Abstract
Although aromatase inhibitors (AIs) are commonly used therapies for breast cancer, their use is limited because they produce arthralgia in a large number of patients. To determine whether AIs produce hypersensitivity in animal models of pain, we examined the effects of the AI, letrozole, on mechanical, thermal, and chemical sensitivity in rats. In ovariectomized (OVX) rats, administering a single dose of 1 or 5 mg/kg letrozole significantly reduced mechanical paw withdrawal thresholds, without altering thermal sensitivity. Repeated injection of 5 mg/kg letrozole in male rats produced mechanical, but not thermal, hypersensitivity that extinguished when drug dosing was stopped. A single dose of 5 mg/kg letrozole or daily dosing of letrozole or exemestane in male rats also augmented flinching behavior induced by intraplantar injection of 1000 nmol of adenosine 5′-triphosphate (ATP). To determine whether sensitization of sensory neurons contributed to AI-induced hypersensitivity, we evaluated the excitability of neurons isolated from dorsal root ganglia of male rats chronically treated with letrozole. Both small and medium-diameter sensory neurons isolated from letrozole-treated rats were more excitable, as reflected by increased action potential firing in response to a ramp of depolarizing current, a lower resting membrane potential, and a lower rheobase. However, systemic letrozole treatment did not augment the stimulus-evoked release of the neuropeptide calcitonin gene-related peptide (CGRP) from spinal cord slices, suggesting that the enhanced nociceptive responses were not secondary to an increase in peptide release from sensory endings in the spinal cord. These results provide the first evidence that AIs modulate the excitability of sensory neurons, which may be a primary mechanism for the effect of these drugs to augment pain behaviors in rats.
Keywords: aromatase inhibitors, letrozole, exemestane, pain, nociception, hypersensitivity, sensory neuron, electrophysiology, excitability, calcitonin gene-related peptide
Introduction
Aromatase inhibitors (AIs) are effective therapies for hormone sensitive breast cancer in postmenopausal women, presumably through their ability to inhibit the biosynthesis of estradiol and estrone (Dowsett et al., 1995; Geisler et al., 2002; Geisler et al., 1998). Studies indicate that up to 50% of breast cancer patients develop pain in joints and/or muscles within weeks to months of initiating AI therapy (Crew et al., 2007; Henry et al., 2007), and this results in approximately one-fourth of these patients discontinuing therapy (Henry et al., 2012). Since there are no objective measures of pathophysiologic changes underlying AI-associated pain, clinical description has mainly focused on subjective evaluation. Patient-reported pain displays large inter-subject variability in the number of joints involved, as well as the intensity and quality of pain, which has been described by patients as “achy”, “stiff”, “tingling”, and “numbness” (Henry et al., 2007). Based on clinical evaluation, patients with AI-induced musculoskeletal pain are diagnosed with a range of conditions, including: tendonitis or tenosynovitis, osteoarthritis, and carpal tunnel syndrome, with multiple rheumatologic conditions diagnosed in a significant fraction of patients (Henry et al., 2007; Moxley, 2010), however, the high frequency of these conditions in post-menopausal women may confound these determinations (Lintermans et al., 2012; Moxley, 2010). Furthermore, it remains unclear whether these conditions contribute to AI-induced pain or are common comorbidities. Therapeutic approaches that effectively alleviate pain associated with inflammatory joint disorders, such as nonsteroidal anti-inflammatory drugs and acetaminophen, are largely ineffective in alleviating AI-induced musculoskeletal pain or improving patient persistence on AI therapy (Crew et al., 2007; Hashem et al., 2013; Martens et al., 2007; Morales et al., 2008). Additionally, factors associated with AI-induced arthralgia identified by clinical studies have been largely inconsistent and mechanisms mediating this side effect remain unknown (Henry et al., 2008).
Evidence suggests that AI-induced arthralgia is linked to the ability of AIs to reduce estrogen concentrations by blocking aromatase, the enzyme that catalyzes the conversion of testosterone to 17β-estradiol (Felson and Cummings, 2005; Henry et al., 2008). This notion is supported by clinical observations that musculoskeletal syndromes develop in patients during periods of low or declining systemic estrogens, such as during the transition into menopause (Berecki-Gisolf et al., 2009; Cecil and Archer, 1925; Meriggiola et al., 2012) or following discontinuation of estrogen replacement therapy (Brunner et al., 2010; Ockene et al., 2005). Studies using male and female rats show that estrogen administration produces antinociception in animal models of pain (Kuba et al., 2005; Liu and Gintzler, 2000; Mannino et al., 2007; Tsao et al., 1999), suggesting that systemic estrogens may be negative regulators of pain.
Although the major source of endogenous estrogen in females is the ovaries, it is well established that males and females synthesize estrogens in other tissues including the central and peripheral nervous systems (Callard et al., 1978; Schaeffer et al., 2010). For instance, estradiol content in the rat central nervous system varies widely between brain regions. Furthermore, while region-specific content does not show a clear correlation with blood estradiol concentrations, regions with relatively high levels of aromatase activity correspond to high estradiol content (Konkle and McCarthy, 2011). The expression of enzymes necessary for estrogen synthesis (Schaeffer et al., 2010) and proteins mediating estrogen signaling cascades (Papka and Storey-Workley, 2002; Takanami et al., 2010; Taleghany et al., 1999) also are found in dorsal root ganglia (DRGs) and in primary sensory neurons. Consequently, aromatase inhibition in these tissues may be an important factor for AI-induced toxicity, particularly when estrogen synthesis by the ovaries is diminished, such as during menopause or following oophorectomy.
Despite the clinical significance of AI-induced arthralgia, few studies have attempted to determine the effects of AIs in animal models of nociception (Evrard and Balthazart, 2004a, b; Fusi et al., 2014; Moradi-Azani et al., 2011). Consequently, we examined the effect of systemically administering letrozole to male and OVX female rats on nociceptive responses to mechanical and noxious thermal stimulation. Previous studies suggest that estradiol attenuates the excitatory effects of the nucleotide adenosine 5′-triphosphate (ATP) (Chaban et al., 2003; Cho and Chaban, 2012; Lu et al., 2013). Therefore, we additionally determined whether systemic administration of letrozole and exemestane (AIs with different mechanisms of action) could alter the nociceptive response to intraplantar injection of ATP. To directly assess the effect of systemic letrozole treatment on sensory neurons, we measured various parameters of excitability in sensory neurons acutely isolated from animals treated with the AI. Finally, we determined whether systemic administration of letrozole could alter the release of calcitonin gene-related peptide (CGRP) from sensory nerve endings in spinal cord slices. We chose to examine CGRP release, in part, because previous studies suggest that estrogens regulate CGRP expression in sensory neurons (Gangula et al., 2000). Our results demonstrate that rats administered aromatase inhibitors exhibit hypersensitivity to mechanical and chemical stimuli and enhanced excitability of sensory neurons. Preliminary findings have appeared in abstract form (Robarge et al., 2011).
Materials and Methods
Animals
Experiments were performed using adult male and female Sprague Dawley rats (150–200 grams; Harlan Laboratories, Indianapolis, IN), housed two to three per cage under a 12-hour dark-light cycle. Ovariectomies were performed by the supplier during which rats were administered 41.7 mg ketamine (i.m.), 8.3 mg/kg xylazine (i.m.) and 3.0 mg/kg ketoprofen (s.q.). Wound clips used to close the surgical incisions were removed 10 days post-surgery, as recommended by the supplier. Male rats were housed for at least one week, while OVX rats were housed for at least two weeks, before beginning experiments. Food and water were available ad libitum. All experiments were performed in accordance with the ethical guidelines for investigation of experimental pain in conscious animals and the guidelines of the Indiana University School of Medicine Institutional Animal Care and Use Committee (Zimmermann, 1983).
Chemicals
Letrozole and exemestane were obtained from United States Pharmacopeia (Rockville, MD). F-12 and DMEM/F-12 media, as well as heat-inactivated horse serum, glutamine, normocin, and penicillin-streptomycin were obtained from Life Technologies (Carlsbad, CA). Papain was obtained from Worthington (Lakewood, NJ). The rabbit anti-rat CGRP antiserum was a gift from Dr. Michael Iadorola (NIH) and its selectivity has been previously characterized (Vasko et al., 1994). All other chemicals and reagents were obtained from Sigma Chemical Company (St. Louis, MO).
Drug administration
In behavioral experiments, on the day of drug injection, letrozole and exemestane powders were dissolved in a 2.5 to 30 percent solution of hydroxypropyl-β-cyclodextrin (HPβCD) in sterile normal saline. AIs were administered by intraperitoneal (i.p.) injections (1 to 30 mg/kg) using a 26G needle. In all experiments, animals in both experimental and control groups received equal amounts of HPβCD in equal injection volumes (5 mL/kg; approximately 1 mL). ATP was prepared in ice-cold sterile phosphate buffered saline (0.01M, pH 7.4) (PBS) and kept on ice until injection. Prior to injection, ATP and PBS vehicle solutions were drawn into a syringe and warmed to room temperature. Injections in the paw were administered intradermally in the mid-plantar footpad using a 31G needle (50 µL injection volume).
Behavioral testing
Experiments were performed between 7 AM and 3 PM during the animals’ light cycle. After a minimum of one week in the animal care facility, animals were acclimated to the testing environment on multiple days for one week preceding any behavioral measurement or drug administration. Prior to each measurement session, animals were acclimated in the testing apparatus for approximately 30 minutes or until grooming or exploratory behavioral ceased. In all experiments, the observer was blinded to treatment and data analysis was completed at the conclusion of the experiment. In animals receiving chronic drug treatment, behavioral measures were assessed prior to the next daily drug administration.
For repeated measures experiments evaluating sensitivity to mechanical and thermal stimuli, baseline responses were obtained by exposing animals to stimuli two to three times on separate days prior to drug administration. The measurement immediately prior to the first drug injection, however, was used as the baseline response. In order to minimize the potential confounding effect of baseline response on drug-induced changes in these longitudinal experiments, both male and OVX rats were rank ordered with respect to baseline mechanical sensitivity, outliers (paw withdrawal threshold greater than 15 or less than 4 grams) were excluded, and the remaining animals were randomized into treatment groups. Approximately 15% of animals evaluated at baseline were excluded from further testing.
The von Frey filament test was used to ascertain mechanical sensitivity. Rats were placed in an elevated cage with a wire mesh bottom that allowed for stimulation of hind paws. Paw withdrawal response to mechanical stimulation was evaluated with calibrated Semmes and Weinstein monofilaments, using a modification of the “up-down method” (0.4 – 26 grams, the lower and upper limit of the test; Stoelting, Wood Dale, IL) (Chaplan et al., 1994). Filaments were applied to the mid-plantar surface of each hind paw, with trials starting with the four gram filament. A trial consisted of three applications of a filament for approximately five seconds, with at least five seconds separating successive stimulation. A positive response was defined as an abrupt paw withdrawal when the stimulus was applied, or paw withdrawal immediately upon removal of the filament. The paw withdrawal threshold (PWT) in grams was the stimulus/filament evoking a paw withdrawal response in at least two of the three stimulations. PWT was measured for both hind paws and averaged to generate final withdrawal threshold at the specified time point.
The Hargreaves test was used to assess thermal sensitivity. Rats were placed in a ventilated acrylic cage atop a flat glass surface. Paw withdrawal latency (PWL) to noxious radiant heat was assessed using an infra-red (IR) heat source (Ugo Basile, Italy) applied to the mid-plantar surface of each hind paw (Hargreaves et al., 1988). Upon initiating the thermal stimulus, an electronic timer was activated. A paw withdrawal (flinching or lifting the paw) was detected by a photocell, which switched off the IR source and timer, yielding the PWL. The heat source intensity was chosen to yield baseline paw withdrawal latencies of 10 seconds in the rats. A cutoff of 30 seconds was used to avoid tissue damage to the paw. At least 30 seconds separated successive trials in the same animal. PWL from each hind paw was measured three times and all six measurements were averaged to generate the final latency in seconds at the specified time point.
Nociceptive behavior in response to ATP was made by observing unrestrained animals on a glass surface covered by an inverted and ventilated four liter plastic beaker. Mirrors were positioned below and behind the beaker for behavioral assessment, regardless of animal orientation. Once acclimated to the testing environment, animals were removed, lightly restrained, and administered an intradermal injection in the left hind paw. Animals were immediately returned to the testing apparatus and behavior was observed. As a measure of spontaneous nocifensive behavior, flinching or shaking of the injected paw or hindquarters was recorded (McGaraughty et al., 2003; Wheeler-Aceto and Cowan, 1991). Each observed behavior was recorded as one flinch and behavioral responses were tallied in one minute intervals for 10 minutes following the injection.
Isolation and cell culture of adult rat sensory neurons
Cultures of sensory neurons for electrophysiological studies were prepared using a protocol developed by Lindsay (Lindsay, 1988) and subsequently modified by Chi and Nicol (Chi and Nicol, 2007). Briefly, rats were sacrificed in a CO2 chamber and dorsal root ganglia (DRG) were dissected into an ice cold solution of Puck’s saline solution composed of (in mM): 171 NaCl, 6.7 KCl, 1.6 NaHPO4, 0.46 KH2PO4, and 6.1 D-glucose, pH 7.4. DRGs were serially digested in F-12 medium containing 10 U/mL of papain (10 min at 37°C in 3% CO2) and 1 mg/mL collagenase IA / 2.5 mg/mL dispase (50 min at 37°C in 3% CO2). Following centrifugation (1000 g for 1 min) and resuspension in F-12 medium (supplemented with 10% heat-inactivated horse serum, 2 mM glutamine, 100 µg/mL normocin, 50 units/mL penicillin, 50 µg/mL streptomycin, 50 µM 5-fluoro-2’-deoxyuridine (FDU), and 150 µM uridine), the ganglia were mechanically dissociated into a single cell suspension using a fire-polished glass pipette. Cells were plated at an approximate density of 7,500 cells per well of a 48-well plate containing plastic coverslips pre-coated with 0.1 mg/mL of poly-D-lysine and 10 µg/mL of laminin. Cells were maintained in culture at 37°C and 3% CO2 for 2 to 6 hours before initiating patch clamp electrophysiology recordings.
Electrophysiological recording
Current clamp recording were performed using the whole-cell patch-clamp technique at room temperature (~23°C) (Hamill et al., 1981; Zhang et al., 2012). Briefly, plastic coverslips containing the sensory neurons were placed inside an open diamond bath chamber (Model RC-25, Warner Instruments, Hamden, CT, USA) containing a solution of normal Ringers composed of (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose (pH 7.4 with NaOH). Glass capillary tubes were used to pull recording pipettes with a Warner Instruments pipette puller (Model G85165T-4). The pipette resistance measured 2–5 MΩ when filled with an intracellular solution composed of the following (in mM): 140 KCl, 5 MgCl2, 4 ATP disodium salt, 0.3 GTP monosodium salt, 2.5 CaCl2, 5 EGTA (free Ca2+ concentration calculated at ~100 nM), and 10 HEPES (pH 7.3 with KOH). The final sodium concentration in the pipette solution was 8.3 mM. After achieving the whole-cell configuration, recordings were acquired with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). Traces from current clamp experiments were filtered at 5 kHz and sampled at 2 kHz. The data were acquired and analyzed using either pCLAMP 8.0 or pCLAMP 10.0.
Upon establishing the whole-cell configuration in either small-diameter (20 µm) or medium-diameter (35 µm) sensory neurons, the cells were held at their resting membrane potentials, which ranged between −45 and −65 mV. Immediately thereafter, the cells were injected with a 500 picoampere (pA) ramp of depolarizing current over 1000 ms and the resulting action potentials (APs) were recorded. The same ramp of current was used in all cells independent of drug treatment to assess alterations in excitability caused by AI exposure. The AP traces were used to calculate all parameters of sensory neuronal excitability except for the rheobase and input resistance, which were determined by injecting a series of 200 millisecond (ms) current steps of variable incremental amplitudes until the firing of a single AP was observed. At the conclusion of each recording session, 300 nM of capsaicin was superfused into the recording chamber and AP firing measured to ascertain whether the neurons were capsaicin-sensitive
Release of calcitonin gene-related peptide from spinal cord tissue
Spinal cord slices were prepared as previously described (Chen et al., 1996). After rats were sacrificed using CO2 asphyxiation and decapitation, a 1 cm segment of the lumbar enlargement was dissected, chopped transversely and parasagitally into 0.3 × 0.3 mm sections, and placed into an individual cylindrical perfusion chamber. Slices were perfused at a flow rate of 0.5 ml/min with HEPES buffer (pH 7.4) supplemented with 200 µM ascorbic acid, 100 µM Phe-Ala, 20 µM bacitracin, and 10 µM phenylmethylsulfonyl fluoride (PMSF), aerated with 95% O2 – 5% CO2, and maintained at 37°C. After perfusing slices for 20 minutes to equilibrate the tissue, perfusate was collected in 3 minute intervals throughout the experiment (1.5 ml/fraction) using an automatic fraction collector. Baseline release was established by perfusing tissue with HEPES for 18 minutes (6 fractions), with the last 3 fractions used to determine basal or resting release. Subsequently, potassium-stimulated release was determined by perfusing tissue for an additional 9 minutes (3 fractions) with HEPES buffer containing 30 mM KCl (substituted for equimolar NaCl). Lastly, slices were perfused with HEPES buffer for 21 minutes (7 fractions) to re-establish basal release. Following tissue perfusion, spinal cord tissue was collected and homogenized in 2 ml of 0.1 N HCl. Homogenates were centrifuged at 3,000 rpm for 20 minutes at 4°C. The supernatants were serially diluted with HEPES buffer and assayed for immunoreactive CGRP (iCGRP) along with perfusate fractions by radioimmunoassay as previously described (Chen et al., 1996). The total iCGRP content in each tissue slice was calculated as content measured in all perfusate fractions plus tissue homogenate.
Statistical analysis
Unless otherwise specified, data are presented as mean ± standard error of the mean (S.E.M.) and statistical significance was set at p < 0.05. Effects of letrozole on PWT and PWL were analyzed using two-way analysis of variance with repeated measures (two-way RM-ANOVA), followed by post hoc two-sample t-tests. One-way RM-ANOVA was used to evaluate whether PWT and PWL changed over time during behavioral testing. Because PWT was measured using logarithmic intervals in pressure exerted by sequential filaments, log10-transformed thresholds were used for data analysis. To compare treatment effects on PWT and PWL between studies, we normalized baseline responses by calculating percent change from baseline for each rat. In experiments evaluating overt nociceptive behavior, numbers of flinches observed in one-minute intervals were compared between treatment groups using the Mann-Whitney U test. Cumulative ATP-induced nociceptive behavior is reported as median (25th percentile, 75th percentile) and was compared between treatment groups using the Mann-Whitney U test. Boxplots depict overt nociceptive behavior as five-number summaries with horizontal lines representing (from top to bottom): 75th percentile + (1.5 × interquartile range) (upper whisker), 75th percentile, median, 25th percentile, 25th percentile - (1.5 × interquartile range) (lower whisker).
In order to determine the various parameters of excitability, the voltage was differentiated with respect to time (dV/dt) and the average of the voltage differential was calculated across the first 100 ms of the ramp stimulus in order to yield a baseline dV/dt. The firing threshold (FT) was the voltage at which the first AP was fired. This was taken as the point at which the dV/dt exceeded the baseline by greater than twenty-fold. The latency to fire was the time point in the ramp stimulus at which the FT was achieved. In order to determine input resistance, a negative current step of 200 ms duration was injected into the cell. The resulting peak potential achieved was subtracted from the resting membrane potential and the difference was divided by the value of the current step in order to yield the final input resistance value. Statistical significance was determined by comparing groups to their respective vehicle controls using two-sample t-tests.
Peptide release from spinal cord tissue is expressed as the amount released per minute in each fraction, divided by the total iCGRP content for that spinal cord sample (percent of total content per minute). Treatment effects were evaluated by comparing peptide release during the three basal fractions to peptide release during the three stimulated fractions. The difference between iCGRP released during exposure to high potassium (stimulated) and iCGRP released during drug or vehicle exposure prior to high potassium stimulation (basal) is termed evoked release. Treatment effects on iCGRP content and release were evaluated using two-sample t-tests.
Results
A single dose of letrozole produces mechanical, but not thermal hypersensitivity in OVX rats
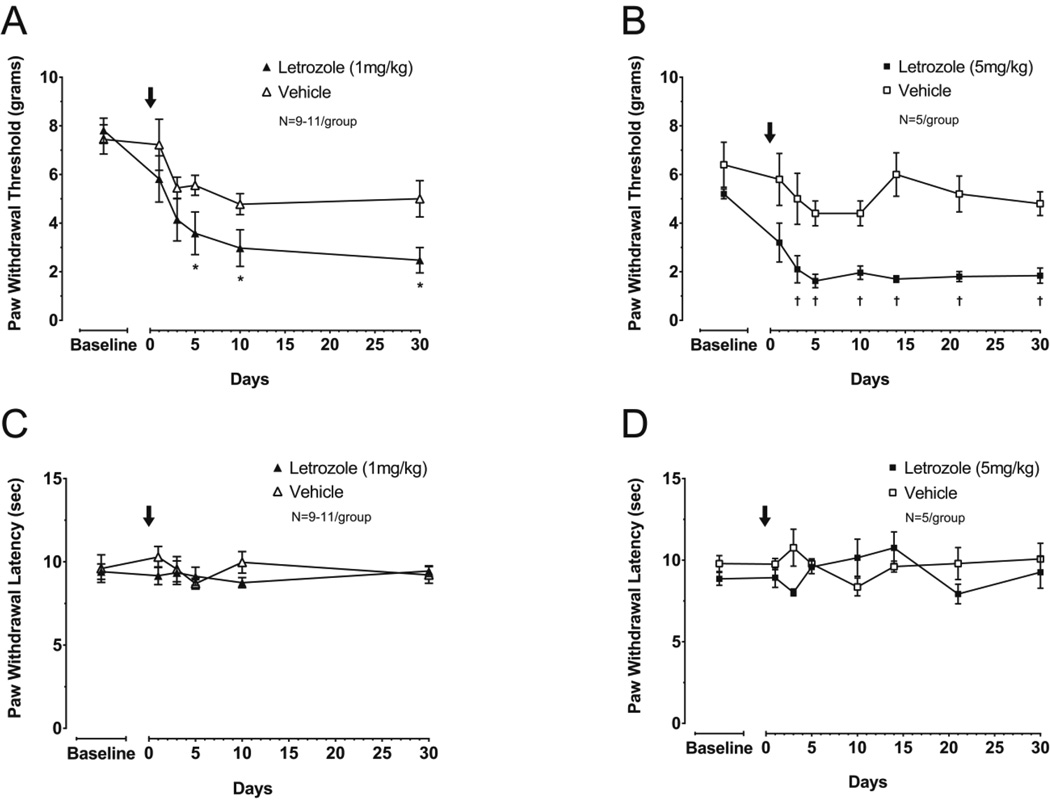
We examined whether AIs alter nociceptive responses in OVX rats by measuring changes in PWT and PWL following treatment with a single dose of 1 mg/kg or 5 mg/kg letrozole. We chose to use a single injection at these doses since they have previous been shown to inhibit aromatase in female rats (Schieweck et al., 1993) and pharmacokinetic studies suggest that the clearance of cyclodextrin-complexed letrozole is 5-fold slower in female rats when compared to males (Wempe et al., 2007). Administration of 1 mg/kg letrozole to OVX rats (n = 11) significantly reduced the response threshold to a mechanical stimulus compared to vehicle-treated controls (100 mg/kg HPβCD; n = 9) at 5, 10 and 30 days after drug administration (two-way RM-ANOVA with post-hoc t-tests, P < 0.05; Figure 1A). In a similar manner, a single injection of 5 mg/kg letrozole (n = 5) produced mechanical hypersensitivity after 3 days compared to vehicle treatment (750 mg/kg HPβCD; n = 5), and this effect was maintained for at least 30 days (two-way RM-ANOVA with post-hoc t-tests, P < 0.05; Figure 1B). Comparing baseline-normalized PWT between letrozole doses did not reveal a significant effect of dose, suggesting PWT was maximally reduced by 1 mg/kg letrozole. In contrast to letrozole-induced mechanical hypersensitivity, response latency to noxious thermal stimulation was not altered at any time points examined after 1 mg/kg or 5 mg/kg letrozole (two-way RM-ANOVA, P > 0.05; Figures 1C and 1D). Additionally, no significant changes in PWL in vehicle treated OVX animals were observed during 30 days of evaluation (one-way RM-ANOVA, P > 0.05).
Figure 1. A single dose of letrozole induces sustained mechanical, but not thermal hypernociception in ovariectomized rats.
Each point represents the mean ± S.E.M. of (A, B) paw withdrawal threshold (PWT) in grams or (C, D) paw withdrawal latency (PWL) in seconds. Letrozole (1 mg/kg: A, C or 5 mg/kg: B, D) or vehicle was administered in a single dose on day 0 (as indicated by the arrows). An asterisk represents significant differences in PWT following treatment with 1 mg/kg letrozole versus vehicle, whereas a cross represents significant differences in PWT following treatment with 5mg/kg letrozole versus vehicle using two-way RM-ANOVA followed by post hoc two-sample t-tests.
Analysis of all vehicle-treated animals for time and vehicle dose showed PWTs were significantly reduced over the course of the experiment (two-way RM-ANOVA, P < 0.05). Consequently, we determined whether this reduction was caused by injection of vehicle or secondary to OVX by measuring PWT in OVX rats treated with a single injection of 750 mg/kg HPβCD (n = 5) or saline (n = 5) (Supplemental Figure 1). PWTs were significantly reduced over 30 days in all rats independent of vehicle or saline injection, suggesting enhanced mechanical sensitivity following ovariectomy is not directly attributable to vehicle administration (two-way RM-ANOVA).
Letrozole produces mechanical hypersensitivity in male rats, without altering thermal sensitivity
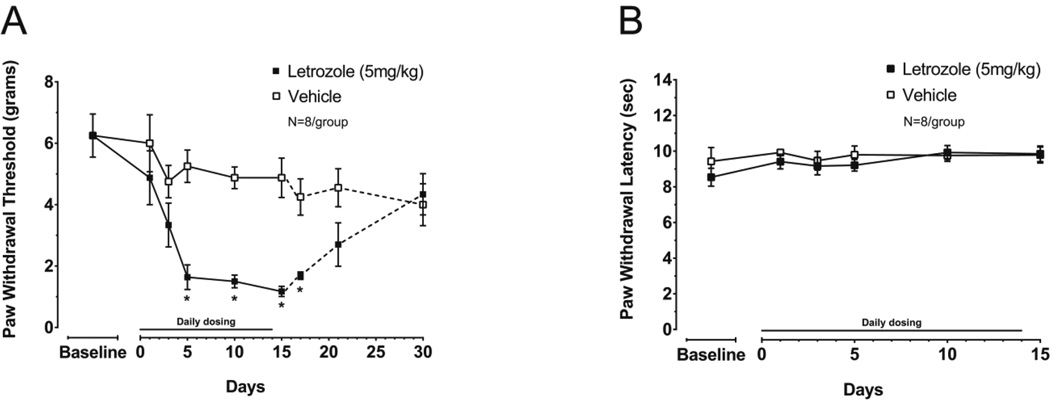
Male rats were treated with 5mg/kg letrozole (n = 8) or vehicle (750 mg/kg HPβCD; n = 8) daily for 15 days and PWT and PWL were measured. When male rats were administered letrozole daily, PWT threshold was significantly reduced five days after initiating drug administration and at days 10 and 15 during treatment (two-way RM-ANOVA with post-hoc t-tests, P < 0.05; Figure 2A). At 5 days of treatment PWT was 5.3 ± 0.5 g in vehicle-treated rats and 1.6 ± 0.4 g in letrozole-treated animals. PWT in vehicle-treated male rats did not significantly change during the treatment period (one-way RM-ANOVA, P > 0.05; Figure 2A). After discontinuing drug administration, animals remained hypersensitive to mechanical stimulation for at least 72 hours. However, no significant difference in PWT between treatment groups was detected 7 days after stopping treatment (experiment day 21), suggesting the effect of letrozole was reversible in males (t-test, P > 0.05; Figure 2A). Treating male rats with 5 mg/kg letrozole for 15 days did not significantly alter PWLs to thermal stimulation when compared to vehicle treated rats (two-way RM-ANOVA, P > 0.05; Figure 2B).
Figure 2. Daily dosing of 5 mg/kg letrozole induces mechanical, but not thermal hypernociception in male rats.
Each point represents the mean ± S.E.M. of (A) paw withdrawal threshold (PWT) in grams or (B) paw withdrawal latency (PWL) in seconds. Letrozole or vehicle was administered daily on days 0 through 14 (15 total doses) as indicated by the horizontal line during which behavior was measured (solid lines). PWT continued to be measured following drug discontinuation (dashed lines in (A)). An asterisk represents significant differences in PWT between treatment groups using two-way RM-ANOVA followed by post hoc two-sample t-tests.
PWTs prior drug or vehicle administration were not significantly different between male rats (n = 16) and OVX female rats (n = 30) (t-test, P = 0.19). Additionally, PWTs measured at day 15 in male rats treated daily with 5 mg/kg letrozole (n = 8) were not significantly different than PWTs measured at day 30 in OVX rats treated with 1 mg/kg letrozole (n = 11; P = 0.07), 5 mg/kg letrozole (n = 5; P = 0.06), or all letrozole treated OVX rats (n = 16; P = 0.06).
Letrozole and exemestane augment ATP-induced flinching in male rats
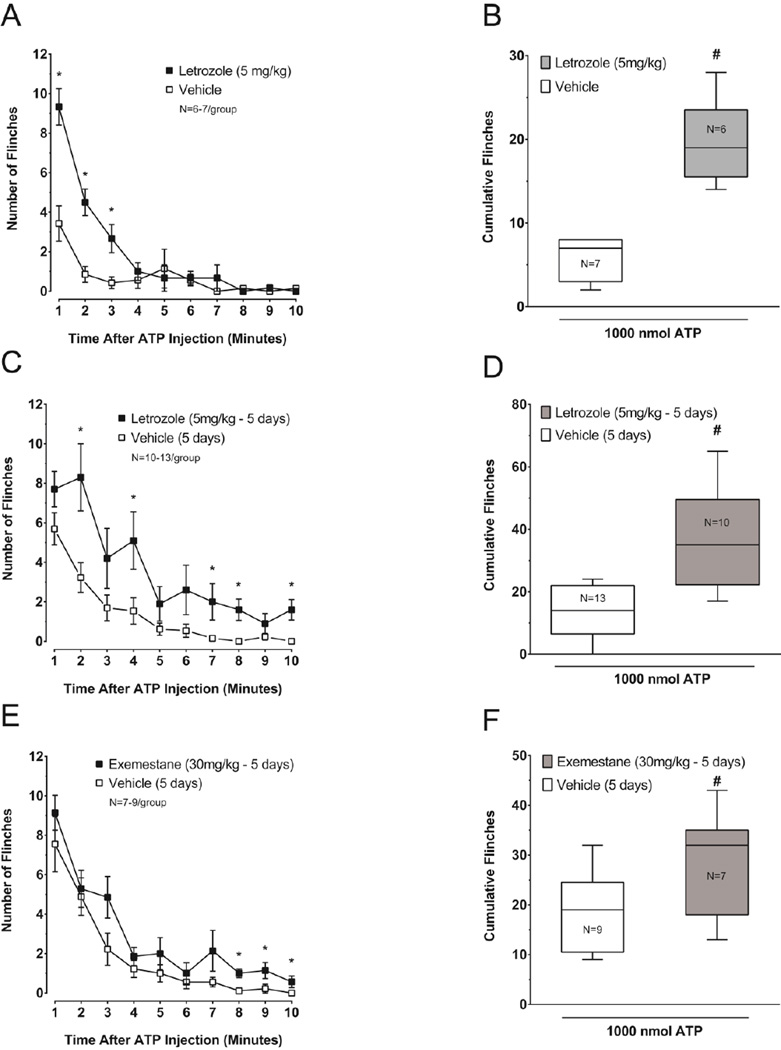
Intradermal injection of purinergic receptor agonists induces overt nociceptive behavior in rats as indicated by hind paw lifting and licking activity.(Bland-Ward and Humphrey, 1997). Consequently, to examine whether letrozole augments ATP-induced nociceptive behavior, male rats were treated with a single dose of vehicle (750 mg/kg HPβCD; n = 7) or with 5 mg/kg letrozole (n = 6) and 3 hours later flinching behavior in response to intraplantar injection of 1000 nmol ATP was observed for 10 minutes. Irrespective of treatment, flinching of the injected paw was observed within one minute following ATP injection (Figure 3A). The flinching rate (flinches per minute) in 11 of 13 rats was maximal during the first two minutes following ATP injection and declined rapidly during the observation period. Flinching evoked by intraplantar ATP was significantly greater in letrozole-treated rats at 1, 2, and 3 minutes after injection of the nucleotide (Mann-Whitney U test, P < 0.05; Figure 3A). Furthermore, letrozole significantly augmented the cumulative number of ATP-induced flinches over 10 min compared to animals treated systemically with the vehicle (Mann-Whitney U test, P < 0.05; Figure 3B).
Figure 3. Letrozole and exemestane augment ATP-induced nocifensive behavior in male rats.
A, C and E: Each point represents the mean ± S.E.M. of flinches observed in one minute intervals after injection of 1000 nmol ATP. In (A), rats were treated acutely with 5 mg/kg letrozole or vehicle as indicated and injected with ATP 3 hours later, whereas in (C) and (E), animals received 5 mg/kg letrozole, 30 mg/kg exemestane, or vehicle daily for 5 days and then were injected with ATP one day after the last injection. An asterisk represents significant differences in the number of flinches observed in one-minute intervals between treatment groups using the Mann-Whitney U test. B, D and F: Cumulative hind paw flinches observed for ten minutes following ATP injections are presented as box plots for each treatment group. The pound symbol indicates significantly different ATP-induced cumulative flinches in rats treated with vehicle versus letrozole or exemestane using the Mann-Whitney U test.
We also determined whether the effect of letrozole on ATP-induced flinching is maintained during chronic administration. For these experiments, male rats were administered AIs or vehicle (HPβCD) daily for five days, a time when letrozole-treated rats in our previous experiments had significantly lowered PWTs. The day following the last injection, we measured flinching in response to 1000 nmol ATP injected in the rat hind paw. In letrozole-treated rats (5 mg/kg; n = 10), significantly more flinches were observed 2, 4, 7, 8, and 10 minutes after ATP injection when compared to vehicle-treated (750 mg/kg HPβCD; n = 13) animals (Mann-Whitney U test, P < 0.05; Figure 3C), which was significantly longer than the effect after a single dose (figure 3A). The chronic letrozole treatment significantly augmented the cumulative ATP-induced flinches observed for 10 min after intraplantar injection of the nucleotide (Mann-Whitney U test, P < 0.05; Figure 3D).
In a series of control experiments, male rats administered saline (n = 8) versus vehicle (750 mg/kg HPβCD; n = 13) daily for five days did not exhibit a significant difference in flinching behavior after injection of 1000 nmol ATP (Mann-Whitney U test, P > 0.05; Supplemental Figure 2). The median (25th percentile, 75th percentile) of cumulative flinches measured for 10 minutes after ATP injection was 17 (9, 24) in saline treated animals versus 14 (6.5, 22) in vehicle treated animals. In addition, flinching behavior in response to intraplantar PBS, the vehicle for intraplantar ATP injections, was measured in male rats following administration of saline (n = 5), vehicle (750 mg/kg HPβCD; n = 5), or 5 mg/kg letrozole (n = 5) for five days (Supplemental Figure 3). Cumulative flinching behavior measured for 10 min after PBS injection was not significantly different between these treatment groups (saline: 1 (0, 4.5), vehicle: 1 (0, 3), and 5 mg/kg letrozole: 2 (0, 3)) demonstrating that letrozole did not sensitize the rats to the intradermal injection procedure (Kruskal-Wallis test, P > 0.05).
We also evaluated whether a mechanism-based inhibitor of aromatase, exemestane (Giudici et al., 1988a) could enhance ATP-induced nocifensive behavior. Male rats were administered vehicle (1000 mg/kg HPβCD; n = 9) or 30 mg/kg exemestane (n = 7) daily for five days. This dose was chosen because it likely produces substantial aromatase inhibition in rats (Giudici et al., 1988b; Zaccheo et al., 1989). The day following the last injection, we measured nociceptive behavior in response to an intraplantar injection of 1000 nmol ATP. The maximal flinching rate of all rats occurred within the first minute of observation and diminished during the 10-minute observation period. Flinching behavior in exemestane-treated rats was greater than in those receiving vehicle at 8, 9 and 10 minutes following ATP injection (Mann-Whitney U test, P < 0.05; Figure 3E). Cumulative flinching over the 10 min after intraplantar injection of 1000 nmol ATP was significantly greater in exemestane-treated rats when compared to vehicle treated animals (Mann-Whitney U test, P < 0.05; Figure 3F).
Systemic letrozole treatment potentiates the excitability of small-diameter and medium-diameter sensory neurons
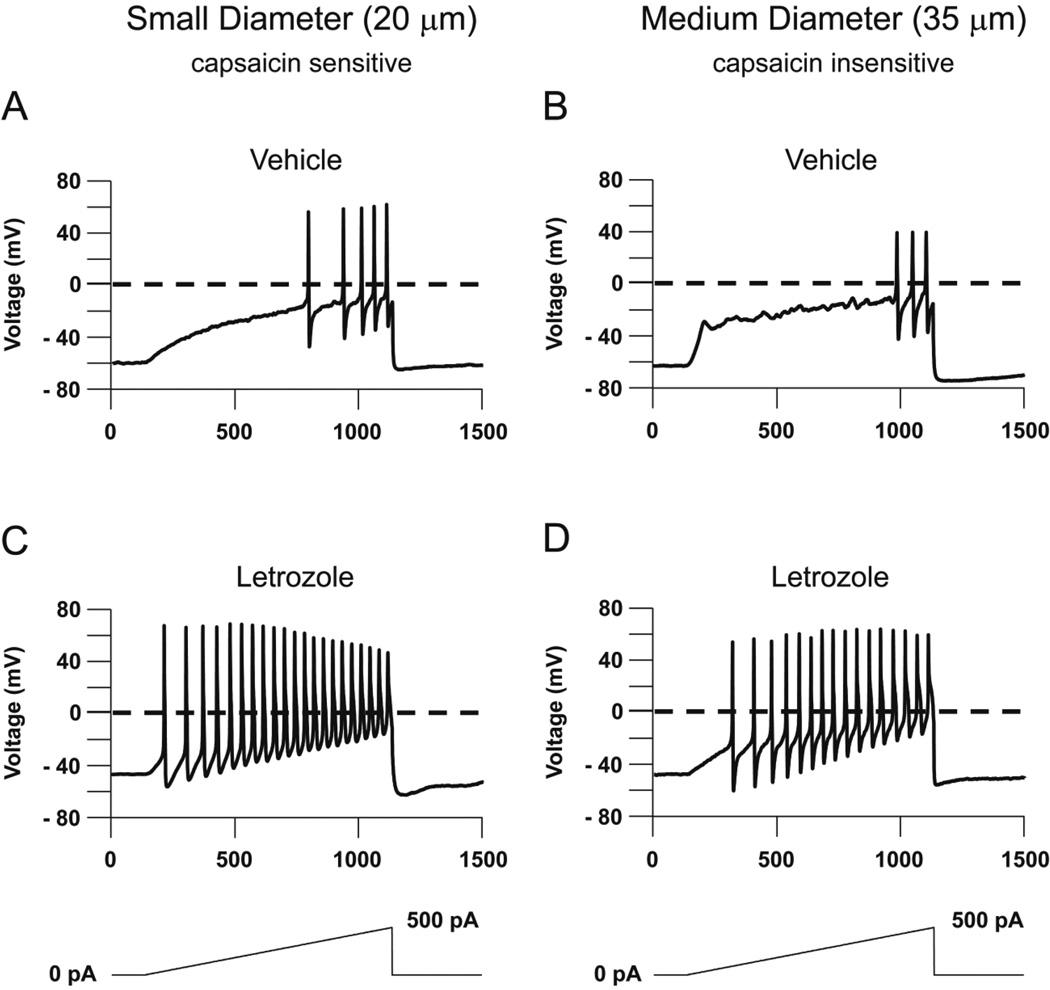
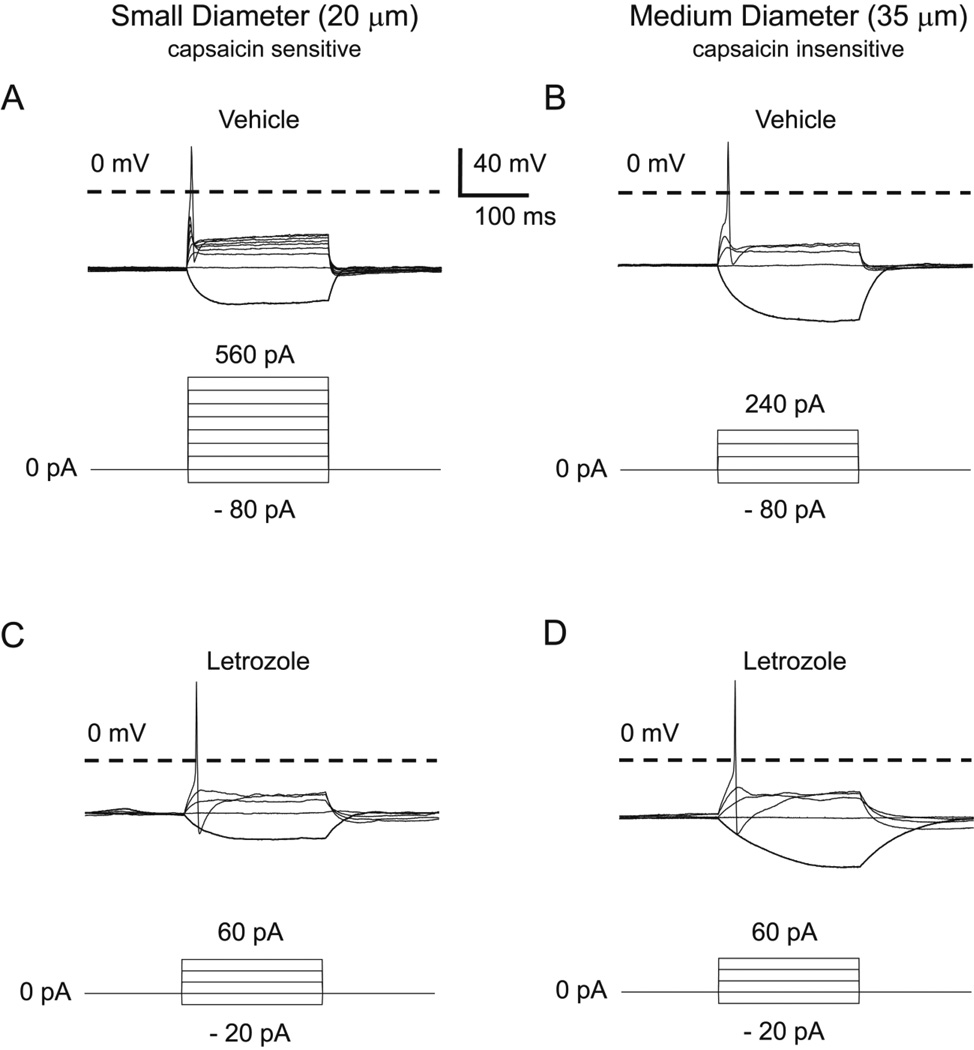
Because an increase in excitability of sensory neurons could contribute to the AI-induced increase in mechanical sensitivity, we examined whether repeated systemic administration of letrozole would augment excitability of small and medium sized sensory neurons. Using neurons isolated from DRGs of male rats treated for 5 days with 5 mg/kg letrozole or vehicle, we determined the number of APs elicited in response to a 500 pA ramp of depolarizing current delivered over 1 second. Representative responses of small and medium diameter neurons from each treatment group are shown in Figure 4 and cumulative results from 6 letrozole-treated and 6 vehicle-treated rats are summarized in Table 1. A representative small diameter neuron from a vehicle-treated rat elicited 5 APs in response to the ramp of depolarizing current (Figure 4A), whereas a representative small-diameter neuron from a letrozole-treated rat fired 21 APs in response to the same stimulus (Figure 4C). In small diameter neurons, the 500 pA ramp elicited significantly more APs in neurons from letrozole-treated rats (22.8 ± 1.2, n = 6) than those from vehicle-treated rats (4.3 ± 0.6, n = 6) (t-test, P < 0.05; Table 1). In a representative medium diameter neuron harvested from a vehicle-treated rat, the ramp of current generated 3 APs (Figure 4B), whereas the same ramp elicited 16 APs in a representative medium diameter neuron from a letrozole-treated rat (Figure 4D). In this neuronal population, the 500 pA ramp elicited significantly more APs in neurons from letrozole-treated rats (14.3 ± 1.7, n = 6) than those from vehicle-treated rats (3.2 ± 1.1, n = 6) (t-test, P < 0.05; Table 1). No medium diameter neurons examined in these experiments were sensitive to capsaicin, whereas all the small diameter neurons displayed capsaicin sensitivity.
Figure 4. Systemic administration of letrozole enhances the number of evoked APs in small- and medium-diameter sensory neurons.
Representative action potentials in response to a 500 pA ramp of depolarizing current over a 1000 ms stimulus. A and B: The top panel shows representative APs from small- (A) and medium-diameter (B) sensory neurons isolated from animals treated with vehicle daily for 5 days. C and D: The bottom panel shows representative APs from small- (C) and medium-diameter (D) sensory neurons isolated from animals administered 5 mg/kg letrozole daily for 5 days.
Table 1.
Effects of systemic letrozole exposure on excitability parameters of sensory neurons a
| Small diameter neurons |
Medium diameter neurons |
|||
|---|---|---|---|---|
| Vehicle | Letrozole | Vehicle | Letrozole | |
| Resting Membrane Potential (mV) | − 65.0 ± 1.5 | − 47.3 ± 0.6 * | − 61.8 ± 1.2 | − 49.4 ± 2.5 * |
| Number of Action Potentials | 4.3 ± 0.6 | 22.8 ± 1.2 * | 3.2 ± 1.1 | 14.3 ± 1.7 * |
| Firing Threshold (mV) | − 7.4 ± 3.8 | − 18.9 ± 1.6 * | − 12.3 ± 3.3 | − 15.1 ± 3.2 |
| Latency to Fire (ms) | 830.5 ± 20.3 | 220.0 ± 21.3 * | 843.9 ± 65.3 | 329.5 ± 52.3 * |
| Rheobase (pA) | 413.3 ± 67.3 | 40.0 ± 5.2 * | 286.7 ± 39.6 | 86.7 ± 24.0 * |
| Input Resistance (MΩ) | 506.8 ± 99.2 | 1068.3 ± 184.8 * | 656.7 ± 120.6 | 932.5 ± 268.8 |
Data are presented as mean ± S.E.M. for small diameter (~20 µm, N=6) and medium diameter (~35 µm, N=6) sensory neurons.
An asterisk indicates a significant difference between treatment groups (two-tailed t-test, p < 0.05).
We next determined whether the minimum current required to elicit a single AP (rheobase) was altered in sensory neurons following systemic letrozole exposure. Representative traces resulting from the rheobase protocol are shown in Figure 5. In sensory neurons isolated from the DRGs of rats treated with vehicle for 5 days, the average rheobase measured in small and diameter neurons was 413.3 ± 67.3 pA and 286.7 ± 39.6, respectively (Table 1). When compared to controls, the rheobase measured in sensory neurons from animals treated for 5 days with 5 mg/kg letrozole were significantly lower (40.0 ± 5.2 pA and 86.7 ± 24.0 in small and medium diameter neurons, Table 1), consistent with the ability of letrozole to enhance the number of evoked APs.
Figure 5. Systemic administration of letrozole attenuates the rheobase in small- and medium-diameter sensory neurons.
Representative traces and current-clamp protocols used to the determine rheobase. A and B: Small- (A) and medium-diameter (B) sensory neurons isolated from rats administered vehicle for 5 days. Current was injected for 200 ms in increments of 80 pA until the firing of a single AP was elicited. C and D: Neurons isolated from rats treated with 5 mg/kg letrozole for 5 days. Small- (C) and medium-diameter (D) neurons were injected with current in increments of 20 pA until the firing of a single AP was elicited.
As summarized in Table 1, several additional electrophysiological parameters were altered in sensory neurons from letrozole-treated animals, indicating that systemic exposure to this drug enhances basal excitability. Resting membrane potentials of small and medium diameter neurons were significantly depolarized in letrozole-treated rats compared to controls (t-test, P < 0.05; Table 1). Additionally, the latency to fire APs following current injection was significantly shorter in all sensory neurons from the letrozole group (t-test, P < 0.05; Table 1). Letrozole exposure also significantly reduced firing thresholds (membrane potentials at which APs initiate) (t-test, P < 0.05); however, this effect was only significant in small diameter neurons (Table 1). Finally, we observed a marked increase in input resistance in small diameter neurons from letrozole treated animals compared to vehicle controls (t-test, P < 0.05), but no change in input resistance was seen in medium diameter neurons (Table 1).
Neuropeptide release from spinal cord slices is not altered in male rats treated with letrozole
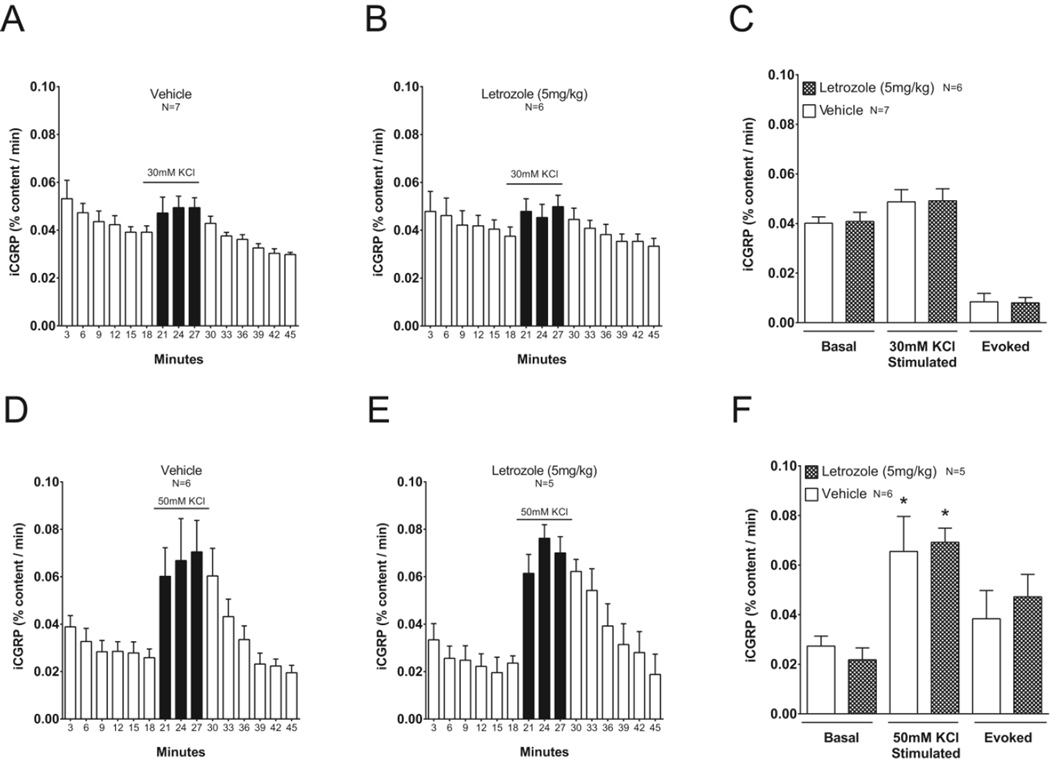
To determine whether letrozole altered release of iCGRP into the spinal cord from sensory neuron central terminals, male rats were treated with 5 mg/kg letrozole (n = 6) or vehicle (750 mg/kg HPβCD; n = 7) daily for 7 to 8 days. PWT was measured at baseline and following 1, 3, and 6 days of treatment in all animals that were used in release experiments. Consistent with our previous results, 5 mg/kg letrozole significantly reduced the PWT compared to vehicle treated controls from 7.0 ± 1.0 grams to 2.7 ± 0.9 grams and 1.6 ± 0.2 at days 3 and 6 following initiation of treatment (two-way RM-ANOVA with post-hoc t-tests, P < 0.05). The day after the last drug dose, we measured iCGRP release from spinal cord slices under basal conditions and during depolarization with 30 or 50 mM KCl. Basal iCGRP release from spinal cord slices isolated from letrozole-treated rats (0.040 ± 0.004 % of total content per min) was not significantly different from vehicle-treated rats (0.040 ± 0.003 % of total content per min) (t-test, P > 0.05; Figures 6A–C). Furthermore, iCGRP release during exposure to 30 mM KCl was not altered in spinal cord slices from rats treated with letrozole compared to vehicle (vehicle: 0.049 ± 0.005 versus letrozole: 0.048 ± 0.005 % of total content per min (t-test, P > 0.05; Figures 6A–C). Consequently, evoked iCGRP release from letrozole treated rats (0.008 ± 0.002 % of total content per min) was not different from that of vehicle treated rats (0.009 ± 0.003 % of total content per min) (t-test, P > 0.05; Figure 6C). Similarly, systemic exposure to 5 mg/kg letrozole for 7 days did not augment iCGRP release from spinal cord slices stimulated with extracellular potassium concentrations sufficient to elicit significant peptide release from basal conditions (t-test, P > 0.05; Figure 6D–F). iCGRP content in the lumbar spinal cord was not different in male rats administered 5 mg/kg letrozole (423.6 ± 35.0 fmol/mg, n = 11) versus vehicle (482.6 ± 40.8 fmol/mg, n = 13) (t-test, P > 0.05).
Figure 6. Release of iCGRP is not altered in spinal cord slices from male rats with letrozole-induced mechanical hypersensitivity.
A, B, D and E: Basal and potassium-stimulated release of iCGRP was measured from spinal cord slices isolated from rats following treatment with vehicle (A, D) or 5 mg/kg letrozole (B, E) for 7–8 days. Open columns represent iCGRP released from spinal cord slices perfused with HEPES buffer alone for successive 3-minute intervals, whereas black columns represent iCGRP released when tissues were perfused with HEPES buffer containing 30 mM (A, B) or 50mM potassium (D, E). The ordinate represents the mean ± S.E.M. of iCGRP released per minute, expressed as the percent of total iCGRP content in the spinal cord slice. C and F: Summary of mean ± S.E.M. of iCGRP release as percent of total content/min during basal (minutes 9–18) and high potassium stimulation (minutes 18–27) in vehicle or letrozole-treated rats from (A - E). Evoked release is calculated as stimulated release minus basal release. Asterisks indicate significant differences in iCGRP release between stimulated and basal conditions using two-sample t-tests.
Discussion
Postmenopausal breast cancer patients undergoing treatment with AIs frequently develop musculoskeletal pain that reduces their quality of life and may lead to treatment discontinuation. Using male and OVX rat models that mimic the low circulating estrogen concentrations observed following menopause, we have shown that systemic administration of AIs induces hypersensitivity to cutaneous mechanical stimuli and augments the nociceptive response to the algogen ATP. Our experiments in OVX rats were conducted two weeks following ovariectomy, when serum estrogen concentrations are significantly depleted (Pfaff, 2002). Of note, serum estradiol concentrations in OVX and male rats have been reported to be between 1 and 3 pg/mL when measured by radioimmunoassay (Kuba et al., 2006; Strom et al., 2008a; Strom et al., 2008b), however the true concentrations may be much lower given the lack sensitivity and specificity of steroid radioimmunoassays in this concentration range (Santen et al., 2007).
We observed that male rats treated with letrozole daily for 15 days showed a significant reduction in the paw withdrawal threshold to mechanical stimuli without any change in withdrawal latency to thermal stimuli. When injections were stopped, mechanical hypersensitivity in letrozole-treated males was reversed by seven days. We also observed mechanical hypersensitivity in OVX rats after a singe dose of 1 or 5 mg/kg letrozole and this effect was maintained for the 30 days of behavioral testing. The drug effect was maintained despite a progressive reduction in PWTs of control OVX rats throughout the testing period, a finding that is consistent with previous studies demonstrating that ovariectomy enhances mechanical sensitivity in animals (Dina et al., 2001; Ma et al., 2011; Sanoja and Cervero, 2005). Our current findings expand on previous work showing AIs alter nociceptive responses in male C57BL6 mice (Fusi et al., 2014). Interestingly, in comparison to a persistent reduction in PWTs observed in letrozole-treated rats, mechanical hypersensitivity reported in mice was transient and reversible during daily chronic AI administration (Fusi et al., 2014). Therefore, differences in the expression and time-course of AI-induced hypersensitivity may be varied depending on species-specific mechanisms mediating AI effects.
Our observed differences in the time course of nociceptive behavior between males and OVX females given the treatment regimens could be secondary to sex differences mediating nociception and analgesia, as have been suggested by many clinical and pre-clinical pain studies (Craft et al., 2004). For example, differences between males and females have been observed in the nociceptive response to capsaicin (Lu et al., 2009), in animal models of chemotherapy-induced peripheral neuropathy (Joseph and Levine, 2003), and in nociceptive changes due to inflammation (Mannino et al., 2007). These differences are often attributed to multiple factors, including the organizational and activational consequences of reproductive hormone differences between sexes. Consequently, treatments that alter endogenous sex steroids may differentially alter nociception in males and females. For example, inhibiting aromatase eliminates one metabolic pathway of androgen metabolism, and therefore AI treatment could alter concentrations of other tissue steroids that might contribute to hypersensitivity. Indeed, AIs are known to increase blood concentrations of the aromatase substrate testosterone in males (Burnett-Bowie et al., 2009; Mauras et al., 2000), but have little effect on testosterone levels in females (Gallicchio et al., 2011; Ingle et al., 2010; Rossi et al., 2009). Alternatively, sex differences seen in the current work could be due to pharmacokinetic differences. Long-term behavioral changes in female rats are consistent with slower elimination of letrozole in this sex, which can produce prolonged tissue exposure and long-term suppression of estrogen synthesis following a single dose of the AI (Wempe et al., 2007). Therefore, in addition to the potential species-specific effects of AIs on hypernociception, our results suggest there also may be sex-dependent effects.
In our experiments, male rats and OVX female rats did not exhibit a thermal hyperalgesia after letrozole treatment. Hypersensitivity to noxious heat applied to the hind paw is often detected in models associated with involvement of inflammatory mediators, such as carrageenan-induced arthritis, collagen-induced arthritis, or nerve growth factor, which lead to functional changes in thermosensitive sensory fibers (Inglis et al., 2007; Malfait et al., 2013; Mills et al., 2013; Zhang et al., 2001). Conversely, heat hypersensitivity is not characteristic of chemotherapy-induced peripheral neuropathy, as modeled in animals (Xiao et al., 2012) or with AI-induced arthralgia. Mechansitically, AI-mediated hypersensitivity may therefore share underlying changes associated with other drug-induced pain syndromes with a significant mechanical, but not thermal component, or there may be commonalities in altered function of the underlying sensory fibers.
Both letrozole and exemestane, which are structurally distinct AIs that inhibit aromatase through different mechanisms, significantly increased flinching behavior induced by intradermal injection of ATP. We chose to examine the effects of ATP since activation of P2Y and P2X nucleotide receptors contributes to inflammatory and neuropathic pain behaviors in rodents (Chen et al., 2005; Cockayne et al., 2000; McGaraughty et al., 2003; Souslova et al., 2000; Tsuda et al., 1999). Previous studies also have shown that systemic estradiol treatment partially attenuates overt nociception in rats induced by ATP (Ma et al., 2011). Estradiol similarly attenuates overt nociception induced by the P2X-selective agonist, α,β-me-ATP, when the compounds are coadministered in the paw (Lu et al., 2013), suggesting that estradiol can modulate ATP-evoked behavior when sufficient local concentrations of the steroid are achieved. We observed that intradermal injection of 1000 nmol ATP rapidly initiated a flinching response of the injected paw that subsided over approximately 10 minutes in control animals, consistent with previous studies of behavior evoked by ATP and ATP analogs (Bland-Ward and Humphrey, 1997; Hamilton et al., 1999). Treating male rats systemically with letrozole and exemestane for five days augmented overt nociceptive responses to intraplantar injection of ATP. These data suggest that AIs enhance the algogenic effects of ATP through a mechanism potentially mediated by estrogen. Our findings are consistent with prior studies showing letrozole treatment in rats and genetic ablation of aromatase in knockout mice enhances formalin-induced nocifensive behavior (Moradi-Azani et al., 2011; Multon et al., 2005). Since overt nociception induced by formalin is attenuated by P2X receptor antagonists and by genetically ablating P2X3 receptors in mice (P2X3−/−), enhanced purinergic receptor sensitivity may underlie the ability of letrozole to potentiate hypernociceptive responses to ATP and to formalin (Cockayne et al., 2000; McGaraughty et al., 2003; Souslova et al., 2000).
AIs are specifically indicated for treating breast cancer in postmenopausal women. Our studies used OVX female and male rats since they have low circulating estrogen concentrations that mimic the blood concentrations observed in women following menopause. Consequently, under these conditions it is more likely that AIs enhance nociception through a mechanism that depletes tissue estrogens rather than circulating levels of the hormone. Although blood estrogen concentrations in ovariectomized rodents are reduced to concentrations observed in males (Davidge et al., 2001; Farrell et al., 1988; Zhao et al., 2004), aromatase expression and activity in extra-gonadal tissues persists following OVX (Zhao et al., 2004). Of note, the capacity for de novo synthesis of estrogens and other steroids in the vertebrate nervous system is well established. In the central nervous system, aromatase is expressed in neuronal cell bodies, presynaptic boutons and postsynaptic neurons and in glia (Hojo et al., 2004; Naftolin et al., 1996; Peterson et al., 2005; Schlinger et al., 1994). Aromatase has also been shown in the dorsal spinal cord of the Japanese quail (Evrard et al., 2000) and aromatase immunoreactivity and synthesis of 3H-estradiol from the steroid precursor 3H-pregnenolone was shown in lumbosacral DRGs from rats (Schaeffer et al., 2010). Aromatase expression in DRG neurons coincides with expression of classical nuclear estrogen receptors, ERα and ERβ, and of GPR30, which are additionally expressed in spinal dorsal horn neurons (Papka and Storey-Workley, 2002; Takanami et al., 2010; Taleghany et al., 1999; Vanderhorst et al., 2005).
We observed an increase in the number of APs fired in response to depolarizing current in sensory neurons isolated from rats treated with 5 mg/kg letrozole for 5 days. In response to a 500 pA ramp of current, letrozole treatment resulted in a 5.3-fold and a 4.5-fold increase in APs fired by small and medium diameter neurons respectively, and these changes were associated with a reduction in the amount of current needed to evoke an AP. Although our experimental design precludes determining whether these neurons are nociceptors, both small diameter and medium diameter sensory neurons respond to mechanical stimulation (Leem et al., 1993). Thus, it is interesting to speculate that the enhanced excitability could be a mechanism that accounts for the pro-nociceptive effects of letrozole. Our electrophysiological observations were made 2 to 6 hours after harvesting neurons; during which time the isolated neurons were not re-exposed to letrozole. Previous electrophysiological studies have shown systemic estrogen administration to OVX rats augments the excitability of trigeminal neurons, when measured 3 to 8 hours after isolation (Flake et al., 2005). These findings suggest that either increasing or decreasing the local or systemic concentrations of estrogens produces long-lasting and persistent changes in the excitability of sensory neurons. These observations are consistent with the persistence of letrozole-induced mechanical hypersensitivity in rats.
The letrozole-induced changes in parameters of excitability strongly suggests that systemic treatment with this AI can enhance activity of sodium channels responsible for initiation of APs (Gurkiewicz et al., 2011). Furthermore, the significant increase in repetitive firing of APs suggests that letrozole exposure downregulates activity of voltage-activated potassium channels that mediate repolarization of the membrane potential (Nicol et al., 1997). Similarly, the increase in input resistance from sensory neurons isolated from letrozole-treated animals indicates a reduction in conductance across leak potassium channels that set the resting membrane potential in sensory neurons (Meuth et al., 2009). Consistent with these findings, there was also a significant depolarization of the resting membrane potential. The fact that letrozole alters excitability of sensory neurons suggests that modifying ion channel activity could prove to be an important therapeutic strategy for treating AI-induced arthralgia in patients. In support of this notion, recent work also suggests that AI-induced pain behaviors in mice are mediated by the TRPA1 (Fusi et al., 2014). Although the mechanism underlying the ability of letrozole to enhance excitability of sensory neurons is unknown, the effects may be mediated by changes in tissue estrogens. There is precedent for this idea since estrogens have been shown to directly interact with ion channels in neurons (McRoberts et al., 2007; Xu et al., 2008). Furthermore, estrogens can alter ion channel expression at the cell surface (Bosch et al., 2013), and indirectly alter ion channel activity by changing the expression and/or activity of ion channel modulators (Diogenes et al., 2006; Kelly and Ronnekleiv, 2009).
Interestingly, we observed that systemic treatment with letrozole enhances excitability of both medium diameter capsaicin insensitive and small diameter capsaicin sensitive sensory neurons. Either activation or inhibition of TRPV1, the pharmacologic target of capsaicin in sensory neurons, does not alter mechanical hypersensitivity in naïve rats; however, TRPV1 sensitization following spinal cord injury and inflammation does enhance sensitivity to mechanical stimuli (Brenneis et al., 2013; Wu et al., 2013). In rat sensory neurons, many TRPV1-expressing neurons co-express the neuropeptide CGRP (Price and Flores, 2007). CGRP is synthesized and released from a number of nociceptive sensory neurons including mechanosensitive C-fibers (Dubin and Patapoutian, 2010; Lawson et al., 2002; Lawson et al., 1997). When released by nociceptors in the periphery, CGRP produces neurogenic inflammation that in turn sensitizes nociceptors in the periphery (Brain and Williams, 1985; Richardson and Vasko, 2002). ERα is known to be co-expressed with CGRP in sensory neurons and estradiol increases CGRP mRNA and CGRP content in a concentration and time-dependent manner (Gangula et al., 2000; Mowa et al., 2003). Additionally, recent studies show CGRP release is stimulated by ex vivo treatment of mouse spinal cord slices with AIs (Fusi et al., 2014). Consequently, we chose to examine whether chronic administration of letrozole to rats would alter the content or release of CGRP from sensory neurons in spinal cord slices taken from these animals. Our results show that basal iCGRP release from spinal cord tissue of letrozole-treated rats was not different than vehicle-treated controls, suggesting that letrozole treatment does not result in persistent activation of CGRP expressing nociceptors. Underlying differences between these findings and those reported in the mouse by Fusi and co-workers are unclear. However, the letrozole concentrations used by Fusi et al. were approximately 500-fold higher than the maximum plasma concentrations achieved by doses that elicited hypersensitivity responses in mice. In addition, our results are consistent with our electrophysiological studies showing a lack of spontaneous AP firing in sensory neurons from letrozole-treated rats (data not shown). Furthermore, we observed no change in the potassium-stimulated release of CGRP in sensory neurons from letrozole-treated rats. While previous studies have provided evidence for the regulation of CGRP expression by exogenous estrogens, we observed no change in total content of iCGRP in vivo after systemic letrozole for up to five days. Taken together, our results do not support altered release of this neuropeptide into the spinal cord as a molecular mediator of letrozole-induced hypersensitivity, but do not preclude a potential effect on other neurotransmitters or other sensory neuron populations. Indeed, a number of mechanosensitive Aδ- and C-fibers do not express CGRP (Dubin and Patapoutian, 2010; Lawson et al., 2002) and thus, the effects we observed on nociception could be mediated by these neurons. Our studies are the first to demonstrate that AI exposure significantly alters the excitability of sensory neurons, although the molecular mechanisms of AI-enhanced excitability and its contribution to AI-induced hypersensitivity remain to be elucidated. Thus, further studies are warranted to identify the mechanism underlying these effects and to determine which subpopulations of neurons are affected by treatment with letrozole or other AIs.
Supplementary Material
Each point represents the mean ± S.E.M. of paw withdrawal threshold (PWT) in grams. Vehicle or saline was administered in a single dose on day 0 (as indicated by the arrow).
A: Each point represents the mean ± S.E.M. of flinches observed in one minute intervals after intraplantar injection of 1000 nmol ATP. Rats received saline or vehicle daily for 5 days and then were injected with ATP one day after the last injection. B: Cumulative hind paw flinches observed for ten minutes following ATP injections are presented as box plots for each treatment group.
A: Each point represents the mean ± S.E.M. of flinches observed in one minute intervals after intraplantar injection of PBS. Male rats were treated with saline, vehicle, or 5 mg/kg letrozole daily for 5 days and then were injected with PBS one day after the last systemic treatment. B: Cumulative hind paw flinches observed for ten minutes following PBS injections are presented as box plots for each treatment group.
Highlights.
Systemic aromatase inhibitor administration alters nociception in male and OVX rats
AI treatment results in mechanical and chemical, but not thermal hypersensitivity
Peripheral sensory neurons display enhanced excitability following AI treatment
AI treatment does not alter CGRP release from sensory nerve terminals in the spinal cord
Acknowledgments
The authors would like to thank Dr. Jill Fehrenbacher, Dr. Grant Nicol, and Dr. Todd C. Skaar for their assistance with experimental design and for technical support.
Disclosures
This publication was made possible, in part, with support from the Indiana Clinical and Translational Sciences Institute (ICTSI), funded in part by Grant Number TR000162 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (JDR). Additional support was provided by a Predoctoral Traineeship Award funded by the Department of Defense Breast Cancer Research Program (W81XWH-10-1-0349) (JDR) and by a Clinical Pharmacology training grant (5T32-GM08425) (DAF, JDR) from the National Institute of General Medical Sciences, National Institutes of Health, Bethesda, MD. In addition, these studies were supported by the Harry and Edith Gladstein Chair in Cancer Genomics, Division of Clinical Pharmacology, Indiana University School of Medicine (DAF), and by a Project Development Team award within the ICTSI NIH/NCRR, Grant Number RR025761 (MRV). These studies were conducted in a facility constructed with the support from Research Facilities Improvement Program, Grant Number C06 RR015481-01 from the National Center for Research Resources, NIH.
Abbreviations
- AI
aromatase inhibitors
- PWT
paw withdrawal threshold
- PWL
paw withdrawal latency
- DRG
dorsal root ganglia
- OVX
ovariectomized
- AP
action potential
- CGRP
calcitonin gene-related peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to declare.
Contributor Information
Jason D. Robarge, Email: jrobarge@iu.edu.
Djane B. Duarte, Email: djane@unb.br.
Behzad Shariati, Email: bshariat@umail.iu.edu.
Ruizhong Wang, Email: rewang@iupui.edu.
David A. Flockhart, Email: dflockha@iupui.edu.
Michael R. Vasko, Email: vaskom@iupui.edu.
References
- Berecki-Gisolf J, Begum N, Dobson AJ. Symptoms reported by women in midlife: menopausal transition or aging? Menopause. 2009;16:1021–1029. doi: 10.1097/gme.0b013e3181a8c49f. [DOI] [PubMed] [Google Scholar]
- Bland-Ward PA, Humphrey PP. Acute nociception mediated by hindpaw P2X receptor activation in the rat. Br J Pharmacol. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Tonsfeldt KJ, Ronnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17beta-estradiol. Mol Cell Endocrinol. 2013;367:85–97. doi: 10.1016/j.mce.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Williams TJ. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol. 1985;86:855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneis C, Kistner K, Puopolo M, Segal D, Roberson D, Sisignano M, Labocha S, Ferreiros N, Strominger A, Cobos EJ, et al. Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. J Neurosci. 2013;33:315–326. doi: 10.1523/JNEUROSCI.2804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner RL, Aragaki A, Barnabei V, Cochrane BB, Gass M, Hendrix S, Lane D, Ockene J, Woods NF, Yasmeen S, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trial. Menopause. 2010;17:946–954. doi: 10.1097/gme.0b013e3181d76953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. The Journal of clinical endocrinology and metabolism. 2009;94:4785–4792. doi: 10.1210/jc.2009-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callard GV, Petro Z, Ryan KJ. Conversion of Androgen to Estrogen and Other Steroids in the Vertebrate Brain. Integrative and Comparative Biology. 1978;18:511–523. [Google Scholar]
- Cecil RL, Archer BH. Arthritis Of The Menopause. JAMA. 1925;84:75–79. [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Barber LA, Dymshitz J, Vasko MR. Peptidase inhibitors improve recovery of substance P and calcitonin gene-related peptide release from rat spinal cord slices. Peptides. 1996;17:31–37. doi: 10.1016/0196-9781(95)02091-8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li GW, Wang C, Gu Y, Huang LY. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain. 2005;119:38–48. doi: 10.1016/j.pain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Chi XX, Nicol GD. Manipulation of the potassium channel Kv1.1 and its effect on neuronal excitability in rat sensory neurons. Journal of neurophysiology. 2007;98:2683–2692. doi: 10.1152/jn.00437.2007. [DOI] [PubMed] [Google Scholar]
- Cho T, Chaban VV. Interaction between P2X3 and oestrogen receptor (ER)alpha/ERbeta in ATP-mediated calcium signalling in mice sensory neurones. J Neuroendocrinol. 2012;24:789–797. doi: 10.1111/j.1365-2826.2011.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2×3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- Davidge ST, Zhang Y, Stewart KG. A comparison of ovariectomy models for estrogen studies. Am J Physiol Regul Integr Comp Physiol. 2001;280:R904–R907. doi: 10.1152/ajpregu.2001.280.3.R904. [DOI] [PubMed] [Google Scholar]
- Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13:2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26:8126–8136. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M, Jones A, Johnston SR, Jacobs S, Trunet P, Smith IE. In vivo measurement of aromatase inhibition by letrozole (CGS 20267) in postmenopausal patients with breast cancer. Clin Cancer Res. 1995;1:1511–1515. [PubMed] [Google Scholar]
- Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. The Journal of clinical investigation. 2010;120:3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard H, Baillien M, Foidart A, Absil P, Harada N, Balthazart J. Localization and controls of aromatase in the quail spinal cord. The Journal of comparative neurology. 2000;423:552–564. doi: 10.1002/1096-9861(20000807)423:4<552::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Balthazart J. Aromatization of androgens into estrogens reduces response latency to a noxious thermal stimulus in male quail. Horm Behav. 2004a;45:181–189. doi: 10.1016/j.yhbeh.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci. 2004b;24:7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell GC, Koltai A, Murray M. Source of raised serum estrogens in male rats with portal bypass. The Journal of clinical investigation. 1988;81:221–228. doi: 10.1172/JCI113299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, Cummings SR. Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum. 2005;52:2594–2598. doi: 10.1002/art.21364. [DOI] [PubMed] [Google Scholar]
- Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. Journal of neurophysiology. 2005;93:1585–1597. doi: 10.1152/jn.00269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusi C, Materazzi S, Benemei S, Coppi E, Trevisan G, Marone IM, Minocci D, De Logu F, Tuccinardi T, Di Tommaso MR, et al. Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat Commun. 2014;5:5736. doi: 10.1038/ncomms6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicchio L, Macdonald R, Wood B, Rushovich E, Helzlsouer KJ. Androgens and musculoskeletal symptoms among breast cancer patients on aromatase inhibitor therapy. Breast Cancer Res Treat. 2011;130:569–577. doi: 10.1007/s10549-011-1611-2. [DOI] [PubMed] [Google Scholar]
- Gangula PR, Lanlua P, Wimalawansa S, Supowit S, DiPette D, Yallampalli C. Regulation of calcitonin gene-related peptide expression in dorsal root ganglia of rats by female sex steroid hormones. Biol Reprod. 2000;62:1033–1039. doi: 10.1095/biolreprod62.4.1033. [DOI] [PubMed] [Google Scholar]
- Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–757. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- Geisler J, King N, Anker G, Ornati G, Di Salle E, Lonning PE, Dowsett M. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4:2089–2093. [PubMed] [Google Scholar]
- Giudici D, Ornati G, Briatico G, Buzzetti F, Lombardi P, di Salle E. 6-Methylenandrosta-1,4-diene-3,17-dione (FCE 24304): a new irreversible aromatase inhibitor. J Steroid Biochem. 1988a;30:391–394. doi: 10.1016/0022-4731(88)90129-x. [DOI] [PubMed] [Google Scholar]
- Giudici D, Ornati G, Briatico G, Buzzetti F, Lombardi P, di Salle E. 6-Methylenandrosta-1,4-diene-3,17-dione (FCE 24304): a new irreversible aromatase inhibitor. J Steroid Biochem. 1988b;30:391–394. doi: 10.1016/0022-4731(88)90129-x. [DOI] [PubMed] [Google Scholar]
- Gurkiewicz M, Korngreen A, Waxman SG, Lampert A. Kinetic modeling of Nav1.7 provides insight into erythromelalgia-associated F1449V mutation. Journal of neurophysiology. 2011;105:1546–1557. doi: 10.1152/jn.00703.2010. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol. 1999;126:326–332. doi: 10.1038/sj.bjp.0702258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hashem MG, Cleary K, Fishman D, Nichols L, Khalid M. Effect of concurrent prescription antiarthralgia pharmacotherapy on persistence to aromatase inhibitors in treatment-naive postmenopausal females. The Annals of pharmacotherapy. 2013;47:29–34. doi: 10.1345/aph.1R369. [DOI] [PubMed] [Google Scholar]
- Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology (Williston Park) 2008;22:1401–1408. discussion 1416, 1424, 1426. [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, Northfelt DW, Olson JE, Perez EA, Desta Z, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010;70:3278–3286. doi: 10.1158/0008-5472.CAN-09-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis JJ, Notley CA, Essex D, Wilson AW, Feldmann M, Anand P, Williams R. Collagen-induced arthritis as a model of hyperalgesia: functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis Rheum. 2007;56:4015–4023. doi: 10.1002/art.23063. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Sexual dimorphism for protein kinase c epsilon signaling in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. 2003;119:831–838. doi: 10.1016/s0306-4522(03)00203-3. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba T, Kemen LM, Quinones-Jenab V. Estradiol administration mediates the inflammatory response to formalin in female rats. Brain Res. 2005;1047:119–122. doi: 10.1016/j.brainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kuba T, Wu HB, Nazarian A, Festa ED, Barr GA, Jenab S, Inturrisi CE, Quinones-Jenab V. Estradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female rats. Horm Behav. 2006;49:441–449. doi: 10.1016/j.yhbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps B, Perl ER. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol. 2002;540:989–1002. doi: 10.1113/jphysiol.2001.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. J Physiol. 1997;505(Pt 1):177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. Journal of neurophysiology. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintermans A, Laenen A, Van Calster B, Van Hoydonck M, Pans S, Verhaeghe J, Westhovens R, Henry NL, Wildiers H, Paridaens R, et al. Prospective study to assess fluid accumulation and tenosynovial changes in the aromatase inhibitor-induced musculoskeletal syndrome: 2-year follow-up data. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012 doi: 10.1093/annonc/mds290. [DOI] [PubMed] [Google Scholar]
- Liu NJ, Gintzler AR. Prolonged ovarian sex steroid treatment of male rats produces antinociception: identification of sex-based divergent analgesic mechanisms. Pain. 2000;85:273–281. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, Burnstock G, Ma B. 17beta-estradiol rapidly attenuates P2×3 receptor-mediated peripheral pain signal transduction via ERalpha and GPR30. Endocrinology. 2013;154:2421–2433. doi: 10.1210/en.2012-2119. [DOI] [PubMed] [Google Scholar]
- Lu YC, Chen CW, Wang SY, Wu FS. 17Beta-estradiol mediates the sex difference in capsaicin-induced nociception in rats. J Pharmacol Exp Ther. 2009;331:1104–1110. doi: 10.1124/jpet.109.158402. [DOI] [PubMed] [Google Scholar]
- Ma B, Yu LH, Fan J, Cong B, He P, Ni X, Burnstock G. Estrogen modulation of peripheral pain signal transduction: involvement of P2X(3) receptors. Purinergic Signal. 2011;7:73–83. doi: 10.1007/s11302-010-9212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait AM, Little CB, McDougall JJ. A commentary on modelling osteoarthritis pain in small animals. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:1316–1326. doi: 10.1016/j.joca.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8:334–342. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Martens HA, Schroder CP, van der Eerden PJ, Willemse PH, Posthumus MD. Severe disabling tendinopathy caused by anastrazole. Rheumatology. 2007;46:1619–1621. doi: 10.1093/rheumatology/kem213. [DOI] [PubMed] [Google Scholar]
- Mauras N, O’Brien KO, Klein KO, Hayes V. Estrogen suppression in males: metabolic effects. The Journal of clinical endocrinology and metabolism. 2000;85:2370–2377. doi: 10.1210/jcem.85.7.6676. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR, Jarvis MF. Effects of A-317491, a novel and selective P2×3/P2×2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol. 2003;140:1381–1388. doi: 10.1038/sj.bjp.0705574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148:1015–1020. doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriggiola MC, Nanni M, Bachiocco V, Vodo S, Aloisi AM. Menopause affects pain depending on pain type and characteristics. Menopause. 2012;19:517–523. doi: 10.1097/gme.0b013e318240fe3d. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Kleinschnitz C, Broicher T, Austinat M, Braeuninger S, Bittner S, Fischer S, Bayliss DA, Budde T, Stoll G, et al. The neuroprotective impact of the leak potassium channel TASK1 on stroke development in mice. Neurobiology of disease. 2009;33:1–11. doi: 10.1016/j.nbd.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Nguyen T, Tanga FY, Zhong C, Gauvin DM, Mikusa J, Gomez EJ, Salyers AK, Bannon AW. Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur J Pain. 2013;17:469–479. doi: 10.1002/j.1532-2149.2012.00202.x. [DOI] [PubMed] [Google Scholar]
- Moradi-Azani M, Ahmadiani A, Amini H. Increase in formalin-induced tonic pain by 5alpha-reductase and aromatase inhibition in female rats. Pharmacology, biochemistry, and behavior. 2011;98:62–66. doi: 10.1016/j.pbb.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Morales L, Pans S, Verschueren K, Van Calster B, Paridaens R, Westhovens R, Timmerman D, De Smet L, Vergote I, Christiaens MR, et al. Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol. 2008;26:3147–3152. doi: 10.1200/JCO.2007.15.4005. [DOI] [PubMed] [Google Scholar]
- Mowa CN, Usip S, Storey-Workley M, Amann R, Papka R. Substance P in the uterine cervix, dorsal root ganglia and spinal cord during pregnancy and the effect of estrogen on SP synthesis. Peptides. 2003;24:761–771. doi: 10.1016/s0196-9781(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Moxley G. Rheumatic Disorders and Functional Disability With Aromatase Inhibitor Therapy. Clin Breast Cancer. 2010 doi: 10.3816/CBC.2010.n.019. [DOI] [PubMed] [Google Scholar]
- Multon S, Pardutz A, Mosen J, Hua MT, Defays C, Honda S, Harada N, Bohotin C, Franzen R, Schoenen J. Lack of estrogen increases pain in the trigeminal formalin model: a behavioural and immunocytochemical study of transgenic ArKO mice. Pain. 2005;114:257–265. doi: 10.1016/j.pain.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. Journal of neurophysiology. 1997;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Barad DH, Cochrane BB, Larson JC, Gass M, Wassertheil-Smoller S, Manson JE, Barnabei VM, Lane DS, Brzyski RG, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–193. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M. Estrogen receptor-alpha and -beta coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci Lett. 2002;319:71–74. doi: 10.1016/s0304-3940(01)02562-9. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings Biological sciences / The Royal Society. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW. Hormones, brain and behavior. San Diego, Calif: Academic Press; 2002. [Google Scholar]
- Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- Robarge JD, Duarte DB, Skaar TC, Fehrenbacher JC, Flockhart DA, Vasko MR. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. The aromatase inhibitor letrozole produces mechanical hypernociception in the rat. Program No. 581.10. 2011. Online. [Google Scholar]
- Rossi E, Morabito A, Di Rella F, Esposito G, Gravina A, Labonia V, Landi G, Nuzzo F, Pacilio C, De Maio E, et al. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: the HOBOE trial. J Clin Oncol. 2009;27:3192–3197. doi: 10.1200/JCO.2008.18.6213. [DOI] [PubMed] [Google Scholar]
- Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: a model of functional abdominal pain. Pain. 2005;118:243–253. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Schaeffer V, Meyer L, Patte-Mensah C, Eckert A, Mensah-Nyagan AG. Sciatic nerve injury induces apoptosis of dorsal root ganglion satellite glial cells and selectively modifies neurosteroidogenesis in sensory neurons. Glia. 2010;58:169–180. doi: 10.1002/glia.20910. [DOI] [PubMed] [Google Scholar]
- Schieweck K, Bhatnagar AS, Batzl C, Lang M. Anti-tumor and endocrine effects of non-steroidal aromatase inhibitors on estrogen-dependent rat mammary tumors. J Steroid Biochem Mol Biol. 1993;44:633–636. doi: 10.1016/0960-0760(93)90270-7. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Amur-Umarjee S, Shen P, Campagnoni AT, Arnold AP. Neuronal and non-neuronal aromatase in primary cultures of developing zebra finch telencephalon. J Neurosci. 1994;14:7541–7552. doi: 10.1523/JNEUROSCI.14-12-07541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2×3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E. Substantial discrepancies in 17beta-oestradiol concentrations obtained with three different commercial direct radioimmunoassay kits in rat sera. Scand J Clin Lab Invest. 2008a;68:806–813. doi: 10.1080/00365510802254638. [DOI] [PubMed] [Google Scholar]
- Strom JO, Theodorsson E, Theodorsson A. Order of magnitude differences between methods for maintaining physiological 17beta-oestradiol concentrations in ovariectomized rats. Scand J Clin Lab Invest. 2008b;68:814–822. doi: 10.1080/00365510802409703. [DOI] [PubMed] [Google Scholar]
- Takanami K, Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Prossnitz ER, Kawata M. Expression of G protein-coupled receptor 30 in the spinal somatosensory system. Brain Res. 2010;1310:17–28. doi: 10.1016/j.brainres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. Journal of neuroscience research. 1999;57:603–615. [PubMed] [Google Scholar]
- Tsao CM, Ho CM, Tsai SK, Lee TY. Effects of estrogen on autotomy in normal and ovariectomized rats. Pharmacology. 1999;59:142–148. doi: 10.1159/000028314. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Ueno S, Inoue K. Evidence for the involvement of spinal endogenous ATP and P2X receptors in nociceptive responses caused by formalin and capsaicin in mice. Br J Pharmacol. 1999;128:1497–1504. doi: 10.1038/sj.bjp.0702960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. The Journal of comparative neurology. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Vasko MR, Campbell WB, Waite KJ. Prostaglandin E2 enhances bradykinin-stimulated release of neuropeptides from rat sensory neurons in culture. J Neurosci. 1994;14:4987–4997. doi: 10.1523/JNEUROSCI.14-08-04987.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wempe MF, Buchanan CM, Buchanan NL, Edgar KJ, Hanley GA, Ramsey MG, Skotty JS, Rice PJ. Pharmacokinetics of letrozole in male and female rats: influence of complexation with hydroxybutenyl-beta cyclodextrin. J Pharm Pharmacol. 2007;59:795–802. doi: 10.1211/jpp.59.6.0006. [DOI] [PubMed] [Google Scholar]
- Wheeler-Aceto H, Cowan A. Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology (Berl) 1991;104:35–44. doi: 10.1007/BF02244551. [DOI] [PubMed] [Google Scholar]
- Wu Z, Yang Q, Crook RJ, O’Neil RG, Walters ET. TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. Pain. 2013;154:2130–2141. doi: 10.1016/j.pain.2013.06.040. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Cheng Y, Keast JR, Osborne PB. 17beta-estradiol activates estrogen receptor beta-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology. 2008;149:5540–5548. doi: 10.1210/en.2008-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccheo T, Giudici D, Lombardi P, di Salle E. A new irreversible aromatase inhibitor, 6-methylenandrosta-1,4-diene-3,17-dione (FCE 24304): antitumor activity and endocrine effects in rats with DMBA-induced mammary tumors. Cancer Chemother Pharmacol. 1989;23:47–50. doi: 10.1007/BF00258457. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89:265–273. doi: 10.1016/s0304-3959(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Kays J, Hodgdon KE, Sacktor TC, Nicol GD. Nerve growth factor enhances the excitability of rat sensory neurons through activation of the atypical protein kinase C isoform, PKMzeta. Journal of neurophysiology. 2012;107:315–335. doi: 10.1152/jn.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Tian Z, Cheng L, Chen B. Electroacupuncture enhances extragonadal aromatization in ovariectomized rats. Reproductive biology and endocrinology : RB&E. 2004;2:18. doi: 10.1186/1477-7827-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each point represents the mean ± S.E.M. of paw withdrawal threshold (PWT) in grams. Vehicle or saline was administered in a single dose on day 0 (as indicated by the arrow).
A: Each point represents the mean ± S.E.M. of flinches observed in one minute intervals after intraplantar injection of 1000 nmol ATP. Rats received saline or vehicle daily for 5 days and then were injected with ATP one day after the last injection. B: Cumulative hind paw flinches observed for ten minutes following ATP injections are presented as box plots for each treatment group.
A: Each point represents the mean ± S.E.M. of flinches observed in one minute intervals after intraplantar injection of PBS. Male rats were treated with saline, vehicle, or 5 mg/kg letrozole daily for 5 days and then were injected with PBS one day after the last systemic treatment. B: Cumulative hind paw flinches observed for ten minutes following PBS injections are presented as box plots for each treatment group.