Abstract
Study Objectives:
To examine the association between sleep apnea and pregnancy outcomes in a large population-based cohort.
Methods:
Population-based cohort study using linked birth and hospital records was conducted in New South Wales, Australia. Participants were all women who gave birth from 2002 to 2012 (n = 636,227). Sleep apnea in the year before pregnancy or during pregnancy was identified from hospital records. Outcomes of interest were gestational diabetes, pregnancy hypertension, planned delivery, caesarean section, preterm birth, perinatal death, 5-minute Apgar score, admission to neonatal intensive care or special care nursery, and infant size for gestational age. Maternal outcomes were identified using a combination of hospital and birth records. Infant outcomes came from the birth record. Modified Poisson regression models were used to examine associations between sleep apnea and each outcome taking into account maternal age, country of birth, socioeconomic disadvantage, smoking, obesity, parity, pre-existing diabetes and hypertension.
Results:
Sleep apnea was significantly associated with pregnancy hypertension (adjusted RR 1.43; 95% CI 1.18–1.73), planned delivery (1.15; 1.07–1.23), preterm birth (1.50; 1.21–1.84), 5-minute Apgar < 7 (1.60; 1.07–2.38), admission to neonatal intensive care/special care nursery (1.26; 1.11–1.44), large-for-gestational-age infants (1.27; 1.04–1.55) but not with gestational diabetes (1.09; 0.82–1.46), caesarean section (1.06; 0.96–1.17), perinatal death (1.73; 0.92–3.25), or small-for-gestational-age infants (0.81; 0.61–1.08).
Conclusions:
Sleep apnea is associated with higher rates of obstetric complications and intervention, as well as preterm delivery. Future research should examine if these are independent of obstetric history.
Citation:
Bin YS, Cistulli PA, Ford JB. Population-based study of sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med 2016;12(6):871–877.
Keywords: sleep-disordered breathing, pregnancy, gestational diabetes, pregnancy-induced hypertension, caesarean section, premature birth, small for gestational age, perinatal death, record linkage, cohort study
INTRODUCTION
Sleep apnea is characterized by pauses in breathing during sleep causing intermittent blood oxygen desaturation and repeated awakening during the night. Snoring, daytime sleepiness and poor daytime function are the main symptoms. Sleep apnea is found disproportionately in men, those of late middle age, and those who are overweight/obese.1 More recently however, sleep apnea has been observed to occur commonly in pregnant women.2,3 While protected by their relative youth and gender, pregnant women are at increased risk for sleep apnea because of the weight gain and hormonal changes associated with pregnancy.4 Nasal congestion, narrowing of the upper airway, increased tongue size relative to the oral cavity, and enlarged neck circumference during pregnancy are all believed to be contributing factors.5–8
In the general population, frank sleep apnea confers long-term risk for cognitive impairment, hypertension, stroke, and cardiovascular mortality.9–14 In pregnant women, sleep apnea has been linked to gestational diabetes, gestational hypertension, preeclampsia and eclampsia, low birthweight infants, intrauterine growth restriction, and neonatal intensive care unit (NICU) admission.2,3,15,16
BRIEF SUMMARY
Current Knowledge/Study Rationale: The evidence for an impact of sleep apnea on pregnancy is limited by weak study designs with small clinical samples and poor measurement of sleep apnea. The current study aimed to examine if clinically significant sleep apnea is prospectively associated with maternal and infant outcomes in a population-based cohort.
Study Impact: The study provides evidence that sleep apnea increases risks for a number of pregnancy outcomes. The findings, together with the greater literature, suggest a large-scale intervention study is needed to determine if treatment of sleep apnea can fully ameliorate these risks.
Despite a number of independent systematic reviews and meta-analyses,2,3,15,16 conclusions about the impact of sleep apnea on pregnancy are limited because the primary studies have been mainly case-control or cross-sectional in nature. Most involve small clinical samples susceptible to selection bias and varying degrees of control for confounding. There are also a number of perplexing results which might be solved by larger and better-designed studies. For instance, some studies report that sleep apnea is associated with low birth-weight infants, while others report no difference in the mean birthweight of babies born to mothers with and without sleep apnea.2,3 Still others show an association between maternal sleep apnea and NICU admission, but paradoxically, sleep apnea does not appear related to preterm birth or Apgar scores of infant condition soon after birth, which would indicate grounds for admission.3
Further, the majority of previous studies have used self-reported snoring as a measure of sleep-disordered breathing, even though snoring alone is a poor indicator of clinically significant sleep apnea.17 Thus the aim of the current study was to examine the association between sleep apnea and maternal and infant outcomes in a population-based cohort of pregnant women, using a diagnosis of sleep apnea from routinely collected health records.
METHODS
Data Sources
New South Wales (NSW) is the most populous state in Australia and sees approximately 93,000 births each year, equating to over 30% of all births in the country.18 Data for this study came from two sets of routinely collected health data: the NSW Peri-natal Data Collection (birth records)19 and the NSW Admitted Patient Data Collection (hospital records).
The birth records describe all births in NSW ≥ 20 weeks' gestation or ≥ 400 g weight. The birth information is collected by the attending midwife or medical practitioner and includes data on maternal health, pregnancy, labor, delivery, and infant characteristics. The hospital records are a census of discharges, transfers and deaths from NSW public and private hospitals and day procedure centers. Diagnoses and procedures associated with each hospital record are coded by trained medical coders according to the Australian Modification of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10-AM)20 and the Australian Classification of Health Interventions.21 Up to 20 diagnoses and 20 procedures associated with each admission can be coded, in addition to the principal diagnosis or primary procedure that is the reason for admission.
The birth and hospital records were linked by the NSW Centre for Health Record Linkage (http://www.cherel.org.au/). Australia does not have a national system of unique individual identifiers so multiple personal identifiers and probabilistic record linkage is used. Probabilistic record linkage involves assigning weights to pairs of records based on the degree of matching on personal identifiers such as names, birth dates, and addresses. Highly-weighted pairs of records are considered matches and lowly-weighted pairs are considered non-matches. Clerical review is conducted of middle-weighted pairs and the process is repeated until there are fewer than 5/1,000 false positives and fewer than 5/1,000 false negatives when identifiers are available.22 The data custodians then remove identifiers to preserve privacy and provide a unique linkage key to researchers to combine relevant records for study.
Ethics approval for the study came from the NSW Population and Health Services Research Ethics Committee.
Study Population and Study Period
The study population were all women who gave birth from 1st January 2002 to 31st December 2012. This included 636,227 unique women and 1,023,357 babies. We selected only the first birth for each woman, so that she appeared only once in the study population and only the first infant from multiple births were counted in the outcomes. We note that a woman's first birth in the study is not equivalent to a woman's first delivery, as she may have birthed prior to 2002, outside of the period for which data were available. Parity was recorded and adjusted for in the analyses. Thus there were 636,227 women and 636,227 infants in the study population.
The study period included the year before pregnancy and the duration of pregnancy up until delivery. This was calculated by subtracting 365 days from the estimated date of conception for each pregnancy, which in turn was estimated by subtracting gestational age at delivery from the delivery date and adding 14 days.
Hospital records were linked to the birth records and hospital admissions in the study period for each woman and these were used to identify the presence of sleep apnea and other medical conditions.
Sleep Apnea
Sleep apnea was defined using the ICD-10-AM diagnosis code G47.3 “Sleep apnoea” which included subcategories of central and obstructive sleep apneas (G47.3x). Women with a sleep apnea code in hospital records in the year before or during pregnancy were considered to have sleep apnea while women without a sleep apnea code during this period were considered not to have sleep apnea. Previous validation of hospital records against medical record review has shown that conditions identified in this way have a specificity of over 99%.23
Outcomes
Outcomes for this study were gestational diabetes, pregnancy hypertension, planned delivery, caesarean section, preterm birth, 5-minute Apgar, admission to the neonatal intensive care unit (NICU) or special care nursery (SCN), perinatal death, and infant size for gestational age.
Gestational diabetes and pregnancy hypertension (including gestational hypertension, preeclampsia, or eclampsia) were derived from a combination of hospital and birth record data which have been previously validated against medical records.24,25
Information on the type of delivery and infant outcomes came from the birth records.
Planned deliveries and caesarean sections were indicated by check box on the birth record. These outcomes have been shown to correspond well to the maternal medical record.26 Planned delivery refers to births without spontaneous labor, i.e., those requiring induction of labor or caesarean section. Planned delivery indicates that a birth required obstetric intervention and is therefore a marker of pregnancy complications. In this context, it likely represents comorbidities with sufficient severity to prompt intervention.
All of the infant outcomes were from the birth record. Preterm birth before 37 completed weeks of gestation was determined from gestational age on the birth record, which in turn is based on the best clinical estimate of gestational age using a combination of early ultrasound and date of last menstrual period. Apgar score at 5 minutes was dichotomized into those considered normal (≥ 7) and those considered low (< 7).27 Admission to the NICU or the SCN was combined, as changes to data collection over time meant the two could not be considered separately. Perinatal death comprised stillbirths and neonatal death up to 28 days after birth. Small for gestational age (SGA) were infants smaller than the 10th percentile of birth weight for their gestational age and sex, and large for gestational age (LGA) comprised infants larger than 90th percentile of birth weight for gestational age and sex.28,29
Covariates
Information on maternal and pregnancy characteristics came from the birth record. These included maternal age (in years), country of birth (Australia/other), socioeconomic disadvantage (quintiles), smoking during pregnancy (any/none), parity (nulliparous/multiparous), and plurality (singleton/multiple). Socioeconomic disadvantage was defined by the Index of Relative Socio-Economic Disadvantage according to a woman's residential postcode. The index is a standard composite measure created by the Australian Bureau of Statistics for geographical areas and takes into account the proportion of residents who have low income, no educational qualifications, are unemployed or employed in unskilled work, live in overcrowded or low rent housing, who have a disability, poor English, no access to a car, and who are in single parent households.30 Quintiles for socioeconomic disadvantage were calculated based on the entire population of women giving birth in New South Wales.
Information on the maternal conditions of chronic hypertension and pre-existing diabetes were derived from a combination of hospital and birth records which have been previously validated against medical records.24,25 Maternal morbid obesity was identified through hospital admissions records during the study period where an ICD-10-AM code of E65 (localised adiposity) or E66 (obesity) was recorded.
Statistical Analysis
Characteristics of women with and without sleep apnea were compared using χ2 tests. Pregnancy outcomes were compared between women with sleep apnea and those without. Modified Poisson regression with robust error variance was used to estimate relative risks with 95% confidence intervals.31 We chose to analyse the data using modified Poisson regression rather than logistic regression since this approach calculates relative risks directly and these are more easily interpreted than odds ratios. The odds ratios from logistic regression approximate relative risks when the outcome is rare, but outcomes in this study are not uncommon so the relative risks would be overestimated if logistic regression was applied. Poisson regression is usually applied to count data, therefore the method has been “modified” to more correctly estimate the standard errors for binary outcomes.31
The analyses for each outcome were performed with and without adjustment for potential confounders. For the outcome of gestational diabetes, only maternal age, country of birth, socioeconomic disadvantage, smoking, obesity, and parity were included in the adjusted model. The diagnosis of gestational diabetes is only applied to women without pre-existing diabetes, hence it was not possible to control for pre-existing diabetes in the model for gestational diabetes. However, we conducted a sensitivity analysis which excluded women with pre-existing diabetes.
For the outcome of pregnancy hypertension, we controlled for chronic hypertension and pre-existing diabetes in addition to maternal age, country of birth, socioeconomic disadvantage, smoking, obesity, and parity. For all other outcomes, the covariates of maternal age, country of birth, socioeconomic disadvantage, smoking, obesity, parity, chronic hypertension, pregnancy hypertension, pre-existing diabetes, and gestational diabetes were included in the adjusted models.
The models for preterm birth were stratified by delivery onset (spontaneous labor vs. planned delivery) and the models for NICU/SCN admission were stratified by gestational age (preterm vs. term) to explore the reasons for the observed associations.
All analyses were conducted using SAS 9.3 (SAS Institute, NC).
RESULTS
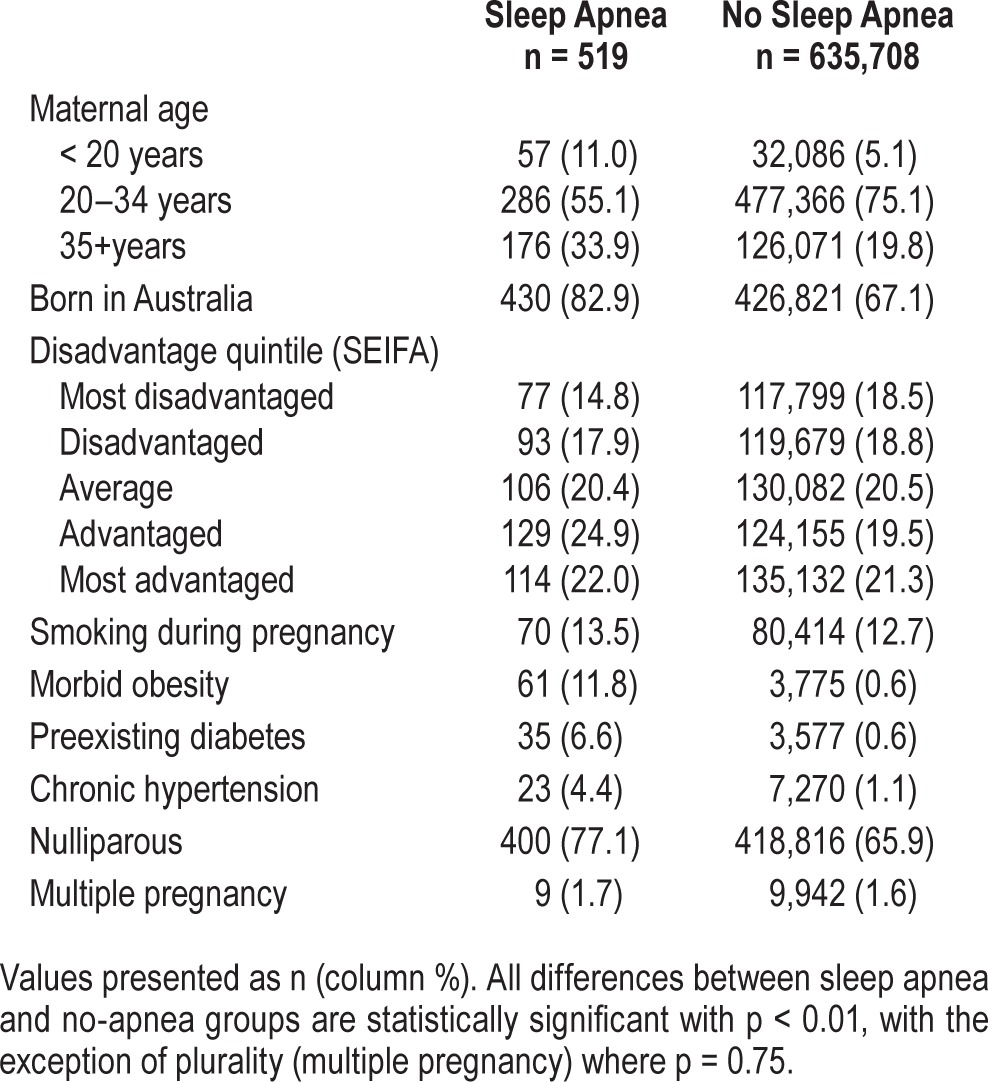
Of the 636,227 women who delivered, 519 (0.08%) had a hospital admission with diagnosis of sleep apnea in the year before or during pregnancy. The characteristics of women with and without a hospital record for sleep apnea are presented in Table 1. Women with sleep apnea were older, more likely Australian-born, and more socioeconomically advantaged than those without sleep apnea. Women with sleep apnea had higher rates of smoking, obesity, pre-existing diabetes, and chronic hypertension than without a diagnosis of sleep apnea. More women with sleep apnea were in their first pregnancy than those without sleep apnea but there was no difference between the two groups on the rate of twin and higher-order pregnancies.
Table 1.
Demographic, health, and pregnancy characteristics of 636,227 pregnant women with and without sleep apnea.
The associations between sleep apnea and the outcomes from the crude and fully adjusted models are presented in Table 2. In the crude models, women with sleep apnea were not more likely to have gestational diabetes than women without sleep apnea, nor were they more likely to have SGA infants. Sleep apnea was significantly associated with the other outcomes, although these associations were attenuated when maternal demographics and health risk factors were included in the models. Sleep apnea remained significantly predictive of pregnancy hyper-tension, planned delivery, preterm birth, low 5-minute Apgar, NICU/SCN admission, and LGA infants. The association between preterm birth and sleep apnea was driven by planned deliveries, while risk of NICU/SCN admissions was significantly increased only in term infants. Perinatal death was a rare outcome and although there was a large elevated risk of perinatal death associated with sleep apnea, this effect did not reach statistical significance in the adjusted model.
Table 2.
Comparison of maternal and infant outcomes in 636,227 pregnant women with and without sleep apnea.
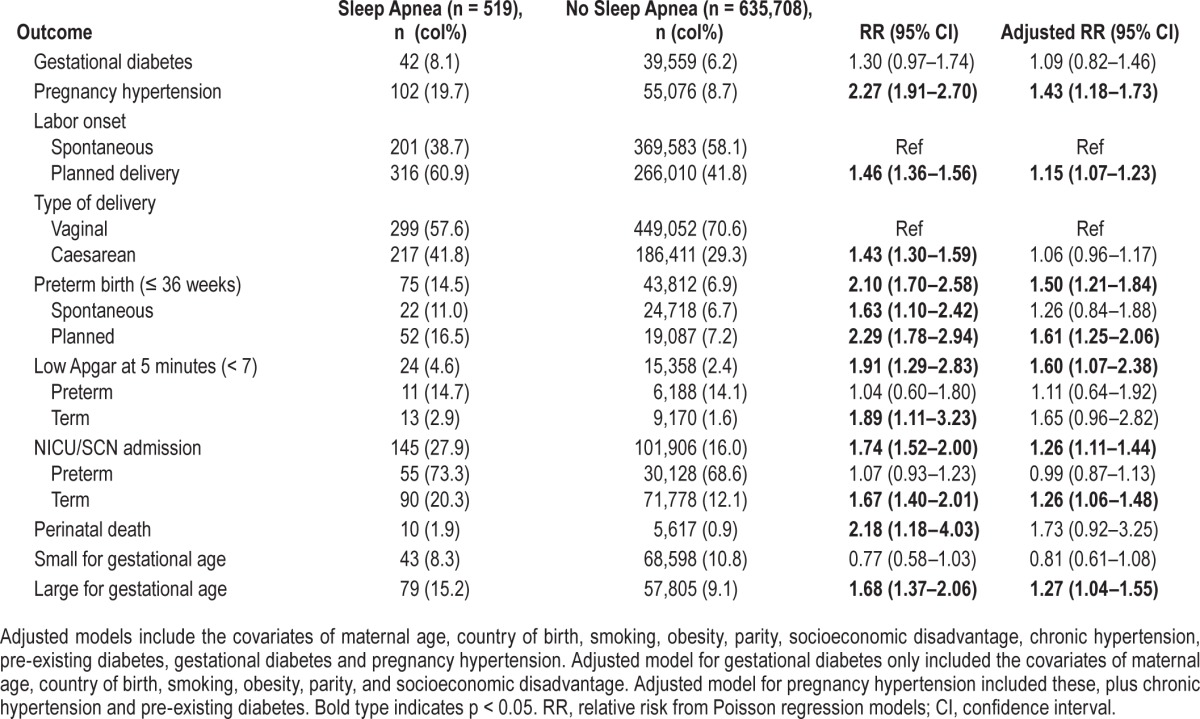
In the sensitivity analysis for gestational diabetes, which excluded women with pre-existing diabetes, the main result was unchanged, i.e. sleep apnea was not significantly associated with risk for gestational diabetes compared to no apnea (RR 1.27; 0.91–1.78).
DISCUSSION
Our study found that a diagnosis of sleep apnea in hospital records in the year before or during pregnancy was associated with adverse pregnancy outcomes in a population-based cohort of women. Women with sleep apnea were more likely to have pregnancy hypertension than women without sleep apnea, but they were not more likely to have gestational diabetes. Sleep apnea was associated with increased risk of planned deliveries and preterm births even after taking into account hypertension, diabetes, and other key risk factors. The infants of women with sleep apnea were more likely to be large for gestational age and to be admitted to the NICU or SCN than those born to women without sleep apnea. However, sleep apnea was not significantly associated with caesarean section, perinatal death, or small-for-gestational-age infants.
Two previous studies have used population health data to examine the association between obstructive sleep apnea (OSA), the most common form of apnea, and pregnancy outcomes: Chen et al. conducted a population-based case-control study using linked health insurance and birth register records in Taiwan,32 and Louis et al. analyzed nationally representative hospital delivery records in the United States.33 These studies and ours are qualitatively different to the studies that have been reviewed in meta-analyses on the topic,2,3,15,16 and hence we will discuss our results with particular reference to these studies.
We found no association between sleep apnea and gestational diabetes, which was surprising given meta-analyses of clinical studies show a two- to three-fold increase in the odds ratio for gestational diabetes associated with sleep-disordered breathing.2,3,15 The two previous population-based studies also reported higher risk of gestational diabetes associated with OSA despite reporting low rates of sleep apnea and controlling only for obesity identified through ICD codes, as in the current study.32,33 It is unclear what might account for this disparity, especially since gestational diabetes was not associated with sleep apnea in our population even before control for confounding. It may be the case that sleep apnea does not contribute independently to risk for gestational diabetes despite the two conditions being commonly comorbid. This would parallel the finding in the general population that sleep apnea does not predict incident diabetes although diabetes does appear to predict subsequent sleep-disordered breathing.34,35 More recent meta-analyses by Ding and colleagues3 and Xu and colleagues16 have found no significant associations between sleep apnea and gestational diabetes in prospective studies despite cross-sectional associations and our findings are consistent with this view.
In contrast, the observed association between sleep apnea and pregnancy hypertension is consistent with both clinical and population-based studies. These have shown that sleep apnea is associated with a greater than two-fold odds ratio for pregnancy-related hypertension, and a roughly two-fold odds ratio for preeclampsia.2,3,32,33 Sleep apnea is similarly predictive of hypertension in the general population11 and treatment of sleep apnea has demonstrated improvements in blood pressure,11,36 which raises the question of whether treatment or prevention of sleep apnea may improve hypertensive disorders in pregnancy. Two small intervention studies have shown that continuous positive airway pressure (CPAP) improves blood pressure in pregnant women with preeclampsia,37,38 although the impact on pregnancy outcomes could not be examined and this will need to be addressed in future research.
A novel finding in the present study was that sleep apnea was not significantly associated with perinatal death, which includes stillbirths and neonatal deaths, although an elevated risk was observed. Clinical studies have been precluded from recruiting mothers with stillbirths due to ethical considerations. However, Louis and colleagues examined the association between OSA and stillbirth in maternal health records and found no significant association.33 That study was able to take into account more maternal comorbidities than the present study, hence the increased risk seen here may be due to residual confounding by maternal cardiovascular, renal, or pulmonary disease. However, that study did not include neonatal deaths, which are included in perinatal mortality here. Given the rarity and gravity of the outcome, the association between sleep apnea and perinatal death warrants further study.
An increase in preterm birth could be one reason for a tendency to more infant deaths. The increased risk for preterm births in mothers with sleep apnea observed in the current study is consistent with previous population and clinical studies.3,32,33 The subgroup analysis showed that the association is mainly driven by planned deliveries, suggesting that sleep apnea is linked to other complications that call for obstetric intervention, but that sleep apnea does not increase the risk of spontaneous preterm delivery. We also found that sleep apnea was associated with a small increase in the risk for planned delivery, although this was not reflected in greater likelihood of caesarean section. This is in contrast to previous US studies which show caesarean section was more likely in mothers who snored39 and those with diagnosed OSA.33
We observed an association between sleep apnea and admission to the NICU/SCN for term but not preterm babies. Pre-term infants may be admitted to the NICU/SCN on the basis of prematurity alone, whilst maternal sleep apnea appears to increase the likelihood of NICU/SCN admission for term infants. In the current study, this is consistent with the tendency to lower Apgar scores for these infants, reflecting poorer condition of the baby soon after birth.
Lastly, we found sleep apnea was associated with infants that were large, rather than small, for gestational age. This was unexpected, as the intermittent hypoxia associated with sleep apnea has been hypothesized to result in smaller infants,40 and in previous studies has been linked to low birthweight2,3 and intrauterine growth restriction.3 However, there are a number of studies that have found no difference in the average weight of infants born to mothers with and without sleep apnea.2,3 We cannot discount the possibility that sleep apnea was associated with larger than expected infants due to residual confounding by obesity since we were only able to account for morbid obesity. It is important to note however that the two previous population-based studies were similarly constrained in their ability to control for obesity but still reported an increased risk for small infants associated with OSA.32,33 These studies did not explore an association with large infants and the results require replication in a cohort able to control for pre-pregnancy BMI.
Strengths and Limitations
Strengths of the present study include the large and population-based sample of mothers and infants, the use of an objective record of sleep apnea as the exposure, and the use of validated information for the outcomes and risk factors. The evidence for a relationship between sleep apnea and maternal outcomes can be considered cross-sectional, while associations between sleep apnea and delivery and infant outcomes are longitudinal, which provides stronger evidence for a causal link between sleep apnea and the outcomes than previous cross-sectionally or retrospectively collected data.
Many previous studies investigating sleep apnea in pregnancy have been limited by selected samples, cross-sectional or case-control study designs. The present study was able to examine outcomes for a population-based cohort of women and their infants, with the advantages of a representative sample and hence greater generalizability, large sample size and the ability to control for multiple risk factors.
Previous studies have relied heavily on self-reported snoring as an indicator of sleep-disordered breathing, although only a small subset of those who snore will have significant sleep apnea.41 The use of objective sleep apnea diagnoses from hospital records improves on previous studies. The hospital records have high specificity (over 99%) for a range of medical conditions when compared to medical record review,23 meaning that the sleep apnea group likely contains true cases of clinically significant sleep-disordered breathing. This is further supported by the fact that 70% of those with sleep apnea had it as the principal diagnosis (and the reason for hospital or clinic admission) and 61% of those with sleep apnea also had a procedure code in the hospital records indicating they underwent a sleep test in the study period.
We included all types of sleep apnea in the current study because the purported effects on pregnancy outcomes derive from symptoms common to both obstructive and central apneas, i.e., pauses in breathing causing hypoxemia. Obstructive sleep apnea accounted for 64.7% of all cases in the current study, 34.0% had sleep apnea that was unspecified, and only 1.2% were coded as having central sleep apnea (see Table S1 in the supplemental material for details).
The rate of sleep apnea determined through hospital records was very low at 0.08%, although studies based on health records in the Taiwan and the United States report similarly low rates (0.03% and 0.01%, respectively).32,33 The hospital data likely underestimates the prevalence of sleep apnea in the study population. We assumed that women without a sleep apnea code in their hospital record did not have sleep apnea. Some of these women may have had sleep apnea that was (i) not recorded on their hospital records or (ii) not recorded because they were not admitted to hospital prior to or during the course of their pregnancy. However, the inclusion of women with unrecognized sleep apnea in the group with “no sleep apnea” should bias the results conservatively towards the null.
We did not have information on the severity of sleep apnea to determine if more severe apnea was correlated with worse pregnancy outcomes. Such information would be important to collect in future studies and would constitute strong evidence for a causal effect of sleep apnea on maternal and infant health.
A related limitation is that we have no information on the treatment of sleep apnea or treatment compliance. It is likely that diagnosed sleep apnea in hospital records would be accompanied by treatment, although we have no way of determining if treatment was provided, or the degree of compliance with treatment. However, treatment and treatment compliance should reduce any association between sleep apnea and pregnancy outcomes resulting in more conservative findings.
We were able to control for a number of major confounding factors, including age, smoking, hypertension, diabetes, and socioeconomic status. Although we attempted to control for the effects of obesity by identifying mothers with a record of morbid obesity (BMI > 30 kg/m2), this was found in < 1% of the sample compared to estimated overweight/obesity rates of 18% to 52% in women of reproductive age from state health surveys.43 Thus we cannot rule out residual confounding by obesity or by unmeasured factors such as obstetric history.
CONCLUSIONS
We found that sleep apnea was associated with pregnancy hypertension, planned delivery, preterm birth, 5-minute Apgar < 7, admission to NICU/SCN, and large-for-gestational-age infants. The results show that women with sleep apnea are at increased risk of adverse outcomes and suggest it may be important to identify and manage sleep apnea in pregnancy.
Future research should investigate if the associations between sleep apnea and pregnancy outcomes are independent of obesity and other obstetric risk factors, and if intervention for sleep apnea can reduce the risk of adverse pregnancy outcomes.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by an Australian National Health and Medical Research Council (NHMRC) Centre for Research Excellence grant (1001066). JBF is supported by an Australian Research Council Future Fellowship (FT120100069). PAC holds an endowed academic chair at the University of Sydney funded by ResMed Inc and has received research and/or equipment support from ResMed Inc, SomnoMed Ltd, Zephyr Sleep Technologies, and Exploramed Inc. PAC has acted as consultant/advisor for Zephr Sleep Technologies, NovoNordisk, and Fisher & Paykel Healthcare. The other authors have no financial conflicts of interest to declare. The funding sources had no involvement in the study design; collection, analysis, and interpretation of the data; or the decision to submit this paper for publication.
ACKNOWLEDGMENTS
The authors thank the New South Wales Ministry of Health for access to the population health data and the Centre for Health Record Linkage (CHeReL) for linkage of the datasets.
ABBREVIATIONS
- CPAP
continuous positive airway pressure
- LGA
large for gestational age
- NICU
neonatal intensive care unit
- NSW
New South Wales
- OSA
obstructive sleep apnea
- SCN
special care nursery
- SGA
small for gestational age
REFERENCES
- 1.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 2.Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;210:52 e1–14. doi: 10.1016/j.ajog.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Ding XX, Wu YL, Xu SJ, et al. A systematic review and quantitative assessment of sleep-disordered breathing during pregnancy and perinatal outcomes. Sleep Breath. 2014;18:703–13. doi: 10.1007/s11325-014-0946-4. [DOI] [PubMed] [Google Scholar]
- 4.Edwards N, Middleton PG, Blyton DM, Sullivan CE. Sleep disordered breathing and pregnancy. Thorax. 2002;57:555–8. doi: 10.1136/thorax.57.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hegewald MJ, Crapo RO. Respiratory physiology in pregnancy. Clin Chest Med. 2011;32:1–13. vii. doi: 10.1016/j.ccm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Izci B, Riha RL, Martin SE, et al. The upper airway in pregnancy and preeclampsia. Am J Respir Crit Care Med. 2003;167:137–40. doi: 10.1164/rccm.200206-590OC. [DOI] [PubMed] [Google Scholar]
- 7.Pilkington S, Carli F, Dakin MJ, et al. Increase in Mallampati score during pregnancy. Br J Anaesth. 1995;74:638–42. doi: 10.1093/bja/74.6.638. [DOI] [PubMed] [Google Scholar]
- 8.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28:1299–305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 9.Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36:203–20. doi: 10.5665/sleep.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017–28. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 11.Duran-Cantolla J, Aizpuru F, Martinez-Null C, Barbe-Illa F. Obstructive sleep apnea/hypopnea and systemic hypertension. Sleep Med Rev. 2009;13:323–31. doi: 10.1016/j.smrv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10:355–62. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendzerska T, Mollayeva T, Gershon AS, Leung RS, Hawker G, Tomlinson G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev. 2014;18:49–59. doi: 10.1016/j.smrv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 15.Luque-Fernandez MA, Bain PA, Gelaye B, Redline S, Williams MA. Sleep-disordered breathing and gestational diabetes mellitus: a meta-analysis of 9,795 participants enrolled in epidemiological observational studies. Diabetes Care. 2013;36:3353–60. doi: 10.2337/dc13-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu T, Feng Y, Peng H, Guo D, Li T. Obstructive sleep apnea and the risk of perinatal outcomes: a meta-analysis of cohort studies. Sci Rep. 2014;4:6982. doi: 10.1038/srep06982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bliwise DL, Nekich JC, Dement WC. Relative validity of self-reported snoring as a symptom of sleep apnea in a sleep clinic population. Chest. 1991;99:600–8. doi: 10.1378/chest.99.3.600. [DOI] [PubMed] [Google Scholar]
- 18.Australian Bureau of Statistics. Births, Australian 2014, Cat. no. 3301.0 2015 [cited 2016 15 April]; Available from: http://www.abs.gov.au/ausstats/abs@.nsf/mf/3301.0.
- 19.NSW Health. NSW Perinatal Data Collection Manual - 2011 Edition. 2010. [cited 2015 1 June]; Available from: http://www0.health.nsw.gov.au/resources/publichealth/mph/pdc_manual_2011.asp.
- 20.National Centre for Classification in Health. Sydney: University of Sydney; 1998. The International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM) [PubMed] [Google Scholar]
- 21.National Centre for Classification in Health. Sydney: University of Sydney; 2010. The Australian Classification of Health Interventions (ACHI) - Seventh Edition. [Google Scholar]
- 22.Centre for Health Record Linkage. Master Linkage Key - Quality Assurance. 2012. [cited 2015 1 June]; Available from: http://www.cherel.org.au/media/24160/qa_report_2012.pdf.
- 23.Hadfield RM, Lain SJ, Cameron CA, Bell J, Morris JM, Roberts CL. The prevalence of maternal medical conditions during pregnancy and a validation of their reporting in hospital discharge data. Aust N Z J Obstet Gynecol. 2008;48:78–82. doi: 10.1111/j.1479-828X.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 24.Roberts CL, Bell JC, Ford JB, Hadfield R, M. MJ. The accuracy of reporting of hypertensive disorders of pregnancy in population health data. Hypertens Pregnancy. 2008;27:285–97. doi: 10.1080/10641950701826695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell JC, Ford JB, Cameron CA, Roberts CL. The accuracy of population health data for monitoring trends and outcomes among women with diabetes in pregnancy. Diabetes Res Clin Pract. 2008;81:105–9. doi: 10.1016/j.diabres.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Roberts CL, Bell JC, Ford JB, Morris JM. Monitoring the quality of maternity care: how well are labour and delivery events reported in population health data? Paediatr Perinat Epidemiol. 2009;23:144–52. doi: 10.1111/j.1365-3016.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- 27.Lain SJ, Algert CS, Nassar N, Bowen JR, Roberts CL. Incidence of severe adverse neonatal outcomes: use of a composite indicator in a population cohort. Matern Child Health J. 2012;16:600–8. doi: 10.1007/s10995-011-0797-6. [DOI] [PubMed] [Google Scholar]
- 28.Roberts CL, Lancaster PA. Australian national birthweight percentiles by gestational age. Med J Aust. 1999;170:114–8. doi: 10.5694/j.1326-5377.1999.tb127678.x. [DOI] [PubMed] [Google Scholar]
- 29.Roberts CL, Lancaster PA. National birthweight percentiles by gestational age for twins born in Australia. J Paediatr Child Health. 1999;35:278–82. doi: 10.1046/j.1440-1754.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- 30.Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, (cat. no. 2033.0.55.001) 2008. [cited 2015 29 June]; Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/2033.0.55.001Main+Features12006?OpenDocument.
- 31.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 32.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206:136, e1–5. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998-2009. Sleep. 2014;37:843–9. doi: 10.5665/sleep.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 35.Barone MT, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91:129–37. doi: 10.1016/j.diabres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Iftikhar IH, Hays ER, Iverson MA, Magalang UJ, Maas AK. Effect of oral appliances on blood pressure in obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2013;9:165–74. doi: 10.5664/jcsm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162:252–7. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 38.Poyares D, Guilleminault C, Hachul H, et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007;9:15–21. doi: 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien LM, Bullough AS, Owusu JT, et al. Snoring during pregnancy and delivery outcomes: a cohort study. Sleep. 2013;36:1625–32. doi: 10.5665/sleep.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med. 2010;16:574–82. doi: 10.1097/MCP.0b013e32833f0d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wheaton AG, Perry GS, Chapman DP, Croft JB. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005-2008. Sleep. 2012;35:461–7. doi: 10.5665/sleep.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medicare Australia. Medicare Item Reports. 2015 25 June 2015 [cited 2015 25 June]; Available from: http://medicarestatistics.humanservices.gov.au/statistics/mbs_item.jsp.
- 43.Australian Bureau of Statistics. 4364.0.55.003 - Australian Health Survey: Updated Results, 2011-2012 2013 7 June 2013 [cited 2015 25 June]; Available from: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/33C64022ABB5ECD5CA257B8200179437?opendocument.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


