Abstract
Objective
To characterise the relationship between simvastatin and risk of acute pancreatitis (AP).
Design
We conducted a retrospective cohort study (2006–2012) on data from an integrated healthcare system in southern California. Exposure to simvastatin was calculated from time of initial dispensation until 60 days following prescription termination. AP cases were defined by ICD-9 CM 577.0 and serum lipase≥3 times normal. Patients were censored at death, last follow-up, and onset of AP or end-of-study. Incidence rate of pancreatitis among simvastatin users was compared with the adult reference population. Robust Poisson regression was used to generate risk ratio (RR) estimates for simvastatin use adjusted for age, gender, race/ethnicity, gallstone-related disorders, hypertriglyceridaemia, smoking and alcohol dependence. Analysis was repeated for atorvastatin.
Results
Among 3 967 859 adult patients (median duration of follow-up of 3.4 years), 6399 developed an initial episode of AP. A total of 707 236 patients received simvastatin during the study period. Patients that received simvastatin were more likely to have gallstone-related disorders, alcohol dependence or hypertriglyceridaemia compared with the reference population. Nevertheless, risk of AP was significantly reduced with simvastatin use, crude incidence rate ratio 0.626 (95% CL 0.588, 0.668), p<0.0001. In multivariate analysis, simvastatin was independently associated with reduced risk of pancreatitis, adjusted RR 0.29 (95% CL 0.27, 0.31) after adjusting for age, gender, race/ethnicity, gallstone disorders, alcohol dependence, smoking and hypertriglyceridaemia. Similar results were noted with atorvastatin, adjusted RR 0.33 (0.29, 0.38).
Conclusions
Use of simvastatin was independently associated with reduced risk of AP in this integrated healthcare setting. Similar findings for atorvastatin suggest a possible class effect.
INTRODUCTION
Acute pancreatitis (AP) is a common cause for hospitalisation with over 280 000 admissions on an annual basis and a rising incidence in the USA.1 Although most cases are attributed to either gallstones or alcohol use, numerous drugs have also been implicated as potentially causing AP. Meanwhile, use of HMG-CoA reductase inhibitors (statins) has greatly increased in recent years. National survey data in the USA estimate the frequency of statin use as high as 50% of men and 36% of women between the ages of 65 years and 74 years between 2004 and 2008.2
Despite the rising incidence of AP and widespread use of statins, the relationship between these medications and AP is poorly understood. Several case reports suggest an increased risk of AP with use of statins, in particular simvastatin3–6 However, more recent literature suggests a possible protective effect7,8 As statins are now among the most widely prescribed medications worldwide, understanding the potential link between these medications and AP is of significant clinical importance.
The objective of the present study was to further characterise the relationship between simvastatin use and risk of AP. Specifically, we sought to determine risk of developing a first episode of AP with use of simvastatin. We also sought to determine whether these effects were agent-specific or potentially a class-effect by comparing the effect of simvastatin with another commonly used statin, atorvastatin.
METHODS
This study was approved by the Institutional Review Board of Kaiser Permanente Southern California (KPSC) and is reported in accordance with the Strengthening reporting of observational studies in epidemiology (STROBE) guidelines.9
Study design, setting and patient population
We conducted a retrospective longitudinal cohort study on data from the KPSC health plan membership from January 2006 to December 2012. The population served by KPSC is socioeconomically diverse and broadly representative of the racial/ethnic groups living in southern California. Members enrol through the Kaiser Foundation Health Plan for prepaid healthcare insurance, including pharmaceutical benefits. KPSC is one of Kaiser Permanente’s largest regions, comprising 15 acute care hospitals and 202 ambulatory medical centres.
We included patients ≥18 years of age as of 1 January 2006. The study start date was selected for historical reasons as this represented the initial date by which comprehensive electronic health data and pharmacy data was concurrently available for all patients enrolled within the KPSC health system. Only patients with at least 28 days of continuous follow-up data were included in order to facilitate adequate medication exposure assessment as well as evaluation for established risk factors for AP. In addition, all patient records were screened for prior history of AP dating back to January 2000 to identify and exclude patients with previous history of acute or chronic pancreatitis. Previous episodes of pancreatitis were identified by either International Classification of Disease Clinical Modification 9th revision (ICD-9 CM) 577.0 or elevation in serum lipase ≥three times upper limit of normal.
Drug exposure
Medication usage was determined by cross-referencing the unique electronic health record number with electronic pharmacy data. Active exposure to medication was defined from the date of initial medication dispensation to 60 days following termination of the prescription. Also for the present study, only individual drugs were evaluated that is, simvastatin or atorvastatin. We included proprietary (Zocor, Lipitor) and generic formulations. However, combined drug formulations such as statin-fibrate for example, niacin-simvastatin (Simcor) were excluded.
Outcome assessment
Cases of AP were identified as hospitalisation at any of the 15 regional KPSC acute care hospitals or claims from outside hospitals for principal diagnosis ICD-9 577.0 and elevation in serum lipase ≥three times upper limit of normal at the time of hospitalisation.
This definition of AP was chosen after evaluating the accuracy of several possible definitions of AP. Specifically, we compared the positive predictive value for a clinical diagnosis of AP using ICD-9 code alone versus incorporating serum lipase levels. Lipase levels were preferentially chosen to increase specificity over isolated serum amylase elevation.10
Confirmation of clinical diagnoses was performed through manual chart abstraction of 100 randomly selected cases identified using each of the respective search criteria. Cases were considered true positive if there was a clinical history consistent with AP, cross-sectional imaging findings compatible with AP, and/or documentation by the primary treatment team of a clinical diagnosis of AP. Through manual chart abstraction, ICD-9 diagnosis of AP was associated with a 55% positive predictive value for clinical AP compared with 95% positive predictive value when ICD-9 diagnosis was combined with serum lipase ≥three times normal.
Data analysis
We performed four sets of analyses to evaluate the relationship between simvastatin use and AP. We also performed a sensitivity analysis to evaluate the impact of diagnostic criteria for AP on effect estimates. Simvastatin was chosen as the primary agent to be analysed based on previous literature suggesting increased risk of pancreatitis with this agent11 In addition, simvastatin was the most widely prescribed statin agent within KPSC during the study period. We then repeated the analysis including atorvastatin (second most commonly prescribed statin agent during the study period) to evaluate for presence of a potential class-wide effect.
Incidence of AP: incidence density was calculated for simvastatin as well as the KPSC adult reference population with patients censored at time of last available follow-up, onset of AP, end-of-study period or death. Relative risk of AP was then calculated based on the incidence-rate ratio.
Independent risk of AP (multivariable analysis): we performed two sets of multivariable analyses to evaluate the effect of either simvastatin or atorvastatin exposure on risk of AP after adjusting for established risk factors for pancreatitis. We generated separate robust Poisson regression models to determine adjusted risk ratios (RRs) for simvastatin and atorvastatin with adjustment for age, gender, race/ethnicity and established risk-factors for AP: gallstone-related disorders, hypertriglyceridaemia (serum triglyceride>1000 mg/dL at any point during study period), smoking and alcohol dependence. Gallstone-related disorders were identified based on ICD-9 code 574.xx. Alcohol dependence was identified based on 303.xx. Smoking was categorised as never-smoker versus ever-smoker. Poisson regression was performed in favour of Cox proportional hazards based on preliminary testing, which indicated that the proportional hazards assumption did not hold for the time-varying covariates included in the model (data not shown).
Effect modification: we performed a series of additional multivariable analyses to evaluate the impact of simvastatin use among specific high-risk populations for AP. Specifically, we performed robust Poisson regression to determine risk of pancreatitis associated with simvastatin use specifically among patients with (1) gallstone disorders, (2) chronic alcohol use and (3) hypertriglyceridaemia. Separate models were constructed for each of the high-risk populations with adjustment for age, gender, race/ethnicity, smoking as well as alternate risk factors for AP as aforementioned.
Simvastatin dose and risk of AP: We performed a subgroup analysis among simvastatin users to evaluate the impact of dose of medication on risk of AP. Since simvastatin is typically prescribed as a chronic medication, we calculated the average-daily dose of medication (cumulative dose of medication/total days of medication exposure). Average-daily dose levels were then divided into strata ranging from 0 mg/day to 10 mg/day, 10 mg/day to 20 mg/day, 20 mg/day to 40 mg/day or >40 mg/day. Risk of AP was then compared across strata (Cochrane-Armitage trend test).
Sensitivity analysis
To determine the extent to which the study results were impacted by our definition of AP, we repeated the primary study analysis (multivariable Poisson regression) using AP defined by ICD-9 code alone. Notably, all cases with serum lipase ≥three times normal were also coded as ICD-9 577.0.
All analyses were performed is SAS statistical software V.9.2 (Cary, North Carolina, USA). All reported p values are two-sided with α level of 0.05 threshold for statistical significance.
RESULTS
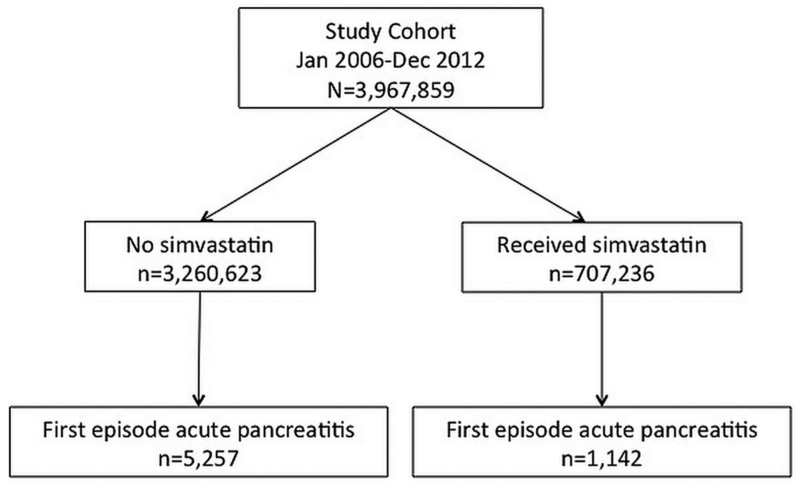
We identified a total of 3 967 859 patients that fulfilled the study inclusion criteria. Details of assembling the study cohort are depicted in figure 1. Overall mean age was 42.1 years, SD 16.2; 51.2% of the cohort was women. Median duration of follow-up was 3.8 years (IQR 1.3, 7.0). A total of 707 236 patients received simvastatin during the study period. Among patients that received simvastatin during the study period, median average daily dose was 20.3 mg/day and median duration of use was 2.3 years. Table 1 depicts the demographic and clinical characteristics of the study cohort stratified by simvastatin usage. Overall, patients that received simvastatin were older and more likely to have additional risk factors for AP compared with the reference population.
Figure 1.
Overview of study cohort stratified by simvastatin use, January 2006–December 2012.
Table 1.
Clinical and demographic features of study cohort
| Simvastatin users N=707 236 |
Reference population N=3 260 623 |
p Value | |
|---|---|---|---|
| Age (mean, SD), years | 57.1 (13.0) | 38.9 (15.0) | <0.0001 |
| Women (%) | 340 815 (48.2) | 1 690 997 (51.9) | <0.0001 |
| Race/ethnicity (%) | <0.0001 | ||
| Non-Hispanic white | 298 309 (42.2) | 868 583 (26.6) | |
| Non-Hispanic black | 69 453 (9.8) | 212 083 (6.5) | |
| Asian | 70 692 (10.0) | 225 818 (6.9) | |
| Hispanic | 203 376 (28.8) | 907 340 (27.8) | |
| Other | 65 406 (9.2) | 1 046 799 (32.1) | |
| Gallstone disorder (%) | 36 779 (5.2) | 76 819 (2.4) | <0.0001 |
| Chronic alcohol (%) | 22 165 (3.1) | 64 498 (2.0) | <0.0001 |
| Hypertriglyceridaemia (%) | 5304 (0.7) | 4574 (0.1) | <0.0001 |
| Follow-up (mean years, SD) | 5.5 (2.1) | 3.5 (2.5) |
Simvastatin use and incidence of AP
A total of 6399 (0.2%) patients developed AP during the study period. Among the patients with AP, 1142 (18%) developed AP while on simvastatin. The crude (unadjusted) incidence rate for AP with simvastatin use was 0.80 (95% CL 0.80, 0.81)/100 000 person-days. Meanwhile, the incidence rate for pancreatitis in the reference population was 1.28 (1.27, 1.28)/100 000 person-days. The crude incidence rate ratio for risk of AP with simvastatin use was 0.626 (95% CL 0.588, 0.668), p<0.0001 compared with the reference population.
Simvastatin use and risk of AP: multivariable analysis
The results of multivariable Poisson regression analysis are presented in table 2. Use of simvastatin was independently associated with reduced risk of AP; adjusted RR 0.29 (95% CL 0.27, 0.31) after adjusting for the effect of age, gender, race/ethnicity, gallstone disorders RR 23.2, alcohol dependence RR 4.8, smoking RR 1.3 and hypertriglyceridaemia RR 12.5. A similar effect was noted for atorvastatin when evaluated separately, adjusted RR 0.33 (0.29, 0.38) (table 3). The combined effect of exposure to either of the two most commonly used statins during the study period, simvastatin or atorvastatin, was RR 0.32 (0.30, 0.34) after adjusting for age, gender, race/ethnicity, gallstone disorder, alcohol, smoking and hypertriglyceridaemia.
Table 2.
Poisson regression analysis: risk of acute pancreatitis associated with simvastatin
| Parameter | Comparison | Risk ratio (95% confidence limit) | P value |
|---|---|---|---|
| Simvastatin use | Yes vs no | 0.29 (0.27, 0.31) | <0.0001 |
| Age, years | 30–39 vs 18–20 | 0.91 (0.82,0.99) | 0.04 |
| 40–49 vs 18–20 | 0.94 (0.86, 1.03) | 0.21 | |
| 50–59 vs 18–20 | 1.23 (1.11, 1.34) | <0.0001 | |
| 60–69 vs 18–20 | 1.69 (1.53,1.86) | <0.0001 | |
| 70+ vs 18–20 | 2.02 (1.82, 2.24) | <0.0001 | |
| Gender | Male vs female | 1.12 (1.07, 1.19) | <0.0001 |
| Race/ethnicity | |||
| Asian vs white | 0.98 (0.89, 1.09) | 0.73 | |
| Black vs white | 1.30 (1.20, 1.42) | <0.0001 | |
| Hispanic vs white | 1.19 (1.12, 1.26) | <0.0001 | |
| Other vs white | 0.17 (0.14, 0.21) | <0.0001 | |
| Hypertriglyceridaemia | Yes vs no | 12.5 (10.8, 14.5) | <0.0001 |
| Gallstone disease | Yes vs no | 23.2 (22.0, 24.5) | <0.0001 |
| Chronic alcohol use | Yes vs no | 4.85 (4.49, 5.23) | <0.0001 |
| Smoking | Ever vs never | 1.22 (1.16, 1.29) | 0.003 |
Acute pancreatitis defined by diagnosis code and serum lipase ≥three times normal.
Table 3.
Poisson regression analysis: risk of acute pancreatitis associated with atorvastatin
| Parameter | Comparison | Risk ratio (95% confidence limit) | p Value |
|---|---|---|---|
| Atorvastatin use | Yes vs no | 0.33 (0.29, 0.38) | <0.0001 |
| Age, years | 30–39 vs 18–20 | 0.87 (0.79, 0.96) | 0.004 |
| 40–49 vs 18–20 | 0.83 (0.76, 0.91) | <0.0001 | |
| 50–59 vs 18–20 | 0.97 (0.88, 1.06) | 0.48 | |
| 60–69 vs 18–20 | 1.16 (1.05, 1.28) | 0.004 | |
| 70+ vs 18–20 | 1.31 (1.19, 1.45) | <0.0001 | |
| Gender | Male vs female | 1.08 (1.03,1.14) | 0.004 |
| Race/ethnicity | |||
| Asian vs white | 0.95 (0.86, 1.05) | 0.33 | |
| Black vs white | 1.29 (1.19,1.41) | <0.0001 | |
| Hispanic vs white | 1.17 (1.10, 1.24) | <0.0001 | |
| Other vs white | 0.17 (0.14, 0.22) | <0.0001 | |
| Hypertriglyceridaemia | Yes vs no | 11.5 (10.1, 13.2) | <0.0001 |
| Gallstone disease | Yes vs no | 23.0 (21.8,24.3) | <0.0001 |
| Chronic alcohol use | Yes vs no | 5.02 (4.66, 5.41) | <0.0001 |
| Smoking | Ever vs never | 1.22 (1.16, 1.29) | <0.0001 |
Acute pancreatitis defined by diagnosis code and serum lipase ≥three times normal.
Impact of simvastatin use among high-risk populations
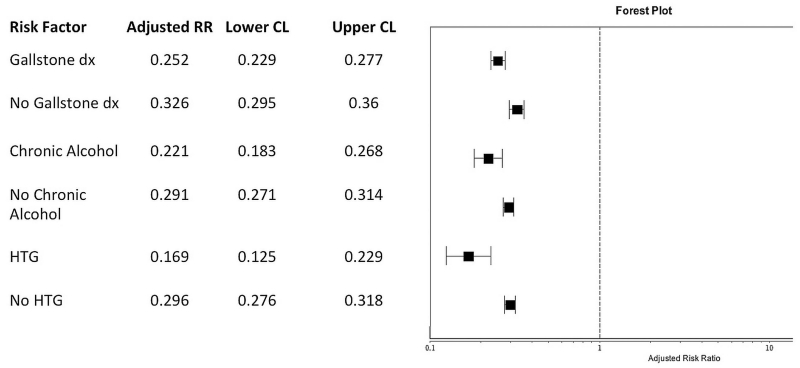
The results of the multivariable analyses stratified according to established risk factors for AP are presented in figure 2. There was a similar reduction in risk across all the high-risk populations (gallstone disorders RR 0.25 (0.23, 0.28), chronic alcohol use RR 0.22 (0.18, 0.27) and hypertriglyceridaemia RR 0.17 (0.13, 0.23) with evidence of effect modification such that patients with hypertriglyceridaemia had marginally increased benefit with simvastatin use compared with those with gallstone disorders. Complete effect estimates including all covariables for each of the high-risk subgroups are presented in online supplemental tables.
Figure 2.
Impact of simvastatin use on risk of acute pancreatitis stratified according to individual risk factors. Risk ratios and 95% confidence limits generated by robust Poisson regression adjusted for age, gender, race/ethnicity, smoking and additional listed risk factors for acute pancreatitis (see online supplemental tables).
Simvastatin dose and risk of AP
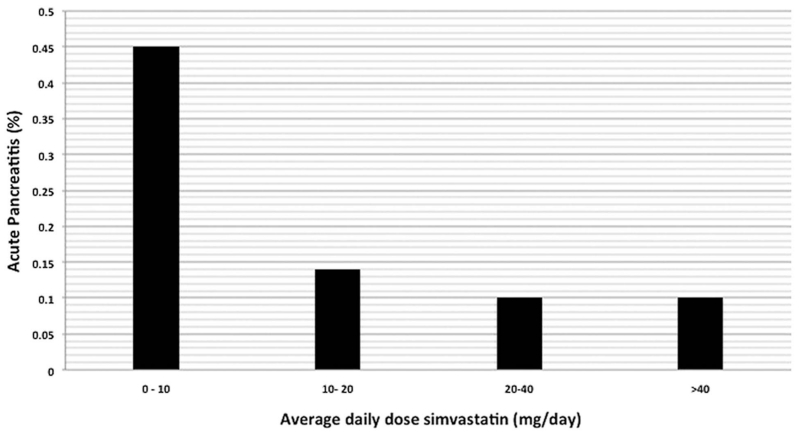
We conducted a subgroup analysis among statin users to identify any potential dose-dependent relationship with respect to risk of AP. Among 707 236 simvastatin users there were 1142 (0.16%) patients that developed AP while on medication. Figure 3 depicts the risk of pancreatitis stratified by average-daily dose of simvastatin. Among simvastatin users, 14.8% received an average daily dose of <10 mg/day, 34.8% received an average daily dose of 10–20 mg/day, 35.3% received an average daily dose of 20–40 mg/day and 15.1% received an average daily dose >40 mg/day. Although overall risk of pancreatitis was less than 1% across all of the strata, there was an inverse relationship between increasing average-daily dose of simvastatin and risk of AP (Cochrane-Armitage trend test, p<0.0001). Of note, there appeared to be a possible threshold effect with average-daily dose levels ≥20 mg having similar levels of risk reduction (figure 2).
Figure 3.
Risk of acute pancreatitis among statin users (n=707 236) stratified by average-daily dose, Cochrane-Armitage trend test, p<0.0001.
Sensitivity analysis
In the present study we defined AP by ICD-9 diagnosis code and serum lipase elevation ≥three times normal. We chose this definition to enhance the specificity of the diagnosis. We repeated the primary study analysis using AP defined only by ICD-9 code irrespective of amylase or lipase values. There were an additional 9286 cases coded as ICD-9 577.0 that did not have serum lipase elevation ≥three times normal. The multivariable analyses including all cases identified by ICD-9 577.0 (n=15 685) are presented in table 4. Simvastatin use was still associated with a significant reduction in risk of AP, adjusted RR 0.69 (0.66, 0.71).
Table 4.
Sensitivity analysis: Poisson regression with acute pancreatitis defined by diagnosis code alone
| Parameter | Comparison | Risk ratio (95% confidence limit) | p Value |
|---|---|---|---|
| Simvastatin use | Yes vs No | 0.69 (0.66, 0.72) | <0.0001 |
| Age, years | 30–39 vs 18–20 | 1.01 (0.95,1.08) | 0.77 |
| 40–49 vs 18–20 | 1.09 (1.03,1.16) | 0.005 | |
| 50–59 vs 18–20 | 1.32 (1.24, 1.40) | <0.0001 | |
| 60–69 vs 18–20 | 1.60 (1.50, 1.72) | <0.0001 | |
| 70+ vs 18–20 | 2.15 (2.01, 2.30) | <0.0001 | |
| Gender | Male vs female | 1.03 (0.99,1.07) | 0.06 |
| Race/ethnicity | |||
| Asian vs white | 0.89 (0.83, 0.95) | 0.0003 | |
| Black vs white | 1.31 (1.24, 1.38) | <0.0001 | |
| Hispanic vs white | 1.09 (1.05, 1.13) | <0.0001 | |
| Other vs white | 0.37 (0.33, 0.40) | <0.0001 | |
| Hypertriglyceridaemia | Yes vs no | 9.64 (8.68, 10.71) | <0.0001 |
| Gallstone disease | Yes vs no | 21.4 (20.7,22.2) | <0.0001 |
| Chronic alcohol use | Yes vs no | 4.85 (4.62, 5.10) | <0.0001 |
| Smoking | Ever vs never | 1.24 (1.20, 1.29) | <0.0001 |
DISCUSSION
We evaluated the relationship between statin use and risk of new onset AP in a large integrated healthcare setting. Our primary analysis focused on simvastatin as previous literature had suggested a potential increased risk of pancreatitis with this agent. Simvastatin was also the most commonly prescribed statin agent within the KPSC health system during the study period. Although there was an increased prevalence of established risk factors for pancreatitis among simvastatin users, we found an overall 38% reduction in the crude incidence of AP among patients taking simvastatin compared with the reference population. In multivariable analysis, after adjusting for the effect of gallstone disorders, chronic alcohol dependence and hypertriglyceridaemia, simvastatin use was associated with marked reduction in risk of developing AP with adjusted RR of 0.29 (70% risk reduction). A similar effect was observed with atorvastatin, adjusted RR 0.33, suggesting a potential class-effect. There was evidence of substantial reduction in risk of pancreatitis among various high-risk populations including those with gallstone disorders, chronic alcohol use or hypertriglyceridaemia. We also identified a potential threshold dose-related effect such that average-daily doses >20 mg/day were associated with a similar level of reduction in risk of AP.
The relationship between simvastatin and AP has been controversial. Previous case reports suggested that several statins might be a cause of AP. A recent literature review identified three case reports of simvastatin-induced AP11; in one case there was documented recurrence of pancreatitis upon medication rechallenge.4 A case potentially linking atorvastatin to AP has also been reported.6 However, as case reports, even with medication rechallenge these studies were limited in their ability to adjust for other causes of AP. In particular, it is conceivable that these patients may have suffered from idiopathic recurrent AP unrelated to use of statins.
More recent data has further called into question whether statins increase the risk of AP. A population-based case-control study from Denmark included 2576 first-time admitted cases of AP and 25, 817 age-matched and gender-matched controls. This study found no association between current statin use and risk of AP (adjusted OR 1.26 (95% CL 0.96, 1.64))12 In addition, a recent meta-analysis of 21 randomised controlled trials evaluating statins and cardiovascular end points (overall 153 414 participants with 465 cases of AP) found an overall reduction in risk of pancreatitis among patients treated with statins compared with placebo (pooled RR 0.79 (95% CL 0.65–0.95), p=0.01).7 While pancreatitis was not the primary outcome for any of the trials included in this meta-analysis, these findings have raised the possibility of a potential protective effect of statins on risk of AP.
In the present study we noted a substantial reduction in risk of pancreatitis with use of either simvastatin or atorvastatin beyond what might have been expected based on these prior studies. There are several explanations for the increased magnitude of effect observed in the present study compared with the aforementioned studies. First, we conducted a longitudinal cohort study and were able to distinguish active exposure to medication based on linkage to electronic pharmacy data. Second, we noted that there was a greater prevalence of established risk factors for AP among simvastatin users. Nevertheless, the incidence of pancreatitis was reduced among simvastatin users. Once we adjusted for the presence of gallstone disorders, chronic alcohol dependence and hypertriglyceridaemia in addition to age-matched and gender, we noted a further reduction in risk of pancreatitis with use of simvastatin.
There were several limitations to the present study. We were unable to determine actual medication intake. Instead, we defined the duration of medication exposure as the time from medication dispensation to 60 days from the time of prescription termination. This definition was chosen to fully account for any residual medications taken after expiration of a filled prescription. In addition, our analyses focused on only the two most commonly prescribed statin agents. It is conceivable that patients receiving treatment with statins could be on a myriad of additional medications that might further modify their risk of developing AP. Cases of AP were identified by ICD-9 code and serum lipase. While this approach was associated with high specificity in our analysis confirmed by high positive predictive value on manual chart review (95%), it is possible that cases were missed due to lack of inclusion of serum amylase or imaging features. We therefore performed a sensitivity analysis broadening the definition of AP to include all patients identified by ICD-9 code 577.0.
Strengths of the present study included use of an integrated data system with linked pharmacy records to establish medication exposure. As a result, we were able to clearly identify episodes of pancreatitis that occurred in the setting of statin use. In addition, we evaluated the relationship between statins and risk of pancreatitis in a longitudinal fashion in a closed network that was able to capture the person-time at risk based on membership status. Further, we combined diagnosis codes and laboratory results to increase the specificity for the diagnosis of AP13
We believe the present study findings are hypothesis generating. Considerations for further research include elucidation of potential mechanisms of action for these agents in relation to the protective machinery of the pancreas. In particular, it will be important to determine whether statins exert their effects on the pancreas via a lipid-mediated or lipid-independent pathway. Statins are known to exert pleiotropic effects with potential anti-inflammatory properties14 Specific anti-inflammatory pathways inhibited by statins in experimental models that may be relevant to AP include blockade of the interleukin 6 mediated Janus kinase/signal transducer (STAT V.3) pathway15 and upregulation of the unfolded protein response helpful in modulating the endoplasmic reticulum stress response16
In addition to further characterising the mechanism of action of statins with respect to pancreatitis, investigation into the role of statins for future therapy in patients may be warranted. Based on the excellent safety profile and substantial preventive effect observed in the present study, secondary prevention trials evaluating a potential role for statin therapy among patients at high risk for developing AP such as those with hereditary or idiopathic recurrent pancreatitis might be considered.
In summary, we have determined that simvastatin and atorvastatin were associated with a substantially reduced risk of AP in this large, population-based study. Further studies are needed to clarify the mechanisms of this effect and whether there exist any potential therapeutic options for these agents in prevention of AP. For the moment, we suggest that AP that occurs in the setting of either simvastatin or atorvastatin should not be attributed to use of these medications.
Supplementary Material
Significance of this study.
What is already known about this subject?
-
▸
Statins are among the most widely prescribed medications worldwide.
-
▸
Case reports suggest that statins may cause acute pancreatitis.
-
▸
The relationship between statins and acute pancreatitis is controversial.
What are the new findings?
-
▸
Simvastatin use was associated with substantially reduced risk of pancreatitis.
-
▸
A similar effect was noted with atorvastatin suggesting a potential class effect.
-
▸
Among simvastatin users, risk of pancreatitis was reduced with increased doses of simvastatin.
How might it impact on clinical practice in the foreseeable future?
-
▸
Acute pancreatitis occurring in the setting of statin use should not be attributed to use of this medication.
-
▸
Further investigation into a potential therapeutic role for these agents in acute pancreatitis should be considered.
Footnotes
Contributors: BUW: study concept and design, data analysis, interpretation of data, manuscript preparation. SP: study concept, data interpretation, critical revision of manuscript, background and mechanistic pathways for simvastatin in acute pancreatitis. I-LAL: data analysis, study design, critical revision of manuscript.
Competing interests None.
Ethics approval Institutional Review Board of Kaiser Permanente Southern California.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplementary Material Supplementary material can be found at: http://gut.bmj.com/content/suppl/2014/04/17/gutjnl-2013-306564.DC1.html
REFERENCES
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–87. e1–3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics . Health US, 2010: in brief. Center for Disease Control and Prevention; Hyattsville, MD: 2011. [Google Scholar]
- 3.Anagnostopoulos GK, Tsiakos S, Margantinis G, et al. Acute pancreatitis due to pravastatin therapy. Jop. 2003;4:129–32. [PubMed] [Google Scholar]
- 4.Pezzilli R, Ceciliato R, Corinaldesi R, et al. Acute pancreatitis due to simvastatin therapy: increased severity after rechallenge. Dig Liver Dis. 2004;36:639–40. doi: 10.1016/j.dld.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.McDonald KB, Garber BG, Perreault MM. Pancreatitis associated with simvastatin plus fenofibrate. Ann Pharmacother. 2002;36:275–9. doi: 10.1345/aph.1A180. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Nautiyal A, Dolan JG. Recurrent acute pancreatitis possibly induced by atorvastatin and rosuvastatin. Is statin induced pancreatitis a class effect? Jop. 2004;5:502–4. [PubMed] [Google Scholar]
- 7.Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308:804–11. doi: 10.1001/jama.2012.8439. [DOI] [PubMed] [Google Scholar]
- 8.Gornik I, Gasparovic V, Gubarev Vrdoljak N, et al. Prior statin therapy is associated with milder course and better outcome in acute pancreatitis—a cohort study. Pancreatology. 2013;13:196–200. doi: 10.1016/j.pan.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol. 2002;97:1309–18. doi: 10.1111/j.1572-0241.2002.05766.x. [DOI] [PubMed] [Google Scholar]
- 11.Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5:648–61. doi: 10.1016/j.cgh.2006.11.023. quiz 4. [DOI] [PubMed] [Google Scholar]
- 12.Thisted H, Jacobsen J, Munk EM, et al. Statins and the risk of acute pancreatitis: a population-based case-control study. Aliment Pharmacol Ther. 2006;23:185–90. doi: 10.1111/j.1365-2036.2006.02728.x. [DOI] [PubMed] [Google Scholar]
- 13.Saligram S, Lo D, Saul M, et al. Analyses of hospital administrative data that use diagnosis codes overestimate the cases of acute pancreatitis. Clin Gastroenterol Hepatol. 2012;10:805–11. e1. doi: 10.1016/j.cgh.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bu DX, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol. 2011;22:165–70. doi: 10.1097/MOL.0b013e3283453e41. [DOI] [PubMed] [Google Scholar]
- 15.Jougasaki M, Ichiki T, Takenoshita Y, et al. Statins suppress interleukin-6-induced monocyte chemo-attractant protein-1 by inhibiting Janus kinase/signal transducers and activators of transcription pathways in human vascular endothelial cells. Br J Pharmacol. 2010;159:1294–303. doi: 10.1111/j.1476-5381.2009.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morck C, Olsen L, Kurth C, et al. Statins inhibit protein lipidation and induce the unfolded protein response in the non-sterol producing nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106:18285–90. doi: 10.1073/pnas.0907117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.