Abstract
Epidermal growth factor receptor (EGFR) signaling has a critical role in oncogenic Kras-driven pancreatic carcinogenesis. However, the downstream targets of this signaling network are largely unknown. We developed a novel model system utilizing murine primary pancreatic ductal epithelial cells (PDECs), genetically engineered to allow time-specific expression of oncogenic KrasG12D from the endogenous promoter. We show that primary PDECs are susceptible to KrasG12D-driven transformation and form pancreatic ductal adenocarcinomas (PDAC) in vivo after Cdkn2a inactivation. In addition, we demonstrate that activation of KrasG12D induces an EGFR signaling loop to drive proliferation. Interestingly, pharmacological inhibition of EGFR fails to decrease KrasG12D-activated ERK or PI3K signaling. Instead our data provide novel evidence that EGFR signaling is needed to activate the oncogenic and pro-proliferative transcription factor c-MYC. EGFR and c-MYC have been shown to be essential for pancreatic carcinogenesis. Importantly, our data link both pathways and thereby, explain the crucial role of EGFR for KrasG12D-driven carcinogenesis in the pancreas.
Keywords: pancreatic cancer, EGFR, Kras, MYC
Introduction
Although novel chemotherapeutic regimens increased the overall survival of patients with advanced pancreatic ductal adenocarcinoma (PDAC), its prognosis remains dismal. The epidermal growth factor receptor (EGFR) signaling pathway plays an outstanding role in the carcinogenesis of the disease 1, 2. Despite being active in only a subgroup of patients, the EGFR inhibitor erlotinib is currently the only known targeted therapeutic for PDAC. EGFR belongs to a receptor tyrosine kinase (RTK) family including ErbB2, ErbB3, and ErbB43. Seven ligands, epidermal growth factor (EGF), transforming growth factor α (TGFα), betacellulin (BTC), heparin-binding EGF-like growth factor (HB-EGF), amphiregulin (ARG), epiregulin (EPR), and epigen (EGN) can induce receptor dimerization and consecutive activation 3. Effectors acting downstream of EGFR in the KrasG12D-induced circuit that drive tumor development in the pancreas remain incompletely understood.
In this study we generated a novel mouse model of PDAC, in which expression of the KrasG12D allele can be induced by a tamoxifen-inducible Cre recombinase in primary pancreatic ductal epithelial cells (PDECs). We provide evidence that KrasG12D-driven proliferation of PDECs depends on an EGFR signaling loop engaging the oncogenic transcription factor c-MYC (MYC afterwards).
Results and Discussion
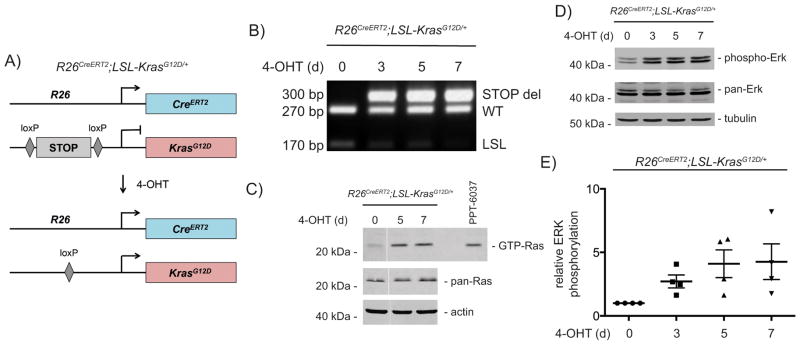
Mutations of the Kras oncogene are one of the earliest genetic events and have been shown to drive carcinogenesis in the pancreas 4. To activate the expression of one allele of oncogenic KrasG12D from the endogenous gene promoter in PDECs, we isolated PDECs from R26CreERT2;LSL-KrasG12D/+ mice (Fig. 1A). These cells show presence of ductal markers and the absence of acinar or endocrine markers (Fig. S1A). PDECs express genes associated with a progenitor state (Fig. S1A). Activation of the Cre recombinase in these cells by 4-hydroxytamoxifen (4-OHT) induced efficient recombination of the Kras locus (Fig. 1B) and more than 90% of the PDECs are recombined after 8 days of 4-OHT treatment (Fig. S1B–D). Expression of oncogenic KrasG12D induced GTP-bound Ras to an extent observed in murine KrasG12D-driven PDAC cell lines (Fig. 1C). In addition, ERK becomes phosphorylated indicating activated canonical Kras signaling (Fig. 1D and 1E).
Figure 1. Activation of canonical Kras signaling in PDECs.
A) Genetic strategy to activate KrasG12D-expression in PDECs (R26CreERT2;LSL-KrasG12D/+). Isolation of PDECs and mouse lines are described in detail in the supplementary material and methods section. All animal studies were conducted in compliance with European guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committees (IACUC) of Technische Universität München, Regierung von Oberbayern, and the University of Pennsylvania. The R26CreERT2 mouse line was described in 48 and LSL-KrasG12D line in 49. B) Genotyping PCR of the indicated PDECs treated with 4-hydroxy-tamoxifen (4-OHT) (200 nM) (Sigma-Aldrich, München, Germany) over time. WT: wild type allele; LSL: Lox-Stop-Lox allele; STOP del: recombined LSL-allele. Primer sequences are depicted in the supplementary material and methods section. C) Ras pull-down assay (Raf-RBD Protein GST beads (Cytoskeleton, Denver, CO, USA)) from vehicle or 4-OHT (200 nM) treated PDECs. The murine KrasG12D-driven PDAC cell line PPT-6037 was used as a positive control. Western blot of pan-Ras expression (clone 10, #05-516, Merck-Millipore, Darmstadt, Germany) (β-actin (Sigma-Aldrich): loading control) Irrelevant lanes were excised and the merger originated from the same gel. D) Western blot of phospho-ERK (Thr202/Tyr204) (#4370, Cell Signaling Technology, Danvers, MA, USA) and pan-ERK (#4696, Cell Signaling Technology) from vehicle or 4-OHT (200 nM) treated PDECs over the indicated time points (α-tubulin (Sigma-Aldrich): loading control). E) Quantification of ERK phosphorylation. PDECs from R26CreERT2;LSL-KrasG12D/+ mice were treated with 4-OHT (200 nM) over time. pan-ERK and phospho-ERK were determined in western blots and quantified using the Odyssey Infrared Imaging System (Li-Cor Biosciences, Bad Homburg, Germany), assuring measurements in the linear range. Shown is the relative ERK phosphorylation of four independent experiments using four individual PDEC lines.
One road to PDAC originates in the pancreatic acinar cells likely via acinar-to-ductal metaplasia (ADM) and pancreatic intraepithelial neoplasia (PanIN) 5. Although the contribution of ductal cells to the carcinogenesis in the pancreas is still a matter of debate 6, available data suggest that ductal cells seem relatively refractory to KrasG12D-driven transformation 7. Therefore, we investigated whether PDECs can form PDAC in vivo. We orthotopically transplanted ex vivo tamoxifen-treated PDECs from R26CreERT2;LSL-KrasG12D/+ mice into the pancreas of immunodeficient mice. However, none of the transplanted mice (n=3) developed PDAC in the investigated time period of 51 days. Furthermore, we detected no pre-malignant lesions in the pancreas of these mice (Fig. S2A). In contrast, it has been reported that transplantation of PDECs, engineered to express KrasG12D, into C57Bl/6 mice, leads to the formation of ductal structures resembling early PanIN lesions 8. Considering low efficacy of KrasG12D-dependent tumor initiation, the number of orthotopically transplanted PDEC cells (1×106 versus 0.15×106 cells) might account for this discrepancy. Indeed, after increasing the number of transplanted PDECs to 7.5×105 cells, formation of PanIN-like structures (lineage label [YFP] and keratin 19 [K19] positive) was detected (Fig. S2B). Besides activating mutations in the Kras gene, the tumor suppressor Cdkn2a is frequently lost in pre-neoplastic lesions. To model the human disease, we isolated PDECs from R26CreERT2;LSL-KrasG12D/+;Cdkn2alox/lox mice (Fig. S2C). Tamoxifen treatment of these cells induced rapid loss of p16Ink4a expression (Fig. S2D) and canonical KrasG12D signaling is activated (Fig. S2E). Orthotopic transplantation of Cdkn2a-deficient PDECs resulted in the development of invasive, proliferative, K19 positive, and metastatic PDAC (Fig. S2F–S2H). Thus, our model system suggests that PDECs contain a cellular population that is susceptible to KrasG12D-induced transformation.
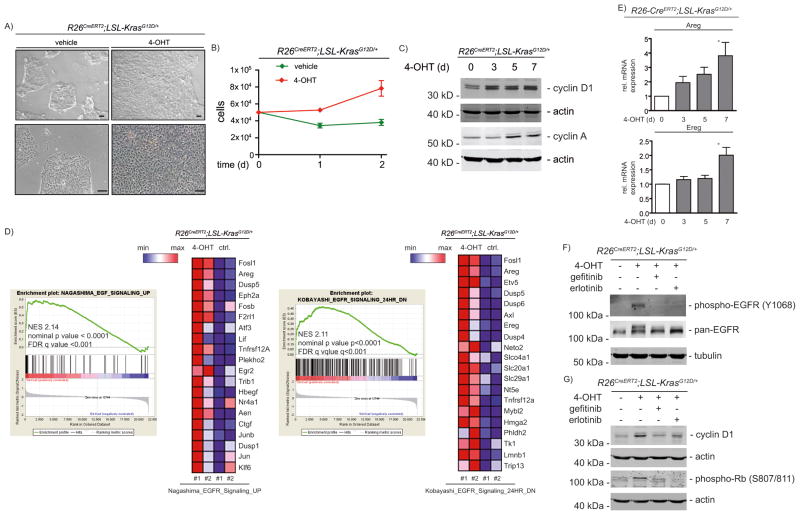
Expression of KrasG12D in PDECs induces proliferation (Fig. 2A and 2B) accompanied by induction of cell cycle genes, like cyclin D1 or cyclin A (Fig. 2C). This is in agreement with observations that KrasG12D prevents premature senescence of PDECs 9 and induces a proliferative response 9–12.
Figure 2. KrasG12D-driven proliferation in PDECs depends on an EGFR-loop.
A) White light microscopic images of PDECs from R26CreERT2;LSL-KrasG12D/+ mice treated for five days with 200 nM 4-OHT or left as vehicle treated controls. Scale bars: 100 μm. B) PDECs from R26CreERT2;LSL-KrasG12D/+ mice were treated with 200 nM 4-OHT or were left as vehicle treated controls. After 7 days, 50.000 PDECs were seeded and cell number was determined for two additional days (two biological replicates performed as triplicates). C) PDECs from R26CreERT2;LSL-KrasG12D/+ mice were treated with 200 nM 4-OHT over time and cyclin D1 ((HD11), sc-246, Santa Cruz Biotechnology, Dallas, Tx, USA) and cyclin A ((H-432), sc-751, Santa Cruz Biotechnology) expression was measured in western blots. Different lysates were blotted to different membranes and loading was controlled by β-actin. D) Enrichment plots of EGFR signatures and corresponding heatmaps (top 20 EGFR controlled genes induced by KrasG12D) from microarrays of vehicle (control: ctrl.) or 4-OHT (200 nM, 3 days) treated PDECs. NES: normalized enrichment score; FDR: false discovery rate. EMBL-EBI ArrayExpress Accession number: E-MTAB-2592. See supplementary material and methods for a description of the microarray analysis and gene set enrichment analysis. E) Relative amphiregulin (Areg) and epiregulin (Ereg) mRNA expression in 4-OHT (200 nM) treated PDECs was determined by qPCR using cyclophilin A mRNA expression as reference. Primers are depicted in supplementary material and methods. One way ANOVA *p-value < 0.05. F) and G) PDECs were treated for 6 days with 4-OHT (500 nM) and erlotinib or gefitinib (10 μM each; LC Laboratories, Woburn, MA, USA) were added for the last 24 hours as indicated. F) phospho-EGFR (#2234, Cell Signaling Technology) and pan-EGFR ((1005), sc-03, Santa Cruz Biotechnology) western blot (α-tubulin: loading controls). G) cyclin D1 and phospho-Rb (#8516, Cell Signaling Technology) western blot. Different lysates were blotted to different membranes and loading was controlled by β-actin.
In order to identify pathways driving KrasG12D induced proliferation, we used gene set enrichment analysis of mRNA expression profiles (GSEA). Several of the gene sets significantly enriched in KrasG12D expressing cells are linked to signatures controlled by the EGFR family (Fig. 2D and Supplemental Table 1). Accordingly, KrasG12D induced expression of the EGFR ligands amphiregulin and epiregulin (Fig. 2D and 2E), arguing for autocrine stimulation. Consistently, in murine PanIN organoids derived from ductal cells of Pdx1-Cre;LSL-KrasG12D/+ mice 13, KrasG12D induced expression of EGFR ligands (Fig. S3). Along with upregulation of EGFR ligands, increased receptor auto-phosphorylation was observed (Fig. 2F). To test whether this EGFR phosphorylation is critical for mutant Kras-regulated proliferation we utilized the EGFR inhibitors erlotinib and gefitinib. Of note, in PDAC models, gefitinib has been demonstrated to be more specific for EGFR than erlotinib 14. Both inhibitors diminished the KrasG12D-induced EGFR phosphorylation (Fig. 2F) and decreased expression of cell cycle regulators, like cyclin D1 (Fig. 2G). A link between the EGFR loop and cyclin D1 was recently described in KrasG12D-driven cancer formation in the pancreas in vivo 1. Importantly, the KrasG12D-mediated inactivation of the Rb-dependent restriction point in the G1-phase of the cell cycle is controlled by EGFR (Fig. 2G).
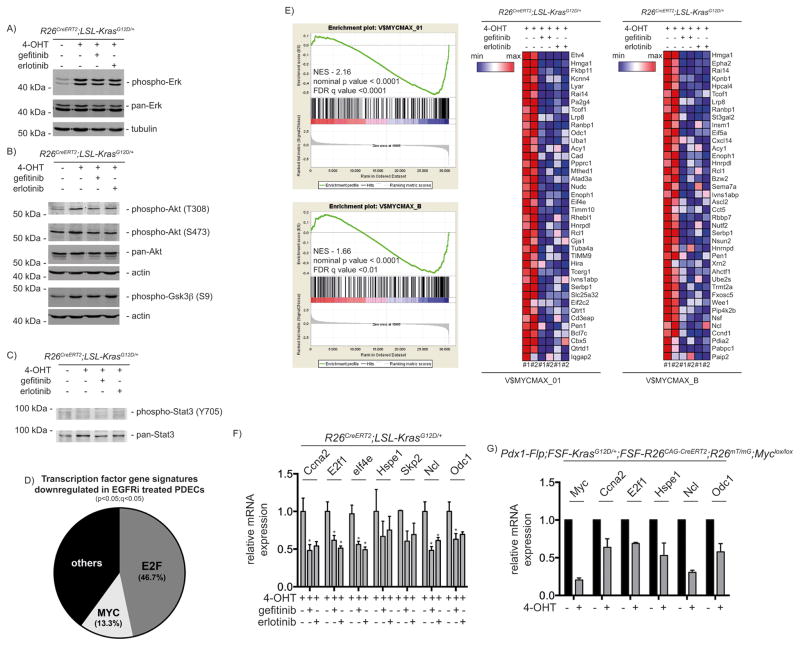
EGFR signaling networks engage ERK-, PI3K- and STAT3-controlled pathways. Although expression of oncogenic KrasG12D induced phosphorylation of ERK, AKT and its substrate GSK3β, both EGFR inhibitors did not distinctly change activation of these pathways (Fig. 3A and 3B). STAT3 phosphorylation was neither induced nor modulated by EGFR inhibitors (Fig. 3C). Involvement of EGFR in Ras induced transformation is context dependent 1, 2, 15 and the context appears to direct the signaling hubs engaged. Indeed, the hypomorphic waved-2 (wa2) EGFR receptor variant reduced AKT activation but not ERK phosphorylation in primary keratinocytes of K5-SOS-F mice 15. In the pancreas, the EGFR-controlled signaling hubs are incompletely understood 16. Despite expression of the KrasG12V oncogene, most acinar cells express low EGFR levels and nuclear phospho-AKT or phospho-STAT3 staining is absent 2. Inflammatory stimuli increase EGFR expression and induce AKT and STAT3 phosphorylation in acinar cells in the context of oncogenic KrasG12V 2, arguing that AKT and STAT3 are part of the EGFR signaling network in this inflammatory context. Furthermore, EGFR signaling increased the signaling output of the canonical Kras pathway to induce acinar-to-ductal metaplasia (ADM) in KrasG12D expressing acinar cells 1. In established Kras-driven PDAC models, the EGFR is inconsistently linked to active ERK, AKT or STAT3 1, 2. Together, these observations clearly demonstrate that the EGFR signaling network is modulated by cell-autonomous (e.g. tumor suppressor status) and non-autonomous (e.g. inflammatory environment) conditions.
Figure 3. MYC is a downstream effector of EGFR.
PDECs were treated for 6 days with 4-OHT (500 nM) and gefitinib or erlotinib (10 μM each) were added for the last 24 hours as indicated. Western blot of A) phospho- and pan-ERK (α-tubulin: loading controls). B) phospho-AKT (#9271 and #9275, Cell Signaling Technology) and -GSK3β (#9323, Cell Signaling Technology) as well as pan-AKT ((C67E7), #4691, Cell Signaling Technology). Different lysates were blotted to different membranes and loading was controlled by β-actin. C) phospho-STAT3 ((D3A7), #9145, Cell Signaling Technology) and pan-STAT3 ((C-20):sc-482, Santa Cruz Biotechnology) western blot. D) Transcription factor gene signatures (TFT-MSigDB) significantly downregulated in EGFR inhibitor (EGFRi) treated PDECs. PDECs were treated as described in A). E) GSEA enrichment plots of MYC signatures and corresponding heatmaps (top 40 MYC controlled genes inhibited by the EGFR inhibitors) from microarrays of PDECs treated as described in A). NES: normalized enrichment score; FDR: false discovery rate. EMBL-EBI ArrayExpress Accession number: E-MTAB-2592. See supplementary material and methods for a description of the microarray analysis and gene set enrichment analysis. F) PDECs were treated as described in A). Relative Ccna2, E2F1, eIf4e, Hspe1, Skp2, Ncl, and Odc1 mRNA expression levels were determined by qPCR using beta-actin mRNA expression as reference. Primers are depicted in supplementary material and methods. One way ANOVA *p-value < 0.05. G) PDECs from 2 months old Pdx1-Flp;FSF-KrasG12D/+;FSF-R26CAG-CreERT2;R26mT/mG;MYClox/lox mice were isolated. In these cells expression of KrasG12D is induced in vivo and expression of floxed genes can be manipulated by the treatment of cells with 4-OHT. The cells were treated with 4-OHT (500 nM) for 24 hours. The green fluorescent protein (GFP)-expressing cells were FACS (fluorescence-activated cell sorting) sorted as recently described 50. Relative Myc, Ccna2, E2f1, Hspe1, Ncl, and Odc1 mRNA expression levels were determined by qPCR using beta-actin mRNA expression as reference and compared to untreated cells, in which expression was set to 1. The Pdx1-Flp, FSF-KrasG12D, and the FSF-R26CAG-Cre-ERT2 mouse lines were described recently in 25. The R26mT/mG mouse line is described in 51 and the MYClox line in 52.
The observation that ERK and AKT remain phosphorylated, despite an inactivation of EGFR auto-phosphorylation and cell cycle progression, suggests that different hubs sense the signal. To identify EGFR-engaged pathways, we again performed transcriptome profiling of EGFR inhibitor-treated PDECs. Enriched gene sets in KrasG12D expressing PDECs with an active EGFR loop were linked to the cell cycle, DNA replication and repair as well as anabolic pathways (Supplemental Table 2). Since we intended to identify an integration of the EGFR loop with the cell cycle machinery, we focused on transcription factors (TFT-MSigDB). We detected that the majority of EGFR-controlled gene sets were linked to the pro-proliferative E2F transcription factor family, corroborating the link of the EGFR loop to the cell cycle (Fig. 3D and Supplemental Table 3). Additionally, we observed six signatures associated with transcription factors of the MYC family (Fig. 3D, 3E, and Supplemental Table 3). Performing a GSEA with curated gene sets (C2) of the MSigDB revealed 18 significant MYC gene sets linked to EGFR (Supplemental Table 4). MYC adopts a prominent role in Ras-driven cancers 17–21. Since MYC is strongly linked to the cell cycle of exocrine progenitors in the pancreas 22, 23 and to the E2F pathway in cell-based PDAC models 24, we investigated the role of MYC. First, we quantified mRNA levels of MYC target genes upon treatment with EGFR inhibitors. Both inhibitors reduced the expression of the MYC target genes Ccna2, E2f1, eIf4e, Hspe1, Skp2, Ncl, and Odc1 (Fig. 3F), indicating robust cross signaling between EGFR and MYC. Second, to demonstrate the regulation of these genes by MYC in the context of murine PDECs, we used a novel dual-recombinase system, allowing the time-specific manipulation of genes 25. We isolated murine PDECs at PanIN stages (2 months old mice) from Pdx1-Flp;FSF-KrasG12D/+;FSF-R26CAG-CreERT2;R26mT/mG;Myclox/lox mice. We treated these PDECs for 24 hours with tamoxifen and sorted GFP expressing cells by FACS to investigate expression of MYC and its target genes. Here, Myc mRNA expression is reduced to 20% compared to untreated controls, accompanied by a reduced mRNA expression of all investigated target genes (Fig. 3G).
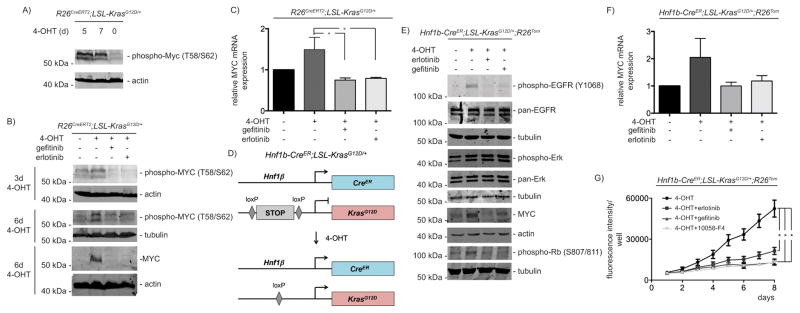
Oncogenic activity of MYC is regulated by phosphorylation of threonine 58 and serine 62 residues at the N-terminal MYC homology box I 26, 27. Indeed, mutant KrasG12D activation induces N-terminal phosphorylation of MYC (Fig. 4A). Increased MYC phosphorylation was detected over time (Fig. 4A and 4B). Furthermore, KrasG12D induces MYC protein expression (Fig. 4B), which is accompanied by a slight induction of Myc mRNA (Fig. 4C). Both EGFR inhibitors prevent KrasG12D-induced MYC protein expression (Fig. 4B) and significantly reduce Myc mRNA expression (Fig 4C). MYC regulation is controlled at multiple levels in PDAC 21. How the EGFR loop is connected to MYC expression is currently unknown and awaits further investigations. Nevertheless, our results show that KrasG12D induces MYC expression, phosphorylation, and MYC target gene expression in an EGFR-dependent manner, suggesting that the KrasG12D-induced EGFR network is engaging MYC as an important effector.
Figure 4. MYC expression is regulated by the autocrine EGFR-loop.
A) Indicated PDECs were treated with 4-OHT (500 nM) over time. Western blot for phospho-MYC (#9401, Cell Signaling Technology) (β-actin: loading control). B) PDECs were treated with 4-OHT (500 nM) as indicated. Gefitinib or erlotinib (10 μM each) were added for the last 24 hours of incubation. Westernblot for phospho-MYC and MYC (#9402, Cell Signaling Technology). Different lysates were blotted to different membranes and loading was controlled by β-actin or α-tubulin as indicated. To detect phosphorylated MYC, PDECs were lysed by directly boiling in protein loading buffer. C) Indicated PDECs were treated with 4-OHT (500 nM) for 6 days. Gefitinib or erlotinib (10 μM each) were added for the last 24 hours of incubation. Relative Myc mRNA expression levels were determined by qPCR using beta-actin mRNA expression as reference. One way ANOVA *p-value < 0.05. D) Genetic strategy to activate KrasG12D-expression in Hnf1β-positive PDECs (Hnf1b-CreER;LSL-KrasG12D/+;R26Tom). The Hnf1b-CreER mouse line was described in 30 and the R26Tom reporter mouse line in 53. E) PDECs from Hnf1b-CreER;LSL-KrasG12D/+;R26Tom mice were treated with 4-OHT (1 μM) for 15 days. Afterwards gefitinib or erlotinib (10 μM each) were added for additional 24 hours or the cells were left as vehicle treated controls. Western blot of phospho-EGFR, pan-EGFR, phospho-ERK and pan-ERK, MYC, and phospho-Rb. Different lysates were blotted to different membranes and loading was controlled by β-actin or α-tubulin as indicated. F) PDECs from Hnf1b-CreER;LSL-KrasG12D/+;R26Tom mice were treated with 4-OHT (1 μM) for 15 days. Afterwards gefitinib or erlotinib (10 μM each) were added for additional 24 hours. Relative Myc mRNA expression levels were determined by qPCR using beta-actin mRNA expression as reference. G) PDECs from Hnf1b-CreER;LSL-KrasG12D/+;R26Tom mice were treated with 4-OHT (1 μM) for 15 days. Afterwards, 2.000 cells per well were seeded in a 96 well plate in quadruplicates (n=4). After 24 hours the cells were treated with erlotinib (10 μM), gefitinib (10 μM), or 10058-F4 (80 μM) or were left as vehicle treated controls. To determine relative growth, fluorescence (excitation: 560 nm, emission 590 nm) was measured daily over 8 days with a BMG FLUOstar OPTIMA Microplate Reader (BMG Labtech, Ortenberg, Germany). One way ANOVA *p-value < 0.05.
Acinar cells cultured in suspension dedifferentiate and activate a ductal gene expression program 5, 28. Therefore, the possibility exists that our PDEC preparations contain dedifferentiated acinar cells. However, in our hands, we were never able to serial passage and subculture acinar cells. This is in agreement with observations that ex vivo acinar cells lack the capacity to proliferate due to a p53-dependent growth arrest 29. Since we subculture and serial passage the PDEC lines (only passage 3 to 8 were used for all experiments), a contamination with acinar cells is unlikely. To further address this point, lineage tracing technology using the ductal marker Hnf1b-CreER mouse line was used 30 (Fig. 4D). First, we activated Cre recombinase in a Hnf1b-CreER;LSL-KrasG12D/+;R26Tom mouse by the i.p. application of tamoxifen in vivo 31. Two weeks after the last tamoxifen administration we isolated PDECs. 87% of these cells express the reporter gene tdTomato (Fig. S4A), arguing for the ductal origin of the prepared cells. To further corroborate the EGFR-MYC loop in the Hnf1β-lineage, we isolated PDEC lines from untreated Hnf1b-CreER;LSL-KrasG12D/+;R26Tom mice. We adapted the tamoxifen treatment regime since 0.5 μM 4-OHT insufficiently recombined the Kras locus in Hnf1b-CreER PDEC lines (Fig. S4B). Even upon an increased 4-OHT dose, only 54% of the cells expressed tdTomato after 7 days (Fig. S4C). However, the fraction of tdTomato expressing cells was increased to 91% after 15 days (Fig. S4D). Then the Kras locus is recombined (Fig. S4E) and the canonical Ras-pathway is activated (Fig. 4E). Additionally to ERK phosphorylation, the EGFR becomes phosphorylated, MYC protein and mRNA expression is induced and the G1-phase restriction point becomes inactivated upon the expression of KrasG12D (Fig. 4E and Fig. 4F). Both EGFR inhibitors prevent EGFR and Rb phosphorylation as well as MYC expression whereas ERK phosphorylation is not influenced (Fig. 4E and Fig. 4F). Together, the lack of proliferation of acinar cells ex vivo and our lineage tracing experiments argue that the described pathway and biology acts in the ductal lineage.
To further demonstrate the impact of the EGFR-dependent loop towards the proliferative capacity of KrasG12D expressing Hnf1b-CreER;LSL-KrasG12D/+;R26Tom PDECs, we measured growth of erlotinib- and gefitinib-treated cells. Both EGFR inhibitors reduce proliferation with a similar potency (Fig. 4G). To compare the effects of the EGFR blockade with the effects of a direct MYC inhibitor, we treated the cells with 10058-F4. This MYC inhibitor prevents the dimerization of MYC with MAX 32. Similar to EGFR inhibitors, 10058-F4 reduces proliferation of KrasG12D expressing PDECs, supporting an important function of MYC downstream of the EGFR.
Overall, PDECs are a valid model to gain mechanistic insights into Kras-driven processes in a specific pancreatic context 6, 8–12, 33. In addition, PDECs are a tool for genetic screening experiments 34. A multipotent subpopulation of adult pancreatic ductal cells capable of reprogramming towards the endocrine lineage was recently described, arguing for a stem cell population in the ductal compartment 35. Consistently, our observations demonstrate that PDECs express progenitor markers and are susceptible to KrasG12D-dependent transformation. In agreement, a non-islet Pdx1-positive PDEC subpopulation in the adult pancreas with a stem-like phenotype was described, harboring tumorigenic and metastatic capacity upon the expression of KrasG12D 36, 37. In line with our data, MYC activation by KrasG12D was observed in this model and MYC was linked to the evolution of pancreatic cancer cells with stem cell-like features and metastatic potential 36, 37. In addition, we analyzed recently published microarray datasets generated from duct and duct-like cells of Pdx1-Cre;LSL-KrasG12D/+ mice 38 at pre-malignant disease stages. Indeed, we observed KrasG12D-induced EGFR- and MYC-signatures using gene set enrichment analysis (Fig. S5), suggesting that the molecular changes occurring in vivo are recapitulated by our in vitro model. Furthermore, our data show that an EGFR-loop contributes to KrasG12D-driven proliferation of PDECs, well in line with recent data from Kras-dependent mouse models 1, 2.
In addition to this EGFR loop, a KrasG12D-activated autocrine loop engaging the insulin like growth factor 1 receptor (IGF1R) in Cdnk2a/Trp53-double deficient PDECs has recently been described 11. Whether EGFR- and IGF1R-dependent loops act in parallel to modulate signaling thresholds and whether the usage of such circuits is determined by tumor suppressive programs, awaits further analysis. However, in vivo findings indicate that the need of EGFR signaling to develop KrasG12D-driven PDACs is bypassed in a p53-deficient background 1, 2, arguing that tumor suppressors determine the need of such loops.
In contrast to the requirement of EGFR for oncogenic Kras-induced pancreatic carcinogenesis, molecular hubs of the EGFR network are incompletely defined. Although the cooperation of Ras and MYC oncogenes to transform cells has been described in the last century 39 and many underlying molecular processes are known 40, our data link for the first time two essential components of Kras-driven transformation in a pancreatic pre-neoplasia equivalent model. Like the EGFR loop, MYC essentially contributes to the carcinogenesis in the pancreas 19–21, 41, 42. Especially, in embryonic stem cell-based genetically engineered mouse models the distinct effect of MYC silencing on disease progression and tumor formation was recently demonstrated 19. MYC is highly expressed in multipotent pancreatic progenitor cells 43 and can autonomously drive tumor initiation and progression in the pancreas 21, 44–46. The EGFR ligand TGFα dramatically accelerates MYC-driven carcinogenesis in the pancreas in vivo 47, which is consistent with our observation of an EGFR-MYC cross signaling. Therefore, connecting EGFR to MYC underscores the importance of the EGFR network in the pancreatic carcinogenesis.
Supplementary Material
Acknowledgments
We thank Drs. S. Hingorani, I. Verma, D. Tuveson, H. Zeng, F. Costantini, T. Jacks, R. DePhino, J. Ferrer, I. de Alboran, and F. Alt for generating or providing mouse lines/plasmids. This work was supported by the Wilhelm-Sander Foundation [2012.084.1 to G.S.], Deutsche Forschungsgemeinschaft (DFG) [SCHN 959/1-2, SCHN 959/2-1 to G.S., GE2289/1 to F.G., SI 1549/2-1 to J.T.S., and SFB824 to G.S. and D.S.], Deutsche Krebshilfe [110908 to G.S., 111273 (Max-Eder Program) to M.R., and 109992 to J.T.S.] and NIH [NIH P30 DK050306 Center for Molecular Studies in Digestive and Liver Diseases (Molecular Pathology and Imaging, Molecular Biology/Gene Expression, Cell Culture, and Transgenic and Chimeric Mouse Cores) to M.R. and A.K.R. and the NIH R01 DK060694 to M.R. and A.K.R.]
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Author Contributions
Concept and design of the research: S.D., M.W., C.S., S.J., F.G., M.R., R.M.S., D.S., A.K.R., G.S.; Realization of research: S.D., M.W., C.S., S.J., F.G., M.R.; Analysis and interpretation of data: all authors; Support with essential reagents/analytical tools: J.T.S., R.R., D.S., A.K.R.; J.T.S., F.G., D.S., M.R., A.K.R. and G.S. obtained funding; S.D., M.W., M.R. and G.S. wrote the paper. All authors discussed the results, commented on the manuscript, revised it critically for important intellectual content and approved the final version of the manuscript.
References
- 1.Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navas C, Hernandez-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318–330. doi: 10.1016/j.ccr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemmon MA, Schlessinger J, Ferguson KM. The EGFR Family: Not So Prototypical Receptor Tyrosine Kinases. Cold Spring Harb Perspect Biol. 2014:6. doi: 10.1101/cshperspect.a020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014;111:817–22. doi: 10.1038/bjc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puri S, Folias AE, Hebrok M. Plasticity and dedifferentiation within the pancreas: development, homeostasis, and disease. Cell Stem Cell. 2015;16:18–31. doi: 10.1016/j.stem.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest. 2011;121:4572–4578. doi: 10.1172/JCI57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JPt, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell. 2010;18:448–458. doi: 10.1016/j.ccr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton JP, Mongeau ME, Klimstra DS, Morris JP, Lee YC, Kawaguchi Y, et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appleman VA, Ahronian LG, Cai J, Klimstra DS, Lewis BC. KRAS(G12D)- and BRAF(V600E)-induced transformation of murine pancreatic epithelial cells requires MEK/ERK-stimulated IGF1R signaling. Mol Cancer Res. 2012;10:1228–1239. doi: 10.1158/1541-7786.MCR-12-0340-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conradt L, Godl K, Schaab C, Tebbe A, Eser S, Diersch S, et al. Disclosure of erlotinib as a multikinase inhibitor in pancreatic ductal adenocarcinoma. Neoplasia. 2011;13:1026–1034. doi: 10.1593/neo.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, et al. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000;102:211–220. doi: 10.1016/s0092-8674(00)00026-x. [DOI] [PubMed] [Google Scholar]
- 16.Perera RM, Bardeesy N. Ready, set, go: the EGF receptor at the pancreatic cancer starting line. Cancer Cell. 2012;22:281–282. doi: 10.1016/j.ccr.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soucek L, Whitfield J, Martins CP, Finch AJ, Murphy DJ, Sodir NM, et al. Modelling Myc inhibition as a cancer therapy. Nature. 2008;455:679–683. doi: 10.1038/nature07260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soucek L, Whitfield JR, Sodir NM, Masso-Valles D, Serrano E, Karnezis AN, et al. Inhibition of Myc family proteins eradicates KRas-driven lung cancer in mice. Genes Dev. 2013;27:504–513. doi: 10.1101/gad.205542.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saborowski M, Saborowski A, Morris JPt, Bosbach B, Dow LE, Pelletier J, et al. A modular and flexible ESC-based mouse model of pancreatic cancer. Genes Dev. 2014;28:85–97. doi: 10.1101/gad.232082.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walz S, Lorenzin F, Morton J, Wiese KE, von Eyss B, Herold S, et al. Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature. 2014;511:483–487. doi: 10.1038/nature13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hessmann E, Schneider G, Ellenrieder V, Siveke JT. MYC in pancreatic cancer: novel mechanistic insights and their translation into therapeutic strategies. Oncogene. 2015 doi: 10.1038/onc.2015.216. [DOI] [PubMed] [Google Scholar]
- 22.Bonal C, Thorel F, Ait-Lounis A, Reith W, Trumpp A, Herrera PL. Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology. 2009;136:309–319. e309. doi: 10.1053/j.gastro.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Nakhai H, Siveke JT, Mendoza-Torres L, Schmid RM. Conditional inactivation of Myc impairs development of the exocrine pancreas. Development. 2008;135:3191–3196. doi: 10.1242/dev.017137. [DOI] [PubMed] [Google Scholar]
- 24.Schild C, Wirth M, Reichert M, Schmid RM, Saur D, Schneider G. PI3K signaling maintains c-myc expression to regulate transcription of E2F1 in pancreatic cancer cells. Mol Carcinog. 2009;48:1149–1158. doi: 10.1002/mc.20569. [DOI] [PubMed] [Google Scholar]
- 25.Schönhuber N, Seidler B, Schuck K, Veltkamp C, Schachtler C, Zukowska M, et al. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat Med. 2014;20:1340–1347. doi: 10.1038/nm.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hann SR. Role of post-translational modifications in regulating c-Myc proteolysis, transcriptional activity and biological function. Semin Cancer Biol. 2006;16:288–302. doi: 10.1016/j.semcancer.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Vervoorts J, Luscher-Firzlaff J, Luscher B. The ins and outs of MYC regulation by posttranslational mechanisms. J Biol Chem. 2006;281:34725–34729. doi: 10.1074/jbc.R600017200. [DOI] [PubMed] [Google Scholar]
- 28.Pinho AV, Rooman I, Reichert M, De Medts N, Bouwens L, Rustgi AK, et al. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut. 2011;60:958–966. doi: 10.1136/gut.2010.225920. [DOI] [PubMed] [Google Scholar]
- 29.Pinho AV, Rooman I, Real FX. p53-dependent regulation of growth, epithelial-mesenchymal transition and stemness in normal pancreatic epithelial cells. Cell Cycle. 2011;10:1312–1321. doi: 10.4161/cc.10.8.15363. [DOI] [PubMed] [Google Scholar]
- 30.Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Jörs S, Jeliazkova P, Ringelhan M, Thalhammer J, Durl S, Ferrer J, et al. Lineage fate of ductular reactions in liver injury and carcinogenesis. J Clin Invest. 2015;125:2445–2457. doi: 10.1172/JCI78585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin X, Giap C, Lazo JS, Prochownik EV. Low molecular weight inhibitors of Myc-Max interaction and function. Oncogene. 2003;22:6151–6159. doi: 10.1038/sj.onc.1206641. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber FS, Deramaudt TB, Brunner TB, Boretti MI, Gooch KJ, Stoffers DA, et al. Successful growth and characterization of mouse pancreatic ductal cells: functional properties of the Ki-RAS(G12V) oncogene. Gastroenterology. 2004;127:250–260. doi: 10.1053/j.gastro.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 34.von Burstin J, Diersch S, Schneider G, Reichert M, Rustgi AK, Schmid RM. Detection of Tumor Suppressor Genes in Cancer Development by a Novel shRNA-Based Method. Mol Cancer Res. 2015;13:863–869. doi: 10.1158/1541-7786.MCR-14-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sancho R, Gruber R, Gu G, Behrens A. Loss of Fbw7 reprograms adult pancreatic ductal cells into alpha, delta, and beta cells. Cell Stem Cell. 2014;15:139–153. doi: 10.1016/j.stem.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ischenko I, Zhi J, Moll UM, Nemajerova A, Petrenko O. Direct reprogramming by oncogenic Ras and Myc. Proc Natl Acad Sci U S A. 2013;110:3937–3942. doi: 10.1073/pnas.1219592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ischenko I, Petrenko O, Hayman MJ. Analysis of the tumor-initiating and metastatic capacity of PDX1-positive cells from the adult pancreas. Proc Natl Acad Sci U S A. 2014;111:3466–3471. doi: 10.1073/pnas.1319911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichert M, Takano S, von Burstin J, Kim SB, Lee JS, Ihida-Stansbury K, et al. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes Dev. 2013;27:288–300. doi: 10.1101/gad.204453.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Lisanti MP, Liao DJ. Reviewing once more the c-myc and Ras collaboration: converging at the cyclin D1-CDK4 complex and challenging basic concepts of cancer biology. Cell Cycle. 2011;10:57–67. doi: 10.4161/cc.10.1.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skoudy A, Hernandez-Munoz I, Navarro P. Pancreatic ductal adenocarcinoma and transcription factors: role of c-Myc. J Gastrointest Cancer. 2011;42:76–84. doi: 10.1007/s12029-011-9258-0. [DOI] [PubMed] [Google Scholar]
- 42.Mazur PK, Herner A, Mello SS, Wirth M, Hausmann S, Sanchez-Rivera FJ, et al. Combined inhibition of BET family proteins and histone deacetylases as a potential epigenetics-based therapy for pancreatic ductal adenocarcinoma. Nat Med. 2015 doi: 10.1038/nm.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Sandgren EP, Quaife CJ, Paulovich AG, Palmiter RD, Brinster RL. Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc Natl Acad Sci U S A. 1991;88:93–97. doi: 10.1073/pnas.88.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grippo PJ, Sandgren EP. Acinar-to-ductal metaplasia accompanies c-myc-induced exocrine pancreatic cancer progression in transgenic rodents. Int J Cancer. 2012;131:1243–1248. doi: 10.1002/ijc.27322. [DOI] [PubMed] [Google Scholar]
- 46.Lin WC, Rajbhandari N, Liu C, Sakamoto K, Zhang Q, Triplett AA, et al. Dormant cancer cells contribute to residual disease in a model of reversible pancreatic cancer. Cancer Res. 2013;73:1821–1830. doi: 10.1158/0008-5472.CAN-12-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandgren EP, Luetteke NC, Qiu TH, Palmiter RD, Brinster RL, Lee DC. Transforming growth factor alpha dramatically enhances oncogene-induced carcinogenesis in transgenic mouse pancreas and liver. Mol Cell Biol. 1993;13:320–330. doi: 10.1128/mcb.13.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 49.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 50.Wirth M, Stojanovic N, Christian J, Paul MC, Stauber RH, Schmid RM, et al. MYC and EGR1 synergize to trigger tumor cell death by controlling NOXA and BIM transcription upon treatment with the proteasome inhibitor bortezomib. Nucleic Acids Res. 2014;42:10433–10447. doi: 10.1093/nar/gku763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 52.de Alboran IM, O’Hagan RC, Gartner F, Malynn B, Davidson L, Rickert R, et al. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity. 2001;14:45–55. doi: 10.1016/s1074-7613(01)00088-7. [DOI] [PubMed] [Google Scholar]
- 53.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.