regions (Garcia-Rill 2015). Moreover, gamma band activity has been reported in the PPN of the mouse in vitro (Ishibashi et al 2015), in the REM sleep-induction region of the rat to which the PPN projects (Brown et al 2006), in the cat in vivo (Steriade et al. 1990), in the region of the PPN in primates when locomoting (Chabardes et al 2015), and in the region of the PPN in humans during stepping (Fraix et al 2013). That is, there is ample evidence for gamma band activity in this region in vitro, in vivo, and across species, including man.
As far as sleep/wake cycles are concerned, PPN neurons increase firing rates during rapid eye movement (REM) sleep ("REM-on"), or both waking and REM sleep ("Wake/REM-on"), and some cells fire only during waking (“Wake-on”) (Datta and Siwek 2002; Sakai et al. 1990; Steriade et al. 1990), suggestive of increased excitation only during activated states. These findings suggest that PPN neurons are mainly active during states marked by high frequency EEG activity such as waking and REM sleep. Injections of glutamate into the PPN of the rat were found to increase both waking and REM sleep, but injections of NMDA increased only waking, while injections of kainic acid (KA) increased only REM sleep (Datta 2002; Datta and Siwek 1997; Datta et al. 2001a, b). Thus, the two states appear to be independently activated by NMDA vs KA receptors. Moreover, the intracellular pathways mediating the two states are different. For example, the CaMKII activation inhibitor, KN-93, microinjected into the PPN of freely moving rats resulted in decreased waking but not REM sleep (Datta et al. 2011). We showed that beta/gamma band oscillations in PPN neurons recorded in vitro were blocked by superfusion of KN-93 (Garcia-Rill et al. 2014), suggesting that some cells manifest their oscillations via the CaMKII pathway. Moreover, the effects of the stimulant modafinil, which are mediated by increased electrical coupling, are modulated by the CaMKII pathway since KN-93 inhibits the action of modafinil (Garcia-Rill et al. 2007, 2014; Urbano et al. 2007).
Conversely, increased ERK1/2 signaling in the PPN is associated with maintenance of sleep via suppression of waking (Desarnaud et al. 2011), while activation of intracellular protein kinase A (PKA) in the PPN instead contributed to REM sleep recovery following REM sleep deprivation (Datta and Desarnaud 2010). Moreover, during REM sleep, pCREB activation in PPN cholinergic neurons was induced by REM sleep, and PPN intracellular PKA activation is mediated by a transcriptional cascade involving pCREB (Datta et al. 2009). These findings suggest that waking in vivo may be modulated by the CaMKII pathway while REM sleep may be modulated by the cAMP/PKA pathway in the PPN (Garcia-Rill et al. 2014; Urbano et al. 2014). In addition, it appears that the cAMP-dependent pathway phosphorylates N-type calcium channels (Hell et al. 1995), while CaMKII regulates P/Q-type calcium channels (Jenkins et al. 2005). Therefore, the presence of P/Q-type calcium channels is related to CaMKII and waking, while the presence of N-type calcium channels is more related to cAMP and REM sleep (Garcia-Rill et al. 2014; Urbano et al. 2014).
N- and P/Q-type calcium channel subtypes both are linked to rapid release of synaptic vesicles (Ishikawa et al. 2005; Reid et al. 2003), but knockout models manifest markedly different phenotypes (Pietrobon 2005). P/Q-type (Cav2.1) knockout animals have deficient gamma band activity in the EEG, abnormal sleep-wake states, ataxia, are prone to seizures (low frequency synchrony), and die by 3 weeks of age (Jun et al. 1999; Llinas et al. 2007). On the other hand, N-type (Cav2.2) knockout animals show few sleep-wake abnormalities but exhibit decreased nociceptive responses, and are otherwise normal (Pietrobon 2005). While the two types of receptors are modulated by G-protein coupled receptors, they require different G-protein subunits (Agler et al. 2003). Intracellularly, protein kinase C (PKC) enhances N-type channel activity but has no effect on P/Q-type channel function (Stea et al. 1995), but CaMKII was shown to modulate P/Q-type channel function (Jiang et al. 2008). That is, the two calcium channel subtypes are modulated by different intracellular pathways, N-type by the cAMP/PK pathway, and P/Q-type via the CaMKII pathway. The implications from all of these results is that there is a “waking” pathway mediated by CaMKII and P/Q-type channels, and a “REM sleep” pathway mediated by cAMP/PK and N-type channels (Figure 1).
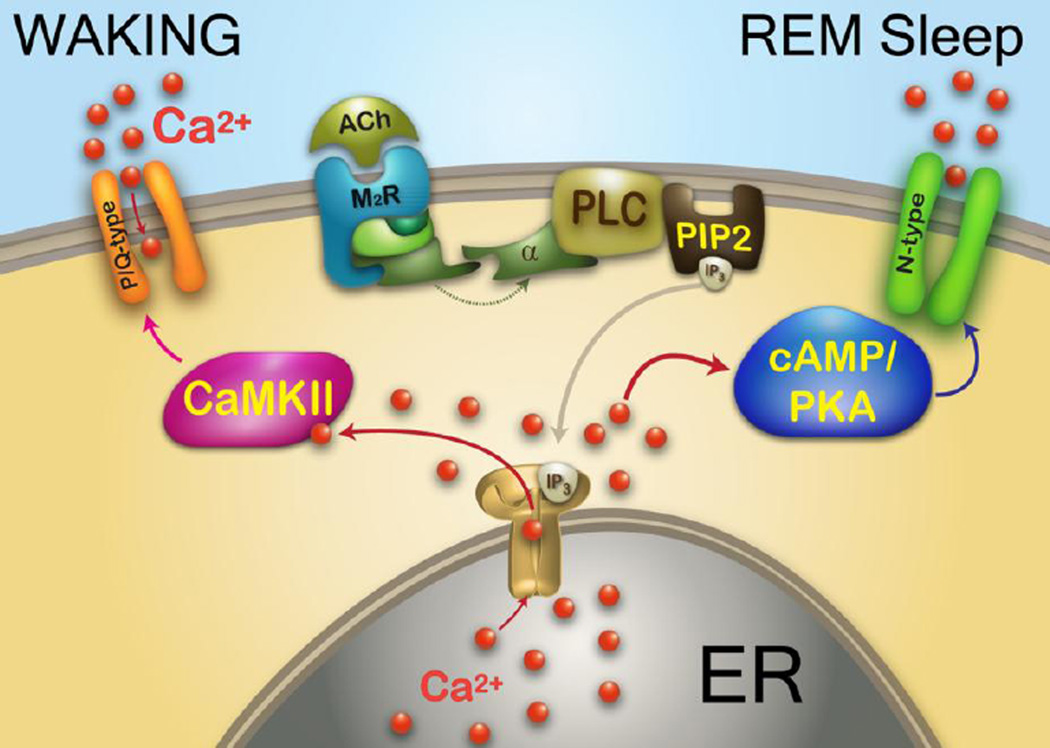
Figure 1. Intracellular pathways and calcium channels differentially related to waking vs REM sleep.
Representation of effects of acetylcholine (ACh) activation of a muscarinic 2 cholinergic receptor (M2R) acting through G protein coupling to phospholipase C (PLC), that in turn cleaves phospholipid phosphatidylinositol biphosphate (PIP2) into inositol triphosphate (IP3). IP3 is released and binds to IP3 receptors in the endoplasmic reticulum (ER) to release calcium (Ca2+). One of the intracellular pathways activated involves CaMKII, which modulates P/Q-type calcium channels and the other pathway involves cAMP/PKA, which modulates N-type calcium channels. The CaMKII/P/Q-type pathway mediates beta/gamma band activity during waking, while the cAMP/PKA/N-type pathway mediates beta/gamma band activity during REM sleep.
We found that all rat PPN cell types manifested beta/gamma oscillations in the presence of synaptic blockers and tetrodotoxin when the membrane potential was depolarized using current ramps (Kezunovic et al. 2011). In fact, intrinsic gamma oscillations are the only property present in every PPN neuron, whether of type I, II, or III, or transmitter type, cholinergic, glutamatergic or GABAergic, while cells around the PPN do not share that property (Kezunovic et al. 2011). More recent studies discovered that in some PPN cells (50%), the N-type calcium channel blocker ω-conotoxin-GVIA (ω-CgTx) reduced gamma oscillation amplitude, while subsequent addition of the P/Q-type blocker ω-agatoxin-IVA (ω-Aga) blocked the remaining oscillations. That is, these cells had N- and P/Q-type channels. In other cells (20%), gamma oscillations were not affected by ω-CgTx, however, ω-Aga blocked the oscillations, suggesting that these cells had only P/Q-type channels. In the rest of the cells (30%), ω-Aga had no effect on gamma oscillations, while ω-CgTx blocked them, suggesting these had only N-type channels. Similar results were found during recordings of voltage-dependent calcium currents. These results confirm the presence of cells in the PPN that manifest gamma band oscillations through only N-type (30%), only P/Q-type (20%), and both N- and P/Q-type (50%) calcium channels (Luster et al. 2014, 2015).
This new cell type classification proposes that some PPN neurons fire only during REM sleep (“REM-on”, N-type only), only during waking (“Wake-on”, P/Q-type only), or during both waking and REM sleep (“Wake/REM-on”, N-type + P/Q-type) (Luster et al. 2014, 2015). Interestingly, PPN cells with N-type channels only manifested oscillations in the 55 Hz range, while cells with P/Q-type channels only exhibited oscillations in the 45 Hz range, while cells with both N- and P/Q-type channels showed oscillations in the 35 Hz range. These results suggest that each channel type may have a preferred oscillation frequency, but when channels coexist they may manifest lower frequencies, perhaps due to kinetic interference, competition, and/or differential location along dendrites (Hyde et al. 2013). Figure 2 provides a diagram of the intracellular “waking” pathway and the “REM sleep” pathway, and their modulation of different calcium channel types. Armed with this information, we can now attempt to selectively modulate waking by affecting P/Q-type calcium channels and/or the CaMKII pathway, or REM sleep by affecting the N-type calcium channels and/or the cAMP/PK pathway.
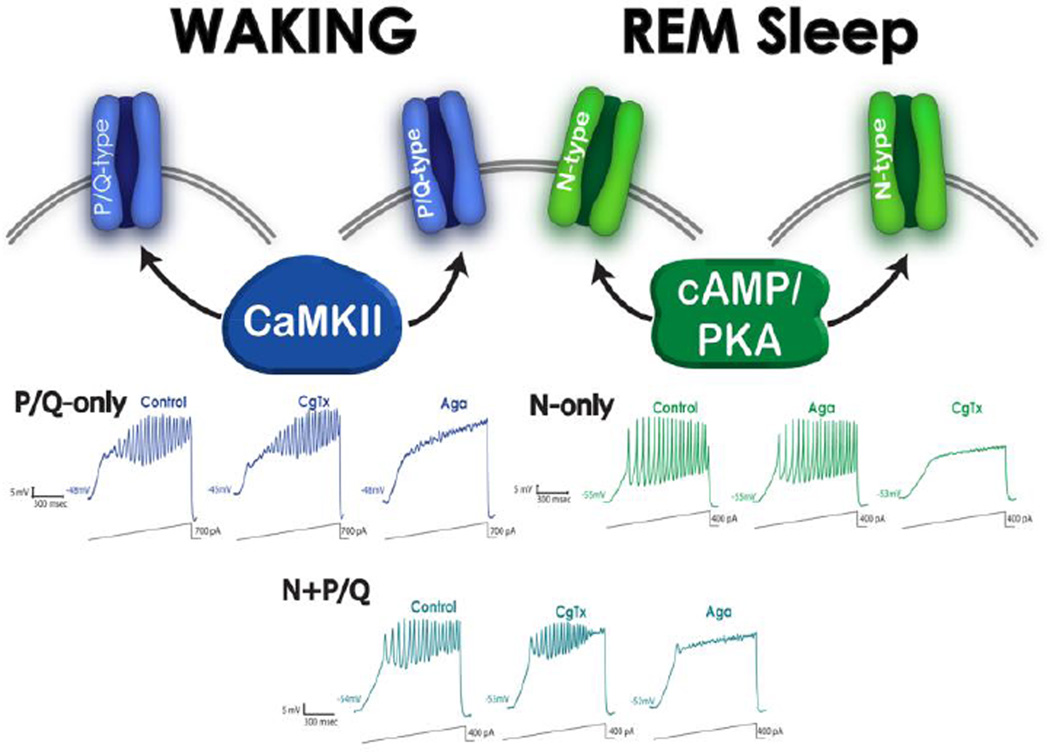
Figure 2. Different PPN cells modulated by N-type channels only, P/Q-type channels only, and both N- and P/Q-type channels.
Top. PPN cells with only P/Q-type calcium channels (20%) are assumed to be “Wake-on” and modulated by CaMKII (left side, blue channels). PPN cells with both N- and P/Q-type calcium channels (50%) are assumed to be Wake/REM-on” and modulated by both CaMKII and cAMP/PKA metabolic pathways (middle, blue and green channels). PPN cells with only N-type calcium channels (30%) are assumed to be “REM-on’ and modulated by the cAMP/PKA pathway (right side, green channels). P/Q-only and N+P/Q cells thus are expected to be active during waking, while N-only and N+P/Q cells are active during REM sleep. Bottom. Left records (blue) show that P/Q-only cells manifested ramp-induced oscillations (first recording) that were not affected by conotoxin (CgTX) (middle recording), but were completely blocked by Aga (right recording). Middle bottom records (blue-green) show that N+P/Q cells manifested ramp-induced oscillations (left recording) that were reduced by CgTx (middle recording), and were further reduced by agatoxin (Aga) (right recording). Right records (green) show that N-only cells manifested ramp-induced oscillations (left recording) that were not affected by Aga (middle recording), but were completely blocked by CgTx (right recording). Data from Luster et al 2015.
3. Implications for REM sleep development
The human newborn exhibits an even distribution of waking, REM sleep and slow wave sleep, spending about 8 hours in each state (Roffwarg et al. 1966). After birth, there is a gradual decrease in REM sleep from about 8 hours at birth to about 1 hour by 15 years of age, beyond which there is a small decrease until senescence. After birth, slow wave sleep may increase transiently, then gradually decrease from 8 hours per day to 6–7 hours per day by 15 years of age. The gain observed in total waking time, from about 8 hours at birth to about 16 hours at maturity, is mostly at the expense of REM sleep duration. In the rat, the decrease in REM sleep occurs between 10 and 30 days of age, declining from over 75% of total sleep time at birth to about 15% of sleep time by 30 days of age (Jouvet-Mounier et al. 1970). We tracked the changes of all relevant transmitter systems active at the level of the PPN between 10 and 30 days and found that some increased, some decreased, and others did not change in relation to the developmental decrease in REM sleep (Garcia-Rill et al. 2008). Therefore, we concluded that there was no specific transmitter system responsible for the developmental decrease in REM sleep.
However, based on the results described above on the presence of two states modulated by two types of calcium channels, we carried out preliminary studies on the expression of N-type vs P/Q-type high threshold, voltage-dependent calcium channels in the PPN over this developmental period (Garcia-Rill et al. 2015). Our findings suggest that there is a marked decrease (>75%) in the expression of N-type calcium channels in the PPN between 10 and 30 days. On the contrary, there was only a small decrease (<35%) in the expression of P/Q-type calcium channels in the PPN between 10 and 30 days. We conclude that the developmental decrease in REM sleep is due at least in part to a marked decrement in the number of N-type calcium channels in the PPN. This suggests that the proportion of PPN cells recorded early in development showing a higher proportion of N-only (REM sleep active) cells may decrease with the developmental decrease in REM sleep to be replaced by a higher proportion of PPN cells with P/Q-only (wake active) cells. Figure 3 shows the relationship between the developmental decrease in REM sleep and developmental changes in N-type vs P/Q-type calcium channel expression. We compared changes in expression of these channels between the PPN and the hippocampus, which showed no change in N-type channel expression between 10 and 30 days, and a small increase in P/Q-type channel expression during the same period. Therefore, the developmental decrease in REM sleep appears related to decreased N-type channel expression specifically in the PPN.
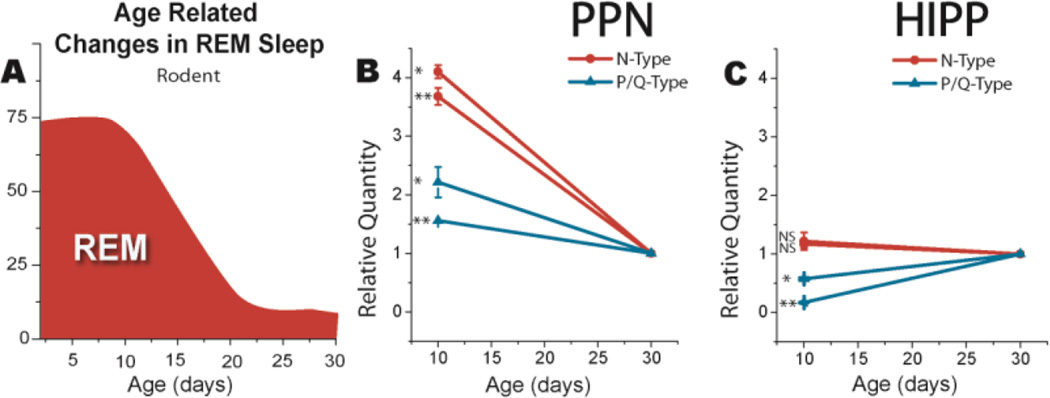
Figure 3. Correlation between the developmental decrease in REM sleep and N-type channel expression.
A) Developmental decrease in REM sleep as a percent of sleep time after Jouvet-Mounier et al (1970). In the rodent, the decrease occurs between 10 and 30 days before assuming adult levels. B) Relative quantity of N-type calcium channel (red lines) and P/Q-type calcium channel (blue lines) expression at 10 vs 30 days in punches from brain slices containing the PPN. Three replications in each of double samples showed that N-type channel expression significantly decreased >75%, while P/Q-type channel expression decreased <35%. C) Relative quantity of N-type calcium channel (red lines) and P/Q-type calcium channel (blue lines) expression at 10 vs 30 days in punches from brain slices containing the hippocampus (HIPP). N-type calcium channel (red lines) expression did not change between 10 and 30 days, while P/Q-type calcium channel (blue lines) expression increased ~50% during the same period in the HIPP. * p<0.05, ** p<0.01, NS not significant. Data from Garcia-Rill et al 2015.
Another implication from the differential control of waking and REM sleep by different calcium channel types is the possibility that the basic rest-activity cycle (BRAC) is mediated by alternation of the two channels. Nathaniel Kleitman proposed that, superimposed on the wake-sleep rhythm, was an ultradian rhythm with a period of ~90 min (Kleitman 1963). This rhythm accounts for the frequency of REM sleep episodes during the night, as well as periodic changes in alertness during the day. There is a reciprocal interaction between the intracellular pathways modulating N-type vs P/Q-type calcium channels, i.e. cAMP and CaMKII, respectively (Mika et al 2015). We hypothesize that this alternation may be due to activation during the troughs preferentially of N-type calcium channels to produce REM sleep during sleeping hours and decreased vigilance during waking hours. Conversely, at the peaks of this rhythm is activation preferentially of P/Q-type calcium channels. This mechanism would explain the manifestation of the BRAC.
4. Cell Clusters
Hebb advanced the concept of cell assemblies to describe a network that is repeatedly activated and thus the synaptic connections among members of the circuit are strengthened (Hebb 1949). Recent studies determined that in the hippocampus there are, according to one group, “patches” of entorhinal cortex cells that play a role in learning and memory (Ray et al 2014). Another group described clusters of cells that may have joint function as “islands” that form a hexagonal lattice over the cortex (Kimura et al 2014). Are there clusters of cells that act together in the PPN to modulate coherence and frequency? We recently hypothesized that the PPN may contain subgroups of functional units (Garcia-Rill et al 2013; Urbano et al 2014).
Anatomically, neurons in the PPN are scattered such that in the pars compacta there are glutamatergic (GLU), cholinergic (ACh), and GABAergic neurons in the ratio of 5:3:2, respectively (Wang & Morales 2009). Studies using calcium imaging in the PPN pars compacta reveal an interesting anatomical organization within the nucleus. Dye coupled PPN neurons, which is indicative of electrical coupling via gap junctions, is evident between spaced pairs of cells throughout the nucleus (Garcia-Rill 2015). These pairs of PPN cells are labeled throughout the nucleus even in the control, unstimulated condition. The spatial separation between couplets suggests that there are clusters of cells throughout the nucleus (Garcia-Rill 2015). Since electrically coupled neurons generally represent GABAergic neurons, we speculate that there are 5 GLU and 3 ACh neurons closely associated with each GABAergic pair. That is, there may be clusters of approximately 10 neurons scattered within the pars compacta that may create a functional subgroup. Much additional evidence is required to support this hypothesis, but it may be possible to dissect such an organization to determine how the nucleus as a whole generates coherent activity at specific frequencies. It is also important to determine how PPN neurons respond to sensory input and how that input generates coherent activity.
Similar functional clustering has been proposed for the hippocampus, especially in relation to epileptic networks (Muldoon et al. 2013). In a study of cell assemblies in the hippocampus, Buzsaki described subsets of about 10 neurons that showed repeated synchronous firing during open field exploration (Buzsaki 2010). Interestingly, the timescale of activity between these neurons had a median of 23 msec, and the peak optimal timescale was ~16 msec, that is, most activity occurred in the 40–60 Hz range. We hypothesize that a similar temporal relationship will be evident among cell clusters in the PPN. As described above, N-only, P/Q-only, and N+P/Q groups each included electrophysiological types I, II, and III. Since it is known that type I cells are non-cholinergic, type II cells are 2/3 cholinergic, and type III cells are 1/3 cholinergic (Garcia-Rill 1991, 2015), it is likely that all transmitter types are represented within each calcium channel group. Thus we postulate that there are the cell clusters of 5 GLU, 3 ACh, and 2 GABA cells, all of which have the N-only calcium channels, representing a cluster firing during REM sleep only (the REM-on cluster). In addition, there are the similar clusters which have only the P/Q-type channels, that fire only during waking (the Wake-on cluster), and clusters which have both the N- and P/Q-type calcium channels, that fire during both waking and REM sleep (the Wake/REM clusters).
5. Implications for Insomnia
We view insomnia not as a sleep disorder, but as a waking disorder, i.e. one in which there is too much waking. Insomnia is called “primary” if it is not related to some other medical or psychiatric condition. Insomnia is a hallmark of a number of psychiatric disorders such as schizophrenia, bipolar disorder, and major depression, in which insomnia is termed “secondary”. Insomnia is also present in a number of neurological diseases. Insomnia includes difficulty falling asleep (prolonged sleep latency), frequent awakenings (difficulty maintaining sleep), and shortened sleep duration (resulting in daytime sleepiness, irritability and fatigue), all of which leads to impairments in daytime functioning. Almost any condition that affects arousal and vigilance can induce insomnia. Recent studies suggest that hyperarousal is present in primary insomnia (Perusse et al. 2013). Given the novel findings described above in which there is a “waking” pathway mediated by CaMKII, and a “REM sleep” pathway mediated by cAMP/PKA (Figure 1), we propose that over expression of P/Q-type calcium channels and/or over activity of the CaMKII-P/Q-type pathway will lead to insomnia. The fact that CaMKII in the PPN is known to modulate the initiation and maintenance of wakefulness (Datta et al. 2011), makes it reasonable to propose that over activity in this pathway or of the calcium channels (P/Q-type) it modulates may lead to increased waking drive.
Treatments for insomnia are palliative and include benzodiazepines and non-benzodiazepine hypnotics, but the risk of physical dependence is high when used chronically. Zolpidem is the most commonly prescribed drug for insomnia and is known to down regulate the CaMKII pathway (Berdyyeva et al. 2014). In order to avoid the abuse potential of such agents, therapy for insomnia could directly address the P/Q-type calcium channel receptor or partially down regulate the CaMKII pathway. Another option could be the use of PPN DBS at high frequency (>100 Hz), perhaps delivered only at night, to decrease waking drive. One strategy for the development of novel treatments for insomnia is the creation of animals that over express P/Q-type calcium channels, preferably by induction. Such animals would be expected to manifest increased waking time correlated with the level of over expression. Agents that modulate such over expression would then be expected to be good candidates for the treatment of insomnia.
6. Implications for Schizophrenia and Bipolar Disorder
Schizophrenia and bipolar disorder are characterized by sleep-wake symptoms such as hyperarousal, increased REM sleep, decreased slow wave sleep, and hallucinations, which are thought to represent REM sleep intrusion into waking (Dement 1967; Garcia-Rill 2009). Reduced gamma band activity has been reported in bipolar disorder (Ozerdem et al. 2011). Aberrant gamma band activity and coherence during cognitive tasks was described in schizophrenia (Ulhass and Singer 2010; 2013). There are also deficits in coherence and maintenance of gamma oscillations in patients with schizophrenia (Wilson et al. 2011). Decreases in gamma band coherence and maintenance can account for many of the symptoms of schizophrenia and bipolar disorder. The positive symptoms include hallucinations, delusions, thought disorder, and agitation, while negative symptoms include lack of affect, anhedonia, and withdrawal. Cognitive symptoms include poor executive function, decreased attention, and disturbed working memory. Cognitive and executive functions are associated with gamma band activity (Eckhorn et al. 1988; Gray and Singer 1989; Philips and Takeda 2009; Singer 1993). In postmortem studies, increased neuronal calcium sensor protein-1 (NCS-1) expression was present in many schizophrenia and bipolar disorder patients, but not in major depression patients (Bergson et al. 2003; Koh et al. 2003). That is, gamma band activity is reduced or disrupted in disorders that show brain NCS-1 over expression.
We recorded PPN neurons in the presence of fast synaptic blockers and terodotoxin with various concentrations of NCS-1 in the recording pipette using whole cell patch clamp (D’Onofrio et al. 2015a). Figure 4A shows that very low concentrations of NCS-1 (0.5 µM) had minor effects, while higher concentrations (1 µM) increased the amplitude of ramp-induced oscillations (not shown), and very high concentrations of NCS-1 (10 µM) blocked the beta/gamma oscillations (Figure 4B). These results suggested that, a) 1 µM NCS-1 appears to be a physiologically effective concentration, and b) 10 µM NCS-1, as would be expected with over expression, down regulates oscillation amplitude. Therefore, NCS-1 over expression may down regulate the ability of PPN cells to generate gamma band oscillations, thus accounting for the gamma band dysregulation present in some patients. However, some patients do not show significant over expression, and also may not show decreased, but rather increased or normal gamma activity (Andreou et al. 2014; Diez et al. 2014). Future clinical trials in patients with schizophrenia or bipolar disorder may benefit from determination of a significant decrease in gamma band activity, which may also help address the heterogeneity of schizophrenia and facilitate the process of identifying more homogeneous groups within the syndrome (Picardi et al. 2012). It is to those patients that pharmacological targeting to increase gamma band activity may be of benefit. We have preliminary evidence suggesting that the stimulant modafinil may indeed compensate to some extent for excessive amounts of NCS-1. We found a partial return of gamma oscillations that were suppressed by high levels of NCS-1 after exposure to modafinil (Garcia-Rill et al. 2014). In addition, PPN DBS could be used to modulate gamma band activity using high stimulation frequencies (>100 Hz) if too high or lower stimulation frequencies (~40 Hz) if too low.
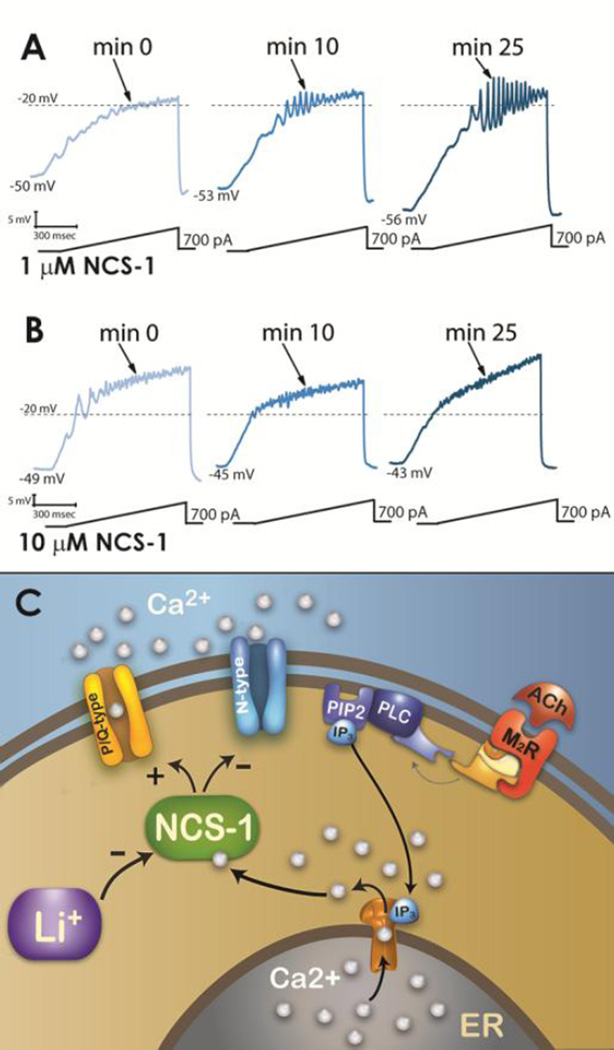
Figure 4. Effects of NCS-1 on PPN neurons amplify oscillations at low concentrations (1 µM), block oscillations at high concentrations (10 µM), while lithium inhibits the effects of NCS-1 on oscillations.
A) Representative 1 sec long current ramp-induced oscillations in a PPN neuron in fast synaptic blockers and tetrodotoxin in the extracellular solution and 1 µM NCS-1 in the recording pipette (left record). After 10 min of NCS-1 diffusing into the cell, the oscillatory activity increased slightly (middle record). However, after 25 min of NCS-1 diffusion both oscillation amplitude and frequency were increased (right record). B) Representative ramp-induced oscillations recorded during 1 sec long current ramps in the presence of fast synaptic blockers and tetrodotoxin and NCS-1 at 10 µM in the recording pipette (left record). After 10 min of NCS-1 diffusing into the cell, the oscillation amplitude increased slightly (middle record). However, testing at 25 min showed a decrease in amplitude compared to both 0 min and 10 min recordings (right record). C) Representation of effects of ACh activation of a M2R acting through G protein coupling to PLC, that in turn cleaves PIP2 into IP3. IP3 is released and binds to IP3 receptors in the ER to release calcium (Ca2+). One of the intracellular pathways activated involves NCS-1, which stimulates P/Q-type calcium channels and somewhat inhibits N-type calcium channels. NCS-1 at low concentrations increases gamma oscillations while NCS-1 at high concentrations blocks them. In addition, NCS-1 over expression is inhibited by 1 mM lithium (Li+), removing the blockade of gamma oscillations and restoring the maintenance of gamma band activity in these cells. Data from D’Onofrio et al 2015a, 2015b.
The mood disturbances in bipolar disorder have been treated effectively using lithium, an ion that remains one of the best treatment options, despite its side effects (Brown and Tracy 2012). Lithium has also been proposed as a neuroprotective agent. Lithium may act by inhibiting the interaction between NCS-1 and inositol 1,4,5-triphosphate receptor protein (InsP) (Schleker et al. 2006). We should note that NCS-1 enhances the activity of InsP (Kasri et al. 2004), which is present in the PPN (Rodrigo et al. 1993). Figure 4C diagrams results showing that lithium reduces ramp-induced oscillations in PPN neurons (D’Onofrio et al. 2015b). That is, lithium may reduce the effects of over expressed NCS-1 in bipolar disorder, thereby normalizing gamma band oscillations mediated by P/Q-type calcium channels modulated by NCS-1. That is, the effects of over expression of NCS-1 in bipolar disorder may be decreased by lithium. As shown above, excessive NCS-1 decreased gamma oscillations (D’Onofrio et al. 2015a), therefore, lithium may prevent the down regulation of gamma band activity and restore normal levels of gamma band oscillations. These findings taken together resolve a 60 year mystery of how lithium works in bipolar disorder. An interesting observation is that NCS-1 down regulates N-type calcium channels, at least in some cell lines (Gambino et al. 2007). This may mean that under some circumstances NCS-1 may inhibit N-type channel function, while promoting P/Q-type channel function.
7. Conclusion
The two most important advances on the physiology of the RAS in the last 10 years were, a) the discovery of electrical coupling in some cells of certain RAS nuclei (Garcia-Rill et al. 2007, 2008; Heister et al. 2008), and b) the finding that every cell in the same nuclei manifests intrinsic membrane gamma oscillations (Simon et al. 2010; Kezunovic et al. 2011; Garcia-Rill et al. 2013). The first advance helps explain how these brain centers maintain the coherence necessary to maintain oscillations at both low and high frequencies. The second advance helps explain how these nuclei induce and maintain gamma band activity necessary for the process of remaining awake and maintaining REM sleep. The PPN simultaneously modulates cortical arousal as well as posture and locomotion. Moreover, in response to sensory inflow, the PPN generates and maintains beta/gamma band activity during waking. These membrane oscillations are mediated by voltage-dependent high threshold N- and P/Q-type calcium channels that are modulated by G proteins. It appears that these two types of channels with separate intracellular pathways are involved in selectively controlling high frequency activity. P/Q-type channels are modulated by CaMKII during waking, while N-type channels are modulated by cAMP/PK during REM sleep (Garcia-Rill et al. 2014). In addition to intrinsic membrane oscillations, the maintenance of gamma band activity requires synaptic connectivity within the nucleus and between regions of the brain. PPN circuitry includes cholinergic, glutamatergic, and GABAergic neurons. Some GABAergic cells are electrically coupled to provide coherence, and the nucleus may include functional cell clusters. However, it is evident that the natural frequency of firing of PPN cells is in the beta/gamma range, which explains the beneficial effects of DBS at these frequencies.
From the moment we awaken, this nucleus ensures that the necessary background of activity is present in order to preconsciously evaluate the world around us (Garcia-Rill et al. 2014). Therefore, this process is embedded in the formulation of our perceptions and actions, and modulates higher-level beta/gamma processing through its projections to the intralaminar thalamus, basal ganglia, hypothalamus, and basal forebrain. That is why it affects functions as disparate as waking and REM sleep, mood and perception, and homeostatic regulation. Consequently, dysregulation in PPN processing will be manifested in motor disorders, psychiatric disorders, neurological disease, as well as sleep disturbances. That is why the PPN is critical to modulating the symptoms of such disorders as PD, insomnia, schizophrenia, and bipolar disorder, among others.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH award P30 GM110702 to Dr. Garcia-Rill. In addition, this work was supported by grants from FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. PICT-2012-1769 and UBACYT 2014-2017 #20120130101305BA (to Dr. Urbano).
References
- Agler HL, Evans J, Colecraft HM, Yue DT. Custom distinctions in the interaction of G-protein b subunits with N-type (Cav2.2) versus P/Q-type (Cav2. 1) Ca2+ channels. J Gen Physiol. 2003;121:495–510. doi: 10.1085/jgp.200208770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C, Nolte G, Leicht G, Polomac N, Hanganu-Opatz IL, Lambert M, Mulert C. Increased resting-state gamma-band connectivity in first-episode schizophrenia. Schizophr Bull. 2015;28:930–939. doi: 10.1093/schbul/sbu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdyyeva T, Otte S, Aluisio L, Ziv Y, Burns LD, Dugovic C, Yun S, Ghosh KK, Schnitzer MJ, Lovenberg T, Bonaventure P. Zolpidem reduces hippocampal neuronal activity in freely behaving mice: a large scale calcium imaging study with miniaturized fluorescence microscopy. PLoS ONE. 2014;9:11, e112068. doi: 10.1371/journal.pone.0112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergson C, Levenson R, Goldman-Rakic P, Lidow MS. Dopamine receptor-interacting proteins: the Ca2+ connection in dopamine signaling. Trends Pharmacol Sci. 2003;24:486–492. doi: 10.1016/S0165-6147(03)00232-3. doi: http://dx.doi.org/10.1016/S0165-6147(03)00232-3. [DOI] [PubMed] [Google Scholar]
- Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles. across the sleep-waking cycle of identified cholinergic, gabaergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J N eurosci. 2014;34:4708–4727. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown KM, Tracy DK. Lithium: the pharmacodynamics actions of the amazing ion. The Adv Psychpharmacol. 2012;3:163–176. doi: 10.1177/2045125312471963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electrophysiological characterization of neurons in the dorsolateral pontine REM sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neurosci. 2006;143:739–755. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanisms of hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci. 2012;109:E2635–E2644. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabardes S, Goetz L, Piallat B, Mathieu H, David O. On the role of the pedunculopontine nucleus and mesencephalic reticular formation in locomotion and waking state in non-human primates. J Neurosci. 2015 doi: 10.1523/JNEUROSCI.2514-15.2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- . Datta S, Desarnaud F. Protein kinase A in the pedunculopontine tegmental nucleus of rat contributes to regulation of rapid eye movement sleep. J Neurosci. 2010;30:12263–12273. doi: 10.1523/JNEUROSCI.1563-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, O’Malley MO, Patterson EH. Calcium/calmodulin kinase II in the pedunculopontine tegmental nucleus modulates the initiation and maintenance of wakefulness. J Neurosci. 2011;23:17007–17016. doi: 10.1523/JNEUROSCI.3981-11.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Spoley EE. Excitation of pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001b;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induce wakefulness and REM sleep. J Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:79–82. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neurosci. 2009;163:397–414. doi: 10.1016/j.neuroscience.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Amer J Physiol Reg Integ Comp Physiol. 2001a;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- Dement WC. Studies on the effects of REM deprivation in humans and animals. Res Publ Assoc Res Nerv Ment Dis. 1967;43:456–467. No doi available. [PubMed] [Google Scholar]
- Desarnaud F, Macone BW, Datta S. Activation of extracellular signal-regulated kinase signaling in the pedunculopontine tegmental cells is involved in the maintenance of sleep in rats. J Neurochem. 2011;116:577–587. doi: 10.1111/j.1471-4159.2010.07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez A, Suazo V, Casado P, Martin-Loeches M, Molina V. Gamma power and cognition in patients with schizophrenia and their first-degree relatives. Neuropsychobiol. 2014;69:120–128. doi: 10.1159/000356970. [DOI] [PubMed] [Google Scholar]
- D’Onofrio S, Kezunovic N, Hyde JR, Luster B, Messias E, Urbano FJ, Garcia-Rill E. Modulation of gamma oscillations in the pedunculopontine nucleus (PPN) by neuronal calcium sensor protein-1 (NCS-1): relevance to schizophrenia and bipolar disorder. J Neurophysiol. 2015a;113:709–719. doi: 10.1152/jn.00828.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio S, Urbano FJ, Garcia-Rill E. Lithium decreases the effects of neuronal calcium sensor protein 1 on pedunculopontine neurons. Neurosci Abst. 2015b;39 doi: 10.14814/phy2.12740. in press. No doi available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraix V, Bastin J, David O, Goetz L, Ferraye M, Benabid AL, Chabardes S, Pollak P, Debu B. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLOS ONE. 2013;8:e83919. doi: 10.1371/journal.pone.0083919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual system? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Gambino F, Pavlowsky A, Begle A, Dupont JL, Bahi N, Courjaret R, Gardette R, Hdjkacem H, Skala H, Poulain B, Vitale N, Humeau Y. IL1-receptor accessory protein-like 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+ -channel and neurite elongation. Proc Nat Acad Sci USA. 2007;104:9063–9068. doi: 10.1073/pnas.0701133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E. Sleep and arousal states: reticular activating system. In: Squire LR, Bloom F, Spitzer N, Gage F, Albright T, editors. New Encyclopedia of Neuroscience. Vol. 8. Oxford, England: Elsevier; 2009. pp. 137–143. [Google Scholar]
- Garcia-Rill E. Waking and the Reticular Activating System in Health and Disease. New York: Academic Press; 2015. p. 330. [Google Scholar]
- Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31:673–690. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Hyde J, Kezunovic N, Urbano FJ, Petersen E. The physiology of the pedunculopontine nucleus- implications for deep brain stimulation. J Neural Transm. 2015;122:225–235. doi: 10.1007/s00702-014-1243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Kezunovic N, D’Onofrio S, Luster B, Hyde J, Bisagno V, Urbano FJ. Gamma band activity in the RAS- intracellular mechanisms. Exptl Brain Res. 2014;232:1509–1522. doi: 10.1007/s00221-013-3794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Kezunovic N, Hyde J, Beck P, Urbano FJ. Coherence and frequency in the reticular activating system (RAS) Sleep Med Rev. 2013;17:227–238. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Mahaffey S, MacNicol M. Correlation between the developmental decrease in REM sleep and N-type calcium channel expression. Neurosci Abst. 2015;39 in press. No doi available. [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Nat Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Shi Y, Xi W, Zhou R, Tan Z, Wang H, Li M, Chen Z, Feng G, Luo M, Huang Z, Duan S. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24:693–698. doi: 10.1016/j.cub.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior: a neuropsychological theory. New York: Wiley; 1949. [DOI] [Google Scholar]
- Hell JW, Yokoyama CT, Breeze LJ, Chavkin C, Catterall WA. Phosphorylation of presynaptic and postsynaptic Ca2+ channels by cAMP-dependent protein kinase in hippocampal neurons. Eur Molec Biol Org J. 1995;13:3036–3044. doi: 10.1002/j.1460-2075.1995.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol. 2013;115:1402–1414. doi: 10.1152/japplphysiol.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Gumenchuk I, kang B, Steger C, Lynn E, Molina NE, Eisenberg LM, Leonard CS. Orexin receptor activation generates gamma band input to cholinergic and serotonergic arousal system neurons and drives an intrinsic Ca2+ -dependent resonance in LDT and PPT cholinergic neurons. Frontiers Neurol. 2015;6:e120. doi: 10.3389/fneur.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Kaneko M, Shin HS, Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J Physiol. 2005;568:199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Christel CJ, Jiao X, Abiria S, Kim KY, Usachev YM, Obermair GJ, Colbran RJ, Lee A. Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:5125–5135. doi: 10.1523/JNEUROSCI.4367-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, Catterall WA. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc Nat Acad Sci USA. 2008;105:341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Jun K, Piedras-Rentería ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams ME, Scheller RH, Tsien RW, Shin HS. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the a1A-subunit. Proc Natl Acad Sci USA. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasri NN, Holmes AM, Bultynk G, Parys JB, Bootman MD, Rietdorf K, Missiaen L, McDonald F, Smedt H, Conway SJ, Holmes AB, Berridge MJ, Roderick Hl. Regulation of InsP3 receptor activity by neuronal Ca2+ -binding proteins. Eur Molec Biol Org J. 2004;23:312–321. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-J. [DOI] [PubMed] [Google Scholar]
- Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur J Neurosci. 2011;34:404–415. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K, Tonegawa S. Island cells control temporal association memory. Science. 2014;343:896–901. doi: 10.1126/science.1244634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N. Sleep and Wakefulness. Chicago, IL: University of Chicago Press; 1953. No doi available. [Google Scholar]
- Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic P, Lidow MS. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci USA. 2003;100:313–317. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DB, Bowden JW, Magoun HW. Effect upon the EEG of acute injury to the brainstem activating system. Electroenceph Clin Neurophysiol. 1949;1:475–486. No doi available. [PubMed] [Google Scholar]
- Llinas RR, Choi S, Urbano FJ, Shin H-S. Gamma band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci. 2007;104:17819–17824. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster B, Hyde J, D’Onofrio S, Urbano FJ, Garcia-Rill E. Mechanisms behind gamma band activity in the pedunculopontine nucleus (PPN) Neurosci Abst. 2014;38:257.20. No doi available. [Google Scholar]
- Luster B, D’Onofrio S, Urbano FJ, Garcia-Rill E. High-threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN)(2015) Physiol Rep. 2015 doi: 10.14814/phy2.12431. in press. No doi available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika D, Richter W, Conti M. A CaMKII/PDE4D negative feedback regulates cAMP signaling. Proc Natl Acad Sci. 2015;112:2023–2028. doi: 10.1073/pnas.1419992112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi G. The sleep-waking cycle. Ergeb Physiol. 1972;64:1–165. doi: 10.1007/3-540-05462-6_1. No doi available. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroenceph Clin Neurophysiol. 1949;1:455–473. No doi available. [PubMed] [Google Scholar]
- Muldoon AF, Soltesz I, Cossart R. Spatially clustered neuronal assemblies comprise the microstructure of synchrony in chronically epileptic networks. Proc Nat Acad Sci. 2013;110:3567–3572. doi: 10.1073/pnas.1216958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem A, Guntenkin B, Atagun I, Turp B, Basar E. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J Affect Disord. 2011;132:325–332. doi: 10.1016/j.jad.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Perusse AD, Turcotte I, St-Jean G, Ellis J, Hudon C, Bastien CH. Types of primary insomnia: is hyperarousal present during napping? J Clin Sleep Med. 2013;9:1273–1280. doi: 10.5664/jcsm.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips S, Takeda Y. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient than efficient visual search in human EEG. Int J Psychophysiol. 2009;73:350–354. doi: 10.1016/j.ijpsycho.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Picardi A, Viroli C, Tarsitani L, Miglio R, de Girolamo G, Dell'Acqua G, Biondi M. Heterogeneity and symptom structure of schizophrenia. Psychiat Res. 2012;198:386–94. doi: 10.1016/j.psychres.2011.12.051. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. Function and dysfunction of synaptic Ca2+ channels: insights from mouse models. Curr Opin Neurobiol. 2005;15:257–265. doi: 10.1016/j.conb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Ray S, Naumann R, Burgalossi A, Tang O, Schmitt H, Brecht M. Grid-layout and theta-modulation of layer 2 pyramidal neurons in medial entorhinal cortex. Science. 2014;343:891–896. doi: 10.1126/science.1243028. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Sakai K, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- Schleker C, Boehmerie W, Jeromin A, DeGray B, Varshey A, Sharma Y, Szigeti-Buck K, Ehrlich BE. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Inv. 2006;116:1668–1674. doi: 10.1172/JCI22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, Williams DK, Garcia-Rill E. Gamma band unit and population responses in the pedunculopontine nucleus. J Neurophysiol. 2010;104:463–474. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Stea A, Soomg TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal Ca2+ channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Steriade M, Constantinescu E, Apostol V. Correlations between alterations of the cortical transaminase activity and EEG patterns of sleep and wakefulness induced by brainstem transections. Brain Res. 1969;13:177–180. doi: 10.1016/0006-8993(69)90152-8. [DOI] [PubMed] [Google Scholar]
- Steriade M, Curro Dossi R, Paré D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Nat Acad Sci USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Paré D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhass PJ, Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dial Clin Neurosci. 2013;15:301–313. doi: 10.31887/DCNS.2013.15.3/puhlhaas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Leznik E, Llinas R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci USA. 2007;104:12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, D’Onofrio S, Luster B, Beck P, Hyde JR, Bisagno V, Garcia-Rill E. Pedunculopontine nucleus gamma band activity- preconscious awareness, waking, and REM sleep. Frontiers Neurol. 2014;5:210. doi: 10.3389/fneur.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinregic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Siason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. Abnormal gamma and beta MEG activity during finger movements in early onset psychosis. Dev Psychobiol. 2011;36:596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrinzo L, Zrinzo LV, Tisch S, Limousin PD, Yousry TA, Afshar F, Hariz MI. Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. 2008;131:1588–1598. doi: 10.1093/brain/awn075. [DOI] [PubMed] [Google Scholar]