Abstract
Study Objectives:
Insomnia, which is the most common sleep disorder, is a significant public health burden. Growing evidence suggests the existence of a relationship between insomnia and hypertension. The aim of this study was to verify the hypothesis that periodic limb movements in sleep (PLMS) are related to increased nocturnal blood pressure (BP) values in patients with insomnia.
Methods:
We retrospectively analyzed polysomnographic recordings of patients with insomnia who were seen in our clinic from January to December 2012. Patients were divided into two groups based on their nocturnal BP values: group I had normal nocturnal BP values (n = 27) and group II (n = 29) had elevated nocturnal BP values. The sleep architecture of the groups was compared.
Results:
The groups did not differ in terms of age, sex, or the prevalence of cardiovascular disorders. However, we found that the number of PLMS was significantly higher in group II than in group I (PLMS index: 18.8 vs. 6.5; p = 0.01).
Conclusions:
Our results suggest that PLMS are related to increased nocturnal BP values in patients with insomnia, which may partly explain the relationship between insomnia and hypertension. Therefore, it is possible that treatment of PLMS may normalize nocturnal BP in patients with insomnia.
Citation:
Sieminski M, Partinen M. A relationship between periodic limb movements in sleep and high nocturnal blood pressure values in patients with insomnia. J Clin Sleep Med 2016;12(6):865–869.
Keywords: insomnia, periodic limb movements, blood pressure
INTRODUCTION
Insomnia, which is the most common sleep disorder with a prevalence ranging from 3.4% to 19.1%, depending on the diagnostic criteria and population studied,1–4 is a significant public health burden. Moreover, a number of recent studies have found a relationship between insomnia and the prevalence of hypertension.5–7 Specifically, patients with insomnia with short sleep durations or hyperarousal have an increased risk of hypertension, which may be explained by hypercortisolemia or increased sympathetic activity associated with insomnia.8,9
Nocturnal blood pressure (BP) values have gained attention recently because it has been shown that high nocturnal BP values, especially the lack of a day-to-night dip in BP, are related to increased cardiovascular risk.10 Therapeutic interventions targeted toward decreasing nocturnal BP values should improve the cardiovascular risk profile. Thus, all possible factors contributing to the increase in nocturnal BP values need to be identified and then eliminated.
Periodic limb movements in sleep (PLMS) are stereotypical, repetitive movements of the lower limbs that appear during sleep. PLMS consist of dorsiflexion of the toes and ankle accompanied by flexion of the knee and sometimes of the hip. Clinically, PLMS resemble the Babinski sign. PLMS last from 0.5 to 10 s and appear in a series of at least four movements with an interval of 5 to 90 s between movements.11,12 Some of these movements are associated with cortical arousal and are called periodic limb movements with arousal (PLMS-A). Interestingly, studies have shown a higher frequency of PLMS in patients with insomnia.13,14 Furthermore, a significant increase in the BP values following PLMS has been observed in both patients with restless legs syndrome (RLS) and healthy subjects.15–17 However, although relationships between insomnia and hypertension as well as between PLMS and increased nocturnal BP values have been shown, to the best of our knowledge, no studies have analyzed whether the presence of PLMS in patients with insomnia is associated with increased nocturnal BP values in this population. Therefore, the aim of this study was to verify the hypothesis that the numbers of PLMS and PLMS-A, as measured by the periodic leg movements in sleep index (PLMS-I, the number of PLMS per hour of sleep) and periodic leg movements in sleep with arousal index (PLMSA-I, the number of PLMS-A per hour of sleep), respectively, are associated with nocturnal BP values in patients with insomnia.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Epidemiologic data show that insomnia is related to an increased risk of hypertension and polysomnographic data show that periodic limb movements lead to an increase in blood pressure. The purpose of this study was to verify whether periodic limb movements are related to increased nocturnal blood pressure values in patients with insomnia.
Study Impact: Periodic limb movements may be a treatable cause of increased blood pressure in patients with insomnia. Therefore, treatment of periodic limb movements is one possible method by which to decrease blood pressure in patients with insomnia, leading to an improvement in the vascular risk profile of this population.
METHODS
We performed a retrospective analysis of the clinical and polysomnographic data from all patients diagnosed with insomnia at the Vitalmed Helsinki Sleep Clinic between January and December 2012. Patients initially presented to the study with complaints of difficulty in initiating or maintaining sleep or too early awakenings leading to impairment in daytime functioning. Patients were diagnosed with insomnia according to the criteria in the International Classification of Sleep Disorders, 2nd edition.18 Patients suffering from insomnia for at least one year and declaring no more than 7 hours of sleep per night were included in the study. All patients underwent an interview with a sleep specialist. Subjects with symptoms suggesting a different or comorbid sleep disorder, such as restless legs syndrome, violent behavior during sleep, sleepwalking, sleep attacks, and/or excessive daytime sleepiness, were not included in the analysis. Body mass index (BMI), self-reported comorbid conditions (e.g., hypertension), and medication in-take were also collected for each patient. Patients with mental illness were included in the study only when no temporal relationship between the psychiatric symptoms and sleep disorders was found. Patients with an apnea-hypopnea index (AHI) ≥ 5, as determined by PSG, were excluded from the analysis.
Patients underwent PSG for a single night. Patients were free of any therapies that could potentially disrupt sleep architecture. Treatments for other conditions, including drugs for hypertension, were stable for ≥ 2 weeks preceding the PSG. All PSG recordings were performed with the SOMNOscreen plus PSG system (Somnomedics, Randersacker, Germany). Data were collected from 22:00 to 06:00 in all patients. Sleep recordings included 4 electroencephalography leads, 2 bilateral electrooculogram leads, bilateral chin electromyographic leads (EMG), and 2 surface EMG leads placed on the left and right anterior tibialis muscles (to record periodic limb movements during both sleep and wakefulness). Respiration was recorded via a nasal cannula, thoracic and abdominal strains, and finger oximetry. Electrocardiograms were recorded via a single precordial lead.
The PSG recording included beat-to-beat BP measurements that were performed automatically, via measurement of the pulse transit time.19 The BP measurements were collected continuously (beat-to-beat) across the whole recording period from “lights-off” to “lights-on.” These measurements were noninvasive and did not disturb sleep. The average value of all measurements across the recording period is referred to here as the “nocturnal blood pressure,” which includes values of BP recorded during both sleep and wakefulness periods during the night. BP values measured during PSG-defined periods of sleep were also averaged and are referred to here as the “sleep blood pressure.” Prior to the start of the PSG recording, the BP value measured from the PSG system was calibrated against a single traditional manual measurement of BP via a cuff sphygmomanometer.
The PSG recordings were scored according to the American Academy of Sleep Medicine guidelines.20 The following sleep parameters were calculated: total sleep time (TST); sleep efficiency; latency to stages 1, 2, slow wave sleep (SWS), and REM sleep; duration of stages 1, 2, SWS, and REM; sleep stage change index (number of transitions between the sleep stages per hour of sleep); wake index (number of awakenings per hour of sleep); duration of wake after sleep onset (WASO); apnea-hypopnea index (AHI); PLMS index (PLMS-I); and PLMS-A index (PLMSA-I).
After the initial nocturnal BP value analysis, patients were divided into 2 groups: group I contained patients (n = 27) with normal mean nocturnal BP (systolic blood pressure (SBP) < 125 mm Hg and diastolic blood pressure (DBP) < 75 mm Hg, in accordance with the American Society of Hypertension position paper21); group II contained patients (n = 29) with elevated mean nocturnal BP (SBP ≥ 125 mm Hg or DBP ≥ 75 mm Hg). Demographic and clinical data and sleep parameters were then compared between the 2 groups. Statistical comparisons between the groups were performed using χ2 tests (categorical variables) or t-tests (continuous variables). A p value less than 0.05 was considered statistically significant.
The protocol for this study was approved by the Independent Bioethical Committee for Scientific Research at the Medical University of Gdansk.
RESULTS
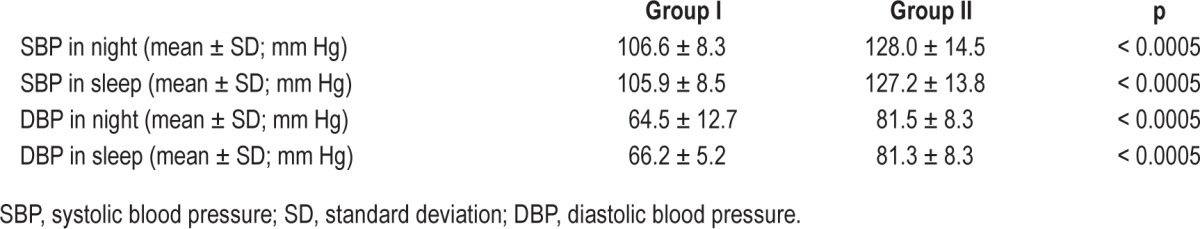
PSG recordings from 56 patients with insomnia were analyzed. Patient demographic and clinical data are presented in Table 1. The groups did not differ significantly in terms of age, sex, or the presence of comorbidities, but differed significantly in terms of nocturnal BP values. Patients in group II exhibited higher nocturnal SBP (nocturnal: 106.6 ± 8.3 mm Hg vs. 128.0 ± 14.5 mm Hg; p < 0.0005; sleep only: 105.9 ± 8.5 mm Hg vs. 127.2 ± 13.8 mm Hg; p < 0.0005) and DBP (nocturnal: 64.5 ± 12.7 mm Hg vs. 81.5 ± 8.3 mm Hg; p < 0.0005; sleep only: 66.2 ± 5.2 mm Hg vs. 81.3 ± 8.3 mm Hg; p < 0.0005) values compared to patients in group I (Table 2).
Table 1.
Demographic and clinical data of all patients included in the study and patients in group I (normal nocturnal blood pressure) and group II (elevated nocturnal blood pressure).
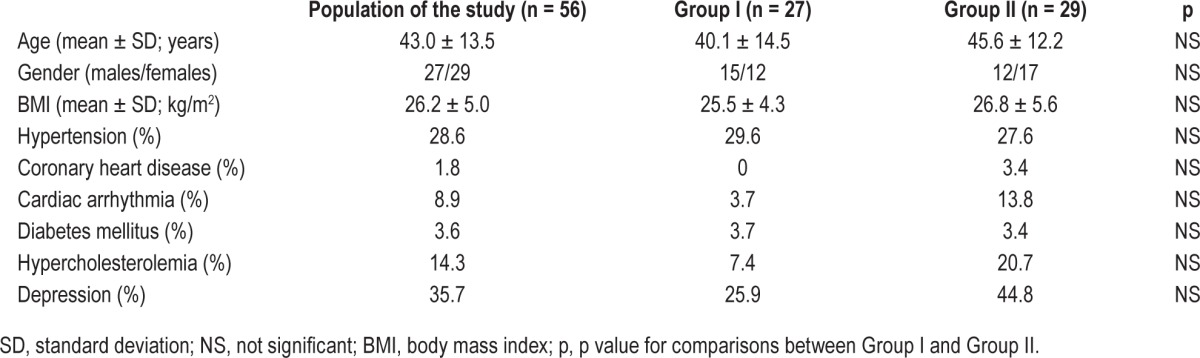
Table 2.
Values of nocturnal blood pressure in groups I and II.
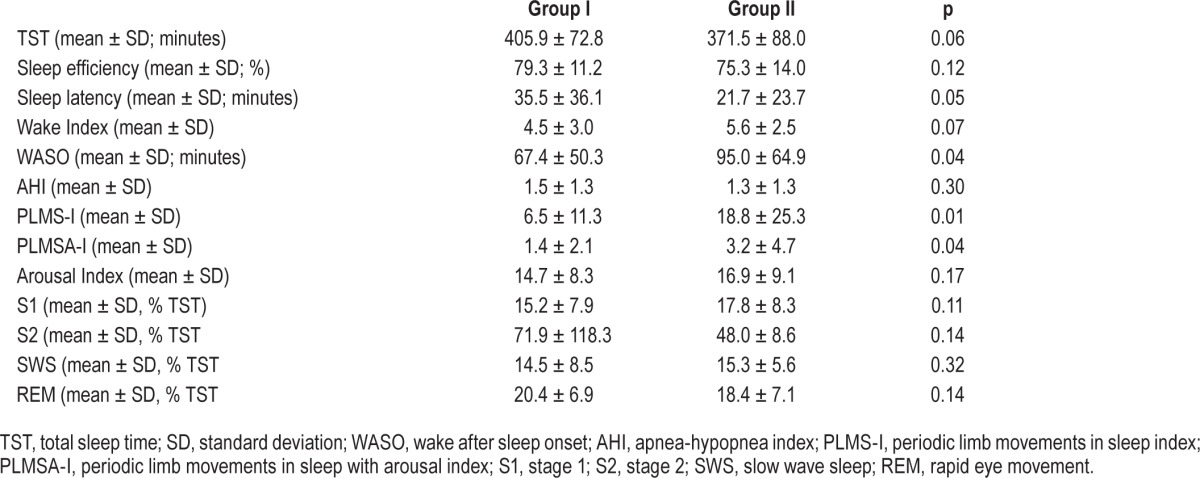
Patients from group II exhibited significantly higher PLMSI (18.8 ± 25.3 vs. 6.5 ± 11.3; p = 0.01) and PLMSA-I (3.2 ± 4.7 vs. 1.4 ± 2.1; p = 0.04) values than did patients in group I. Moreover, patients in group II had more WASO (95.0 ± 64.9 min vs. 67.4 ± 50.3 min; p = 0.04) than patients in group I. No other differences in sleep architecture between the groups were found. Sleep architecture data are presented in Table 3.
Table 3.
Sleep parameter data from patients in groups I and II.
DISCUSSION
In this study, nearly one-third of the patients with insomnia included in our study suffered from hypertension. Furthermore, half of the patients with insomnia exhibited increased nocturnal BP. Patients with high BP values also had more PLMS and PLMS-A. No other differences in sleep architecture between groups was observed except for slightly more WASO in the patients with increased nocturnal BP values. The groups did not differ in terms of age, sex, BMI, or the prevalence of cardiovascular disorders.
Several published studies have found that insomnia is associated with increased risk for hypertension. For instance, Vgontzas et al.7 found that hypertension is more prevalent in patients with insomnia. Specifically, the multivariate logistic regression analysis performed in that study revealed that insomnia was associated with a significantly higher risk for hypertension, with an odds ratio (OR) ranging from 2.41 to 2.76. Moreover, the authors found that insomnia combined with an objective short sleep duration led to a five-fold higher risk for hypertension.7 Fernandez-Mendoza et al.5 performed a prospective study on 786 subjects and found that chronic insomnia was significantly associated with an increased risk of developing hypertension (OR ranging from 2.24 to 2.66). Insomnia combined with short sleep duration was associated with a significantly increased risk of developing hypertension during the follow-up (OR ranging from 3.75 to 4.50).5 Additionally, Li et al.6 showed that insomnia combined with physiological hyperarousal led to a three-to-fourfold increase in the risk of hypertension. Meng et al.,22 after performing a meta-analysis of 11 prospective cohort studies, concluded that short sleep duration and symptoms of insomnia increased the risk of hypertension. Our finding that hypertension was present in 28.6% of patients with insomnia in our cohort is consistent with these studies.
An increased risk of hypertension in patients with insomnia may be related to the higher values of nocturnal BP and a lack of the nocturnal BP dip in patients with insomnia. Lanfranchi et al.23 found that patients with insomnia had significantly higher nocturnal SBP and blunted nocturnal dips in SBP compared to good sleepers. Higher BP was also associated with higher activity in the beta frequency band, which is a neurophysiological feature of cortical activation, on electroencephalographs recorded from patients with insomnia. Interestingly, in that study, only two significant differences in sleep architecture between patients with insomnia and good sleepers were observed: good sleepers had slightly higher PLMS-I values and a higher percentage of REM sleep.23 In our study, we found that half of the patients with insomnia had increased nocturnal BP values, which is in consistent with the observations of Lanfranchi et al.
Several studies have reported relationship between increased nocturnal BP values and altered sleep architecture. For example, Loredo et al.24 found that the percentage of stage 4 sleep was positively correlated with the nocturnal BP dip, whereas the percentage of WASO was negatively correlated with the nocturnal BP dip. The authors concluded that deeper and less fragmented sleep is associated with the nocturnal BP dip.24 Moreover, Pedullà et al.25 found that hypertensive patients with higher nocturnal BP values (i.e., non-dippers) had lower percentages of stage 4 sleep and more microarousals. From these results, the authors concluded that the lack of a nocturnal dip is associated with central sympathetic hyperactivity, which alters sleep.25 Carrington et al.26 also found that repetitive arousals were associated with increases in nocturnal BP. Together these studies suggest that more microarousals are associated with higher (i.e., non-dipped) BP values. Our finding that patients with insomnia with high nocturnal BP values have more PLMS and PLMS-A is consistent with these studies, as PLMS are associated with arousals during sleep.
Our observation that a higher number of PLMS was associated with higher nocturnal BP values is also consistent with the studies that have analyzed the relationship between PLMS and BP. For example, Siddiqui et al.15 found that both PLMS and PLMS-A are followed by a significant increase in SBP and DBP in patients with RLS. In particular, increases in BP were highest after PLMS-A (which are comparable to respiratory-related leg movements).15 Similar results were obtained by Pennestri et al.16 who also observed a significant increase in BP following PLMS and PLMS-A in patients with RLS. In that study, the mean increase in SBP was 25 mm Hg after PLMS-A and 18 mm Hg after PLMS and the mean increase of DBP was 12.6 mm Hg after PLMS-A and 9.0 mm Hg after PLMS.16 A recent study has shown that this increase in SBP and DBP following PLMS and PLMS-A is present not only in patients with RLS, but also in healthy subjects although the magnitude of increase is less in healthy subjects than in patients with RLS.17 These findings suggest that patients with PLMS are exposed to serial, significant increases in BP during the night, which may lead to overall higher nocturnal BP values and blunted nocturnal dipping. In this context, our finding that patients with insomnia with increased nocturnal BP values have higher indices of PLMS and PLMS-A is not surprising. It should be noted, however, that PLMS are not a risk factor for developing insomnia and do not lead to an increase in insomnia severity. Nevertheless, PLMS are present in some patients with insomnia and thus may have an impact on the course of nocturnal sleep. Our results suggest that their presence influences nocturnal fluctuations in BP.
Our study has several limitations. First, the PSG study was conducted in a sleep laboratory; sleeping in such an unnatural environment may alter both sleep and BP. However, laboratory-based PSG recordings allowed us to collect continuous beat-to-beat measurements of BP throughout the night without interfering with the patient's sleep. Thus, we can be confident that our PSG measurements are representative of the natural fluctuations in BP across the night. Second, we did not perform a 24-hour BP measurement. Thus, we were unable to analyze circadian BP patterns, and cannot draw conclusions regarding the relationship between the number of PLMS and the presence of the nocturnal BP dip in patients with insomnia. Third, the sample size in our study was small. However, we used strict criteria to select cases, which eliminated the potential for overlap of other sleep disorders. Thus, our findings are representative of patients with insomnia without comorbid sleep disorders. Although our sample size was sufficient to draw significant conclusions, a larger sample size would strengthen our findings. Fourth, we did not include insomnia scales or sleep diaries in this study. Future studies should include additional clinical data to provide a more complete picture of insomnia. Variables such as severity of insomnia, type of insomnia (i.e., differentiation between patients with difficulty in initiating sleep from patients with difficulty in maintaining sleep) should be considered in conjunction with PSG parameters. Future studies should also consider the biological background of insomnia, e.g., assessment of the patient's hormonal status, to further examine connections between insomnia, sleep architecture and BP.
Our conclusion that patients with insomnia with increased nocturnal BP values have more PLMS adds new evidence in support of the relationship between insomnia and BP. This relationship needs to be precisely determined in future studies, given the prevalence of insomnia and the clinical significance of hypertension. Further studies confirming our results may lead to therapeutic trials that focus on normalizing nocturnal BP via the treatment of PLMS.
DISCLOSURE STATEMENT
This was not an industry supported study. Markku Partinen worked as a Consultant for UCB; received Grant Support from Jazz Pharma; worked as a Speaker for Leiras-Takeda and Orion Pharma; and received investigational drug from Biopharma. None of those activities was related to this article. Mariusz Sieminski has no conflicts of interest to declare. There was no off-label or investigational use of drugs in this study. This work was performed at the Vitalmed Helsinki Sleep Clinic, Helsinki, Finland and at the Department of Adults' Neurology, Medical University of Gdansk, Gdansk, Poland.
ACKNOWLEDGMENTS
The authors thank their co-workers from Vitalmed Helsinki Sleep Clinic for their help in collecting the material.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- BP
blood pressure
- DBP
diastolic blood pressure
- EMG
electromyography
- NS
not significant
- OR
odds ratio
- PLMS
periodic limb movements in sleep
- PLMS-A
periodic limb movements in sleep with arousal
- PLMS-I
periodic limb movements in sleep index
- PLMSA-I
periodic limb movements is sleep with arousal index
- PSG
polysomnography
- REM
rapid eye movements
- RLS
restless legs syndrome
- SBP
systolic blood pressure
- SD
standard deviation
- SWS
slow wave sleep
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Pallesen S, Sivertsen B, Nordhus IH, Bjorvatn B. A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Med. 2014;15:173–9. doi: 10.1016/j.sleep.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Beck F, Richard JB, Leger D. [Insomnia and total sleep time in France: prevalence and associated socio-demographic factors in a general population survey] Rev Neurol (Paris) 2013;169:956–64. doi: 10.1016/j.neurol.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Hermes E, Rosenheck R. Prevalence, pharmacotherapy and clinical correlates of diagnosed insomnia among Veterans Health Administration service users nationally. Sleep Med. 2014;15:508–14. doi: 10.1016/j.sleep.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Benbir G, Demir AU, Aksu M, et al. Prevalence of insomnia and its clinical correlates in a general population in Turkey. Psychiatry Clin Neurosci. 2015;69:543–52. doi: 10.1111/pcn.12252. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–35. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Vgontzas AN, Fernandez-Mendoza J, et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension. 2015;65:644–50. doi: 10.1161/HYPERTENSIONAHA.114.04604. [DOI] [PubMed] [Google Scholar]
- 7.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–9. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Ferri R, Gschliesser V, Frauscher B, Poewe W, Hogl B. Periodic leg movements during sleep and periodic limb movement disorder in patients presenting with unexplained insomnia. Clin Neurophysiol. 2009;120:257–63. doi: 10.1016/j.clinph.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RM, Pollak CP, Weitzman ED. Periodic movements in sleep (nocturnal myoclonus): relation to sleep disorders. Ann Neurol. 1980;8:416–21. doi: 10.1002/ana.410080413. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 17.Pennestri MH, Montplaisir J, Fradette L, Lavigne G, Colombo R, Lanfranchi PA. Blood pressure changes associated with periodic leg movements during sleep in healthy subjects. Sleep Med. 2013;14:555–61. doi: 10.1016/j.sleep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders, 2nd ed: diagnostic and coding manual. [Google Scholar]
- 19.Gesche H, Grosskurth D, Kuchler G, Patzak A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol. 2012;112:309–15. doi: 10.1007/s00421-011-1983-3. [DOI] [PubMed] [Google Scholar]
- 20.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2012. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. www.aasmnet.org. [Google Scholar]
- 21.Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2010;4:56–61. doi: 10.1016/j.jash.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36:985–95. doi: 10.1038/hr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep. 2009;32:760–6. doi: 10.1093/sleep/32.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep. 2004;27:1097–103. doi: 10.1093/sleep/27.6.1097. [DOI] [PubMed] [Google Scholar]
- 25.Pedulla M, Silvestri R, Lasco A, et al. Sleep structure in essential hypertensive patients: differences between dippers and non-dippers. Blood Press. 1995;4:232–7. doi: 10.3109/08037059509077600. [DOI] [PubMed] [Google Scholar]
- 26.Carrington MJ, Trinder J. Blood pressure and heart rate during continuous experimental sleep fragmentation in healthy adults. Sleep. 2008;31:1701–12. doi: 10.1093/sleep/31.12.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]


